Abstract
Chemical synthesis is a powerful method for precise modification of the structural and electronic properties of proteins. The difficulties in the synthesis and purification of peptides containing transmembrane segments have presented obstacles to the chemical synthesis of integral membrane proteins. Here, we present a modular strategy for the semi-synthesis of integral membrane proteins in which solid phase peptide synthesis is limited to the region of interest, while the rest of the protein is obtained by recombinant means. This modular strategy considerably simplifies the synthesis and purification steps that have previously hindered the chemical synthesis of integral membrane proteins. We develop a sumo-fusion and proteolysis approach for obtaining the N-terminal cysteine containing membrane spanning peptides required for the semi-synthesis. We demonstrate the feasibility of the modular approach by the semi-synthesis of full-length KcsA K+ channels in which only regions of interest, such as the selectivity filter or the pore helix, are obtained by chemical synthesis. The modular approach is used to investigate the hydrogen bond interactions of a tryptophan residue in the pore helix, tryptophan 68, by substituting it with the iso-steric analog, β-(3-benzothienyl)-L-alanine (3BT). A functional analysis of the 3BT mutant channels indicates that the K+ conduction and selectivity of the 3BT mutant channels are similar to the wild type, but the mutant channels show a three-fold increase in Rb+ conduction. These results suggest that the hydrogen bond interactions of tryptophan 68 are essential for optimizing the selectivity filter for K+ conduction over Rb+ conduction.
Introduction
Understanding the relationship between the structure of a protein and its function requires the ability to modify the protein structure. This is generally accomplished by traditional site directed mutagenesis (SDM). The protein modifications that are possible using SDM are however limited by the set of naturally occurring amino acids. Chemical synthesis of a protein, on the other hand, permits the incorporation of a large number of unnatural amino acids and peptide backbone modifications which enables us to generate precise modification of the structural and electronic properties of the protein molecule.(1) Similar protein modifications are not possible using SDM which makes chemical synthesis an important asset in structure-function investigations.
The development of the native chemical ligation (NCL) methodology has made the chemical synthesis of small to medium sized proteins technically feasible.(2) In the NCL reaction, a peptide with a N-terminal Cys reacts with a peptide with a C-terminal thioester to link the two peptides with a native peptide bond at the ligation site.(3) The NCL reaction therefore enables assembly of the protein from synthetically accessible peptide building blocks. Most reports of chemical synthesis of proteins have however dealt with soluble proteins, while the application of chemical synthesis to integral membrane proteins has been limited.(4) The major factor that complicates the synthesis of an integral membrane protein is the synthesis and purification of peptide segments that contain a transmembrane segment.(5, 6) A "total-synthesis" approach in which all the peptide building blocks required are generated using solid phase peptide synthesis (SPPS) is therefore practical, only for the synthesis of relatively small membrane proteins.(7–9)
Due to the limitations of a total-synthesis approach, we have pursued a semi-synthetic approach in which the membrane protein is assembled from peptide building blocks obtained using both SPPS and recombinant means. Our interests are focused on K+ channels, integral membrane proteins that allow the rapid and selective permeation of K+ ions across biological membranes.(10) We have previously reported the semi-synthesis of the K+ channel, KcsA.(11, 12) In this semi-synthesis, a truncated form of the KcsA channel was obtained by the NCL reaction of a chemically synthesized peptide and a recombinantly expressed peptide thioester followed by in vitro folding to the native state. This semi-synthetic strategy was used for the modification of the selectivity filter (the ion binding sites) using chemical synthesis.(13, 14) However this strategy suffers from the limitations that it does not provide the full-length KcsA channel and more importantly, the strategy does not permit the use of SPPS for manipulating all regions of interest in the KcsA channel. We were faced with this limitation in our investigations of the pore helix of the KcsA channel.
The selectivity filter region in a K+ channel is preceded by a short helix referred to as the pore helix.(15) Residues in the pore helix have extensive hydrogen bond and van der Waal's interactions with the selectivity filter. Mutations of pore helix residues affect conduction properties and "C-type" inactivation, a gating process of the selectivity filter.(16–18) The pore helix has only been investigated by conventional mutagenesis. The precise alterations that are possible using chemical synthesis has the potential to provide additional insights into the exact role of the pore helix in channel structure and function. The pore helix in the KcsA channel comprises residues 62–74.(15) Using the previously reported semi-synthetic strategy to manipulate the pore helix, will therefore require the chemical synthesis of a peptide ~70 amino acids in length. The SPPS of peptides of this length, especially peptides that encompass a transmembrane segment, is extremely challenging.
Here, we develop a modular approach for the semi-synthesis of the KcsA channel that provides the full length channel and allows us to use chemical synthesis to manipulate all the functionally important regions such as the selectivity filter or the pore helix. The modular approach therefore eliminates the limitations of the previously described semi-synthesis. The modular approach that we develop should find general applicability in the semi-synthesis of other integral membrane proteins. We use the modular semi-synthesis to investigate the H-bond interactions of a Trp residue in the pore helix of the KcsA channel. Our investigations demonstrate that the H-bond interactions of Trp68 optimize the selectivity filter for K+ conductions over Rb+ conduction through the channel.
Results and Discussion
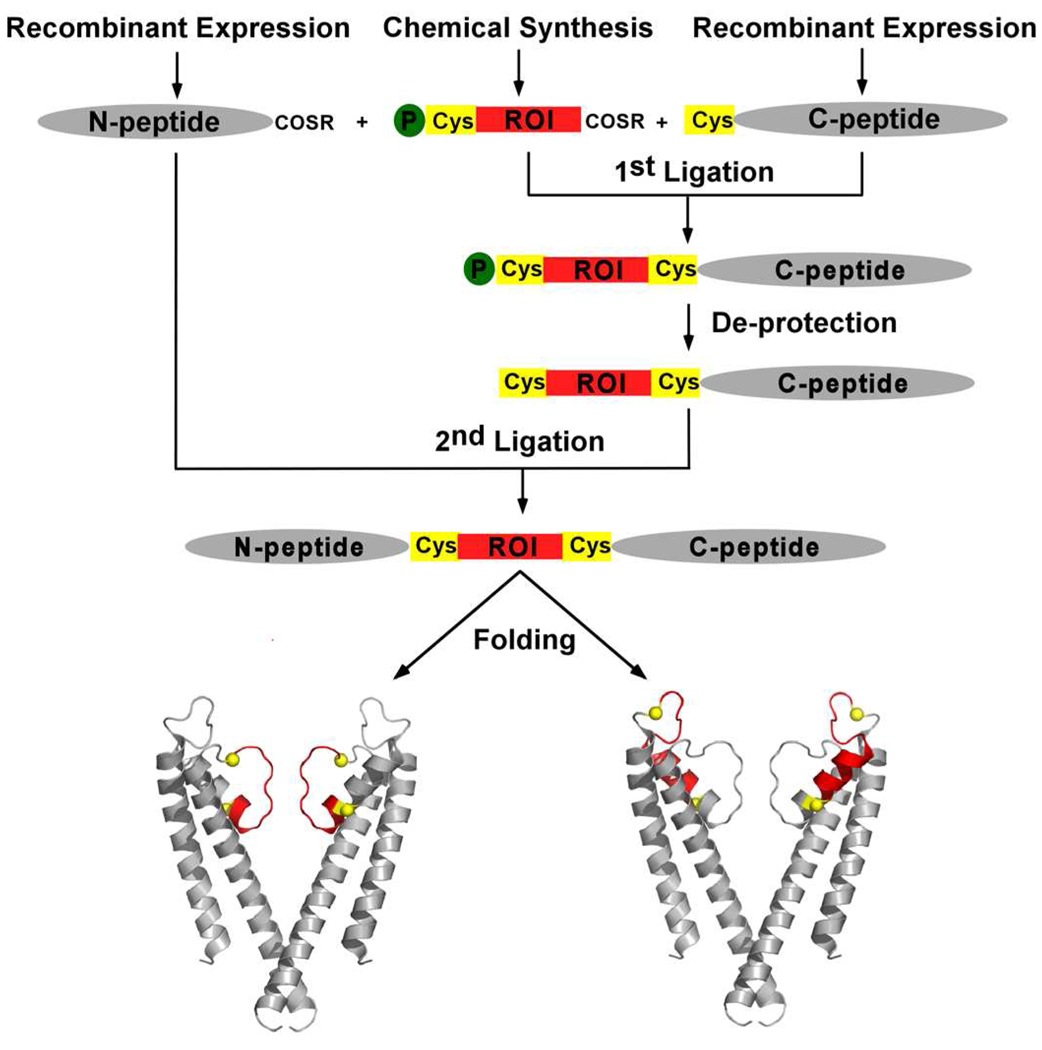
The modular strategy for the semi-synthesis is based on the observation that regions of interest (ROI) in the KcsA channel, such as the amino acids forming the ion binding sites in the selectivity filter (residues 75–79) or the residues that comprise the pore helix (residues 62–74), are contained within a short ~10–20 amino acid stretch. The synthesis and purification of peptides of this size is relatively easy and can be carried out in high yields. In our synthetic strategy, we divide the KcsA channel into three segments, a central peptide that corresponds to the ROI and the protein segments N- and C-terminal to the ROI that we refer to as the N-peptide and the C-peptide respectively (Fig. 1). SPPS is used only for the central ROI while the N and C-peptides are obtained by recombinant means. The use of SPPS for the ROI allows us to take advantage of chemical synthesis to modify the ROI while the use of recombinant means for obtaining the N- and C-peptides liberates us from the size limits of SPPS. An advantage of using recombinant means for obtaining the N- and C-peptides is that the peptides obtained by recombinant means are easier to purify compared to peptides obtained by SPPS.(11) The KcsA polypeptide is then assembled by two sequential NCL reactions. For the NCL reaction, the N-peptide has to bear a C-terminal thioester and the C-peptide has to carry an N-terminal Cys (N-Cys) while the ROI peptide should possess both a C-terminal thioester and a N-Cys. Following assembly, the KcsA polypeptide is folded to the native tetrameric state using the lipid based folding protocol that we have previously established for the in vitro folding of the KcsA channel.(19)
Fig. 1. The modular semi-synthesis of the KcsA channel.
The KcsA polypeptide is synthesized by two sequential ligation reactions. The first ligation reaction between a recombinantly expressed C-peptide and a chemically synthesized region of interest (ROI) peptide yields the intermediate peptide. The Thz protecting group (green sphere) on the N-terminal Cys of the intermediate peptide is removed and the de-protected intermediate peptide is ligated to a recombinantly expressed N-peptide thioester to yield the KcsA polypeptide. The KcsA polypeptide is folded in vitro to the native state. The modular strategy can be used to assemble semi-synthetic KcsA channels in which the selectivity filter (left) or the pore helix (right) is obtained by chemical synthesis. The protein segments obtained by chemical synthesis are colored red while the protein segments obtained by recombinant means are colored grey. The ligation sites are represented by yellow spheres.
The first step in the semi-synthesis is to identify two positions flanking the ROI at which Cys residues can be introduced to serve as the ligation sites. We identified residues 70 and 82, which flank the selectivity filter, as positions at which Cys substitutions are well tolerated and were therefore selected as the ligation sites when the ROI is the selectivity filter.(14, 20) Similarly, positions 54 and 70 were selected as the ligation sites when the ROI is the pore helix. A semi-synthetic KcsA channel in which the selectivity filter is obtained by SPPS is referred to as KcsA(70,82) while a semi-synthetic KcsA channel in which the pore helix is obtained by SPPS is referred to as KcsA(54,70).
Our modular semi-synthetic strategy calls for a recombinant approach for generating membrane spanning N-Cys peptides and peptide thioesters. The general approach that has previously been used for obtaining an N-Cys peptide consists of appending a Factor Xa or a tobacco etch virus (TEV) protease cleavage site N-terminal to the Cys residue and generating the N-Cys peptide by proteolysis.(21, 22) However, these proteases have only been used for obtaining N-Cys peptides corresponding to soluble proteins. Our tests of these proteases for generating the membrane spanning N-Cys peptides required for the semi-synthesis of the KcsA channel were not successful. We observed low levels of cleavage and subsequently obtained only very low yields of the desired peptide (data not shown). We hypothesized that the lack of successful cleavage is due to the hydrophobic nature (due to the presence of a transmembrane segment) of the peptides which causes aggregation thereby preventing access of the protease to the cleavage site. Attempts to improve accessibility of the protease to the cleavage sites, for example, through the use of ionic detergents or refolding in the presence of the protease were not successful.
As the commonly used strategies failed to provide the desired N-Cys peptide, we investigated the sumo protease for this purpose. The sumo protease cleaves proteins that are fused to the C-terminus of the sumo protein and the cleavage takes place following the Gly-Gly residues at the C-terminus of sumo (Fig. 2A).(23) The cleavage is independent of the residue at the N-terminus of the fusion protein (except Pro) and can therefore be used to obtain peptides with a N-terminal Cys.(23) As the sumo protein is highly expressed and water soluble, we expected that the fusion of the sumo protein to the membrane spanning polypeptide will increase the expression and solubility of the fusion protein.(24)
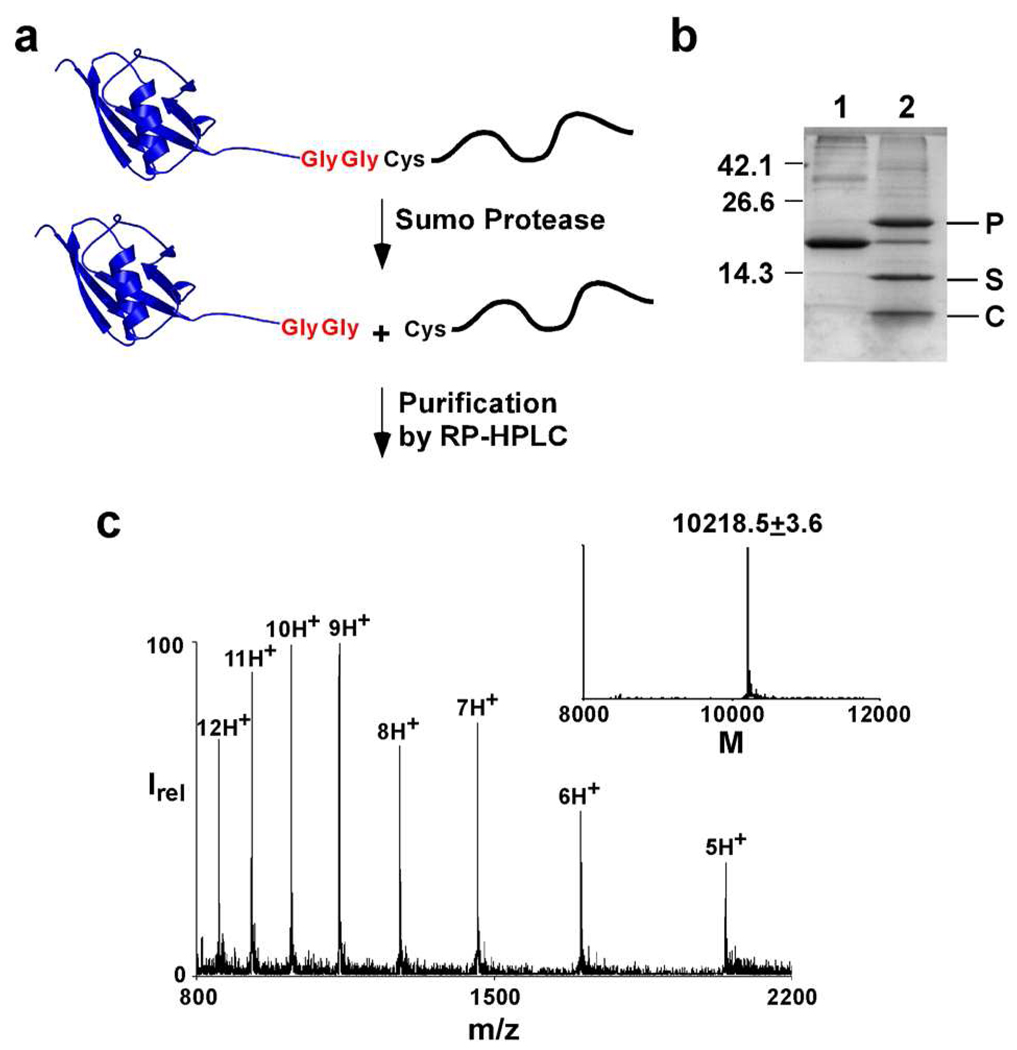
Fig. 2. The Sumo fusion-proteolysis strategy for obtaining membrane spanning N-Cys peptides.
a) The peptide of interest is expressed as a sumo fusion. The fusion protein is cleaved by the sumo protease to generate the desired N-Cys peptide that is purified by RP-HPLC. b) SDS-PAGE of the sumo fusion protein (lane 1) and the proteolysis reaction (lane 2) with P, sumo protease; S, sumo protein; C, N-Cys KcsA (82–160) C-peptide. c) ES-MS of the purified N-Cys KcsA peptide (82–160). Inset, reconstructed spectrum (Expected mass = 10220.6).
We generated a fusion protein in which residues 82–160 of KcsA with a Cys substitution at position 82 were fused after the C-terminal Gly-Gly residues of the sumo protein. A His6 tag was introduced at the C-terminus of the fusion protein for easy purification. As anticipated, high levels of expression of the fusion protein was observed. The fusion protein was purified using immobilized metal affinity chromatography and cleavage of the purified protein by the sumo protease was tested. Complete and rapid cleavage of the fusion protein (cleavage was generally complete within 1 h) was observed resulting in the desired peptide (Fig. 2B). The 82–160 N-Cys peptide released by proteolysis was purified using RP-HPLC and identity of the peptide was confirmed by ES-MS (Fig. 2C). This procedure provided ~2 mg of the purified 82–160 N-Cys peptide per L of bacterial culture. The 70–160 N-Cys peptide required for the semi-synthesis of KcsA(54,70) was also obtained using the same approach.
We have previously reported on a “sandwich-fusion” strategy for obtaining the N-peptide thioester corresponding to residues 1–69 required for the semi-synthesis of KcsA(70,82).(11) The sandwich fusion strategy also provided the N-peptide thioester (residues 1–53) required for the semi-synthesis of KcsA(54,70) in good yield.
The ROI peptides corresponding to the selectivity filter (residues 70–81, the filter peptide) or the pore helix (residues 54–69, the helix peptide) were synthesized using SPPS. The ROI peptides bear a thioester at the C-terminus and a protected Cys residue at the N-terminus. The N-terminal Cys residue is protected to prevent cyclization and/or polymerization of the ROI peptide during the first ligation reaction. The 1,3-thiazolidine-4-carboxo (Thz) group was selected for protection of the N-Cys due to the ease with which the Cys residue can be de-protected for use in the subsequent ligation step.(25) The small size of the ROI peptide enables easy synthesis and purification of the filter and the pore peptides.
The KcsA(70,82) or the KcsA(54,70) polypeptides were assembled in the C to N direction by two sequential ligation reactions. In the first ligation reaction to assemble the KcsA(70,82) polypeptide, the chemically synthesized pore peptide thioester (residues 70–81) was ligated to the C-terminal peptide (residues 82–160) to form the intermediate peptide (residues 70–160). A survey of reaction conditions indicated that rapid ligation took place in the presence of detergents such as SDS (sodium dodecylsulfate) or Fos-12 (n-dodecylphosphocholine) (Fig. 3A). Fos-12 was selected as it does not interfere with the subsequent RP-HPLC purification step. After the ligation reaction, the Thz protecting group at the N-Cys of the intermediate peptide was removed by treatment with methoxylamine and the de-protected intermediate peptide was purified by RP-HPLC and confirmed by ES-MS (Fig. 3B).
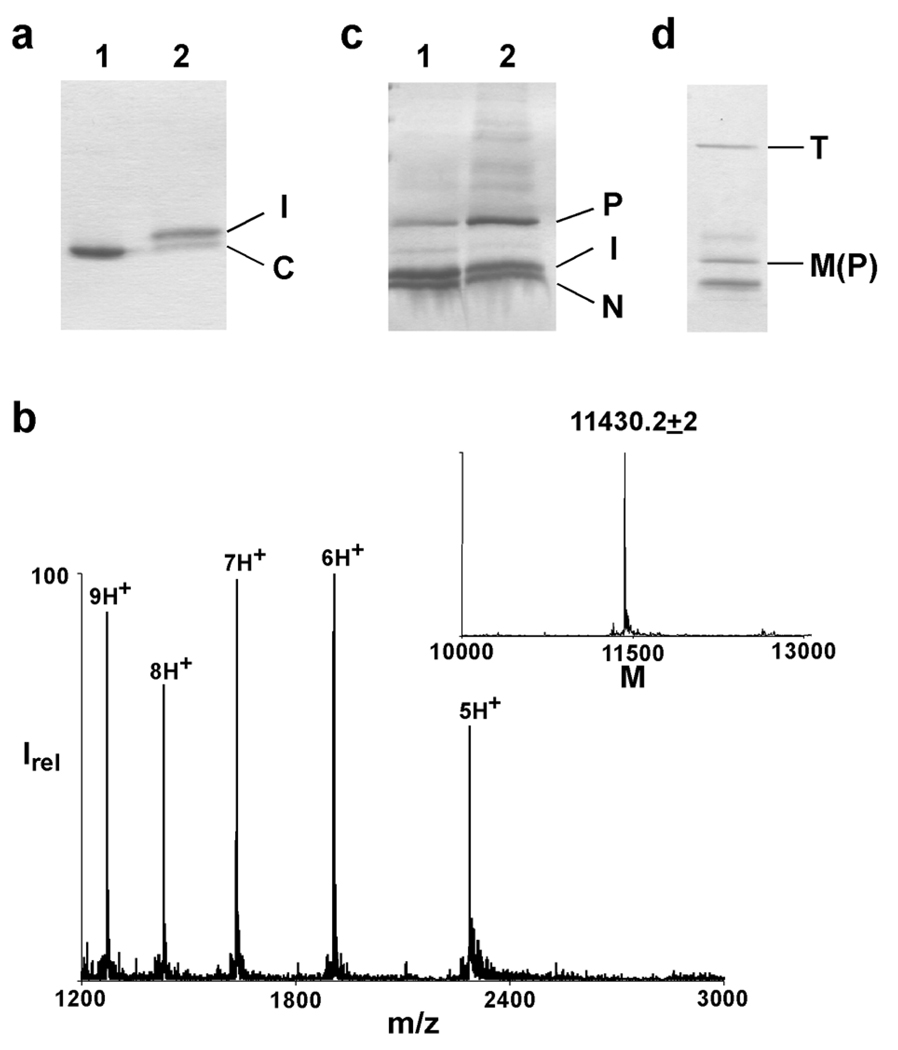
Fig. 3. Semi-synthesis of KcsA(70,82).
a) SDS-PAGE of the first ligation reaction between the C-peptide (C, residues 82–160) and the filter peptide (residues 70–81) to form the intermediate peptide (I, residues 70–160) at 0 min (lane 1) and 24 h (lane 2). b) ES-MS of the intermediate peptide. Inset, reconstructed spectrum (Expected mass = 11431.9). c) SDS-PAGE of the second ligation reaction between the N-peptide thioester (N, residues 1–69) and the intermediate peptide to form the KcsA polypeptide at 0 min (lane 1) and 24 h (lane 2). d) SDS-PAGE showing the folding of semi-synthetic KcsA by lipids. The unfolded monomeric (M, which corresponds to the KcsA polypeptide, P) and the folded tetrameric KcsA (T) are indicated.
In the second ligation reaction, the intermediate peptide (residues 70–160) was ligated to the N-peptide thioester (residues 1–69) to obtain the full length KcsA polypeptide. Analysis of commonly used ligation reaction conditions indicated a very low extent of reaction, even in the presence of detergents such as SDS or Fos-12 that were beneficial for the first ligation step. Based on published reports that the ligation reaction between membrane spanning peptides is enhanced in the presence of lipid vesicles, we tested the ligation reaction after incorporating the N-peptide thioester and the intermediate peptide into POPC (1-Palmitoyl-2-Oleoyl-3-Glycreo-Phosphatidylcholine) lipid vesicles.(26, 27) In POPC vesicles, we observed a rapid ligation reaction that proceeded to around 50% completion (Fig. 3C). The lipid vesicle enhanced ligation strategy was therefore used for the second ligation reaction between the intermediate peptide and the N-peptide thioester to obtain the KcsA(70,82) polypeptide.
The final step of the semi-synthesis is the in vitro folding of the KcsA polypeptide to the native state. We used the lipid based procedure for the in vitro folding reaction.(11, 19) The folding reaction was carried out on the reaction mixture itself without purification of the KcsA polypeptide from the reaction mixture. The reduced yield of the folding reaction obtained using the reaction mixture was offset by the losses that were encountered during the purification of the KcsA polypeptide prior to the folding reaction. Folding was confirmed by the identification of a tetrameric protein band on SDS-PAGE (Fig. 3D). The presence of a His6 tag at the C-terminus enabled the separation of the folded and the unfolded proteins from the lipid vesicles using immobilized metal affinity chromatography. The folded tetrameric protein was then separated from the unfolded molecules and the un-reacted peptides by size-exclusion chromatography. Starting with 4 mg of the C-peptide, the first ligation step followed by de-protection and RP-HPLC purification gave ~1.5 mg of the intermediate peptide, which after the second ligation step, in vitro folding and purification provided 0.1 mg of the folded semi-synthetic KcsA(70,82) channel.
The semi-synthetic KcsA(54,70) channel was assembled using the recombinantly expressed N-peptide thioester (residues 1–53) and C-peptide (residues 70–160) along with a chemically synthesized helix peptide (residues 54–69). The assembly, in vitro folding and purification of the KcsA(54,70) channel were carried out using the same protocols that were used for generating the semi-synthetic KcsA(70,82) channel.
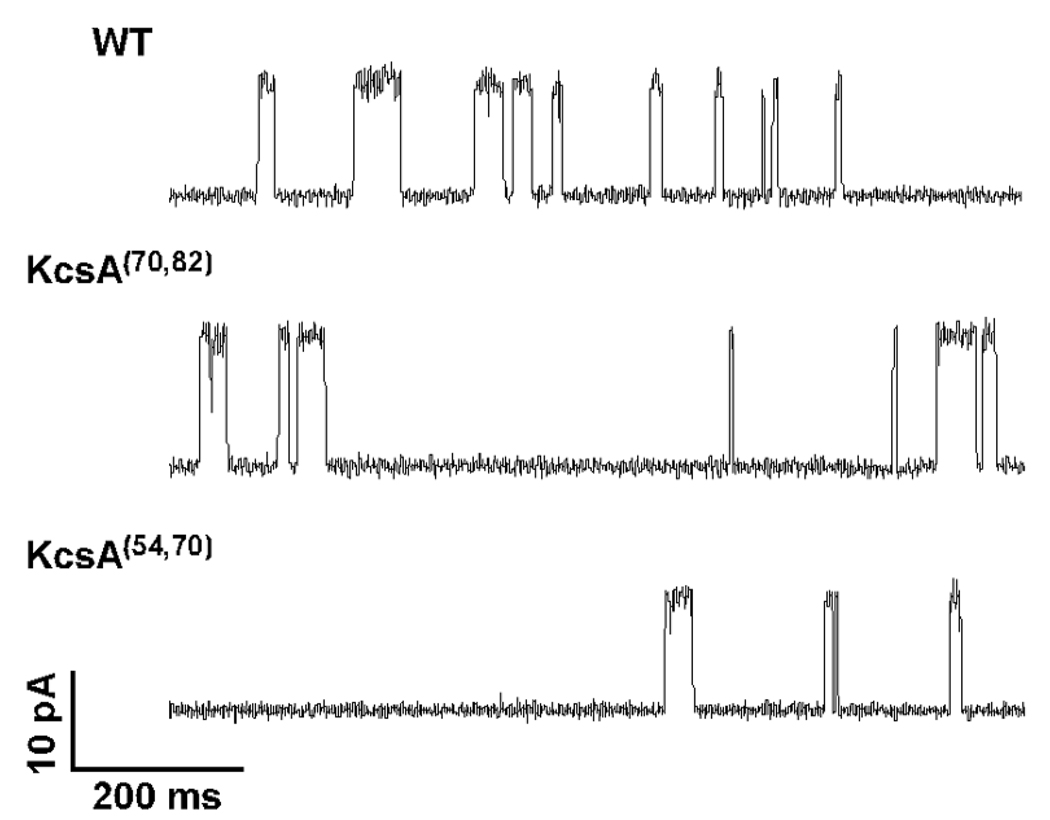
For functional analysis, the purified semi-synthetic KcsA channels were incorporated into planar lipid bilayers as previously described.(19) Channel activity for the semi-synthetic KcsA channels was readily detected. Representative single channel traces for the wild-type channel and the semi-synthetic channels are shown in Fig. 4. The chord conductance for the semi-synthetic channels measured at +150 mV is similar to the chord conductance for the wild-type channel (80±2 pS for KcsA(70,82), 81±10 pS for KcsA(54,70) and 85±9 pS for wild-type KcsA).
Fig. 4. Single channel activity of semi-synthetic KcsA.
Single channel traces for the wild-type (WT) KcsA channel, the semi-synthetic KcsA(70,82) and KcsA(54,70) channels recorded at +150 mV in 10 mM succinate/150 mM KCl (pH 4.0) inside and 10 mM HEPES/150 mM KCl (pH 7.5) outside are shown.
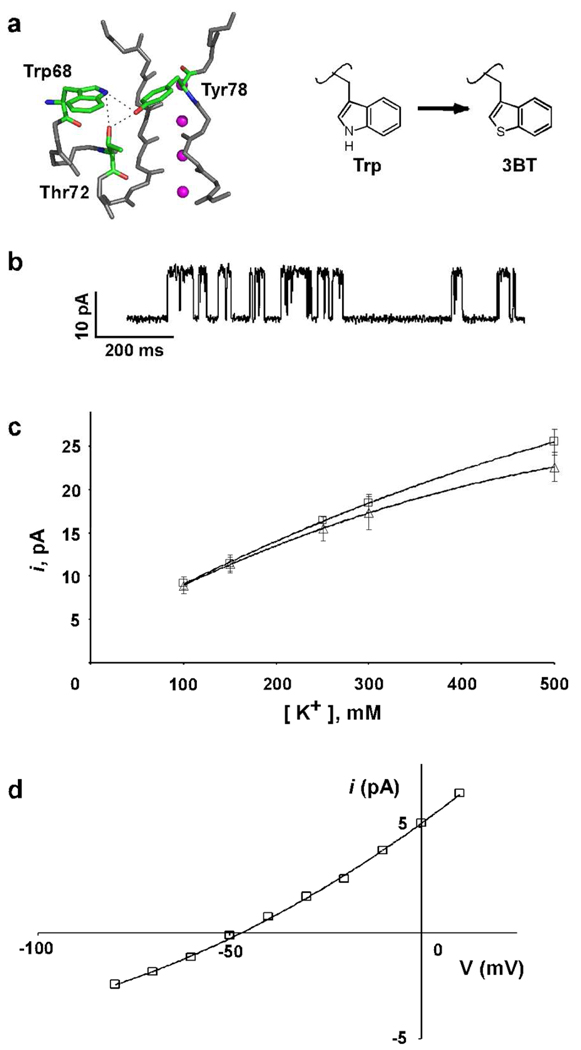
The modular semi-synthesis approach was used to investigate H-bond interactions of Trp68 in the pore helix. The crystal structure of the KcsA channel indicates that Trp68 forms H-bonds interactions with Thr72 at the base of the pore helix of the same subunit and Tyr78 in the selectivity filter of the adjacent subunit (Fig 5A).(28) To investigate the H-bond interactions of Trp68, we replaced it with β-(3-benzothienyl)-L-alanine (3BT). The 3BT side chain is iso-steric to the Trp side chain but the indole N-H in the Trp side chain, which participates in H-bond interactions, is replaced by a sulfur atom in the 3BT side chain (Fig. 5A). Thus with the Trp68→3BT substitution, the H-bond interactions are disrupted while the perturbation of the VDW interactions of the side chain are expected to be minimal. This is in contrast to the natural aromatic amino acid substitutions of Tyr or Phe that perturb both the H-bond and the VDW interactions of the side chain. The 3BT substitution therefore provides us with a precise method to evaluate the role of the H-bond interactions of Trp side chain.
Fig. 5. The KcsA(Trp68→3BT) channel.
a) Close-up view of the selectivity filter. The peptide backbone of residues 68–80 and the side chains of residues Trp68 and Thr72 from one subunit and Tyr78 from an adjacent channel subunit that are involved in a H-bond network are shown in stick representation (pdb code: 1k4c). K+ ions are shown as magenta spheres. b) Representative single channel traces of the KcsA(Trp68→3BT) channels recorded at +150 mV in 10 mM succinate/150 mM KCl (pH 4.0) inside and 10 mM HEPES/150 mM KCl (pH 7.5) outside. c) Single channel current at +150 mV as a function of the K+ ion concentration for KcsA(Trp68→3BT) (Δ) and wild-type KcsA(□). Solid lines have no theoretical meaning. d) Macroscopic currents recorded using 10 mM succinate and 150 mM KCl (pH 4.0) as the internal solution, and 10 mM HEPES, 20 mM KCl, and 130 mM NaCl (pH 7.5) as the external solution are plotted against the voltage applied to determine the reversal potential (calculated reversal potential = −51 mV, measured = −48.3±2.2 mV).
To substitute Trp68 with 3BT, a helix peptide (residues 54–69) thioester with the desired substitution was synthesized. The Trp68→3BT substituted helix peptide was used to assemble the mutant KcsA channels using the modular semi-synthetic approach. Functional analysis of the Trp68→3BT KcsA channel in planar lipid bilayers indicated that the mutant channels were functional (Fig. 5B). Comparison to the wild-type channel indicates that the conductance for the mutant channels (at +150 mV in 150 mM K+) is similar to the wild-type channel with a conductance of 76±6 pS for the mutant channels. The K+ conductance-concentration profile for the mutant channel is also similar to the wild-type channel (Fig. 5C) except that the conductance of the mutant channel is slightly lower at higher K+ concentrations. Reversal potential measurements were used to determine the effect of the 3BT substitution on K+/Na+ selectivity.(29) With 150 mM K+ in the internal solution and 130 mM Na+ and 20 mM K+ in the external solutions, the current reverses at −48.3±2.2 mV which is very near the calculated Nernst potential of −51 mV for the K+ gradient (Fig. 5D). This indicates that the Trp68→3BT KcsA channel is selective for K+ over Na+ and we can therefore conclude that the H-bond interactions of the Trp68 side chain are not essential for K+ over Na+ discrimination.
K+ channels also conduct the slightly larger Rb+ ions but at lower rates than K+, and the Rb+ conductance of the wild-type KcsA channel is lower than the K+ conductance.(30, 31) Further, the Rb+ conductance-concentration profile shows no appreciable increase with Rb+ concentration for concentrations > 100 mM, which is different from the profile observed for K+.(30, 31) These observations suggest that the selectivity filter of K+ channels is optimized for conduction of K+ over Rb+. Crystallographic analysis of ion binding to the selectivity filter of the KcsA channel have revealed differences in the occupancy of K+ and Rb+ ions in the selectivity filter. K+ ions show equal occupancy at the four ion binding sites while Rb+ ions only occupy sites 1,3 and 4 with negligible occupancy at site 2.(28, 30) The structural reason for the differences in the binding of K+ and Rb+ ions to the selectivity filter or the permeation properties of these ions is presently not clear.
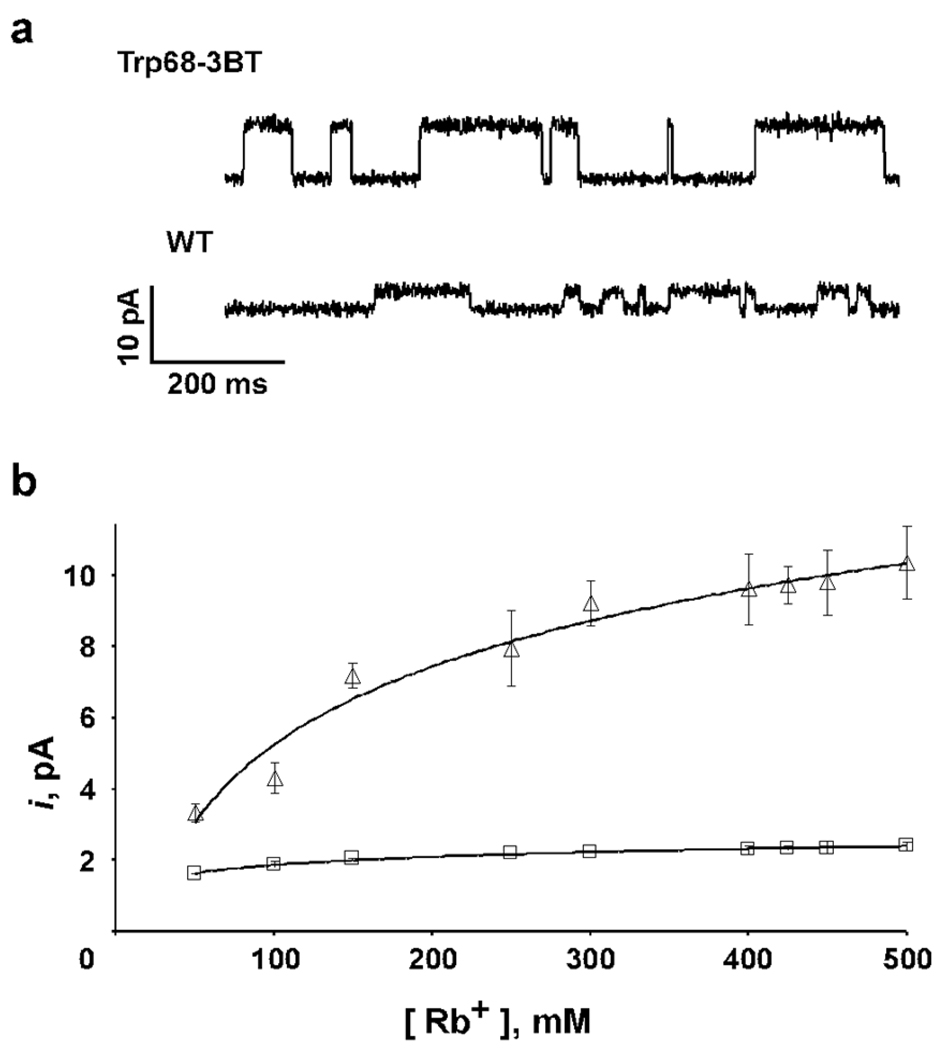
A single channel trace for the Trp68→3BT channel in 150 mM Rb+ is shown in Fig. 6A. Comparison to the wild-type indicates that the single channel conductance for the 3BT mutant channel is ~3 fold higher with a chord conductance of 49±3 pS at +150 mV in 150 mM Rb+ for the 3BT mutant channels compared to 14±2 pS for the wild-type channel. The conductance-concentration profile for the 3BT mutant in Rb+ shows an increase in conductance with concentration in contrast to the wild-type protein (Fig. 6B). These observations indicate that the Trp68→3BT substitution, while essentially silent for K+ conduction, increases the rate of Rb+ conduction through the selectivity filter. Based on these results, we can conclude that (in the wild-type channel) the H-bond interactions of the Trp68 side chain serve to limit Rb+ conduction through the selectivity filter. Ion conduction through the selectivity filter is a multi-step process involving the binding of ions to the selectivity filter, transit through and the release of ions from the selectivity filter. While we cannot presently attribute the effect of the Trp68→3BT substitution to a specific step in the conduction process, we speculate that the disruption of the H-bonds of Trp68 mainly affects the process of transit of Rb+ ions through the selectivity filter.
Fig. 6. Rb+ conduction by KcsA(Trp68→3BT) channels.
a) Representative single channel traces of the KcsA(Trp68→3BT) or wild-type KcsA channels recorded at +150 mV in 10 mM succinate/150 mM RbCl (pH 4.0) inside and 10 mM HEPES/150 mM RbCl (pH 7.5) outside. b) Single channel current at +150 mV as a function of the Rb+ ion concentration for KcsA(Trp68→3BT) (Δ) and wild-type KcsA(□). Solid lines have no theoretical meaning.
In conclusion, we have developed a modular approach for the semi-synthesis of the KcsA K+ channel in which the region of interest is obtained by SPPS while the rest of the protein is obtained by recombinant means. We have developed a sumo fusion strategy for obtaining the membrane spanning N-Cys peptides and used a sandwich fusion strategy for obtaining the membrane spanning thioester peptides required for the semi-synthesis. We have used the modular strategy to assemble semi-synthetic KcsA channels in which either the selectivity filter or the pore helix was obtained by SPPS.
Using this modular approach, we substituted Trp68 in the pore helix of KcsA with 3BT to disrupt the H-bond interactions of the side chain. Functional analysis of the Trp68→3BT mutant demonstrated an increased rate of Rb+ conduction but only a marginal effect on K+ conduction. This result indicates that the H-bond interactions of Trp68 optimize the selectivity filter for K+ conduction over Rb+. This study provides a specific example of how the interactions between the residues forming ion binding sites and the surrounding protein residues modulate ion conduction through the selectivity filter. We expect that the modular semi-synthetic approach developed herein will be useful in future investigations to understand the structural and functional roles of the other non-covalent interactions of residues in the K+ selectivity filter and the pore helix. Further, we anticipate that the modular approach described will also be applicable to the semi-synthesis of other integral membrane proteins.
Experimental Methods
Expression and purification of N-Cys peptides
The sumo fusion proteins consisted of KcsA channel residues (82–160 with Tyr82→Cys or residues 70–160 with Val70→Cys) after the Gly98 of the yeast sumo protein.(23) The fusion proteins also contained the Ala98→Gly substitution which increases the open probability of the KcsA channel.(12) A thrombin site followed by a His6 purification tag was introduced following the KcsA channel sequences. The protein construct was expressed in Escherichia coli BL21(DE3) cells. For protein purification, the cells were lysed by sonication and the fusion protein was solubilized by the addition of N-lauryl-sarcosine to 1% (w/v). Triton-X-100 was then added to 2% (v/v) and the sumo fusion protein was purified using a Co2+ affinity column (Clontech). The purified protein was cleaved using a 1/50 ratio of the sumo protease in the presence of 2 mM DTT. The sumo protease used was expressed and purified as previously described.(32) The N-Cys peptides that were liberated by the sumo protease were purified by RP-HPLC and confirmed by ES-MS [observed mass for 82–160 N-Cys peptide = 10218.5±3.6, expected = 10220.6; observed mass for 70–160 N-Cys peptide = 11490.4±1.6, expected = 11491.9].
Expression and purification of N-peptide thioesters
A sandwich fusion strategy was used for expression of the N-peptide thioesters.(11) The fusion proteins consisted of glutathione-S-transferase at the N-terminus followed by a thrombin site, a strep tag, KcsA channel residues 1–69 or 1–53 followed by the gyrA intein-chitin binding domain. Expression of the fusion protein in inclusion bodies was carried out in Escherichia coli BL21(DE3) cells as previously described.(14) Isolation of the N-peptide thioester from the inclusion bodies was carried out as previously described.(14) The N-peptide thioester was purified by RP-HPLC and confirmed by ES-MS [observed mass for 1–69 thioester peptide = 8554.4±1.9, expected = 8555.2; observed mass for 1–53 thioester peptide = 6871.1±0.7, expected = 6871.2].
Chemical Synthesis of ROI peptides
The sequence of the filter peptide is Thz-CETATTVGYGDL-COSR. The sequence of the helix peptide is Thz-CPGAALISYPDALWWA-COSR. The Trp residue underline was substituted with 3-BT to generate the Trp68→3BT helix peptide. Synthetic peptide thioesters were assembled by manual solid phase peptide synthesis on a S-propylamide derivatized 4-methylbenzylhydralamine (MBHA) resin according to the in situ neutralization/2-(1H-benzo-triazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) activation protocol for Boc-solid phase peptide synthesis.(33, 34) Following synthesis, the peptides were globally de-protected and cleaved from the resin using anhydrous HF at 4 °C as previously described. All the peptides were purified by RP-HPLC and confirmed by ES-MS [observed mass for the filter peptide = 1328.3 (M+H), expected = 1328.5 (M+H); observed mass for helix peptide = 1833.6 (M+H), expected = 1832.8 (M+H); observed mass for Trp68→3BT helix peptide = 1849.8 (M+H), expected = 1849.8 (M+H)].
Assembly of the semi-synthetic KcsA channels
Semi-synthesis of the KcsA channel involved the assembly of the polypeptide by two sequential native chemical ligation reactions followed by in vitro folding of the KcsA polypeptide to the native state. The first ligation reaction was carried out between the C-peptide and the ROI thioester peptide. A 10-fold molar excess of the thioester peptide was used and the ligation reaction was carried out in 0.1 M sodium phosphate buffer, pH 7.4, 1% Fos-12. The reaction was initiated by the addition of thiophenol to 2% and incubated at 37 °C. The reaction was monitored by SDS-PAGE and was usually complete (~90%) in 24 hours. The intermediate peptide that is formed carries a Thz protected Cys residue at the N-terminus. The N-Cys was de-protected by treatment with 0.25 M methoxylamine, 5 mM TCEP, 10 mM methionine, overnight at room temperature.(25) The de-protected intermediate peptide was purified by RP-HPLC and confirmed by ES-MS [KcsA(70,82) intermediate peptide: observed = 11430.2±2.1, expected = 11431.9; KcsA(54,70) intermediate peptide: observed = 13206.3±2.1, expected = 13207.9; KcsA(54,70) intermediate peptide with the Trp68→3BT substitution: observed = 13223.2±1.9, expected = 13224.9].
The second ligation reaction between the intermediate peptide and the corresponding N-peptide thioester was carried out in POPC (1-Palmitoyl-2-Oleoyl-Glycero-3-Phosphatidylcholine) lipid vesicles. The peptides at ~1 mg/mL were reconstituted into POPC lipid vesicles (10 mg/mL in 0.1 M Sodium Phosphate, pH 7.4) and the ligation reaction was initiated by the addition of sodium mercaptoethanesulfonic acid (MESNA) to 0.1 M. The reaction was carried out at 37 °C and monitored by SDS-PAGE. The reaction generally proceeded to around 50% completion after overnight incubation.
Folding reactions were carried out on the second ligation reaction mixtures without further purification. For folding, the POPC lipid vesicles which contain the ligation product was solubilized by the addition of SDS to 1% and reduced by the addition of DTT to 0.1 M. Folding of the KcsA polypeptide was initiated by a 10-fold dilution into Soybean phosphatidylcholine vesicles (Avanti Polar Lipids, 20 mg/mL in 50 mM 2-morpholinoethanesulfonic acid pH 6.4, 0.3 M KCl, 10 mM DTT). The mixture was sonicated in a water bath sonicator and then incubated at room temperature. The extent of folding was monitored by the appearance of a tetramer band on SDS-PAGE.(19)
Purification of semi-synthetic KcsA channels
For purification of the folded semisynthetic KcsA channels, the folding reaction was initially dialyzed against 50 mM Tris pH 7.5, 150 mM KCl for removal of DTT. The folding mixture was then solubilized by the addition of 40 mM decylmaltoside (DM, Anatrace) and the proteins were purified from the lipids on a Co2+ affinity column (Clontech). The folded tetrameric semi-synthetic KcsA channels were separated from the unfolded monomeric protein and the un-ligated peptides by size exclusion chromatography on a Superdex 200 column using a running buffer consisting of 50 mM Tris pH 7.5, 150 mM KCl and 10 mM DM. The semi-synthetic KcsA channels were reconstituted into lipid vesicles as previously described.(19)
Electrophysiological studies
The recombinant and semi-synthetic KcsA channels were reconstituted into lipid vesicles composed of 1-Palmitoyl-2-Oleoyl-Glycero-3-Phosphatidylethanolamine (POPE) (7.5 mg/mL) and 1-Palmitoyl-2-Oleoyl-Glycero-3-Phosphatidylglycerol (POPG) (2.5 mg/mL) at a protein to lipid ratio of 1:400 for singe channel measurements and a ratio of 1:50 for multi-channel, macroscopic currents used for reversal potential measurements. Channel activity was measured using planar lipid bilayers as previously described.(20) For the reversal potential measurements, the leak currents were determined by blocking the current through the channel with 5µM agitoxin2, which was added to the external side.(35) Recombinant agitoxin2 was expressed and purified as previously described.(36)
Acknowledgements
We thank Drs. Roderick MacKinnon, Tom Muir, Jeffrey Karpen and Thomas Scanlan for critical comments on the manuscript. This project was initiated by FIV in the laboratories of Drs. MacKinnon and Muir and their support for the project in the initial stages is acknowledged. The research was supported by grants to FIV from the NIH (GM087546), a Scientist Development Grant from the American Heart Association (0835166N) and a Pew Scholar Award.
References
- 1.Kent S. Chemical synthesis of peptides and proteins. Annu. Rev. Biochem. 1988;57:957–989. doi: 10.1146/annurev.bi.57.070188.004521. [DOI] [PubMed] [Google Scholar]
- 2.Dawson PE, Kent SBH. Synthesis of native proteins by chemical ligation. Annu. Rev. Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 3.Dawson PE, Muir TW, Clarklewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 4.Olschewski D, Becker CF. Chemical synthesis and semisynthesis of membrane proteins. Mol. Biosyst. 2008;4:733–740. doi: 10.1039/b803248c. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ECB, Kent SBH. Studies on the insolubility of a transmembrane peptide from signal peptide peptidase. J. Am. Chem. Soc. 2006;128:7140–7141. doi: 10.1021/ja058377y. [DOI] [PubMed] [Google Scholar]
- 6.Stanley AM, Fleming KG. The process of folding proteins into membranes: challenges and progress. Arch. Biochem. Biophys. 2008;469:46–66. doi: 10.1016/j.abb.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi E, Ingenito R, Simon RJ, Pessi A. Engineering and chemical synthesis of a transmembrane protein: The HCV protease cofactor protein NS4A. J. Am. Chem. Soc. 1999;121:7698–7699. [Google Scholar]
- 8.Kochendoerfer GG, Salom D, Lear JD, Wilk-Orescan R, Kent SBH, DeGrado WF. Total chemical synthesis of the integral membrane protein influenza A virus M2: Role of its C-terminal domain in tetramer assembly. Biochem. 1999;38:11905–11913. doi: 10.1021/bi990720m. [DOI] [PubMed] [Google Scholar]
- 9.Clayton D, Shapovalov G, Maurer J, Dougherty D, Lester H, Kochendoerfer G. Total chemical synthesis and electrophysiological characterization of mechanosensitive channels from Escherichia coli and Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U S A. 2004;101:4764–4769. doi: 10.1073/pnas.0305693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacKinnon R. Potassium channels and the atomic basis of selective ion conduction (Nobel lecture) Angew. Chem., Int. Ed. 2004;43:4265–4277. doi: 10.1002/anie.200400662. [DOI] [PubMed] [Google Scholar]
- 11.Valiyaveetil F, MacKinnon R, Muir T. Semisynthesis and folding of the potassium channel KcsA. J. Am. Chem. Soc. 2002;124:9113–9120. doi: 10.1021/ja0266722. [DOI] [PubMed] [Google Scholar]
- 12.Valiyaveetil FI, Sekedat M, Muir TW, MacKinnon R. Semisynthesis of a functional K+ channel. Angew. Chem., Int. Ed. 2004;43:2504–2507. doi: 10.1002/anie.200453849. [DOI] [PubMed] [Google Scholar]
- 13.Valiyaveetil FI, Sekedat M, MacKinnon R, Muir TW. Glycine as a D-amino acid surrogate in the K(+)-selectivity filter. Proc Natl Acad Sci U S A. 2004;101:17045–17049. doi: 10.1073/pnas.0407820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valiyaveetil F, Sekedat M, MacKinnon R, Muir T. Structural and functional consequences of an amide-to-ester substitution in the selectivity filter of a potassium channel. J. Am. Chem. Soc. 2006;128:11591–11599. doi: 10.1021/ja0631955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle DA, Cabral JM, Pfuetzner RA, Kuo AL, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 16.Perozo E, MacKinnon R, Bezanilla F, Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993;11:353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 17.Cordero-Morales JF, Jogini V, Lewis A, Vasquez V, Cortes DM, Roux B, Perozo E. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 2007;14:1062–1069. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 18.Alagem N, Yesylevskyy S, Reuveny E. The pore helix is involved in stabilizing the open state of inwardly rectifying K+ channels. Biophys. J. 2003;85:300–312. doi: 10.1016/S0006-3495(03)74475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valiyaveetil F, Zhou Y, MacKinnon R. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochem. 2002;41:10771–10777. doi: 10.1021/bi026215y. [DOI] [PubMed] [Google Scholar]
- 20.Heginbotham L, LeMasurier M, Kolmakova-Partensky L, Miller C. Single streptomyces lividans K(+) channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 1999;114:551–560. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlanson DA, Chytil M, Verdine GL. The leucine zipper domain controls the orientation of AP-1 in the NFAT.AP-1.DNA complex. Chem. Biol. 1996;3:981–991. doi: 10.1016/s1074-5521(96)90165-9. [DOI] [PubMed] [Google Scholar]
- 22.Tolbert T, Wong C. New methods for proteomic research: preparation of proteins with N-terminal cysteines for labeling and conjugation. Angew. Chem., Int. Ed. 2002;41:2171–2174. doi: 10.1002/1521-3773(20020617)41:12<2171::aid-anie2171>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genomics. 2004;5:75–86. doi: 10.1023/B:JSFG.0000029237.70316.52. [DOI] [PubMed] [Google Scholar]
- 24.Zuo X, Li S, Hall J, Mattern M, Tran H, Shoo J, Tan R, Weiss S, Butt T. Enhanced expression and purification of membrane proteins by SUMO fusion in Escherichia coli. J. Struct. Funct. Genomics. 2005;6:103–111. doi: 10.1007/s10969-005-2664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang D, Kent SBH. A one-pot total synthesis of crambin. Angew. Chem., Int. Ed. 2004;43:2534–2538. doi: 10.1002/anie.200353540. [DOI] [PubMed] [Google Scholar]
- 26.Hunter CL, Kochendoerfer GG. Native chemical ligation of hydrophobic [corrected] peptides in lipid bilayer systems. Bioconjug. Chem. 2004;15:437–440. doi: 10.1021/bc049959s. [DOI] [PubMed] [Google Scholar]
- 27.Otaka A, Ueda S, Tomita K, Yano Y, Tamamura H, Matsuzaki K, Fujii N. Facile synthesis of membrane-embedded peptides utilizing lipid bilayer-assisted chemical ligation. Chem. Commun (Camb) 2004:1722–1723. doi: 10.1039/b404008b. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Morais-Cabral J, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 29.Hille B. Ion Channels of Excitable Membranes. Third ed. Sunderland, MA: Sinauer Associates, Inc; 2001. [Google Scholar]
- 30.Morais-Cabral JH, Zhou YF, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 31.LeMasurier M, Heginbotham L, Miller C. KcsA: it's a potassium channel. J. Gen. Physiol. 2001;118:303–314. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- 33.Camarero JA, Cotton GJ, Adeva A, Muir TW. Chemical ligation of unprotected peptides directly from a solid support. J. Pept. Res. 1998;51:303–316. doi: 10.1111/j.1399-3011.1998.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 34.Schnolzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int. J. Pept. Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 35.Valiyaveetil FI, Leonetti M, Muir TW, MacKinnon R. Ion selectivity in a semisynthetic K+ channel locked in the conductive conformation. Science. 2006;314:1004–1007. doi: 10.1126/science.1133415. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]