Abstract
Pharmacogenetics is the study of gene-drug interactions. In the post-Human Genome era, and with the realization that personal genotypes differ by millions of bases (gene polymorphisms), the application of genetics to explain inter-individual differences in clinical drug response seems to offer great promise. Here, recent basic and translational developments in pharmacogenetic profiling of β-blocker response in heart failure are reviewed in the context of the possible consequences of such advances on drug development and clinical therapeutics.
Introduction
Six years after completing the draft Human Genome, routine clinical genetic testing for cardiovascular disease is focused mainly upon diagnosing rare familial hypertrophic and dilated cardiomyopathies. Inter-individual genetic variability of ~3–4 million single nucleotide polymorphisms (SNPs) per genome suggests that personal gene variation can impact cardiovascular disease, but identifying specific genetic risk factors and disease modifiers using genome wide association techniques has proven quite challenging(1). On the other hand, delineating specific genetic interactions that affect drug efficacy has born more fruit because polymorphism discovery and evaluation can be based upon a thorough understanding of the biology of drug availability and action. Not with standing well-known limitations of candidate gene approaches, cardiovascular pharmacogenomics research has successfully catalogued a number of relevant gene variants, demonstrated their functional consequences in experimental systems, and reproducibly shown their impact on drug availability or therapeutic effects in clinical studies. Altered β-blocker pharmacodynamics have been described with common genetic polymorphisms of β-adrenergic receptors (βAR) and the G-protein coupled receptor kinase that regulates βAR signaling that modify the effectiveness of β-blockers at their primary drug target. Likewise, altered β-blocker pharmacokinetics have been elucidated for loss-of-function polymorphisms in the major hepatic cytochrome P450 enzyme that inactivates lipophilic β-blockers such as metoprolol. Based on these results, it is reasonable to expect that cardiovascular practice in the coming years will include selection of optimal pharmaco therapeutics directed in some instances by individual genotype. This article provides an overview of our current understanding of the pharmacogenetics of β-adrenergic blocking agents. For another class of cardiovascular drugs for which pharmacogenetic interactions are likely to important, the interested reader is referred to a recent editorial summarizing the current state of the art incytochrome P450 2C19 polymorphisms that impactanti-platelet therapy with clopidogrel versus prasugrel(2).
What are the genetic influences on response to β-blockers?
β-blockers are prescribed to millions of Americans for heart failure, angina, after myocardial infarction, and for hypertension. Except in heart failure, the therapeutic value of β-blockers resides in their ability to decrease cardiac work by lowering heart rate (negative chronotropism) and the force of cardiac contraction (negative inotropism). Depressing myocardial work favorably affects the mismatch between oxygen supply and demand that leads to angina, infarction, and some arrhythmias. Likewise, β-blockers blunt the hyper-adrenergic component of hypertension. In contrast, it would seem that negative inotropism caused by β-blockers would be counter-productive in heart failure, since cardiac output is already insufficient to meet normal physiological demands. Nevertheless, β-blockers are one of only two classes of drugs (the other being angiotensin converting enzyme inhibitors) that prolong life in heart failure (3, 4). The clear mortality benefit and associated improvements in ventricular performance(5) that are thought to result from antagonism of β1-adrenergic receptor (β1AR) mediated cardiotoxicity in the context of pathologically increased systemic and myocardial catecholamines. Since β2AR effects in the heart are widely considered to be potentially beneficial, β-blocker treatment of clinical heart failure tends to focus on β1AR inhibition, and preserving the actions of vasodilatory and bronchodilatory β2ARs. Other differences in β-blocker pharmacological profile, i.e. whether they act like neutral antagonists or inverse agonists, whether or not they exhibit intrinsic sympathomimetic (partial agonist) activity, and whether they are water or lipid soluble, also have the potential to modify β-blocker efficacy in heart failure, and therefore to determine their susceptibility to specific pharmacogenomic interactions. To date, functionally significant polymorphisms in three different genes have reproducibly impacted β-blocker treatment effects in human studies, the β1AR Arg389Gly polymorphism (6), the G-protein Receptor Kinase 5 (GRK5) Gln41Leu polymorphism(7), and multiple loss-of-function polymorphisms of the cytochrome P450 enzyme, CYPD2D6 (8). Each are described in detail below.
β1 adrenergic receptor Arg389Gly polymorphism
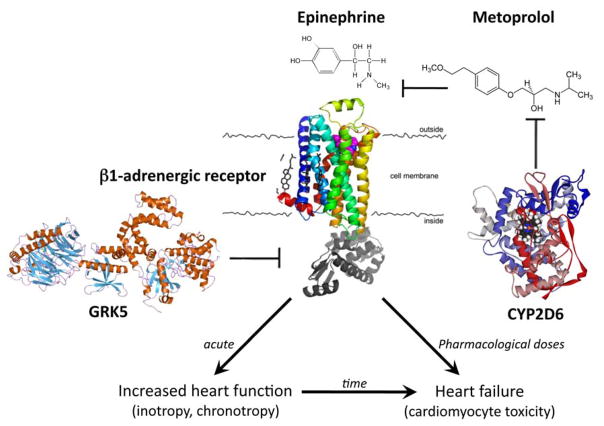
β1ARs mediate the positive cardiac inotropic (contractility), lusitropic (relaxation), and chronotropic (heart rate) effects of sympathetic catecholamines. They are the primary myocardial targets for β-blockers, which competitively inhibit agonist binding and thereby prevent catecholamine-stimulated receptor signaling (Figure). β1ARs are highly polymorphic, with multiple non-synonymous polymorphisms (i.e. polymorphisms that encode a change in amino acid sequence). The Arg to Gly substitution at amino acid 389 is generally common in multiple ethnic groups(9–11), and the functional consequences of this polymorphism have been characterized using recombinant expression of the Arg and Gly receptors in cultured cells. In comparison with β1AR Arg389, the Gly389 receptor couples less efficiently to the Gαs signaling protein that transmits activated membrane receptor responses to intracellular effectors and targets(6, 12). Since agonist-stimulated signaling is diminished, homologous desensitization is also impaired, relative to β1AR Arg389 (13). In aggregate, cell-based studies showed that Gly389 receptors have similar signaling activity to desensitized Arg389 receptors.
Figure. Modulating factors on β1-adrenergic receptor signaling.
The endogenous catecholamines epinephrine and norepineprhine (not shown) bind to and stimulate β1-adrenergic receptors (β1AR), thereby increasing heart rate (positive chronotropy) and myocardial contraction (positive inotropy). At high doses or over prolonged periods, catecholamine stimulation contributes to heart failure by injuring cardiomyocytes. β-blockers, such as metoprolol, competitively inhibit epinephrine/norepinephrine binding to β1AR, which interrupts cardiotoxic catecholamine signaling and prolongs life in heart failure. The Arg389 polymorphism of β1AR makes it hyper-responsive to agonist stimulation and inhibition by β-blockers, compared to Gly389. Lipophilic β-blockers like metoprolol, but not hydrophilic β-blockers, are metabolized and cleared from the circulation by hepatic cytochrome P450 enzyme, CYP2D6. Multiple loss of function CYP2D6 polymorphisms create “poor metabolizers” that exhibit increased metoprolol levels and clinical effects. G-protein receptor kinase 5 (GRK5) desensitizes agonist-bound β1AR by disrupting intracellular signal transduction. The Leu41 polymorphism of GRK5 is more efficient at receptor desensitization, which mimics the effects of pharmacological β-blockade.
Transgenic mice were created expressing either the Arg389 or Gly389 variant of human β1AR in the heart to examine the consequence of the different receptor signaling activities on the intact heart, and to explore whether pharmacological β-blockers would have different inhibitory effects on cardiac inotropy and chronotropy stimulated by the two receptors(14). For these studies, the non-selective (i.e. inhibits both β1AR and β2AR) β-blocker, propranolol, was used. When propranolol was acutely infused to isolated perfused working hearts or when chronically administered orally to live mice, mouse hearts expressing β1AR Arg389 exhibited typical negative inotropy or chronotropy, whereas these responses were blunted or absent in mice expressing the Gly389 variant. Together, the experimental work in cells and mice expressing recombinant human β1AR indicate that the Gly389 receptor is intrinsically less active, and is less susceptible to inhibition by β-blockers, than the Arg389 receptor.
The pharmacological effects of polymorphic β1AR have been examined in several small human studies. Infusion of the β1AR-selective agonist, dobutamine, in normal subjects showed diminished inotropic and chronotropic responses in individuals who were homozygous for Gly389, compared to homozygous Arg389 subjects(15). Importantly, inhibition of these effects by the β-blocker bisoprolol was less effective in Gly389 than Arg389 subjects. Three small studies of systolic heart failure have directly examined at the effect of β1AR 389 genotype on cardiac functional improvement with β-blocker treatment. The change in left ventricular ejection fraction as a result of at least 6 months of treatment with the same dose of the combined α-blocker and β-blocker, carvedilol, was assessed by β1AR 389 genotype in a small heart failure study cohort(14). Gly389 homozygotes showed no significant change in ejection fraction, whereas Arg389 homozygotes exhibited a substantial functional improvement (0.93 ± 1.7% vs 8.7 ± 1.1%, respectively). These general findings were independently replicated in another small study (16). In both of these reports, heterozygous individuals (i.e. β1ARArg/Gly389) either had intermediate responses to carvedilol or acted more like Arg389 homozygotes. Similar results have also been described with the β1AR-selective blocker, metoprolol(17). Taken together, these findings in small human cohorts are remarkably similar to those from propranolol-treated transgenic mice(14). From a purely phenomenological basis, the data indicate that the Arg389 receptor appears hypersensitive to agonist stimulation, and is therefore exquisitely sensitive to pharmacological β-blockade. Looked at from the opposite perspective, the Gly389 receptor functions almost as if it were already “β-blocked”.
A novel experimental platform supports the findings of functional differences between Arg389 and Gly389 β1AR, and has provided additional insight on both the mechanisms involved, and on different pharmacogenetic interactions for β-blockers used in heart failure. In the first description of intra-molecular Fluorescence Resonance Energy Transfer (FRET) to explore differences in drug effect on polymorphic β1ARs, Rochais et al examined the molecular configuration of Arg389 and Gly389 receptors in response to agonist (norepinephrine) and the three β-blockers currently used to treat heart failure (metoprolol, bucindolol, and carvedilol) (18). Utilizing the different conformations of agonist and β-blocker occupied receptor as a readout for inverse agonist activity (which generates a receptor conformation that turns off all signaling), they observed that the effects of metoprolol and bisoprolol did not differ between Arg389 and Gly389 receptor. In contrast, the Arg389 receptor was much more sensitive to inverse agonism by carvedilol than was the Gly389 receptor. These studies demonstrate that the β1AR 389 polymorphism can determine specific responses to individual β-blockers.
The potential for the β1AR 389 polymorphism to determine β-blocker response in human heart failure was revealed by Liggett and Bristow (19). They integrated laboratory studies of myofiber contractile responses from Arg389 or Gly389 hearts with a mortality analysis of heart failure in patients stratified both by β1AR389 genotype and by treatment with the non-selective and highly sympatholytic β-blocker, bucindolol. When the contractile properties of non-failing right ventricular trabeculae were studied in organ baths, those from individuals carrying one or two Gly389 alleles (termed “carriers”) showed depressed contractility compared to homozygous Arg389 hearts. Similar, but smaller, differences were seen in trabeculae from failing hearts. Of most interest, forskolin-contracted trabeculae from failing Arg389 or Gly389 hearts showed equivalent negative inotropic responses to carvedilol, whereas the experimental β-blocker bucindolol decreased contractile force only in Arg389 hearts (i.e. it acted as an inverse agonist in this group only). Although the experimental design used forskolin to pre-contract the muscle fibers, which completely bypassed βAR signaling to directly stimulate downstream adenylyl cyclase, the results support the idea that intrinsic pharmacological properties of β-blockers are an important factor in pharmacogenomic interactions.
The clinical arm of this study consisted of an analysis of mortality/hospitalization benefit from bucindololin the genetic substudy of the BEST trial(20), examining outcomes in placebo- and bucindolol-treated subjects stratified by β1AR Arg/Gly389 genotype. Kaplan-Meier survival analysis identified a single treatment group with improved survival compared to the other groups, Arg389 homozygotes receiving bucindolol. In contrast, subjects with one or two Gly389 alleles had identical survival with placebo or bucindolol. In summary, Arg389 homozygotes act as bucindolol responders, whereas Gly 3889 carriers did not. The upshot of these findings is that genetic testing can be used to personalize therapy with agents, within a broad class of indicated therapeutics, that have the optimal pharmacological profile for a given individual’s genotype/phenotype.
G-protein Receptor Kinase 5 Gln41Leu polymorphism
GRKs desensitize βARs, thereby modulating adrenergic responses under conditions such as heart failure wherein βAR signaling pathways are chronically activated by persistently elevated catecholamines (21). GRK-mediated desensitization therefore represents an endogenous protective response that is analogous to that provided by β-blocker treatment (Figure). Given the critical regulatory function of GRKs on the primary target of β-blockers, it is not surprising that the same conceptual approach described for β1ARs (identification of polymorphic variants by human screening, functional analysis of recombinant variants in cultured cells and genetically manipulated mice, and genotype-phenotype association studies in human heart failure) has likewise revealed significant pharmacogenomic interactions.
Two GRKs are expressed at high levels in myocardium, GRK2 (originally designated the “β-adrenergic receptor kinase” or “β-ARK”) and GRK5(22, 23). Comprehensive GRK2 and GRK5 exomic resequencing of several dozen ethnically diverse human genomic DNAs did not detect any common non-synonymous polymorphisms of GRK2, but did identify four amino acid changing variants in GRK5. Three were extremely rare, but a Gln to Leu substitution at amino acid 41 was uniquely common in African Americans(7). Cell-based studies have shown that the Leu41 variant exhibited enhanced activity for β1AR and β2AR, although the intrinsic kinase activity was not altered, consistent with a modification in one of the kinase’s regulatory domains(7, 24). Thus, the GRK5 Leu41 variant more efficiently desensitizes βAR signaling, and acts (as do β-blockers) to suppress βAR signaling.
Isolated perfused hearts from transgenic mice expressing human GRK5 Gln41 or Leu41 in the heart exhibited similar decreases in inotropic response to the non-selectve βAR agonist, isoproterenol, compared to non-transgenic mice(7). This is the expected result from simply increasing overall myocardial GRK levels in both mouse lines. However, Leu41 transgenic hearts demonstrated accelerated desensitization of isoproterenol-stimulated cardiac inotropism, compared to Gln41 overexpressing hearts. This result mimicked both the cell culture results with recombinant polymorphic GRK5 and the functional consequences of β-blockade. For this reason, the effects of GRK5 Leu41, GRK5 Gln41, and pharmacological β-blockade with propranolol were compared in an experimental model of heart failure created by chronic catecholamine excess, continuous infusion of isoproterenol administered via subcutaneously implanted osmotic mini-pump. Left ventricular remodeling (increased echocardiographic end-systolic and end-diastolic dimensions) is rapid and pronounced in this model, and β-blocker therapy largely prevents the cardiomyopathy. Similar to propranolol, GRK5 Leu41-expressing mice were protected from chronic βAR stimulation, whereas GRK5 Gln41 mice developed the characteristic cardiomyopathic dilation and depressed ejection performance(7).
Since the data in experimental systems indicated that GRK5 Leu41 and β-blockers both decreased βAR signaling and were cardioprotective under conditions of chronic agonist stimulation, the effects of GRK5 genotype were examined on mortality in a small pilot study of human heart failure, stratified by β-blocker treatment status. Since the GRK5 Leu41 polymorphism is prevalent only in African Americans (~40% carry one or two alleles, whereas fewer than 2% of Caucasians do), this study was limited to 375 African Americans with ischemic or non-ischemic heart failure. As with the β1AR 389 polymorphism, a case-control analysis showed similar Leu41 allele frequencies in non-affected and heart failure populations. Thus, GRK5 Leu41 is not an independent risk factor for developing heart failure. However, among patients not treated with β-blockers, study subjects carrying one or two GRK5 Leu41 alleles had strikingly longer transplant-free survival times than subjects homozygous for Gln41. Hazards modeling after adjusting for age and sex showed that the protective effect of GRK5 Leu41 was comparable to that of β-blockers.
Examining the combined effects of β1AR 389 and GRK5 41 polymorphisms on the β-blocker mortality benefit in heart failure
The concordance of β1AR Gly389 and GRK5 Leu41 effects in cell culture, transgenic mice, and early studies of human heart failure suggested that gene-drug interactions can significantly impact inter-individual variability of β-blocker treatment response. Given their different frequencies in populations with different genetic ancestry, we were specifically interested in whether these two genetic polymorphisms might contribute to reported differences in β-blocker mortality effect between Caucasians and African Americans with heart failure. While an overall mortality benefit for β-blocker therapy has been repeatedly demonstrated, the results of sub-group analyses of African Americans have been inconsistent. In part this relates to study design and power, but we considered that population differences in the prevalence of the β1AR Gly389 and GRK5 Leu41 polymorphisms that alter heart failure outcome could also be playing a role. To test this hypothesis, we examined mortality as a function of β1AR and GRK5 genotype in a large bicenter heart failure cohort of 2,460 subjects that included 711 African Americans(11). Within this cohort, 82% of subjects were being treated with β-blockers (largely carvedilol and metoprolol), permitting us to assess outcome as a function of genotype and (non-randomized) β-blocker treatment status.
When the combined results of all subjects were analyzed without regard to geographic ancestry, β-blocker treatment significantly improved mean survival time (P=0.0008) and protected against heart failure mortality (age- and sex-adjusted hazard ratio = 0.78, P=0.003). Sub-group analysis confirmed the β-blocker survival benefit in Caucasians (P=0.0004), but it was not significant in African Americans (P=0.327). We examined reasons for the apparent difference in β-blocker effect by comparing outcomes between Caucasians and African Americans within the untreated or β-blocker-treated groups. In subjects not treated with β-blockers, survival times were similar between African Americans and Caucasians, whereas in subjects taking β-blockers, African Americans had shorter survival times than Caucasians (P=0.0005). Thus, the absence of a significant β-blocker treatment effect in this African American heart failure study cohort was attributable to a greater mortality benefit from β-blocker therapy in Caucasians.
We then compared heart failure survival times as a function of β1AR 389 or GRK5 41 genotype in addition to geographic ancestry and β-blocker treatment status. β1AR Gly389 was associated with shorter survival in β-blocker untreated Caucasians only (P=0.03), consistent with a previously reported modest benefit for the Arg389 genotype (10).β1AR 389 genotype did not affect the mortality benefit from carvedilol or metoprolol. For GRK5 Leu41, which is almost exclusively expressed in individuals of African ancestry, the numbers in Caucasians were too small to power a comparative analysis between races. However, within African Americans with heart failure, GRK5 Leu41 was associated with significantly longer survival times in subjects not being treated with β-blockers (P=0.01), which is consistent with the previous report (7). Thus, in this heart failure cohort the β1AR 389 polymorphism was associated with altered outcome only in Caucasians, and the GRK5 41 polymorphism that is observed almost exclusively in African Americans also altered outcome. Since the differences in β-blocker effect on outcome between populations with differing geographic ancestry originally seen in our study failed to incorporate these genetic factors, we re-examined our data comparing heart failure outcome between Caucasians and African Americans only in subjects with β1AR Arg/Arg389 and GRK5 Gln/Gln genotype (i.e. lacking the functional polymorphic alleles). When survival was matched for genotype in this manner, the mortality benefit for β-blocker therapy was significant in both Caucasians, (P=0.005) and African Americans (p=0.037), and the previously observed differences between survival times in β-blocker treated subjects disappeared. These results demonstrate the importance of accounting for relevant genotypes when examining drug effect. Taken together, the totality of the data with the β1AR 389 and GRK5 41 polymorphisms indicates that enhanced βAR desensitization in the face of catecholamine excess, which is observed in the β1AR Arg389 and GRK5 Leu41 variants, is the key functional characteristic protecting against early mortality in β-blocker untreated heart failure.
Loss-of-function polymorphisms of CYPD2D6
Lipophilic β-blockers, such as metoprolol, undergo extensive hepatic metabolism and are cleared from the body by cytochrome P450 enzymes, especially CYP2D6 (Figure)(25). These metoprolol metabolites are functionally inactive at the concentrations achieved in vivo(26). Therefore, the rate of metoprolol metabolism by CYP2D6 is a primary determinant of active drug levels. To date, over 80 allelic variants of CYP2D6 have been described (http://www.cypalleles.ki.se/), and functionally significant CYP2D6 polymorphisms interact to confer distinct human pharmacokinetic phenotypes. Scoring systems have been proposed that relate complex genotypes to metabolizer phenotype and account for the relative function of different allelic variants and their combinatorial interactions (27). For the purposes of this review, however, we will use the traditional system in which pharmacokinetc profiles are assigned by the ability to metabolize specific CYP2D probe drugs and are broadly related to genotype (8, 28). In this system phenotypes are classified as ultrarapid metabolizers (~5% of Caucasians), extensive metabolizers (~70% of Caucasians), intermediate metabolizers (~15% of Caucasians), and poor metabolizers (~10% of Caucasians)(29). Ultrarapid and extensive metaboliers have two fully functional CYP2D6 alleles, intermediate metabolizers have the equivalent of one functional allele (in many instances representing two hypofunctional alleles), and poor metabolizers have no functional alleles(29, 30). Altered drug clearance as a result of CYP2D6 polymorphisms has striking consequences on metoprolol pharmacokinetics(31), with the elimination half-life ranging from 2.8 hours in extensive metabolizers to 7.5 hours in poor metabolizers(25). Indeed, multiple studies in normal subjects, hypertensive patients, and individuals with heart failure have demonstrated the strong relationship between CYP2D6 genotype, metabolizer phenotype, and metoprolol concentrations, even after chronic treatment at standard doses (32, 33). Accordingly, the possible consequences of altered metabolic clearance include altered drug efficacy (because of insufficient drug levels in extensive metabolizers, or increased drug levels in poor metabolizers) or increased toxicity (from high drug levels in poor metabolizers).
The preponderance of evidence supporting an effect of CYP2D6 genotype on metoprolol treatment effect derive from three recent studies, two in hypertension and one after myocardial infarction(34–36). Clinical efficacy of metoprolol was assessed as the change in heart rate and diastolic or mean arterial blood pressure after treatment. In each of these studies, patients with no fully functional CYP2D6 alleles (i.e. poor metabolizers) exhibited enhanced negative chronotropism, and in the case of the hypertensive cohortsa greater hypotensive effect, from metoprolol. However, poor metabolizers represent only a small fraction of the general population (7–10% of the Caucasian population), and so it is understandable that these findings have not been reproduced in smaller studies that lacked sufficient power to independently examine this subgroup. When these types of analyses have compared metoprolol effect in subjects with two functional CYP2D6 alleles (i.e. extensive metabolizers [70% of population]) versus combined intermediate and poor metabolizers (15 and 10% of the population, respectively), differences have not been significant(33). Thus, while there is little doubt that CYP2D6 genotype can determine metabolizer status and affect metoprolol levels, increased drug effect is clearly significant only in poor metabolizers who represent a small fraction of the population. Since evaluating metoprolol efficacy can be as simple as recording pulse and blood pressure during dose titration, there appears to be little indication for antecedent genotyping to gauge optimal drug dosing in this instance.
The other potential application for CYP2D6 genotyping is to indentify poor metabolizers who, due to impaired drug clearance, may develop metoprolol side effects at standard doses. Support for this approach was provided in a retrospective study describing increased prevalence of poor metabolizers (38%) among patients who discontinued metoprolol because of adverse effects(37). Subsequent reports have failed to establish an unambiguous relationship between CYP2D6 genotype/phenotype and adverse events from metoprolol treatment, despite showing strong associations with plasma levels (17, 31, 38). Again however, the poor metabolizer group tended to be under-represented in these studies, and this is the group that has displayed a higher incidence of side effects from other drugs cleared in a CYP2D6-dependent manner (39–41). Given the wide therapeutic window and safety profile for metoprolol and other lipophilic β-blockers, any conclusion regarding the utility of genetic testing in the functionally brittle heart failure population to identify poor metabolizers who might receive alternate drug dosing or increased surveillance requires properly powered prospective studies in this disease.
Summary
The goal of this review was to examine potential opportunities for genotype-directed drug treatment in heart failure, so-called preprescription genotyping. A critical need is to define the necessary steps to begin implementation of genotype-directed therapy. Specifically regarding β-blockers, their use in heart failure and after myocardial infarction is a class I indication based on rigorous evidence for a group mean effect(42). A highly regarded colleague and expert in the area has suggested that pharmacogenetics may never be applied to β-blocker therapy, not with standing the scientific evidence for pharmacodynamic and pharmacokinetic interactions, because of the undeniable group benefit. It is his belief that necessary proof of concept studies cannot be performed because it would be unethical to modify established life-prolonging treatment regimens in order to explore possible benefits of targeted therapeutics based on individual genotype. Indeed, in the case of heart failure, there are really only three β-blockers that are accepted as reducing mortality and morbidity (43), and it is not clear under what conditions it would be permissible, even under carefully controlled experimental conditions, to substitute a different β-blocker for one of these old standards, or even to exchange the same β-blocker in a different formulation. One approach would be to perform initial prospective pharmacogenetic evaluations in a different patient population, such as hypertensives, where there is greater latitude to prescribe a variety of β-blockers, and in whom typical endpoints of drug effectivess (change in heart rate and blood pressure) can be evaluated in the near and long-term. Another possibility is to evaluate individual treatment responses in heart failure patients using these same surrogate endpoints of drug effectiveness in short-term trials. By way of example, clinically stable and well-compensated heart failure study subjects selected by genotype for specific analyses could be withdrawn from their standard β-blockers (typically carvedilol or metoprolol XL) for a few weeks under careful supervision in order to assess chronotropic and inotropic responses or plasma levels after administration of one of the newer generation of β-blockers. Normal treatments could then be resumed after this relatively brief interruption. If the results from such studies demonstrated specific gene-drug interactions, they could support trials comparing β-blocker effectiveness in subject randomized either to being guided, or not guided, by genotype, and using intermediate term endpoints such as change in left ventricular ejection fraction after 6 months(5). These issues represent some formidable challenges that will need to be addressed through the coordinated efforts of practicing clinicians, geneticists, pharmacologists, and an understanding of the complexities involved on the parts of institutional review boards and data safety monitoring committees.
Acknowledgments
Supported by NIH P50 HL077101 and R01HL087871.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorn GW, Cresci S. The mechanistic imperative for pharmacogenomics. Pharmacogenomics. 2008 Jul;9(7):801–3. doi: 10.2217/14622416.9.7.801. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM, Stein CM. Clopidogrel and the concept of high-risk pharmacokinetics. Circulation. 2009 Apr 28;119(16):2127–30. doi: 10.1161/CIRCULATIONAHA.109.865907. [DOI] [PubMed] [Google Scholar]
- 3.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 4.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 5.Fauchier L, Pierre B, de Labriolle A, Babuty D. Comparison of the beneficial effect of beta-blockers on mortality in patients with ischaemic or non-ischaemic systolic heart failure: a meta-analysis of randomised controlled trials. Eur J Heart Fail. 2007 Nov;9(11):1136–9. doi: 10.1016/j.ejheart.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999 Apr 30;274(18):12670–4. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 7.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, et al. A GRK5 polymorphism that inhibits β-adrenergic receptor signaling is protective in heart failure. Nat Med. 2008;14(5):510–7. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997 Feb;60(2):284–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006 Jul 25;103(30):11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacanowski MA, Zineh I, Li H, Johnson BD, Cooper-DeHoff RM, Bittner V, et al. Adrenergic gene polymorphisms and cardiovascular risk in the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Journal of Translational Medicine. 2008;6:11. doi: 10.1186/1479-5876-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresci S, Kelly RJ, Cappola TP, Diwan A, Dries D, Kardia SL, et al. Clinical and Genetic Modifiers of Long-Term Survival in Heart Failure. Journal of American College of Cardiology. 2009 doi: 10.1016/j.jacc.2009.05.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph SS, Lynham JA, Grace AA, Colledge WH, Kaumann AJ. Markedly reduced effects of (−)-isoprenaline but not of (−)- CGP12177 and unchanged affinity of beta-blockers at Gly389-beta1-adrenoceptors compared to Arg389-beta1-adrenoceptors. Br J Pharmacol. 2004 May;142(1):51–6. doi: 10.1038/sj.bjp.0705753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathz DA, Gregory KN, Fang Y, Brown KM, Liggett SB. Hierarchy of polymorphic variation and desensitization permutations relative to β1- and β2-adrenergic receptor signaling. J Biol Chem. 2003;278:10784–9. doi: 10.1074/jbc.M206054200. [DOI] [PubMed] [Google Scholar]
- 14.Mialet-Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, et al. β1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9(10):1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 15.Bruck H, Leineweber K, Temme T, Weber M, Heusch G, Philipp T, et al. The Arg389Gly beta1-adrenoceptor polymorphism and catecholamine effects on plasma-renin activity. J Am Coll Cardiol. 2005 Dec 6;46(11):2111–5. doi: 10.1016/j.jacc.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar P, Chen L, Semmler AB, Parsonage WA, Kaumann AJ. Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through ‘affinity states’ and polymorphism. Clin Exp Pharmacol Physiol. 2007 Oct;34(10):1020–8. doi: 10.1111/j.1440-1681.2007.04730.x. [DOI] [PubMed] [Google Scholar]
- 17.Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS, et al. beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure. Clin Pharmacol Ther. 2005 Mar;77(3):127–37. doi: 10.1016/j.clpt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Rochais F, Vilardaga JP, Nikolaev VO, Bunemann M, Lohse MJ, Engelhardt S. Real-time optical recording of beta1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007 Jan;117(1):229–35. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, et al. A polymorphism within a conserved β1-adrenergic receptor motif alters cardiac function and b-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103(30):11288–93. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BEST Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344(22):1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 21.Kaye DM, Smirk B, Finch S, Williams C, Esler MD. Interaction between cardiac sympathetic drive and heart rate in heart failure: modulation by adrenergic receptor genotype. J Am Coll Cardiol. 2004 Nov 16;44(10):2008–15. doi: 10.1016/j.jacc.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 22.Kunapuli P, Benovic JL. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A. 1993;90:5588–92. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–41. [PubMed] [Google Scholar]
- 24.Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB. A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors. Pharmacogenet Genomics. 2008 Aug;18(8):729–32. doi: 10.1097/FPC.0b013e32830967e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lennard MS, Silas JH, Freestone S, Ramsay LE, Tucker GT, Woods HF. Oxidation phenotype--a major determinant of metoprolol metabolism and response. N Engl J Med. 1982 Dec 16;307(25):1558–60. doi: 10.1056/NEJM198212163072505. [DOI] [PubMed] [Google Scholar]
- 26.Regardh CG, Johnsson G. Clinical pharmacokinetics of metoprolol. Clin Pharmacokinet. 1980 Nov;5(6):557–69. doi: 10.2165/00003088-198005060-00004. [DOI] [PubMed] [Google Scholar]
- 27.Kirchheiner J. CYP2D6 phenotype prediction from genotype: which system is the best? Clin Pharmacol Ther. 2008 Feb;83(2):225–7. doi: 10.1038/sj.clpt.6100455. [DOI] [PubMed] [Google Scholar]
- 28.Chou WH, Yan FX, Robbins-Weilert DK, Ryder TB, Liu WW, Perbost C, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem. 2003 Apr;49(4):542–51. doi: 10.1373/49.4.542. [DOI] [PubMed] [Google Scholar]
- 29.Raimundo S, Fischer J, Eichelbaum M, Griese EU, Schwab M, Zanger UM. Elucidation of the genetic basis of the common ‘intermediate metabolizer’ phenotype for drug oxidation by CYP2D6. Pharmacogenetics. 2000 Oct;10(7):577–81. doi: 10.1097/00008571-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Zanger UM, Fischer J, Raimundo S, Stuven T, Evert BO, Schwab M, et al. Comprehensive analysis of the genetic factors determining expression and function of hepatic CYP2D6. Pharmacogenetics. 2001 Oct;11(7):573–85. doi: 10.1097/00008571-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Fux R, Morike K, Prohmer AM, Delabar U, Schwab M, Schaeffeler E, et al. Impact of CYP2D6 genotype on adverse effects during treatment with metoprolol: a prospective clinical study. Clin Pharmacol Ther. 2005 Oct;78(4):378–87. doi: 10.1016/j.clpt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Rau T, Heide R, Bergmann K, Wuttke H, Werner U, Feifel N, et al. Effect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatment. Pharmacogenetics. 2002 Aug;12(6):465–72. doi: 10.1097/00008571-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Sharp CF, Gardiner SJ, Jensen BP, Roberts RL, Troughton RW, Lainchbury JG, et al. CYP2D6 genotype and its relationship with metoprolol dose, concentrations and effect in patients with systolic heart failure. Pharmacogenomics J. 2009 Jun;9(3):175–84. doi: 10.1038/tpj.2009.9. [DOI] [PubMed] [Google Scholar]
- 34.Rau T, Wuttke H, Michels LM, Werner U, Bergmann K, Kreft M, et al. Impact of the CYP2D6 genotype on the clinical effects of metoprolol: a prospective longitudinal study. Clin Pharmacol Ther. 2009 Mar;85(3):269–72. doi: 10.1038/clpt.2008.218. [DOI] [PubMed] [Google Scholar]
- 35.Bijl MJ, Visser LE, van Schaik RH, Kors JA, Witteman JC, Hofman A, et al. Genetic variation in the CYP2D6 gene is associated with a lower heart rate and blood pressure in beta-blocker users. Clin Pharmacol Ther. 2009 Jan;85(1):45–50. doi: 10.1038/clpt.2008.172. [DOI] [PubMed] [Google Scholar]
- 36.Goryachkina K, Burbello A, Boldueva S, Babak S, Bergman U, Bertilsson L. CYP2D6 is a major determinant of metoprolol disposition and effects in hospitalized Russian patients treated for acute myocardial infarction. Eur J Clin Pharmacol. 2008 Dec;64(12):1163–73. doi: 10.1007/s00228-008-0525-3. [DOI] [PubMed] [Google Scholar]
- 37.Wuttke H, Rau T, Heide R, Bergmann K, Bohm M, Weil J, et al. Increased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effects. Clin Pharmacol Ther. 2002 Oct;72(4):429–37. doi: 10.1067/mcp.2002.127111. [DOI] [PubMed] [Google Scholar]
- 38.Zineh I, Beitelshees AL, Gaedigk A, Walker JR, Pauly DF, Eberst K, et al. Pharmacokinetics and CYP2D6 genotypes do not predict metoprolol adverse events or efficacy in hypertension. Clin Pharmacol Ther. 2004 Dec;76(6):536–44. doi: 10.1016/j.clpt.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Brockmoller J, Kirchheiner J, Schmider J, Walter S, Sachse C, Muller-Oerlinghausen B, et al. The impact of the CYP2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin Pharmacol Ther. 2002 Oct;72(4):438–52. doi: 10.1067/mcp.2002.127494. [DOI] [PubMed] [Google Scholar]
- 40.Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003 Oct;160(10):1830–5. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- 41.Wedlund PJ, de LJ. Cytochrome P450 2D6 and antidepressant toxicity and response: what is the evidence? Clin Pharmacol Ther. 2004 May;75(5):373–5. doi: 10.1016/j.clpt.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) Journal of American College of Cardiology. 2001;38(7):2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 43.Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med. 2001 Apr 3;134(7):550–60. doi: 10.7326/0003-4819-134-7-200104030-00008. [DOI] [PubMed] [Google Scholar]