Abstract
Background and Objective
Following stroke, subjects retain the ability to adapt interlimb symmetry on the split-belt treadmill. Critical to advancing our understanding of locomotor adaptation and its usefulness in rehabilitation is discerning whether adaptive effects observed on a treadmill transfer to walking over ground. We examined whether aftereffects following split-belt treadmill adaptation transfer to overground walking in healthy persons and those poststroke.
Methods
Eleven poststroke and 11 age-matched and gender-matched healthy subjects walked over ground before and after walking on a split-belt treadmill. Adaptation and aftereffects in step length and double support time were calculated.
Results
Both groups demonstrated partial transfer of the aftereffects observed on the treadmill (P < .001) to overground walking (P < .05), but the transfer was more robust in the subjects poststroke (P < .05). The subjects with baseline asymmetry after stroke improved in asymmetry of step length and double limb support (P = .06).
Conclusions
The partial transfer of aftereffects to overground walking suggests that some shared neural circuits that control locomotion for different environmental contexts are adapted during split-belt treadmill walking. The larger adaptation transfer from the treadmill to overground walking in the stroke survivors may be due to difficulty adjusting their walking pattern to changing environmental demands. Such difficulties with context switching have been considered detrimental to function poststroke. However, we propose that the persistence of improved symmetry when changing context to overground walking could be used to advantage in poststroke rehabilitation.
Keywords: Stroke, Locomotion, Rehabilitation, Adaptation
Humans are remarkably adept at modifying their walking pattern to accommodate changing task demands. For example, when walking on a split-belt treadmill with the belts moving at 2 different speeds, subjects adjust the spatial and temporal relationships of their legs through trial-and-error practice to reestablish symmetry of parameters such as step length and limb phasing.1-3 In healthy subjects this adaptation induces an aftereffect, resulting in walking asymmetry when the belt speeds are returned to normal.1,2 A similar adaptive phenomenon occurs when wearing a robotic gait orthosis that provides viscous resistance to the leg. Subjects adapt their walking kinematics to reestablish a normal walking pattern in the presence of the resistance and then demonstrate aftereffects when the resistance is removed.4
Recent evidence suggests that such locomotor adaptation is influenced by damage to the cerebellum, but not by damage to cerebral structures.3,5,6 Locomotor adaptation on a split-belt or circular treadmill is impaired in humans with damage to the cerebellum.5,6 In cats, nitric oxide deprivation, which is thought to play a role in long-term depression in the cerebellum, abolishes locomotor adaptive capacity.7 In contrast, following cerebral damage due to stroke, subjects retain the ability to adapt interlimb symmetry on the split-belt treadmill.3 After only 15 minutes of split-belt treadmill walking, stroke subjects demonstrate aftereffects in double support and step length that improve the symmetry of these variables. These findings have led to the suggestion that exploiting adaptive mechanisms may have potential as a rehabilitative strategy after damage to cerebral structures poststroke.
Critical to advancing our understanding of locomotor adaptation and its usefulness in rehabilitation is discerning whether adaptive effects observed on a treadmill transfer to walking over ground. Previous studies have shown that many factors can influence the transfer of an adaptation, such as the overlap of body parts used during adaptation, the similarity of the movements, and the implicit or explicit nature of the adaptation.1,8-11 Generally, it is thought that if overlapping or shared neural circuits are adapted during the movement, then training in one context should cause aftereffects in both contexts.10,11
To what extent are shared neural circuits involved in treadmill and overground walking? Recent evidence indicates that when the direction of walking is the same in the 2 conditions, similar neural networks may be involved.1 This would suggest that as long as walking occurs in the same direction on the treadmill and over ground, overlapping neural circuits may be involved and transfer from the treadmill to over ground would be robust. However, the context in which walking occurs is quite different between the treadmill and overground conditions. When on the treadmill, subjects do not move through space as during overground walking. They may also hold on to a static safety railing and wear a safety harness. These contextual differences include sensory information, such as the lack of optic flow during treadmill walking. It is possible that these identifiable differences in task context would be enough to limit transfer of adaptation from the treadmill to a more natural overground walking environment.
The purpose of this study was to determine whether aftereffects following a split-belt treadmill adaptation will transfer to overground walking in healthy persons and those poststroke. Such knowledge is important for the application of locomotor adaptation to the rehabilitation training of individuals following stroke. We hypothesized that both groups would transfer an adapted walking pattern from the split-belt treadmill to overground walking. Preliminary findings were published in abstract form.12
Methods
Subjects
Eleven individuals who had sustained a single, unilateral stroke more than 6 months prior to the study (2 women and 9 men) and 11 age-matched and gender-matched healthy control subjects were recruited to participate in the study (Table 1). Ages of stroke subjects varied between 35 and 70 years, with a mean age of 55 years. The distribution of lesion location was roughly equal between hemispheres among subjects (6 left and 5 right; Table 1). All subjects gave their informed consent prior to participating, and a human subjects committee approved the study. Subjects were excluded if they had other neurologic conditions, orthopedic conditions affecting the legs or back, uncontrolled hypertension, pacemaker or automatic defibrillator, active cancer, radiological and/or physical examination evidence of damage to the cerebellum, or were unable to complete the task. Subjects who customarily wear an ankle–foot orthosis were allowed to wear it during testing.
Table 1. Stroke Subject Characteristics.
| Subject | Age (Years) | Lesion Location | LE Fugl-Meyer Score | Asymmetric Step Length (S), Double Support (DS) | Fast Overground Walking Speed (m/s) | AFO | Monofilament Thresholda | Time Since Stroke |
|---|---|---|---|---|---|---|---|---|
| S1 | 35 | Right parietal hemorrhagic | 33/34 | 1.58 | No | >6.65 | 10 months | |
| S2 | 54 | Right hemisphere | 31/34 | 1 | No | 3.61 | 24 months | |
| S3 | 47 | Left hemisphere, hemorrhagic | 15/34 | S | 0.819 | Yes | 4.56 | 128 months |
| S4 | 49 | Left basal ganglia hemorrhagic | 21/34 | S, DS | 0.84 | No | >6.65 | 49 months |
| S5 | 59 | Left hemisphere | 25/34 | 1.12 | No | 4.31 | 79 months | |
| S6 | 62 | Left caudate head, ant. limb internal capsule | 28/34 | S | 0.66 | Yes | 3.61 | 20 months |
| S7 | 61 | Left hemisphere | 26/34 | 0.99 | No | 4.31 | ||
| S8 | 70 | Right parietal hemorrhagic | 32/34 | DS | 1.51 | No | 4.31 | 12 months |
| S9 | 57 | Left posterior temporoparietal | 22/34 | S, DS | 1.32 | No | 3.61 | 35 months |
| S10 | 58 | Right hemisphere | 26/34 | 1.55 | No | 6.65 | 8 months | |
| S11 | 52 | Right hemisphere | 21/34 | 1.4 | No | 2.83 | 81 months |
Abbreviations: LE, lower extremity; AFO, ankle–foot orthosis.
Normal = 3.61 g.
Clinical Examination
All hemiparetic subjects underwent a clinical examination including measurement of lower extremity performance on the Fugl-Meyer test, fast walking speed, cutaneous pressure sensitivity, and proprioception. The lower extremity section of the Fugl-Meyer test assesses the coordination, reflexes, and the ability to move in and out of synergy.13 Fast walking speed was measured as the average of 3 trials along a 9-m walkway. Pressure sensitivity of the great toe was tested using graded monofilaments. The lowest gram filament that could correctly be detected on 4 out of 5 trials was recorded. Proprioception was tested at the great toe, ankle, knee, and hip by moving the joint approximately 10° and asking the subject to determine the direction of movement. The number of correct responses out of 5 trials was recorded, and the percentage of trials correct at the great toe was used for subsequent analysis.
Testing Paradigm
The testing paradigm consisted of both treadmill and overground walking. For the treadmill portion, subjects were asked to walk on a custom-built treadmill (Woodway USA, Waukesha, WI) consisting of 2 separate belts, each with its own motor that permitted the speed of each belt (ie, each leg) to be controlled independently. The speed of the belts was unique for each subject and was determined using a subject's overground fast walking speed. The “slow” speed was calculated by dividing the patient's overground fast walking speed in half, and the “fast” speed equaled the overground fast walking speed. During different testing periods, subjects walked on the treadmill with the 2 belts either moving at the same speed (“tied” configuration) or at different speeds (“split-belt” configuration). During the tied configuration, treadmill belt speeds were set at the subject's predetermined slow speed. In the split-belt configuration, one treadmill belt was set at the subject's slow speed whereas the other was set at the fast speed. The leg assigned to the fast belt in stroke subjects was determined by visual analysis of step length asymmetry during overground walking. Based on our previous study, we know that to improve symmetry in subjects with asymmetric steps, we need to train the leg with the shortest step length on the fast belt.3 Therefore, in subjects with step length asymmetry, the leg with the shortest step length was assigned to the fast belt. In subjects with no apparent step length asymmetry, the nonparetic leg was assigned to the fast belt. Matched controls were randomly assigned to either their right or left leg on the fast belt.
All subjects participated in 1 testing session consisting of 6 testing periods. In the “Overground Baseline period,” subjects walked over ground at their self-selected gait speed for 5 to 10 trials depending on tolerance. One trial equaled 1 pass down a 9-m walkway. In the “Treadmill Baseline period,” subjects walked on the treadmill with the belts tied at their slow speed for 2 minutes. In the “Adaptation period,” the treadmill belts were split (one belt fast and the other belt slow) for 15 minutes. After 10 minutes of Adaptation, the belts were briefly (1 minute) returned to the tied slow configuration (“Catch Trial”). Following this, the belts were split for another 5 minutes to complete the total 15 minutes of Adaptation. In the “Overground Postadaptation period,” all subjects walked over ground for 10 trials. Figure 1A illustrates this experimental paradigm. The treadmill belts were stopped between each period. Subjects were given rest breaks every 5 minutes during the adaptation periods, or more frequently as requested. One of the stroke survivors was only able to complete 10 total minutes of Adaptation due to fatigue.
Figure 1. Illustration of the Paradigm, Marker Locations, and Parameter Calculations.
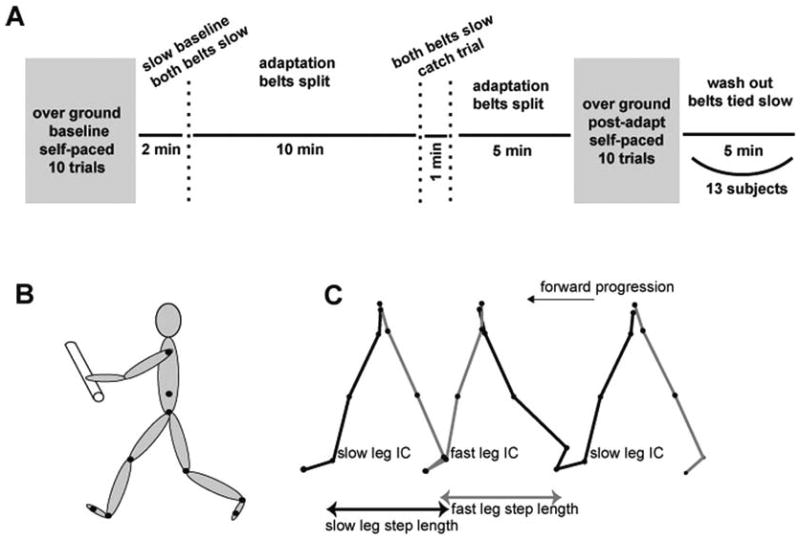
Note: A, Time course for the experimental paradigm showing Baseline, Adaptation, and Postadaptation periods in overground and treadmill walking. B, Illustration of marker locations. C, Illustration of parameter calculations. Step length depicted in overground walking with forward progression. IC indicates initial contact.
Four subjects with hemiparesis and 9 control subjects completed an extra period of treadmill walking at the end of the paradigm described above. These subjects walked on the treadmill in the belts-tied configuration for 5 minutes following the final trial of overground walking (“Washout period”). This allowed us to test for washout of the treadmill aftereffect due to overground walking.
Prior to data collection, subjects walked on the treadmill briefly in the tied condition at their slow speed. They were not given any practice in the split-belt configuration, although they were told that the 2 belts would move at 2 different speeds at some point during the testing. For safety purposes while walking on the treadmill, all subjects held onto a front handrail and wore a ceiling-mounted safety harness around the upper chest. The harness did not support body weight or interfere with subjects' walking. During overground walking, subjects were guarded closely by an experimenter. Subjects were transported in a wheelchair between the treadmill and overground runway so that no walking other than that collected by the motion capture system occurred between the treadmill and overground periods.
During testing, subjects were alerted when the treadmill was going to start, but they were not informed about belt speeds or coupling. Subjects were instructed to look straight ahead and refrain from looking down at the belts while walking to minimize the effect of visual information about belt speeds. An examiner stood by to monitor compliance with this instruction.
Data Collection
Computerized gait analysis was performed using OPTOTRAK (Northern Digital, Waterloo, Ontario, Canada) sensors that were used to record 3-dimensional position data from both sides of the body (Figure 1B). Infrared emitting diodes were placed bilaterally (Figure 1B) on the foot (fifth metatarsal head), ankle (lateral malleolus), knee (lateral joint space), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process). Foot contacts were determined using 4 contact switches per foot: 2 placed on the forefoot and 2 on the heel. Voltages reflecting treadmill belt speeds were recorded directly from treadmill motor output. Marker position and analog data (foot switches and treadmill speed) were synchronized and sampled simultaneously using OPTOTRAK software at 100 and 1000 Hz, respectively.
Data Analysis
Three-dimensional marker position data were low-pass filtered at 6 Hz. Custom software written in MATLAB (MathWorks Inc, Natick, MA) was used for all subsequent analyses. Based on our previous work, we measured spatial and temporal walking parameters that were expected to change more gradually using adaptive mechanisms.2 These adaptive parameters were step length and the percentage time in double support (Figure 1C). These measures were calculated for both limbs during all testing periods. Step length was calculated as the anterior–posterior distance between the ankle markers at the time when each foot contacted the ground (Figure 1C). The percentage time in double limb support was the time that both feet were in contact with the floor expressed as a percentage of the stride time for each leg.
To determine the transfer of aftereffects observed on the treadmill to overground walking we calculated a Transfer Index:
where Oafter is the mean of the first 5 strides in the Overground Postadaptation period, Obase is the mean of the first 5 strides in the Overground Baseline period, TMcatch is the mean of the first 5 strides during the Catch Trial, and TMbase is the mean of the first 5 strides in the Treadmill Baseline period.
To determine the extent to which walking over ground washed out aftereffects on the treadmill, we calculated a washout percentage:
where TMcatch is the mean of the first 5 strides during the Catch Trial, TMwash is the mean of the first 5 strides in the Washout period, and TMbase is the mean of the first 5 strides in the Treadmill Baseline period.
Baseline asymmetry in the group of stroke survivors was determined individually for each subject. A stroke survivor was deemed asymmetric if the average of 5 strides in the Treadmill Baseline period exceeded 2 standard deviations of the mean asymmetry in the healthy control subjects during the same period. These calculations were completed separately for double support and step length.
To compare results across walking periods and between groups, we used repeated-measures analysis of variance with a between-subjects factor of group (control and stroke) and a within-subjects (repeated measures) factor of testing period (Treadmill or Overground Baseline, Adaptation, Catch Trial, and Overground Postadaptation). Statistical analyses were completed using the averages of the first 5 strides (after the treadmill reaches steady state speed) in the Baseline period, the Adaptation period, and the Catch Trial and Washout periods. For the overground walking periods, averages over the first 4 strides in the Overground Baseline and Overground Postadaptation periods were used. This represents the number of strides collected on the first 2 passes down the walkway over ground. Separate analyses were completed for dependent variables of step length and time in double support. When the analysis of variance yielded significant results, post hoc analyses were completed using Tukey's honest significant difference test.
Due to the small sample size, nonparametric statistics were used to compare differences between groups for the Washout percentage. They were also used to compare differences between the Overground Baseline and Overground Postadaptation periods in the group of stroke subjects with baseline step length or double support asymmetry.
Spearman R correlations were performed to test for relationships between ordinal clinical measures (eg, lower extremity Fugl-Meyer score, proprioception, and sensation) and the magnitude of the step length and double support transfer index. Pearson product moment correlations were performed to test for relationships between fast walking speed and the magnitude of the step length and double support transfer index. The level of statistical significance for all measures was set at P < .05, and all statistical calculations were completed using Statistica (StatSoft, Tulsa, OK) software.
Results
For all results, we refer to the leg on the slower versus faster moving belt during the split-belt portion of the paradigm as the “slow” or “fast” leg, respectively. Consistent with our previous work, all stroke survivors could adapt to walking on the split-belt treadmill and showed aftereffects on the treadmill during the Catch Trial period.3
Transfer of Interlimb Coordination Adaptation to Overground Walking
Figure 2A and B shows examples of 2 parameters that adapt over the course of split-belt walking. Early in split-belt adaptation, the step length parameter and the double support parameter are asymmetric but then adapt to symmetry (ie, zero) over many steps (Figure 2A and B). These example subjects also illustrate the reverse asymmetry (ie, aftereffect) when they returned to tied belts in the Catch Trial period. There was typically a slightly smaller aftereffect during the Overground Postadaptation period. Figure 2C and D shows group data for these 2 parameters. For both parameters, there was a significant effect of testing period (P < .0001) but no group effect. In other words, the stroke group did not differ in adaptive ability compared with the control group. Post hoc tests showed significant changes from Treadmill Baseline to the Adaptation and Catch Trial periods (all P < .001), indicating that the subjects stored an aftereffect when walking on the treadmill. Importantly, there was also a change from Overground Baseline to Overground Postadaptation (all P < .05), which indicates a transfer of the aftereffect to overground walking when examining differences between testing periods across groups.
Figure 2. Adapted Parameters.

Note: A, B, Step length (A) and double support time (B) values for sequential strides over ground and on the treadmill from a typical control (top row) and matched stroke (bottom 2 rows) subject across all testing periods. Filled black circles indicate strides over ground, and filled gray circles indicate strides on the treadmill representing the difference between the legs (fast leg minus slow leg) in step length and double support time values. C, D, Average step length (C) and double support time (D) differences for the stroke subjects over ground (open circles) and on the treadmill (open triangles) and for the control group over ground and on the treadmill (filled circles and triangles, respectively). Each data point represents values averaged over the first 5 strides from the early or late portions of each testing period used in statistical analysis for each control and stroke subject individually and then averaged across all subjects in a group. Error bars indicate ±1 standard error. Asterisk indicates a significant difference between points. E, F, Step length (E) and double support time (F) differences for individual stroke subjects over ground during the Overground Baseline and Postadaptation periods. Each data point represents values averaged over the first 4 strides from each testing period for an individual stroke subject.
To give some sense of the variability and decay of the overground aftereffect, we calculated initial and final recorded step length difference. The initial step length difference over ground was 0.05 ± 0.01 and 0.02 ± 0.02 m for the stroke and control group, respectively. By their last recorded stride (∼25th actual stride), the average step length difference was 0.004 ± 0.02 and 0.01 ± 0.01 m for the stroke and control group, respectively. When compared with the baseline step length difference over ground (−0.01 ± 0.02 and 0.002 ± 0.02, stroke and control, respectively), it seems that by the 25th stride both groups are essentially back to baseline step length difference.
Group Differences in Adaptation Transfer and Washout
We compared whether the 2 groups differed in transfer of aftereffects from treadmill to overground walking. Figure 3A shows that there was indeed a significant difference between groups in the Transfer Index for double support, with the subjects poststroke showing larger overground aftereffects (P < .05). A trend toward a similar difference was found for step length (P = .07). Thus, stroke survivors appeared to transfer the adapted pattern to a greater extent from the treadmill to overground walking compared with controls.
Figure 3. Transfer Index and Washout.
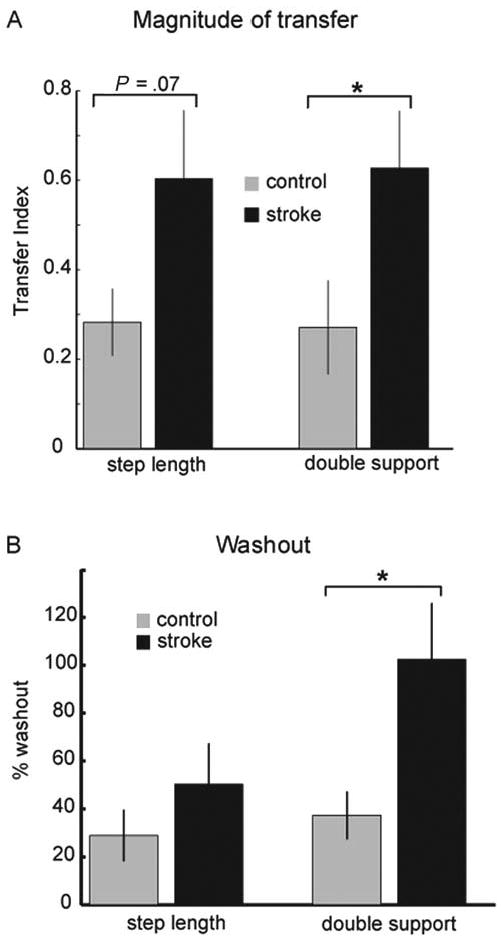
Note: A, Transfer index for control subjects (gray bars) and stroke survivors (black bars) for step length (leftmost 2 bars) and double support. The transfer index indicates the amount of adaptation transfer from the treadmill to overground walking in each group. For both adapted parameters, this value is greater in the stroke survivors. Error bars indicate ±1 standard error. Asterisk indicates a significant difference between groups. B, Percentage washout of the treadmill aftereffect caused by overground walking for the control subjects (gray bars) and the stroke survivors (black bars) for step length (2 leftmost bars) and double support. Data for this figure are from a subset of 4 stroke survivors and 9 control subjects who participated in this portion of the study. Error bars indicate ±1 standard error. Asterisk indicates a significant difference between groups.
We then tested whether walking over ground washed out what was learned on the treadmill. If so, it would suggest that subjects were using the same adapted neural circuitry for controlling walking on the treadmill and over ground. We found that the Washout period aftereffect was indeed smaller than that measured during the Catch Trial (P < .01 and P = .001 for step length and double support, respectively). Figure 3B shows the stroke group washed out more of the adapted pattern than the control group; this difference was significant for the double support parameter (P < .05).
Changes in Asymmetry
Some of our stroke subjects showed asymmetries in step length and/or double support times during baseline walking. We have previously shown that we can improve asymmetries after adaptation on the split-belt treadmill.3 Here we tested whether those improvements would transfer to overground walking.
People with hemiparesis can show step length asymmetries in either direction.14-16 To reestablish symmetric walking, the training pattern (ie, which leg is on the fast belt during Adaptation) must therefore be based on the subject's initial asymmetry.3 Thus, subjects who take a longer paretic step during Baseline are trained with the paretic leg on the slow belt to induce an aftereffect that leads to greater symmetry, and vice versa. In contrast, when double support time is asymmetric, it is generally due to longer double support period at the end of hemiparetic stance. Table 1 shows that 4 subjects met our criteria for asymmetry of step length at baseline: 2 with a longer paretic step and 2 with a longer nonparetic step. Three subjects were found to have baseline double support asymmetries, all with more time spent at the end of paretic leg stance.
Figure 4A illustrates an example of the type of improvement we see in step length asymmetry for a stroke subject who, at baseline, takes a shorter step with his nonparetic leg. By purposely increasing this asymmetry through further lengthening of the paretic step length and shortening of the nonparetic step length during the early Adaptation period, we present a situation that drives adaptation of motor commands to ultimately reduce the asymmetry. Thus, in the Overground Postadaptation or Catch Trial period we see aftereffects that result in symmetrical step lengths on the 2 legs. Importantly, this was true for overground walking, as well as walking on the treadmill. Results of the nonparametric testing revealed there was a strong trend (P = .06) toward a difference in asymmetry in the Overground Baseline period compared with the Overground Postadaptation period and in the Treadmill Baseline period compared with Catch Trial. Figure 4B and C show that as a group the asymmetric stroke survivors became more symmetric after adaptation. Figure 4D and E presents the magnitude of overground aftereffects (aftereffect minus baseline) separately for the group of asymmetric and symmetric stroke survivors. There were no significant differences between these subgroups in the magnitude of the overground aftereffects for either step length (Figure 4D) or double support (Figure 4E).
Figure 4. Changes in Asymmetry.
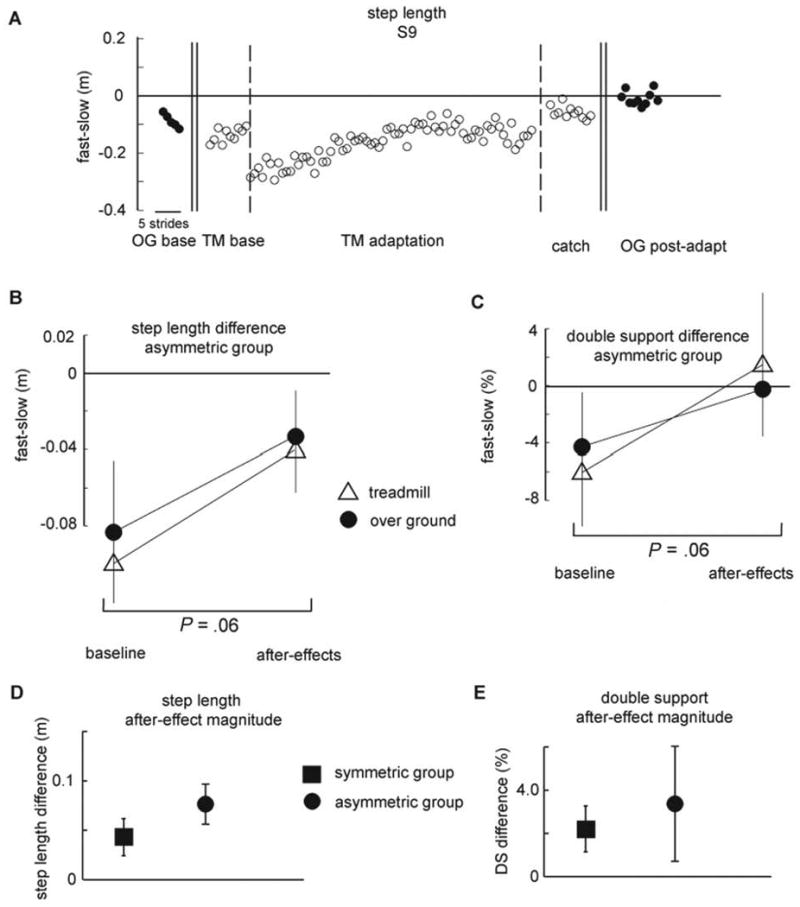
Note: A, Step length of a stroke survivor. Step length difference (fast – slow) shown for consecutive strides in all periods. Note the marked baseline asymmetry over ground and on the treadmill when the belts are tied, the increase in asymmetry when the belts are split (because the paretic leg is on the fast belt, thus exaggerating the baseline asymmetry), and the symmetry when the belts are tied in the Catch Trial and the transfer of this symmetry to overground walking in the Overground Postadaptation period. B, C, Changes in step length and double support from Baseline to either the Catch Trial (for the treadmill) or the Overground Postadaptation periods for subjects who demonstrated significant (see Methods) baseline asymmetry. Data from the treadmill are indicated by the open triangles, and data from overground walking are indicated by the filled circles. Note the similar improvement in asymmetry from the Baseline to Catch Trial or Baseline to Overground Postadaptation period for both treadmill and overground walking. Each data point represents values averaged over the first 5 strides from the represented period. Error bars indicate ±1 standard error. D, E, The magnitude of overground aftereffects for subjects who demonstrated significant baseline asymmetry (squares) and those who were not asymmetric at baseline (circles). Each data point represents values averaged over the first 5 strides during Overground Postadaptation normalized to overground Baseline. Error bars indicate ±1 standard error.
Impairment Level and Aftereffect Magnitude
An important question is whether any of the stroke survivors' impairments relate to the transfer of the adaptation from the treadmill to overground walking. Our subjects had widely varying sensory and motor impairments (Table 1). There were no significant correlations between lower extremity impairment scores (ie, Fugl-Meyer score, sensation, proprioception) or fast walking speed and the adaptation transfer of step length or double support to overground walking (all P > .05).
Discussion
We demonstrated that a locomotor adaptation following split-belt treadmill walking partially transfers to overground walking in both healthy control subjects and persons poststroke. This finding suggests that some overlapping or shared neural circuits that control locomotion for different environmental contexts (treadmill vs over ground) are adapted during split-belt treadmill walking. However, the lack of complete transfer suggests that some aspects of the control of overground walking were not influenced by the treadmill adaptation.
Equally important, and somewhat surprising, is the finding that the adaptation transfer was larger in the stroke survivors when compared with the control subjects. This robust transfer of decreased asymmetry in the stroke survivors following split-belt treadmill walking provides additional support for the previous suggestion that asymmetric walking patterns post-stroke could be remediated using the split-belt treadmill as a long-term rehabilitation strategy.3
Why Is There Partial Transfer?
Previous studies investigating adaptation transfer have shown that there are many factors that can influence transfer, such as the overlap of body parts used during adaptation, the similarity of the movements, and the implicit or explicit nature of the adaptation.1,8-11 It has been suggested that the amount of transfer depends on the degree to which shared or overlapping neural control involved in both contexts is adapted.1,10,11 Some locomotor adaptation studies have shown minimal transfer to new demands or environmental contexts.17,18 Other studies involving conventional or circular treadmill training have shown robust aftereffects in overground walking, but all overground tests were done with eyes closed, and the handrail was not used.19-21 We suspect that transfer of the adapted pattern should be greatest when subjects walk in contexts that are most similar to that experienced during training. Thus, closing the eyes and refraining from handrail use during treadmill walking might reduce context cues that allow subjects to switch patterns. In the present study, subjects were tested with eyes open and holding onto the handrail during treadmill walking, so it is not surprising that control subjects transferred a modest 30% (Figure 3A). What is surprising is that the patients transferred much more, typically 60% (Figure 3A). One interpretation is that damage to the cerebrum affects the use of context cues to switch control of locomotor patterns.
The Role of Cerebral Inputs in Locomotor Adaptation Transfer
Cerebral damage in human adults does not impair the ability to adapt to walking on a split-belt treadmill.3 Persons with cerebral damage due to unilateral stroke can adapt interlimb coordination during split-belt walking and show aftereffects when the treadmill belts are returned to the same speed.3 However, stroke survivors are impaired in other locomotor tasks, namely, those that are visually guided and/or require cognitive attention.22-25 For example, obstacle avoidance during walking requires cerebral control in both cats and humans.22-25 During obstacle avoidance, patients show a persistent pattern of step lengthening to clear the obstacle, even when switching to a step-shortening strategy may be more appropriate,22 indicating that cerebral control is important for changing the movement pattern to meet changing environmental demands.
Here, we tested for transfer of aftereffects to overground walking. This meant that there was a change in the environmental context of walking. Control subjects more readily switched walking patterns with the change in context from the treadmill to overground walking (ie, limited transfer of aftereffect). The fact that the stroke survivors did not change walking patterns, and instead generalized the adapted pattern, may indicate a deficit in using environmental context cues to switch motor patterns. Thus, switching the walking pattern from that used on a treadmill to that used to move freely over ground in a different room may require cerebral mechanisms.
The ability to switch movement patterns based on environmental context demonstrates a high level of motor skill. In terms of locomotion, this skill is critical for function in the community, where environmental demands are varied and changing.26 It is well known that stroke survivors who have recovered some basic locomotor ability often continue to have difficulty with community locomotion.27,28 This is thought to occur because they have difficulty changing their pattern to accommodate to the variety of environmental demands encountered during community ambulation, although this hypothesis has not been formally tested.27,28 Results from the present study could be interpreted as evidence to support this hypothesis. However, this “deficit” in context switching may be used to advantage by allowing subjects to experience and practice a more symmetric walking pattern (ie, on or off the treadmill) during rehabilitation.
Although we hypothesize that a deficit in context switching is what caused greater transfer of the adapted motor pattern poststroke, there are other plausible explanations. First, the new pattern may have transferred over ground in the patients because it represents an improvement, and theoretically may benefit the system in terms of energy cost, balance, or efficiency. It is also possible that the pattern transfers because stroke survivors have diminished limb sensation or proprioception and are unable to detect context switches completely. This latter explanation seems somewhat unlikely given the lack of correlation between these sensory impairments and the magnitude of adaptation transfer.
Gait Asymmetry and Using Motor Adaptation in Rehabilitation
Previous studies have demonstrated that aftereffects of a locomotor or visuomotor adaptation can improve task performance in persons poststroke.29,30 This is the first study, however, to demonstrate that this improved performance can be transferred to a real world task, in this case, overground walking. In the present study, improvements in step length and double support asymmetry following split-belt treadmill adaptation transferred to overground walking.
Asymmetry of step length following stroke is related to decreased propulsive force of the hemiparetic limb, decreased work and power of the hemiparetic plantarflexors, decreased walking speed, and increased hemiparetic severity.31-34 Asymmetric double support time is related to decreased speed.15 Thus, improvements in these asymmetries may affect both impairment and function poststroke.
Previous studies of locomotor training poststroke have demonstrated the importance of task specificity of training.35,36 Additionally, previous studies indicate that, although very similar, there are differences in the locomotor pattern observed between treadmill and overground walking poststroke.37-39 Hence, to maximize improvements in walking poststroke through task-specific training, an ideal rehabilitation intervention would include overground walking. In the present study, we found improvements (ie, reductions in asymmetry) in treadmill and overground walking. Thus, repetitive practice of the improved walking pattern following split-belt treadmill adaptation can be undertaken over ground, providing for optimal locomotor task–specific training. This makes the utility of this type of locomotor adaptation training particularly appealing for rehabilitation. To add to this appeal is the finding that the magnitude of the adaptation transfer was not correlated with the degree of sensorimotor impairment or walking speed. This suggests that this type of locomotor adaptation training may be successfully used across a broad spectrum of patients. However, the majority of our subjects were beyond the level of household ambulation according to Perry's classification of walking disability.40 Therefore, future studies should include a greater number of subjects with very slow walking speeds to expand our understanding of the relationship between walking impairment and adaptation.
To determine the role of split-belt locomotor adaptation in rehabilitation, further research is needed to investigate the effects of long-term training. Current investigations are underway to examine whether training over weeks can lead to long-term learning of improved step length symmetry following split-belt treadmill walking poststroke and to investigate the impact of feedback about symmetry during and after split-belt treadmill walking.
Acknowledgments
We wish to thank J. Choi for her contribution to analyses and interpretation and A. Torrie for her assistance with data collection.
This work was supported by NIH grant K01HD050582 awarded to D. S. Reisman and NIH grant R01HD048740 awarded to A. J. Bastian. A. J. Bastian's facilities were supported in part by an NIH NCRR Extramural Research Facilities Construction Grant RR15488.
Footnotes
For reprints and permission queries, please visit SAGE's Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–1062. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 2.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 3.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(pt 7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95:766–773. doi: 10.1152/jn.00473.2005. [DOI] [PubMed] [Google Scholar]
- 5.Earhart GM, Fletcher WA, Horak FB, et al. Does the cerebellum play a role in podokinetic adaptation? Exp Brain Res. 2002;146:538–542. doi: 10.1007/s00221-002-1238-y. [DOI] [PubMed] [Google Scholar]
- 6.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci U S A. 1996;93:13292–13297. doi: 10.1073/pnas.93.23.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol. 2006;4:e316. doi: 10.1371/journal.pbio.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci. 2004;24:8084–8089. doi: 10.1523/JNEUROSCI.1742-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol. 2004;92:2497–2509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- 11.Savin DN, Morton SM. Asymmetric generalization between the arm and leg following prism-induced visuomotor adaptation. Exp Brain Res. 2008;186:175–182. doi: 10.1007/s00221-007-1220-9. [DOI] [PubMed] [Google Scholar]
- 12.Reisman DS, Wityk R, Bastian AJ. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Split-belt treadmill walking adaptation in post-stroke hemiparesis. Program No. 756.4. [Google Scholar]
- 13.Hsueh IP, Hsu MJ, Sheu CF, Lee S, Hsieh CL, Lin JH. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabil Neural Repair. 2008;22:737–744. doi: 10.1177/1545968308315999. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22:51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- 16.Roth EJ, Merbitz C, Mroczek K, Dugan SA, Suh WW. Hemiplegic gait. Relationships between walking speed and other temporal parameters. Am J Phys Med Rehabil. 1997;76:128–133. doi: 10.1097/00002060-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 17.McVea DA, Pearson KG. Long-lasting, context-dependent modification of stepping in the cat after repeated stumbling-corrective responses. J Neurophysiol. 2007;97:659–669. doi: 10.1152/jn.00921.2006. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds RF, Bronstein AM. The moving platform aftereffect: limited generalization of a locomotor adaptation. J Neurophysiol. 2004;91:92–100. doi: 10.1152/jn.00495.2003. [DOI] [PubMed] [Google Scholar]
- 19.Anstis S. Aftereffects from jogging. Exp Brain Res. 1995;103:476–478. doi: 10.1007/BF00241507. [DOI] [PubMed] [Google Scholar]
- 20.Earhart GM, Melvill Jones G, Horak FB, Block EW, Weber KD, Fletcher WA. Transfer of podokinetic adaptation from stepping to hopping. J Neurophysiol. 2002;87:1142–1144. doi: 10.1152/jn.00588.2001. [DOI] [PubMed] [Google Scholar]
- 21.Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW. Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Exp Brain Res. 1998;120:377–385. doi: 10.1007/s002210050411. [DOI] [PubMed] [Google Scholar]
- 22.Den Otter AR, Geurts AC, de Haart M, Mulder T, Duysens J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res. 2005;161:180–192. doi: 10.1007/s00221-004-2057-0. [DOI] [PubMed] [Google Scholar]
- 23.Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Drew T, Jiang W, Kably B, Lavoie S. Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol. 1996;74:426–442. [PubMed] [Google Scholar]
- 25.Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- 26.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther. 2002;82:670–681. [PubMed] [Google Scholar]
- 27.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair. 2008;22:649–660. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 28.Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke. 2005;36:1457–1461. doi: 10.1161/01.STR.0000170698.20376.2e. [DOI] [PubMed] [Google Scholar]
- 29.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res. 2006;168:368–383. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti Y, Rode G, Pisella L, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–169. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 33.Buurke JH, Nene AV, Kwakkel G, Erren-Wolters V, Ijzerman MJ, Hermens HJ. Recovery of gait after stroke: what changes? Neurorehabil Neural Repair. 2008;22:676–683. doi: 10.1177/1545968308317972. [DOI] [PubMed] [Google Scholar]
- 34.Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2009;29:129–137. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87:1580–1602. doi: 10.2522/ptj.20060310. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–691. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 37.Bayat R, Barbeau H, Lamontagne A. Speed and temporal-distance adaptations during treadmill and overground walking following stroke. Neurorehabil Neural Repair. 2005;19:115–124. doi: 10.1177/1545968305275286. [DOI] [PubMed] [Google Scholar]
- 38.Harris-Love ML, Forrester LW, Macko RF, Silver KH, Smith GV. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. 2001;15:105–112. doi: 10.1177/154596830101500204. [DOI] [PubMed] [Google Scholar]
- 39.Hesse S, Konrad M, Uhlenbrock D. Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Arch Phys Med Rehabil. 1999;80:421–427. doi: 10.1016/s0003-9993(99)90279-4. [DOI] [PubMed] [Google Scholar]
- 40.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]


