A rich literature dating back to the early twentieth century has characterized the cognitive morbidity associated with the epilepsies and the association of this morbidity with the cause, course, and treatment of the disorder. Critical reviews over the decades have cataloged the links between cognitive disorders and specific clinical features of the epilepsies including their etiology; seizure frequency and severity; complications of the disorder (e.g. status epilepticus); antiseizure medications; and electroencephalographic abnormalities, such as the type, frequency, and distribution of interictal epileptiform and slow wave activity.1–6
The understanding of the neuropsychological consequences of the epilepsies evolved in concert with advancements in the wider worlds of cognitive psychology and epilepsy. First, early studies were typically but not exclusively limited to the evaluation of intelligence. Assessment of higher cognitive functions was in its formative years7 and with the introduction of the Binet-Simon scales, and especially their adaption for use in the United States (eg, the early Vineland translation and the later Stanford-Binet revision), characterization of intellectual status in epilepsy followed quickly.8 As a deeper understanding of human cognition developed, and newer tests and measures became available to assess those concepts, appreciation of the cognitive correlates of epilepsy expanded apace. Second, much of the early literature came from very limited segments of the population of people with epilepsy, typically from specialized institutions (or colonies) serving the more complicated and severely affected individuals.3 Over time more representative portions of the population were sought out and investigated, which yielded a less biased but still imperfect characterization of the relationship between epilepsy and intelligence and broader cognitive status.2,9,10 The focus of research has continued to be on persons with epilepsy presenting to specialized tertiary care medical centers, although more representative population-based studies of cognition are available, particularly among children with epilepsy.11–14 Third, classification and taxonomy of the epilepsies developed from early rudimentary systems to the evolving and increasingly sophisticated international classification of epileptic seizures and syndromes.15,16 The neuropsychological features of these syndromes and their primary cognitive signatures have developed accordingly.6,11,17–20 Finally, as medical technology evolved patients with epilepsy have been studied with increasing sophistication to understand the underlying neurobiology of cognitive impairment in epilepsy, a primary focus of this article.
A critical and fundamental feature of the neuropsychology of epilepsy literature is that throughout its history a primary focus has been the relationship of cognition and cognitive disorders to core clinical features of the epilepsies including but not limited to the age of onset of epilepsy, etiology, seizure type and syndrome, medications, duration of epilepsy, and electroencephalographic features.21–26 Unequivocal associations between these clinical characteristics and neuropsychological impairments have been reported and repeatedly replicated through the decades, but the neurobiologic mechanisms through which they exert their effects have been investigated less intensively. A new literature is now under way, one linking cognitive abnormalities directly to indices of structural, functional, metabolic, and other neurobiological markers of cerebral integrity, independent of their association with clinical epilepsy characteristics. These trends are reviewed in the material to follow. The initial focus is on temporal lobe epilepsy (TLE) as a model with which to address the core points, because this form of localization-related epilepsy has been very carefully studied from both a cognitive and imaging standpoint. Some pertinent historical issues are touched on first, followed by more detailed reviews of the cognitive and neuroimaging abnormalities that have been found in TLE, followed by an overview of studies examining direct structure-function relationships in TLE and other epilepsies.
THE UNIQUE CONTRIBUTION OF TLE
Psychomotor epilepsy or TLE has provided an especially important window into the neuropsychology of epilepsy. The term “psychomotor epilepsy” was used beginning in the 1930s to describe relatively poorly understood spells that some had called psychic equivalents.27,28 The early electroencephalographic features were characterized in the context of clinical attacks wherein “….the patient, though he may perform apparently conscious acts, is not subject to command; he may exhibit involuntary tonic movements; he may display psychomotor disturbances….and on recovery he has complete amnesia for the events which occurred in the attack.”27,29,30 These seizures were later found to be associated with an anterior temporal lobe spike focus,30,31 and consideration of epilepsy surgery for these “nonlesional” patients developed early on in Chicago32 and Montreal.33
Early surgical centers routinely incorporated cognitive assessments in their evaluations, which were performed under the supervision of Donald Hebb34 and Brenda Milner35,36 at the Montreal Neurological Institute, Ward Halstead37 at the University of Illinois, and Victor Meyer at the Maudsley Hospital.38,39 The neuropsychology of epilepsy benefitted enormously from these early opportunities to assess patients before and after surgery and to correlate cognitive changes with detailed preoperative histories, well-characterized surgical resections, careful neuropathologic examinations of resected tissue, and eventually quantitative neuroimaging.5
THE PALM DESERT CONFERENCES ON EPILEPSY SURGERYAND THE NEUROPSYCHOLOGY OF EPILEPSY
Important events for the neuropsychology of TLE were the Palm Desert Conferences on Epilepsy Surgery.40 Before these international conferences there were varying opinions regarding the operational definition of surgical candidacy. Various reasonable criteria were proposed, including seizure frequency and severity, the number of failed medications, or the degree of social and occupational disability; but consensus remained to be achieved. At the second Palm Desert Conference a focus was placed on “surgically remediable syndromes,” among which mesial TLE (mTLE) was prominent.40 If a syndrome was surgically curable, it should be identified and treated with minimal delay.41
This conceptualization had an important impact not only on the thinking regarding optimal patient selection and surgical timing, but it also facilitated increasingly careful characterization of mTLE.42 At that time details regarding the neuropsychological features of mTLE were few in number and the primary cognitive correlates were viewed as largely linked to material-specific memory impairment demonstrated either through formal cognitive assessment or the Wada Test. A stated contraindication to the syndrome of mTLE was the presence of generalized cognitive compromise, all of this was a reasonable early characterization.41 For neuropsychologists who saw a steady stream of surgical candidates with well characterized mTLE, however, the neuropsychological correlates were viewed as considerably more complex. Memory asymmetry was indeed seen in a proportion of patients, an asymmetry linked to the degree of neuronal loss and sclerosis in the affected hippocampus,43 but this pattern often occurred in the context of more distributed cognitive impairment. Formal investigations of the ability of neuropsychological tests to localize and lateralize the ictalonset area revealed variable discriminatory power44 and these results were viewed by some as indicative of the limitations of neuropsychological assessment. The material to follow, however, provides an overview of the distributed nature of cognitive abnormality in mTLE in the context of the distributed nature of identified structural abnormalities in mTLE, and their important associations.
THE DISTRIBUTED NATURE OF COGNITIVE IMPAIRMENT IN MTLE
By definition the syndrome of mTLE is a disorder of childhood-adolescent onset.45 A core finding, clear from some of the earliest cognitive studies and routinely reported through the decades, is that an early age of onset of recurrent seizures is associated with a pernicious impact on a broad array of cognitive functions. This effect was reported as early as 192446 and subsequently confirmed in studies of adult patients with diverse seizure types,10,47–50 and even reported in neuropsychological studies of younger patients with complex partial and other types of seizures.51–53
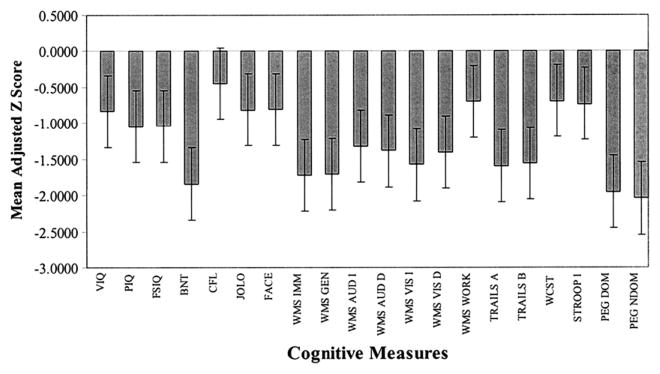
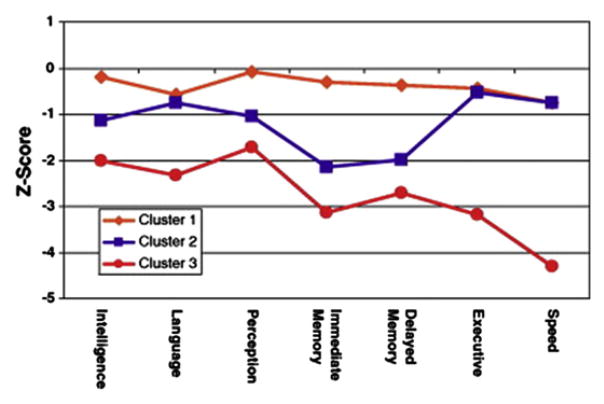
When the comprehensive neuropsychological status of patients with mTLE with confirmed hippocampal sclerosis was examined compared with healthy controls or patients with other TLE syndromes, such as so-called “MRI-negative TLE,” mTLE patients exhibited a pattern of distributed cognitive impairment affecting not only memory, but also intelligence quotient (IQ), executive functions, language, sensorimotor, and other abilities (Fig. 1).54–57
Fig. 1.
Mean adjusted (age, gender, education) z scores for patients with TLE compared with healthy control subjects. (From Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 2004;62:1736–42.)
This average profile of distributed neuropsychological impairment in mTLE, obtained in the context of a focal epileptogenic lesion whose resection results in excellent outcome, was unexpected. The testable hypothesis was that just as memory impairment was related to neuropathology in the hippocampus, perhaps the more widespread neuropsychological abnormalities might be secondary to more diffusely existing neuroanatomic abnormality that could be detected by quantitative neuronimaging techniques, and that individual variability in neuropsychological abnormalities might be associated with individual patterns of anatomic abnormality.54
THE DISTRIBUTED NATURE OF ANATOMIC ABNORMALITIES IN CHRONIC TLE
The cumulative neuroimaging literature, focusing here on structural neuroimaging, has shown that anatomic abnormalities in mTLE can be extensive. For reasons that remain to be understood, mTLE seems to be associated with abnormalities in surprisingly diverse neuronal systems, a pattern consistent with the generalized and distributed average neuropsychologic profile of TLE (see Fig. 1).
Initial quantitative MRI volumetric and voxel-based morphometry (VBM) studies identified atrophy of the hippocampus,58–61 along with reports of abnormalities in related structures including entorhinal cortex,60–63 fornix,64 parahippocampal gyrus,61 amygdala,60,61 basal ganglia,65,66 and thalamus.65,67,68 More distant extrahippocampal temporal lobe58,69–72 and extratemporal lobe regions69,73 were implicated including the lateral temporal cortex, frontal lobe, and the cerebellum.74,75 With this degree of distributed atrophy, it is not surprising that reductions in overall total cerebral tissue volume were reported.56,69,70,76–78
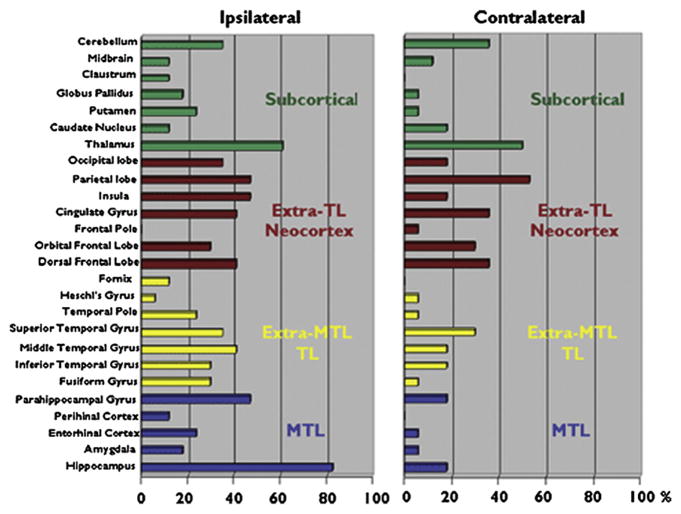
With few exceptions68,79 most of the early volumetric investigations examined one or a limited number of structures rather than characterizing a broad and diverse range of regions as to the presence and degree of abnormality. Examination of several regions in the same patient group facilitated appreciation of the distribution and relative degree of structural burden carried by many patients.68,79 In that regard, a very helpful summary of the presence and distribution of structural abnormality associated with TLE is provided by Keller and Roberts.80 In their review they surveyed 26 brain regions examined in VBM investigations of TLE compared with healthy controls. Their summary (Fig. 2) demonstrates the proportion of studies revealing abnormalities in mesial, extramesial temporal lobe, subcortical, and extratemporal lobe cortical regions. The presence and distribution of these abnormalities would suggest that cognitive abnormalities might extend beyond memory to involve diverse cognitive abilities.
Fig. 2.
Twenty-six brain regions found to be significantly reduced in volume in patients with TLE relative to healthy controls. The results are presented ipsilateral and contralateral to the epileptogenic focus. MTL, medial temporal lobe; TL, temporal lobe. (From Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 2008;49:741–57.)
DISTRIBUTED ABNORMALITIES IN CORTICAL SURFACE FEATURES IN TLE
Another example of the potential structural consequences of mTLE is provided by quantitative characterization of the cortical mantle, including indices of gyrification, cortical depth, and surface area. These indices provide important information concerning normal and abnormal brain development, the effects of normal aging, and disease impact.
Lee and colleagues70 were among the first to examine cortical surface features (sulcal curvature) in unilateral TLE, the bulk of evidence awaiting the development of more sophisticated image processing systems. Accumulating evidence has shown that patients with unilateral TLE exhibit bilateral and diffuse abnormalities in sulcal and gyral curvature, cortical depth, and cortical complexity.56,81–84
Oyegbile and colleagues56 examined cortical surface features in an initial cohort of 96 patients with TLE and 82 healthy controls. They found patients with unilateral TLE to exhibit abnormalities in whole-brain gyral and sulcal curvature with increased surface cerebrospinal fluid (CSF) volume. These cortical surface feature abnormalities were generalized in nature and evident both contralateral and ipsilateral to the side of temporal lobe seizure onset.
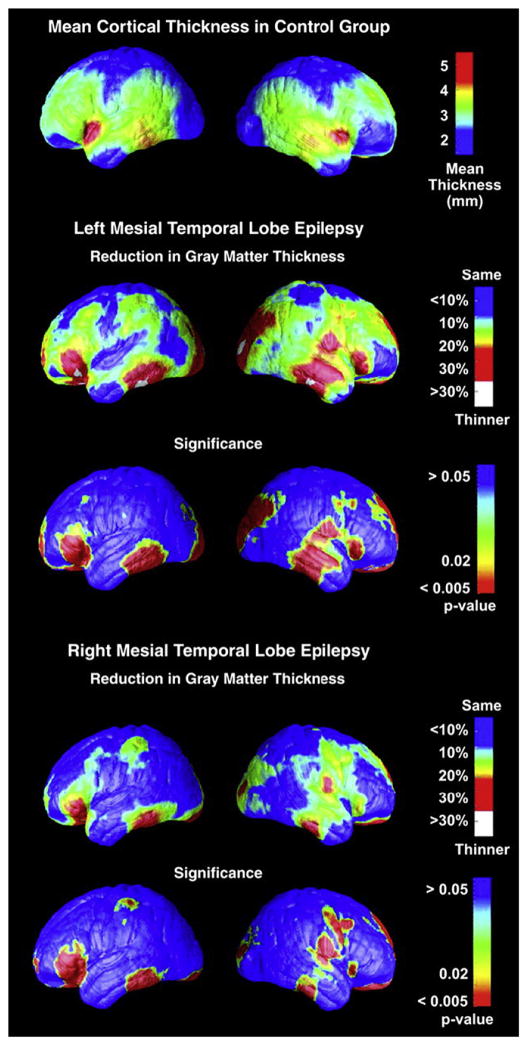
Lin and colleagues85 examined cortical thickness and cortical complexity in an extremely carefully defined patient cohort. Their cohort of patients exhibited at least three ictally monitored seizures demonstrating unilateral temporal lobe onset, hippocampal sclerosis demonstrated by histopathology, and class 1 outcome 2 years following surgery. Comparing 15 left and 15 right mTLE patients with 19 healthy controls, they found both left and right mTLE groups to have regions with up to 30% bilateral decrease in average cortical thickness, with significant thinning of the bilateral frontal poles, frontal operculum, orbitofrontal, lateral temporal, and occipital regions, right angular gyrus, and primary sensorimortor cortex surrounding the central sulcus (Fig. 3). Examining cortical complexity, the left TLE group showed significantly reduced complexity across all left and right hemisphere lobes except the right frontal lobe. The right TLE group exhibited significantly reduced cortical complexity across all lobes except the right frontal and parietal lobes. A very diffuse and bilateral pattern of abnormality was therefore evident in the cortical mantle in patients with unilateral mTLE.
Fig. 3.
Cortical thickness maps: regional reduction in mTLE groups. (From Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex 2007;17:2007–18.)
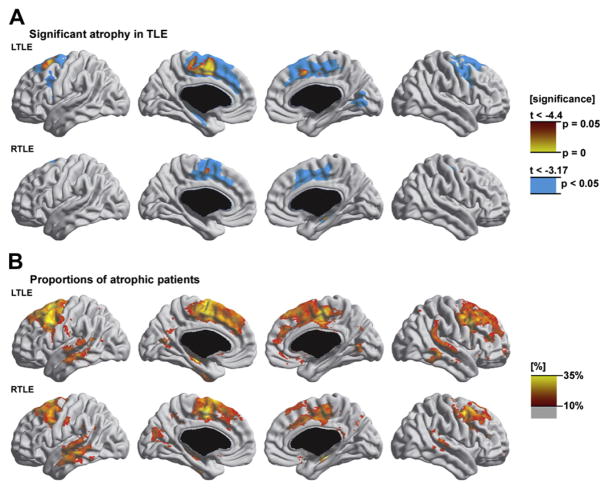
Subsequent studies have elaborated and extended these findings. Bernhardt and colleagues83 examined 110 patients with TLE (56 left, 54 right) and 45 controls, and Fig. 4 summarizes the findings. Thinning was evident in bilateral frontocentral (superior, middle, and medial frontal gyrus; precentral gyrus; paracentral lobule); cingulate; and contralateral medial occipitotemporal regions. The most severe frontal thinning occurred in the ipsilateral precentral regions. In addition, there was severe cortical thinning in the hippocampus and parahippocampal regions ipsilateral to side of seizure onset. Contralateral to the seizure focus, there was cortical thinning in the medial occipitotemporal gyrus. In right TLE the severity of atrophy was similar and the pattern resembled that found in left TLE, but less widespread.
Fig. 4.
Atrophy in TLE. Significant atrophy in TLE (A). Proportions of atrophic patients (B). (From Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage 2008;42:515–24.)
McDonald and colleagues82 compared 21 patients with TLE with 21 healthy controls. Bilateral cortical thinning (5%–15%) was reported in the lateral temporal lobes (Heschl’s gyrus) and frontal lobes (precentral, paracentral, pars opercularis). Unilateral cortical thinning was evident in the left superior temporal gyrus and sulcus and medial orbital cortex; and the right middle temporal gyrus and lateral orbitofrontal cortex. If analyses were restricted to mTLE patients, then the parietal cortex was also affected.
Mueller and colleagues84 used 4-Tesla MRI and examined 35 controls and 15 patients with mTLE and 16 non-mTLE patients. Both groups exhibited widespread temporal and extratemporal cortical thinning. The mTLE group showed significant thinning in ipsilateral temporal lobe (entorhinal cortex, parahippocampal fusiform gyrus, temporopolar–anterior superior temporal region, retrosplenial region) and bilateral precentral-postcentral regions, superior frontal, transverse temporal, precuneus, and prestriate regions.
In summary, widespread temporal and extratemporal lobe abnormalities in cortical surface features seem present, both contralateral and ipsilateral to the side of seizure onset, in mTLE in particular and TLE more generally.
DISTRIBUTED ABNORMALITIES IN WHITE MATTER AND WHITE MATTER TRACTS IN TLE
In addition to gray matter abnormalities, a decrease in white matter volume is present in chronic TLE. Traditional volumetric studies have demonstrated distributed cerebral white matter abnormalities in patients with unilateral TLE, with abnormalities evident both contralateral and ipsilateral to the side of seizure onset, affecting temporal and extratemporal regions.86 Whole-brain VBM analyses have also demonstrated temporal and extratemporal abnormalities, although the findings are much less extensive and consistent when compared with gray matter VBM studies. McMillan and coworkers87 found that left TLE patients had reduced white matter volume in the left temporal lobe, corpus callosum, and bilateral prefrontal cortex. In patients with right TLE, only right temporal lobe and fornix showed decreased volume. Bernasconi and colleagues88 found that both right and left TLE individuals had reduced white matter volume in the temporal lobes, ipsilateral to side of seizure onset and the body of the corpus callosum. The right TLE individuals had additional white matter reduction in the postcentral regions. Their study did not, however, reveal frontal lobe white matter abnormalities.
Although these white matter volumetric studies further elucidate the widespread structural abnormalities in TLE, they do not provide measures of specific white matter tract coherence or the overall integrity of networks connecting different brain regions. Recent compelling evidence from functional imaging, lesional, and behavioral studies suggest that widespread and coordinated networks are required for complex cognitive function.89 These distributed networks are linked by projection, association, and commissural white matter fiber tracts that connect cortical-subcortical, cortical-cortical, and interhemispheric regions.90
In macaque monkeys, interrupting major afferent pathways from the basal forebrain to the temporal lobe, without removal of the medial temporal structures, produces severe amnesia.91 In addition, simply disconnecting the parietal lobe from the frontal lobe functionally, as can be done by white matter stimulation during surgery, leads to profound neglect in humans.92 In certain cases, loss or disruption of cerebral connections can produce a greater magnitude of deficits than localized lesions, suggesting the importance of an integrated structural network in normal cognitive functions. This concept has led to a unifying hypothesis that disconnection between important cortical and subcortical regions impairs information transfer and contributes to cognitive impairments.93 Indeed, such disconnection models can now be tested, using quantitative imaging techniques, such as diffusion tensor imaging (DTI), to interrogate the integrity of white matter tracts.94
In DTI, the primary measure of white matter integrity is fractional anisotropy (FA), which is determined by the directional magnitude of water diffusion in three-dimensional space.95 Tightly packed white matter fascicles provide structural coherence, which results in water diffusion in a preferred direction (high FA). In contrast, white matter fascicles that have poor structural organizations allow water to diffuse more randomly (low FA). Other measures of white matter integrity include mean diffusivity (MD) or apparent diffusion coefficient, which calculates bulk water diffusion characteristic in the intracellular and extracellular water compartments.96 Using these water diffusion parameters, studies have evaluated the coherence of specific white matter tracts and whole-brain white matter connectivity.
Initial DTI studies used a region of interest approach to investigate specific white matter tracts in the limbic network. One of the first DTI tractography studies evaluated the integrity of fornix and cingulum in patients with mTLE. In these fiber tracts, Concha and colleagues97 observed diffusion abnormalities ipsilateral and contralateral to the side of seizure onset. Postulating a more diffuse epileptogenic network in TLE, other investigations have extended this initial finding to frontal-temporal (uncinate fasciculus and arcuate fasciculus),98–100 temporal-occipital (inferior longitudinal fasciculus),101 frontal-occipital (inferior frontal occipital fasciculus),101 and interhemispheric (corpus callosum) connections.102–104 Parallel to the gray matter region of interest studies, these DTI studies have also shown extensive bilateral abnormalities in cortical-cortical, cortical-subcortical, and interhemispheric connections, despite unilateral seizure onset.
More recently, whole-brain voxelwise analysis techniques of DTI data have been developed. These analyses allow mapping of white matter profiles and delineate systemic differences between TLE patients and healthy individuals, without a priori bias for specific tracts or brain regions. DTI data have poor anatomic resolution, which makes spatial matching across multiple subjects particularly challenging. Two methods have been developed to overcome these technical difficulties: VBM and tract-based spatial statistics. In patients with mTLE, both methods revealed extensive bilateral white matter diffusion abnormalities, particularly in the temporal and frontal lobes ipsilateral to the side of seizure onset.105–107 Tract-based spatial statistics seemed to be more a sensitive method by detecting more extensive white matter changes when compared with traditional VBM methods in the same patient population.106
TLE: A LOCALIZATION-RELATED DISORDER?
The essential theme is that although the primary epileptic zone may be contained within the confines of the hippocampus and temporal lobe, considerable anatomic abnormality exists outside this region affecting a myriad of cortical, subcortical, and cerebellar regions and their direct and indirect connectivity. Importantly, the sum of these distributed structural abnormalities, not to mention associated abnormalities in other aspects of brain integrity, such as metabolism and blood flow, may result in a cumulative cognitive and behavioral burden that may be substantial on average, but with a mosaic that may be highly individualized depending on the underlying architecture of a given patient’s structural and functional pathology.
LINKING DISTRIBUTED COGNITIVE IMPAIRMENTS WITH DISTRIBUTED NEUROIMAGING ABNORMALITIES:THE MOSAIC OF STRUCTURE-FUNCTION RELATIONSHIPS IN CHRONIC TLE
A developing literature has begun to characterize the links between cognition and the diverse regions of anatomic abnormality in TLE. This section touches on representative examples of these links. Although the focus is on associations between cognition and structural abnormalities, additional investigations albeit smaller in number have examined associations between cognition and behavior with other measures of brain integrity (eg, metabolism, functional MRI [fMRI] activation), and a few representative examples of those studies are included.
Hippocampus
Early studies demonstrated the expected relationship between memory performance and hippocampal pathology characterized by neuronal loss and sclerosis.108 Subsequent studies demonstrated a link between hippocampal volumes and memory performance before epilepsy surgery, and preoperative left hippocampal volumes were also predictive of the risk of preoperative to postoperative memory change.58,109,110 Abnormalities in left hippocampal volume have also been shown to be associated with some language-based abilities including confrontation naming111 and fluency.112
Thalamus
The degree to which the thalamus is affected in chronic mTLE is increasingly recognized and there is now a demonstrated link between atrophy of the thalamus and performance on measures of memory and intelligence in TLE.113,114 In addition, prior fluorodeoxyglucose positron emission tomography research demonstrated that verbal memory was affected in the context of ipsilateral thalamic hypometabolism in patients with left TLE.115
Basal Ganglia
Negative symptoms including affective flattening, alogia and avolition, anergia, apathy, anhedonia, and loss of social drive have been reported in patients with TLE.116,117 These patients also exhibit cognitive and psychosocial correlates that are not seen in patients with TLE without negative symptoms, but neuroimaging correlations with cortical regions were nonspecific and limited only to increased total CSF.116,117 Geary and coworkers118 hypothesized that basal ganglia and anterior cingulate regions of interest play a role in the expression of negative symptoms in epilepsy and compared a matched group of TLE patients with and without negative symptoms with healthy controls (N = 22 per group). They found that TLE patients with negative symptoms exhibited significantly reduced volumes in the putamen and globus pallidus, that these volumetric abnormalities were independent of self-reported depression, and that there were specific significant relationships between alogia with volumes of the putamen and globus pallidus, and affective flattening with volume of the putamen.118
Frontal Lobe
There has been considerable interest in the neural basis of reported impairments in executive function among patients with TLE and their relevance to “secondary” frontal lobe or frontostriatal pathology. Keller and colleagues119 examined prefrontal and hippocampal volume using sterology and VBM in 30 controls and 26 left and 17 right TLE patients. Assessment of executive functions included evaluation of response inhibition, working memory, and lexical fluency. They found volume reduction of the ipsilateral hippocampus as expected, but also volume reduction in regions of the prefrontal cortex. In addition, there were significant associations between prefrontal cortex and executive function including working memory with all prefrontal regions examined except right dorsal prefrontal cortex; lexical fluency with left dorsal prefrontal cortex, whole left prefrontal cortex, and left hippocampus; and response inhibition with left ventral prefrontal cortex). Along related lines, an earlier positron emission tomography investigation of patients with TLE showed that extension of hypometabolism into the ipsilateral frontal lobe was associated with cognitive consequences.120
Examining 36 patients with unilateral mTLE using VBM, Bonilha and colleagues121 addressed the extrahippocampal correlates of memory performance. In addition to the contributions of hippocampus, entorhinal, and perirhinal cortices to general and verbal memory, they found that atrophy of the cingulate and orbitofrontal cortex was also associated with disrupted memory performance.
Temporal Lobe
In addition to the investigations of memory and the integrity of the hippocampus reviewed previously, there have been several investigations of temporal lobe structure and memory for unfamiliar and familiar faces.122,123 These studies include bilateral investigation of T2 relaxation time in hippocampus, amygdala, and fusiform gyrus, showing that worse immediate but not delayed memory was correlated with greater differences in T2 values between left and right fusiform gyrus and hippocampus. Griffith and colleagues124 examined the relationship between [18F] fluorodeoxyglucose positron emission tomography and performance on a task of famous face recognition, naming, and generation of semantic information in 12 patients with TLE. Strong relationships between all aspects of the Famous Faces Task and the left temporal pole were revealed, whereas Famous Faces Task correlations with the right temporal pole were not significant. These findings indicated that the left temporal pole was associated with lexical and semantic retrieval of knowledge of famous persons in patients with TLE.
Cerebellum
The traditional view of cerebellar function is that it contributes primarily to movement and motor control; however, converging animal and human studies indicate that the cerebellum contributes to a variety of higher cognitive abilities, including specific types of memory.125,126 Human memory is composed of multiple systems, each mediating specific forms of learning and each dependent on different neuronal networks for efficient operation.127,128 A clear behavioral and anatomic distinction exists between the conscious recollection of facts and events (explicit or declarative memory) versus memories that are inaccessible to conscious recollection but that are expressed through changes in skills, habits, and other forms of simple associative learning (implicit or procedural memory).127,128 Classical conditioning, a fundamental form of implicit associative learning, is one type of procedural memory, and conditioning of the eyeblink response is the most commonly investigated conditioning paradigm. The neural circuitry underlying this associative learning has been well characterized and shown to be dependent on the cerebellum.129 There is now evidence that cerebellar atrophy in TLE is associated with compromised classical eyeblink conditioning.130
Cortical Surface Features
Several studies have demonstrated distributed abnormalities in the cortical mantle of patients with unilateral TLE. Only one investigation has examined the association of cognition with cortical surface features (gyral and sulcal curvature, cortical area, and thickness).56 Among TLE patients, measures of cortical curvature (gyral and sulcal) were significantly associated with performance IQ, verbal and visual memory, simple and complex psychomotor processing, and speeded fine motor dexterity. Total surface area was associated only with verbal IQ.
Amygdala
Abnormalities of the amygdala have been shown to have a relationship with psychopathology both in children131 and adults with epilepsy.132 In addition, early onset right mTLE has been shown to be associated with poor facial recognition133 or for facial emotional processing to be affected following right anterior temporal lobectomy.134
CSF
Links between measures of CSF and cognition are uncommon but robust. Indices of total CSF have been associated with measures of cognition including total impairment index.135,136 Total CSF has also been found to be related to measures of negative symptoms (eg, apathy, anhedonia) but not positive symptoms (eg, hallucinations) or depression in TLE.117
Cerebrum Volume
Investigating 28 adult patients with lateralized TLE, Baxendale and coworkers137 found 15 to exhibit extrahippocampal abnormalities on quantitative MRI analysis. Thirteen of the patient group overall had global or bilateral memory impairment. Bilateral memory deficits were significantly associated with both the presence of cerebral abnormalities and poor postoperative seizure control (P<.05). They concluded that disproportions in the regional distribution of gray and white matter in patients with hippocampal sclerosis may form the structural basis of global memory disturbance in patients with TLE.
White Matter Volumes: Cerebrum
Traditional volumetric measures of cerebral white matter volume have been found to have significant associations with multiple cognitive domains including nonverbal intelligence, memory, executive function, and psychomotor processing speed.138 In addition, total cerebral white matter volumes have been shown to be associated with reaction time and mental scanning efficiency.139
White Matter Volume: Corpus Callosum
Volume of the corpus callosum has been found to be reduced in TLE, particularly those with childhood onset.77,140 Volume of the corpus callosum was found to be significantly related to measures of nonverbal problem solving, immediate memory, complex psychomotor processing, and speeded fine motor dexterity.140
White Matter Microstructure: DTI
Recent studies have examined relationships between specific white matter regions or tracts and aspects of cognition and behavior. Flugel and colleagues141 evaluated diffusion characteristics of TLE patients with (N = 18) and without (N = 20) interictal psychosis and correlated diffusion measures with neuropsychological test scores. The investigators sampled frontal and temporal white matter regions and found that TLE patients with interictal psychosis had greater white matter compromise, as measured by FA, in these brain regions. Further, FA reductions were correlated with cognitive dysfunction and increased negative symptoms. Diehl and colleagues142 compared the integrity of the uncinate fasciculus, an important frontotemporal white matter tract, between healthy controls (N = 10) and patients with lateralized TLE (N = 28). They demonstrated that TLE patients have bilateral uncinate fasciculus diffusion abnormalities. In patients with left TLE, the integrity of the left uncinate fasciculus was related to verbal memory performances, whereas the integrity of the right uncinate fasciculus was associated with visual memory scores. McDonald and colleagues143 examined the relationship between cognitive performances and the integrity of several white matter tracts including the uncinate fasciculus, arcuate fasciculus, fornix, parahippocampal cingulum, inferior frontooccipital fasciculus, and corticospinal tract in 17 patients with TLE and 17 healthy controls. Verbal memory and language performances were correlated with MD and FA values of multiple cortical-to-cortical association tracts and limbic projection tracts, particularly in the left hemisphere. Further, cognitive performance was related to anatomic derangements in connections that were germane to the specific cognitive task, such as the relationship between arcuate fasciculus and language scores. These studies demonstrated a clear association between abnormal white matter connections and adverse cognitive outcomes and supported the disconnection model of cognitive dysfunction in patients with TLE.
Multimodality Imaging
Several studies have used fMRI to examine language reorganization in TLE and found that patients with left TLE have a greater propensity for bilateral language representation.144,145 It was unclear, however, whether such functional reorganization was associated with structural asymmetry in the language network. Recent investigations have combined fMRI and DTI tractography to answer this question.146 In these studies, patients underwent fMRI tasks, such as reading comprehension and verb generation, to activate language areas in the inferior frontal and superior temporal lobes. DTI tractography was then used to delineate the structural connections between fMRI-defined language areas. Using this combined imaging technique, Powell and colleagues147 found that left TLE patients had reduced left hemisphere and increased right hemisphere white matter connections, when compared with controls and right TLE patients. Further, a greater degree of lateralization to the left hemisphere was correlated with greater decline in naming function after a dominant hemisphere temporal lobe surgery.148 The abnormal structural lateralization found in DTI was congruent with fMRI activation patterns and established the important relationship between structure and function in brain regions salient for language processing.
There is no question that anatomic abnormalities in TLE are distributed in nature throughout the brain and have clinical relevance through their association with cognition or behavior. The unique and nonoverlapping associations between specific cognitive abilities and anatomic areas remain to be further determined.
THE LIMITATIONS OF MODAL COGNITIVE PROFILES
Modal or average cognitive profiles clearly help to convey a sense of the overall cognitive burden associated with TLE, an average burden that seems surprisingly onerous. Similarly, modal or average neuroimaging profiles convey a similar impression regarding the neuroanatomic burden. The symmetry between these modal cognitive and neuroimaging profiles helps to make the cognitive pathology understandable. Although helpful, these average profiles are just that, averages, and may not be particularly representative of individual patients. There is considerable variability across patients in their patterns of both cognitive and structural abnormalities and the authors have argued that one way to understand this variability is through the study of so-called “cognitive phenotypes.”149–151
Using cluster analysis and analyzing age- and education-adjusted cognitive domain scores, the authors identified three neuropsychological profile types in adult TLE (Fig. 5). One group was characterized by relatively preserved mentation (47% of sample); a second group demonstrated more generally affected mentation but with particular memory impairment (24% of sample); and a third group exhibited diffuse cognitive impairment with especially impaired memory, executive function, and motor-psychomotor processing speed (29% of sample).149
Fig. 5.
Cognitive Phenotypes in TLE. (From Hermann B, Seidenberg M, Lee EJ, et al. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 2007;13:12–20.)
Within the context of an average profile of generally affected mentation (see Fig. 1), discrete underlying cognitive profile types could be identified. In addition, these groups exhibited varying cognitive prognoses over a subsequent 4-year interval. Most interestingly, these groups also had distinct patterns of associated underlying neuroanatomic abnormality.
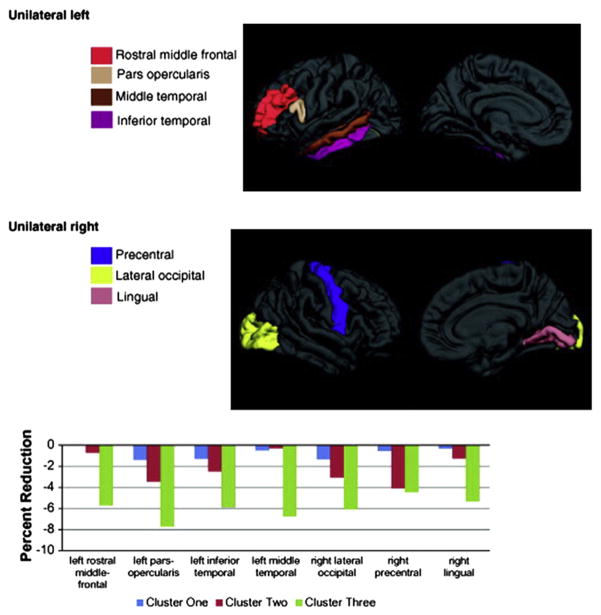
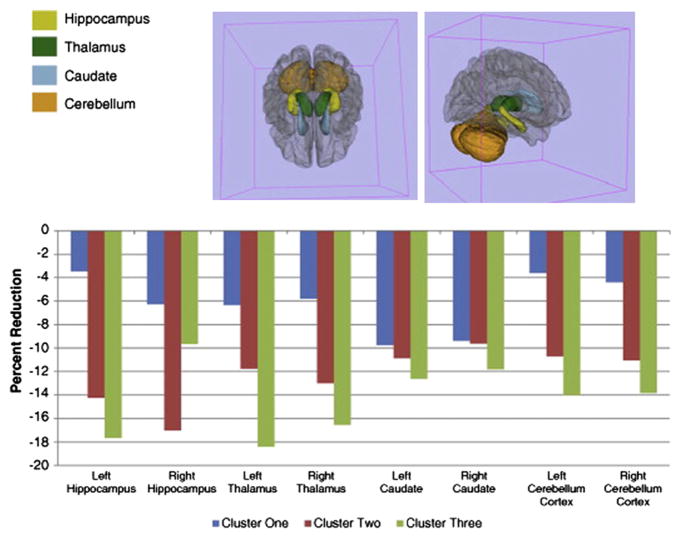
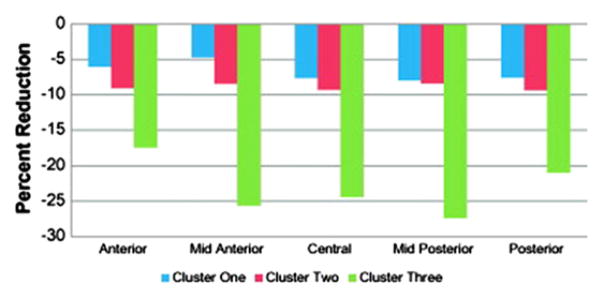
Specifically, depicted in the accompanying figures and bar graphs are patterns of anatomic abnormality associated with these cognitive phenotypes. Abnormalities in cortical thickness are evident across the cognitive phenotypes detected bilaterally in several areas (Fig. 6), unilaterally in others (Fig. 7), along with volumetric abnormalities in subcortical structures and cerebellum (Fig. 8), and targeted white matter tracts (corpus callosum) (Fig. 9). The link between cognitive profile and corresponding neuroanatomic abnormality is telling and speaks to the anatomic reality of identified neuropsychological profiles in TLE.150 Buried within modal profiles of both cognition and anatomic abnormality are groups that vary substantially in their cognitive and associated anatomic status.
Fig. 6.
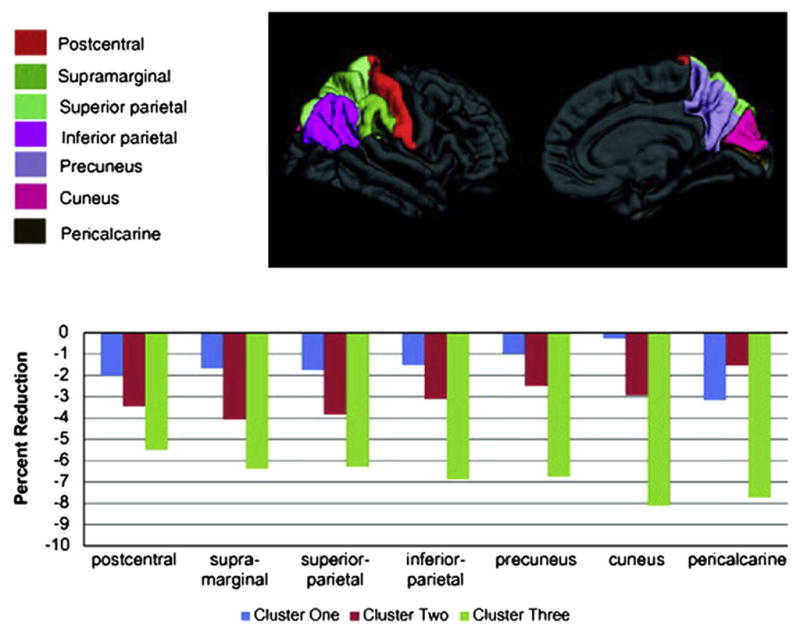
Bilateral reductions in cortical thickness across cognitive phenotypes. (From Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15(4):445–51.)
Fig. 7.
Unilateral reductions in cortical thickness across cognitive phenotypes. (From Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15(4):445–51.)
Fig. 8.
Volumetric reductions in subcortical and cerebellar regions across cognitive phenotypes. (From Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15(4):445–51.)
Fig. 9.
Volumetric reductions of the corpus callosum across cognitive phenotypes. (From Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 2009;15(4):445–51.)
IS TLE UNIQUE?
The evidence presented indicates that patients with chronic TLE often exhibit cognitive and quantitative neuroimaging abnormalities that extend beyond the primary zone of seizure onset with significant associations between specific “extratemporal” structural abnormalities and cognitive impairments. These patterns may have their origin in considerable part from the effects of epilepsy and its causes on neurodevelopment as well as progressive abnormalities in a subset of patients. There is suggestive evidence that similar patterns may be evident in other epilepsy syndromes (eg, juvenile myoclonic epilepsy). Early neuropsychological investigations reported impairments in higher level executive functions consistent with the primary thalamofrontal pathophysiology of juvenile myoclonic epilepsy.152–154 Some recent reports suggest more distributed neuropsychologic impairment,155 findings that become understandable in the context of recently reported widely distributed cortical thinning in juvenile myoclonic epilepsy (Fig. 10).156 The degree to which extrathalamofrontal abnormalities are linked to distributed neuropsychologic impairments and the presence and characteristics of phenotypes of structure-function abnormalities remain to be investigated in this and other epilepsy syndromes.
Fig. 10.
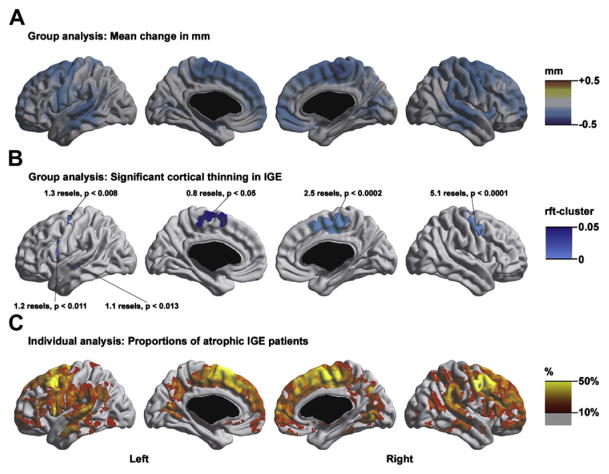
Group and individual analyses of cortical thickness. (A) Absolute differences in cortical thickness (in millimeters) between idiopathic generalized epilepsy (IGE) patients and controls. (B) Areas of significant cortical thinning in IGE compared with controls. Peak positions and resolution elements (ie, resels) of significant clusters after random field theory (rft) correction are shown (cluster threshold t ≤ −3.2, cluster extent threshold 0.8 resels). (C) Individual analysis. At each vertex, the corresponding proportion of atrophic patients with a thickness z-score of ≤ −2 SD with respect to healthy controls is shown. Only fractions above 10% are displayed. In controls, no vertex displayed a prevalence of atrophy above 10%. Individual analysis showed widespread atrophy in more than 10% of patients; up to 40% of them had atrophy localized in the same areas detected by the group analysis. (From Bernhardt BC, Rozen DA, Worsley KJ, et al. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage 2009;46:373–81.)
It is hypothesized that within most if not all epilepsy syndromes there exists a distribution of cognitive phenotypes that are linked to structural, metabolic, and other neuroimaging features. These structural abnormalities will lend shape to the patients’ neuropsychologic profile. In addition to these structural abnormalities with associated neurobehavioral consequences will be the added the perhaps more variable influence of factors such as antiseizure medications and their particular adverse cognitive profiles,21 variations in epileptiform and other waveform abnormalities,157–160 complications of the disorder,25 and postictal effects. In addition, familial susceptibilities and other factors will influence cognitive status.161,162
EPILEPSYAND COGNITION: BRIDGING THE OLD AND NEW LITERATURES
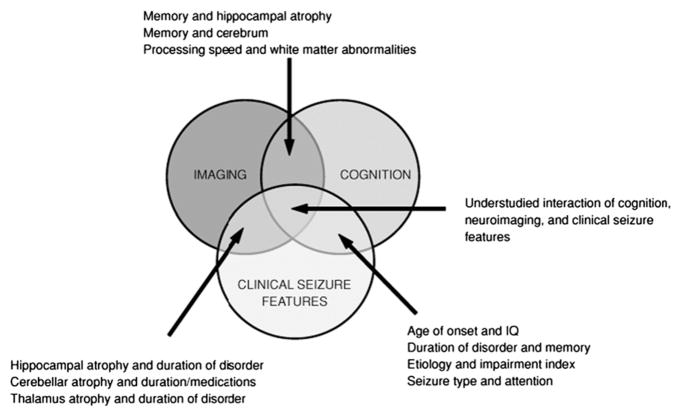
This article describes a developing architecture of cognitive impairment in the epilepsies, moving from the long established traditional focus on the relationship between neuropsychological status and clinical epilepsy characteristics (eg, seizure frequency, seizure severity, duration of epilepsy, age of onset, etiology, medications), to one focusing on interrelationships between underlying anatomic, metabolic, and other neurobiologic correlates of the epilepsies with critical cognitive and behavioral functions. This is a paradigm shift that has a strong influence on the understanding of the neuropsychology of epilepsy. The older literature, however, is reliable, well understood, and clinically meaningful. Is there some way to understand the intersection of the cognition and clinical seizure feature and the cognition and neuroanatomy literatures (Fig. 11), and are there methods that might help inform the understanding of the neuroanatomic pathways through which clinical seizure features exert their impact on cognition in epilepsy?
Fig. 11.
The intersection of imaging, cognition, and clinical epilepsy characteristics. (From Oyegbile TO, Bhattacharya A, Seidenberg M, et al. Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. Epilepsia 2006;47:143–52.)
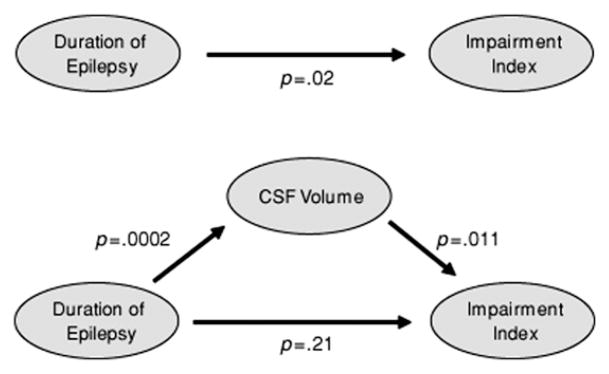
In a first step to address this issue, the authors tested a mediator-moderator model to determine how overall cognitive impairment, total CSF, and duration of epilepsy were associated and whether duration of epilepsy affected cognition directly or through a structural neuroimaging marker (brain atrophy reflected in total CSF) (Fig. 12). It was found that total CSF mediated the relationship between duration of epilepsy and cognitive impairment.136 That is, the longer the duration of epilepsy, the more abnormal the total CSF, with resulting greater cognitive impairment. The impact of duration of epilepsy on cognition was mediated by the degree of overall brain atrophy reflected in total CSF. This represents a crude initial approach to a complex problem, but this statistical approach may have some use in understanding these complex relationships and the move toward a more unified understanding of the neuropsychologic consequences of the epilepsies.
Fig. 12.
Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. (From Oyegbile TO, Bhattacharya A, Seidenberg M, et al. Epilepsia 2006;47(1):143–52.)
CONCLUSION
The landscape of cognitive impairment is clearly in transition from a long-standing focus on the relationship between cognitive function and clinical epilepsy features to one linking cognitive impairment to a multitude of neuroimaging parameters. Whether it will be possible to derive a broad understanding of cognition, clinical epilepsy features and neuroimaging markers remains to be determined. This represents an interesting research challenge for the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folsom A. Psychological testing in epilepsy. Epilepsia. 1952;1:15–22. [Google Scholar]
- 2.Keating LE. A review of the literature on the relationship of epilepsy and intelligence in school children. J Ment Sci. 1960;106:1042–59. doi: 10.1192/bjp.106.444.1042. [DOI] [PubMed] [Google Scholar]
- 3.Tarter RE. Intellectual and adaptive functioning in epilepsy: a review of 50 years of research. Dis Nerv Syst. 1972;33:763–70. [PubMed] [Google Scholar]
- 4.Trimble MR, Thompson PJ. Neuropsychological and behavioral sequelae of spontaneous seizures. Ann N Y Acad Sci. 1986;462:284–92. doi: 10.1111/j.1749-6632.1986.tb51263.x. [DOI] [PubMed] [Google Scholar]
- 5.Novelly RA. The debt of neuropsychology to the epilepsies. Am Psychol. 1992;47:1126–9. doi: 10.1037//0003-066x.47.9.1126. [DOI] [PubMed] [Google Scholar]
- 6.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–72. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 7.Boake C. From the Binet-Simon to the Wechsler-Bellevue: tracing the history of intelligence testing. J Clin Exp Neuropsychol. 2002;24:383–405. doi: 10.1076/jcen.24.3.383.981. [DOI] [PubMed] [Google Scholar]
- 8.Wallin JEW. Eight months of psycho-clinical research at the New Jersey state village for epileptics, with some results from the Binet-Simon testing. Epilepsia. 1912;A3:366–80. [Google Scholar]
- 9.Angers WP. Intelligence quotients of institutionalized and non-institutionalized epileptics. J Psychol Studies. 1962;13:152–6. [Google Scholar]
- 10.Lennox WG. Epilepsy and related disorders. Boston: Little, Brown and Co; 1960. [Google Scholar]
- 11.Berg AT, Langfitt JT, Testa FM, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–14. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 12.Hackett R, Hackett L, Bhakta P. Psychiatric disorder and cognitive function in children with epilepsy in Kerala, South India. Seizure. 1998;7:321–4. doi: 10.1016/s1059-1311(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 13.Hoie B, Mykletun A, Sommerfelt K, et al. Seizure-related factors and non-verbal intelligence in children with epilepsy: a population-based study from Western Norway. Seizure. 2005;14:223–31. doi: 10.1016/j.seizure.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Hoie B, Sommerfelt K, Waaler PE, et al. The combined burden of cognitive, executive function, and psychosocial problems in children with epilepsy: a population-based study. Dev Med Child Neurol. 2008;50:530–6. doi: 10.1111/j.1469-8749.2008.03015.x. [DOI] [PubMed] [Google Scholar]
- 15.Tuxhorn I, Kotagal P. Classification. Semin Neurol. 2008;28:277–88. doi: 10.1055/s-2008-1079332. [DOI] [PubMed] [Google Scholar]
- 16.Seino M. Classification criteria of epileptic seizures and syndromes. Epilepsy Res. 2006;70(Suppl 1):S27–33. doi: 10.1016/j.eplepsyres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Nolan MA, Redoblado MA, Lah S, et al. Intelligence in childhood epilepsy syndromes. Epilepsy Res. 2003;53:139–50. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 18.Nolan MA, Redoblado MA, Lah S, et al. Memory function in childhood epilepsy syndromes. J Paediatr Child Health. 2004;40:20–7. doi: 10.1111/j.1440-1754.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Macallister WS, Schaffer SG. Neuropsychological deficits in childhood epilepsy syndromes. Neuropsychol Rev. 2007;17:427–44. doi: 10.1007/s11065-007-9048-4. [DOI] [PubMed] [Google Scholar]
- 20.Hommet C, Sauerwein HC, De Toffol B, et al. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev. 2006;30:85–96. doi: 10.1016/j.neubiorev.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev. 2007;17:413–25. doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- 22.Klove H, Matthews CG. Psychometric and adaptive abilities in epilepsy with differential etiology. Epilepsia. 1966;7:330–8. doi: 10.1111/j.1528-1157.1966.tb03812.x. [DOI] [PubMed] [Google Scholar]
- 23.Matthews CG, Klove H. MMPI performances in major motor, psychomotorand mixed seizure classifications of known and unknown etiology. Epilepsia. 1968;9:43–53. doi: 10.1111/j.1528-1157.1968.tb04956.x. [DOI] [PubMed] [Google Scholar]
- 24.Dodrill CB. Neuropsychological aspects of epilepsy. Psychiatr Clin North Am. 1992;15:383–94. [PubMed] [Google Scholar]
- 25.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–4. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Aldenkamp AP, Bodde N. Behaviour, cognition and epilepsy. Acta Neurol Scand Suppl. 2005;182:19–25. doi: 10.1111/j.1600-0404.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs FA, Gibbs EL, Lennox WG. Epilepsy: a paroxysmal cerebral dysrhythmia. Brain. 1937;60:377–88. doi: 10.1016/s1525-5050(02)00050-1. [DOI] [PubMed] [Google Scholar]
- 28.Dejong RN. Psychomotor or temporal lobe epilepsy: a review of the development of our present concepts. Neurology. 1957;7:1–14. doi: 10.1212/wnl.7.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs FA, Gibbs EL, Lennox WG. Cerebral dysrhythmias of epilepsy: measures for their control. Arch Neurol Psychiatr. 1938;39:298–314. [Google Scholar]
- 30.Jasper H, Kershman J. Electroencephalographic classification of the epilepsies. Arch Neurol Psychiatr. 1941;45:903. [Google Scholar]
- 31.Gibbs EL, Gibbs FA, Fuster B. Psychomotor epilepsy. Arch Neurol Psychiatr. 1948;60:331–9. doi: 10.1001/archneurpsyc.1948.02310040002001. [DOI] [PubMed] [Google Scholar]
- 32.Hermann BP, Stone JL. A historical review of the epilepsy surgery program at the University of Illinois Medical Center: the contributions of Bailey, Gibbs, and collaborators to the refinement of anterior temporal lobectomy. J Epilepsy. 1989;2:155–63. [Google Scholar]
- 33.Feindel W, Leblanc R, de Almeida AN. Epilepsy surgery: historical highlights 1909–2009. Epilepsia. 2009;50(Suppl 3):131–51. doi: 10.1111/j.1528-1167.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- 34.Hebb DO, Penfield W. Human behavior after extensive bilateral removal from the frontal lobes. Not Found In Database. 1940;44:421–38. [Google Scholar]
- 35.Milner B. Intellectual function of the temporal lobes. Psychol Bull. 1954;51:42–62. doi: 10.1037/h0054728. [DOI] [PubMed] [Google Scholar]
- 36.Milner B, Penfield W. The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc. 1955;80:42–8. [PubMed] [Google Scholar]
- 37.Halsted WC. Some behavioral aspects of partial temporal lobectomy in man. Proceedings of the association for research in nervous and mental disease; Baltimore (MD): Williams & Wilkins; 1958. pp. 478–89. [PubMed] [Google Scholar]
- 38.Meyer V, Yates AJ. Intellectual changes following temporal lobectomy for psychomotor epilepsy: preliminary communication. J Neurol Neurosurg Psychiatr. 1955;18:44–52. doi: 10.1136/jnnp.18.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bladin PF. Murray Alexander Falconer and the Guy’s-Maudsley hospital seizure surgery program. J Clin Neurosci. 2004;11:577–83. doi: 10.1016/j.jocn.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Engel J. Surgical treatment of the epilepsies. 2. New York: Raven Press; 1993. [Google Scholar]
- 41.Engel J., Jr Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992) Neurology. 1993;43:1612–7. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- 42.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 43.Sass KJ, Spencer DD, Kim JH, et al. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40:1694–7. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- 44.Williamson PD, French JA, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–7. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 45.Janszky J, Janszky I, Ebner A. Age at onset in mesial temporal lobe epilepsy with a history of febrile seizures. Neurology. 2004;63:1296–8. doi: 10.1212/01.wnl.0000140701.40447.88. [DOI] [PubMed] [Google Scholar]
- 46.Fox JT. The response of epileptic children to mental and educational tests. Br J Med Psychol. 1924;4:235–48. [Google Scholar]
- 47.Dikmen S, Matthews CG, Harley JP. The effect of early versus late onset of major motor epilepsy upon cognitive-intellectual performance. Epilepsia. 1975;16:73–81. doi: 10.1111/j.1528-1157.1975.tb04723.x. [DOI] [PubMed] [Google Scholar]
- 48.Dikmen S, Matthews CG, Harley JP. Effect of early versus late onset of major motor epilepsy on cognitive-intellectual performance: further considerations. Epilepsia. 1977;18:31–6. doi: 10.1111/j.1528-1157.1977.tb05584.x. [DOI] [PubMed] [Google Scholar]
- 49.Dodrill CB, Matthews CG. The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am Psychol. 1992;47:1139–42. doi: 10.1037//0003-066x.47.9.1139. [DOI] [PubMed] [Google Scholar]
- 50.Glosser G, Cole LC, French JA, et al. Predictors of intellectual performance in adults with intractable temporal lobe epilepsy. J Int Neuropsychol Soc. 1997;3:252–9. [PubMed] [Google Scholar]
- 51.O’Leary DS, Seidenberg M, Berent S, et al. Effects of age of onset of tonic-clonic seizures on neuropsychological performance in children. Epilepsia. 1981;22:197–204. doi: 10.1111/j.1528-1157.1981.tb04102.x. [DOI] [PubMed] [Google Scholar]
- 52.O’Leary DS, Lovell MR, Sackellares JC, et al. Effects of age of onset of partial and generalized seizures on neuropsychological performance in children. J Nerv Ment Dis. 1983;171:624–9. doi: 10.1097/00005053-198310000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Schoenfeld J, Seidenberg M, Woodard A, et al. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 54.Hermann BP, Seidenberg M, Schoenfeld J, et al. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 55.Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 56.Oyegbile T, Hansen R, Magnotta V, et al. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–37. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- 57.Marques CM, Caboclo LO, da Silva TI, et al. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav. 2007;10:477–85. doi: 10.1016/j.yebeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Lencz T, McCarthy G, Bronen RA, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992;31:629–37. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- 59.Cendes F, Andermann F, Gloor P, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–25. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 60.Salmenpera T, Kalviainen R, Partanen K, et al. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res. 2001;46:69–82. doi: 10.1016/s0920-1211(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 61.Bernasconi N, Bernasconi A, Caramanos Z, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–9. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 62.Bernasconi N, Bernasconi A, Andermann F, et al. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology. 1999;52:1870–6. doi: 10.1212/wnl.52.9.1870. [DOI] [PubMed] [Google Scholar]
- 63.Bonilha L, Rorden C, Halford JJ, et al. Asymmetrical extra-hippocampal grey matter loss related to hippocampal atrophy in patients with medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatr. 2007;78:286–94. doi: 10.1136/jnnp.2006.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuzniecky R, Bilir E, Gilliam F, et al. Quantitative MRI in temporal lobe epilepsy: evidence for fornix atrophy. Neurology. 1999;53:496–501. doi: 10.1212/wnl.53.3.496. [DOI] [PubMed] [Google Scholar]
- 65.DeCarli C, Hatta J, Fazilat S, et al. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43:41–5. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- 66.Dreifuss S, Vingerhoets FJ, Lazeyras F, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–41. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 67.Natsume J, Bernasconi N, Andermann F, et al. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- 68.Szabo CA, Lancaster JL, Lee S, et al. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2006;27:2155–60. [PMC free article] [PubMed] [Google Scholar]
- 69.Marsh L, Morrell MJ, Shear PK, et al. Cortical and hippocampal volume deficits in temporal lobe epilepsy. Epilepsia. 1997;38:576–87. doi: 10.1111/j.1528-1157.1997.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee JW, Andermann F, Dubeau F, et al. Morphometric analysis of the temporal lobe in temporal lobe epilepsy. Epilepsia. 1998;39:727–36. doi: 10.1111/j.1528-1157.1998.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 71.Jutila L, Ylinen A, Partanen K, et al. MR volumetry of the entorhinal, perirhinal, and temporopolar cortices in drug-refractory temporal lobe epilepsy. AJNR Am J Neuroradiol. 2001;22:1490–501. [PMC free article] [PubMed] [Google Scholar]
- 72.Moran NF, Lemieux L, Kitchen ND, et al. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain. 2001;124:167–75. doi: 10.1093/brain/124.1.167. [DOI] [PubMed] [Google Scholar]
- 73.Hermann B, Seidenberg M, Bell B, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–71. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- 74.Sandok EK, O’Brien TJ, Jack CR, et al. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia. 2000;41:1315–20. doi: 10.1111/j.1528-1157.2000.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 75.Sisodiya SM, Moran N, Free SL, et al. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Ann Neurol. 1997;41:490–6. doi: 10.1002/ana.410410412. [DOI] [PubMed] [Google Scholar]
- 76.Theodore WH, DeCarli C, Gaillard WD. Total cerebral volume is reduced in patients with localization-related epilepsy and a history of complex febrile seizures. Arch Neurol. 2003;60:250–2. doi: 10.1001/archneur.60.2.250. [DOI] [PubMed] [Google Scholar]
- 77.Weber B, Luders E, Faber J, et al. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–54. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ronan L, Murphy K, Delanty N, et al. Cerebral cortical gyrification: a preliminary investigation in temporal lobe epilepsy. Epilepsia. 2007;48:211–9. doi: 10.1111/j.1528-1167.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 79.Pulsipher DT, Seidenberg M, Morton JJ, et al. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav. 2007;11:442–9. doi: 10.1016/j.yebeh.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–57. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 81.Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 82.McDonald CR, Hagler DJ, Jr, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49:794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- 83.Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage. 2008;42:515–24. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- 84.Mueller SG, Laxer KD, Barakos J, et al. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage. 2009;46(2):353–9. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17(9):2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 86.Seidenberg M, Kelly KG, Parrish J, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–30. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- 87.McMillan AB, Hermann BP, Johnson SC, et al. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–74. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Bernasconi N, Duchesne S, Janke A, et al. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–23. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Gaffan D. Against memory systems. Philos Trans R Soc Lond B Biol Sci. 2002;357:1111–21. doi: 10.1098/rstb.2002.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Filley C. The behavioral neurology of white matter. New York: Oxford University Press; 2001. [Google Scholar]
- 91.Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia. 2001;39:51–70. doi: 10.1016/s0028-3932(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 92.Thiebaut de Schotten M, Urbanski M, Duffau H, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–8. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- 93.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 94.Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 95.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–85. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 96.Gass A, Niendorf T, Hirsch JG. Acute and chronic changes of the apparent diffusion coefficient in neurological disorders: biophysical mechanisms and possible underlying histopathology. J Neurol Sci. 2001;186(Suppl 1):S15–23. doi: 10.1016/s0022-510x(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 97.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57:188–96. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- 98.Rodrigo S, Oppenheim C, Chassoux F, et al. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy: initial findings. Eur Radiol. 2007;17:1663–8. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- 99.Lin JJ, Riley JD, Juranek J, et al. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–70. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto R, Okada T, Mikuni N, et al. Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008;255:1703–11. doi: 10.1007/s00415-008-0005-9. [DOI] [PubMed] [Google Scholar]
- 101.Ahmadi ME, Hagler DJ, Jr, McDonald CR, et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol. 2009 June 9; doi: 10.3174/ajnr.A1650. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arfanakis K, Hermann BP, Rogers BP, et al. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–9. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- 103.Concha L, Gross DW, Wheatley BM, et al. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–9. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- 104.Concha L, Beaulieu C, Collins DL, et al. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatr. 2009;80:312–9. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- 105.Thivard L, Lehericy S, Krainik A, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–90. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 106.Focke NK, Yogarajah M, Bonelli SB, et al. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–37. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 107.Schoene-Bake JC, Faber J, Trautner P, et al. Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage. 2009;46:569–76. doi: 10.1016/j.neuroimage.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Sass KJ, Sass A, Westerveld M, et al. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol. 1992;14:662–72. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- 109.Trenerry MR, Jack CR, Jr, Ivnik RJ, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- 110.Trenerry MR, Jack CR, Jr, Cascino GD, et al. Gender differences in post-temporal lobectomy verbal memory and relationships between MRI hippocampal volumes and preoperative verbal memory. Epilepsy Res. 1995;20:69–76. doi: 10.1016/0920-1211(94)00060-a. [DOI] [PubMed] [Google Scholar]
- 111.Seidenberg M, Geary E, Hermann B. Investigating temporal lobe contribution to confrontation naming using MRI quantitative volumetrics. J Int Neuropsychol Soc. 2005;11:358–66. [PubMed] [Google Scholar]
- 112.Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8:593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Seidenberg M, Hermann B, Pulsipher D, et al. Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. J Int Neuropsychol Soc. 2008;14:384–93. doi: 10.1017/S1355617708080399. [DOI] [PubMed] [Google Scholar]
- 114.Stewart CC, Griffith HR, Okonkwo OC, et al. Contributions of volumetrics of the hippocampus and thalamus to verbal memory in temporal lobe epilepsy patients. Brain Cogn. 2009;69:65–72. doi: 10.1016/j.bandc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rausch R, Henry TR, Ary CM, et al. Asymmetric interictal glucose hypometabolism and cognitive performance in epileptic patients. Arch Neurol. 1994;51:139–44. doi: 10.1001/archneur.1994.00540140045013. [DOI] [PubMed] [Google Scholar]
- 116.Getz K, Hermann B, Seidenberg M, et al. Negative symptoms and psychosocial status in temporal lobe epilepsy. Epilepsy Res. 2003;53:240–4. doi: 10.1016/s0920-1211(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 117.Getz K, Hermann B, Seidenberg M, et al. Negative symptoms in temporal lobe epilepsy. Am J Psychiatry. 2002;159:644–51. doi: 10.1176/appi.ajp.159.4.644. [DOI] [PubMed] [Google Scholar]
- 118.Geary B, Hermann B, Seidenberg M, et al. Anatomic correlates of negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci. 2009;21(2):152–9. doi: 10.1176/appi.neuropsych.21.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keller SS, Baker G, Downes JJ, et al. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav. 2008;49(5):741–57. doi: 10.1016/j.yebeh.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Jokeit H, Seitz RJ, Markowitsch HJ, et al. Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain. 1997;120(Pt 12):2283–94. doi: 10.1093/brain/120.12.2283. [DOI] [PubMed] [Google Scholar]
- 121.Bonilha L, Alessio A, Rorden C, et al. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp. 2007;28(12):1376–90. doi: 10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bengner T, Malina T, Lindenau M, et al. Face memory in MRI-positive and MRI-negative temporal lobe epilepsy. Epilepsia. 2006;47:1904–14. doi: 10.1111/j.1528-1167.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 123.Bengner T, Siemonsen S, Stodieck S, et al. T2 relaxation time correlates of face recognition deficits in temporal lobe epilepsy. Epilepsy Behav. 2008;13:670–7. doi: 10.1016/j.yebeh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Griffith HR, Richardson E, Pyzalski RW, et al. Memory for famous faces and the temporal pole: functional imaging findings in temporal lobe epilepsy. Epilepsy Behav. 2006;9(1):173–80. doi: 10.1016/j.yebeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 125.Botez MI, Botez T, Elie R, et al. Role of the cerebellum in complex human behavior. Ital J Neurol Sci. 1989;10:291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- 126.Schmahmann JD. The cerebellum and cognition. New York: Academic Press; 1997. [Google Scholar]
- 127.Eichenbaum H, Chhen N. From conditioning to conscious recollection: memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- 128.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–6. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 129.Wooduff-Pak D, Steinmetz J. Eyeblink classical conditioning: applications in humans. New York: Plenum Press; 2000. [Google Scholar]
- 130.Hermann B, Seidenberg M, Sears L, et al. Cerebellar atrophy in temporal lobe epilepsy affects procedural memory. Neurology. 2004;63:2129–31. doi: 10.1212/01.wnl.0000145774.89754.0c. [DOI] [PubMed] [Google Scholar]
- 131.Daley M, Siddarth P, Levitt J, et al. Amygdala volume and psychopathology in childhood complex partial seizures. Epilepsy Behav. 2008;13:212–7. doi: 10.1016/j.yebeh.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tebartz Van Elst L, Baeumer D, Lemieux L, et al. Amygdala pathology in psychosis of epilepsy: a magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125:140–9. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- 133.Meletti S, Benuzzi F, Rubboli G, et al. Impaired facial emotion recognition in early-onset right mesial temporal lobe epilepsy. Neurology. 2003;60:426–31. doi: 10.1212/wnl.60.3.426. [DOI] [PubMed] [Google Scholar]
- 134.McClelland S, Garcia RE, Peraza DM, et al. Facial emotion recognition after curative nondominant temporal lobectomy in patients with mesial temporal sclerosis. Epilepsia. 2006;47:1337–42. doi: 10.1111/j.1528-1167.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 135.Focke NK, Thompson PJ, Duncan JS. Correlation of cognitive functions with voxel-based morphometry in patients with hippocampal sclerosis. Epilepsy Behav. 2008;12:472–6. doi: 10.1016/j.yebeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 136.Oyegbile TO, Bhattacharya A, Seidenberg M, et al. Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. Epilepsia. 2006;47:143–52. doi: 10.1111/j.1528-1167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 137.Baxendale SA, Sisodiya SM, Thompson PJ, et al. Disproportion in the distribution of gray and white matter: neuropsychological correlates. Neurology. 1999;52:248–52. doi: 10.1212/wnl.52.2.248. [DOI] [PubMed] [Google Scholar]
- 138.Hermann BP, Seidenberg M, Bell B, et al. Extratemporal quantitative MRI volumetrics and neuropsychological function in temporal lobe epilepsy. J Int Neuropsychol Soc. 2003;9:353–62. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- 139.Dow C, Seidenberg M, Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav. 2004;5:919–25. doi: 10.1016/j.yebeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 140.Hermann B, Hansen R, Seidenberg M, et al. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 141.Flugel D, Cercignani M, Symms MR, et al. Diffusion tensor imaging findings and their correlation with neuropsychological deficits in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia. 2006;47:941–4. doi: 10.1111/j.1528-1167.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 142.Diehl B, Busch RM, Duncan JS, et al. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- 143.McDonald CR, Ahmadi ME, Hagler DJ, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waites AB, Briellmann RS, Saling MM, et al. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 145.Janszky J, Mertens M, Janszky I, et al. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–7. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- 146.Powell HW, Parker GJ, Alexander DC, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 147.Powell HW, Parker GJ, Alexander DC, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–21. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 148.Powell HW, Parker GJ, Alexander DC, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatr. 2008;79:327–30. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hermann B, Seidenberg M, Lee EJ, et al. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13:12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- 150.Dabbs K, Jones J, Seidenberg M, et al. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15(4):445–51. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paradiso S, Hermann BP, Somes G. Patterns of academic competence in adults with epilepsy: a cluster analytic study. Epilepsy Res. 1994;19:253–61. doi: 10.1016/0920-1211(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 152.Devinsky O, Gershengorn J, Brown E, et al. Frontal functions in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:243–6. [PubMed] [Google Scholar]
- 153.Piazzini A, Turner K, Vignoli A, et al. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia. 2008;49:657–62. doi: 10.1111/j.1528-1167.2007.01482.x. [DOI] [PubMed] [Google Scholar]
- 154.Sonmez F, Atakli D, Sari H, et al. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–36. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 155.Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, et al. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007;10:263–7. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 156.Bernhardt BC, Rozen DA, Worsley KJ, et al. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. 2009;46:373–81. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 157.Dodrill CB, Wilkus RJ. Relationships between intelligence and electroencephalographic epileptiform activity in adult epileptics. Neurology. 1976;26:525–31. doi: 10.1212/wnl.26.6.525. [DOI] [PubMed] [Google Scholar]
- 158.Wilkus RJ, Dodrill CB. Neuropsychological correlates of the electroencephalogram in epileptics: I. Topographic distribution and average rate of epileptiform activity. Epilepsia. 1976;17:89–100. doi: 10.1111/j.1528-1157.1976.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 159.Koop JI, Fastenau PS, Dunn DW, et al. Neuropsychological correlates of electroencephalograms in children with epilepsy. Epilepsy Res. 2005;64:49–62. doi: 10.1016/j.eplepsyres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 160.Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2003;2:725–30. doi: 10.1016/s1474-4422(03)00584-2. [DOI] [PubMed] [Google Scholar]
- 161.Iqbal N, Caswell HL, Hare DJ, et al. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: a preliminary controlled experimental video-EEG case series. Epilepsy Behav. 2009;14:516–21. doi: 10.1016/j.yebeh.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 162.Clarke T, Strug LJ, Murphy PL, et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2258–65. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]