Abstract
Objective
To evaluate the efficacy of a behavioral plus nutrition education intervention, Be-In-CHARGE!, compared to nutrition education (NE) alone, on calorie intake and weight gain in children with cystic fibrosis (CF) and pancreatic insufficiency.
Design
Randomized controlled trial
Setting
CF Centers in the Eastern, Midwestern and Southern United States
Participants
79 children ages 4 to12 years, below the 40th percentile weight for age were recruited. 67 completed the intervention and 59 completed a 24 month follow-up assessment.
Outcome Measures
Primary outcomes were change from pre- to post-treatment in calorie intake and weight gain. Secondary outcomes were change from pre- to post-treatment on % Estimated Energy Requirement (EER), and body mass index z-score (BMIZ). These outcomes were also examined 24 months post-treatment.
Results
The behavioral plus nutrition education intervention had a statistically greater average increase on the primary and secondary outcomes of calorie intake (872 vs. 489 cal/d), %EER (148% vs. 127%), weight gain (1.47 vs. 0.92kg), and BMIZ (0.38 vs. 0.18) at post-treatment than NE. At 24 month follow-up, children in both conditions maintained an EER around 120% and did not significantly differ on any outcomes.
Conclusions
Behavioral plus nutrition education intervention is more effective at increasing dietary intake and weight over a brief 9 week period, however across the 24 month follow-up both treatments achieved similar outcomes. Implications for standard nutritional care are discussed.
Weight gain in children with cystic fibrosis (CF) is associated with improved lung functioning over time.1, 2 While CF Foundation Consensus Conferences on nutrition have repeatedly endorsed the goal of “normal growth” for patients with CF,3, 4 the mean highest median weight for age percentile reached by pediatric patients with CF is the 42nd and is achieved by 3 years of age. After this age there is a steady decline in nutritional status across childhood and adolescence.5 The 2002 Nutrition Consensus statement recommended the consumption of 120 to 150% of the Required Dietary Allowance for energy per day,3 yet only 12 to 16% of children with CF meet these recommendations.6 Diet is the most frequently cited treatment problem for parents of children with CF7 and growing evidence indicates that parent and child mealtime behaviors impact nutritional status.8-11
While early treatment of suboptimal growth in CF is recommended, there are only a handful of studies that address both nutritional deficits and mealtime behavior problems. These studies show behavioral intervention, in combination with nutrition education, is effective in increasing caloric intake and weight gain in children with CF; however, the literature is limited by lack of control groups12, 13 and small sample sizes.14-16 We therefore conducted a multi-site, randomized clinical trial comparing behavioral plus nutrition education to nutrition education. Primary outcomes were differences from pre-to post-treatment in caloric intake and weight. Secondary outcomes were percent Estimated Energy Requirement and body mass index z-score. We hypothesized that children receiving the behavioral plus nutrition education intervention would have a significantly greater increase in these outcomes than children receiving nutrition education. In order to examine long-term effects of this intervention, participants were followed two years post-treatment. We hypothesized that children receiving the behavioral plus nutrition education intervention would have a significantly greater increase from pre-treatment through 24 month follow-up on these outcomes.
Methods
Participants
Subjects were recruited from five CF Centers located in the Eastern, Midwestern, and Southern US. The study was approved by the institutional review board at each medical center. Written informed consent and assent was obtained. Inclusion criteria were: ages 4 to 12 years; diagnosis of CF by sweat test; pancreatic insufficiency; and weight for age or for height < 40th percentile. Exclusion criteria included medical condition that would affect growth or diet (eg., insulin dependent diabetes); prescribed medication that would affect growth or appetite (eg., steroids); significant developmental delay or mental health diagnosis of depression or psychosis (parent or child); positive sputum culture for Burkholderia cepacia; FEV1 <40% of predicted; or receiving enteral or parenteral nutrition.
Recruitment and Randomization
Medial records of children ages 4 to 12 years were reviewed for inclusion/exclusion criteria. Families of children meeting criteria were sent a letter introducing the study. As enrollment was rolling, letters were sent to 20 potential participants at a time in the order they appeared on the Center roster of patients. A follow up call was made by the study staff 10 days after the mailing to describe the study in detail, verify inclusion/exclusion criteria, and invite participation. If a family agreed, a home visit was scheduled to get informed consent and collect questionnaire data (not reported here) and families were asked about time conflicts with treatment sessions (morning or afternoon). If a family had a schedule conflict they were assigned to the time of day without a conflict. Families with no schedule conflicts were assigned to morning or afternoon sessions by a coin flip. Once all families who agreed to participate had been assigned to time of day, the time of day (morning/afternoon) was then randomized to treatment arm by coin flip by the research assistant and postdoctoral fellow together. This yielded a group size of 2 to 5 families per treatment condition. Families were told the study was comparing two approaches to improving nutrition, one that focused on diet and one that focused on diet and child behavior. Families were never explicitly told which treatment they had been assigned.
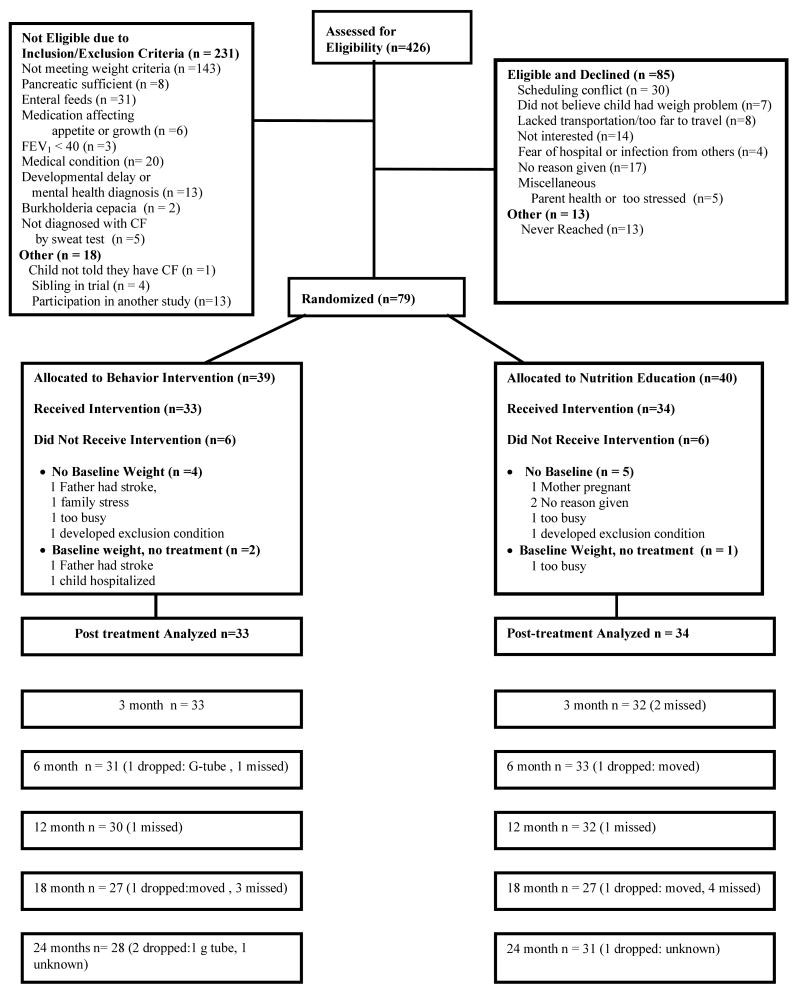
In three prior studies of this behavioral intervention, an effect size of 1.58 standard deviation units for weight gain was achieved. Because a potentially efficacious, alternative treatment was used the effect size was estimated more conservatively to be 0.90 standard deviation units. A sample size of 25 subjects per group with a significance level of 0.05 yielded 87% power, therefore 79 children were recruited between 1996 and 1999 to allow for an estimated 60% retention rate across the 27 months of the study. As shown in Figure 1, 426 medical charts were reviewed, of these 249 were excluded, 231 did not meet inclusion criteria or had an exclusion criteria. An additional 18 were excluded for other reasons including participation in another research study where weight was an outcome (n =13), had a sibling who had been randomized to the trial (n = 4), and one child who the physician excluded because the child had not been told they had CF. Of the 177 who met eligibility, 164 could be contacted by phone and 85 declined participation and 79 agreed and were randomized to treatment.
Figure 1.
CONSORT Flow diagram of participants randomized to Behavioral plus Nutrition Education (Be-In-CHARGE!) and Nutrition Education and assessed at each time point.
Study Design
Subjects in each condition attended a 90 minute baseline session (Session 1, pre-treatment), followed two weeks later by 5 weekly group sessions (Sessions 2-6), and finally followed two weeks later by a post-treatment assessment/review session (Session 7). Thus, there was a span of 9 weeks between pre- and post-treatment data collection. Participants were followed up at 3, 6, 12, 18 and 24 months post-treatment. Parents and children were seen in simultaneous, but separate groups. The group sessions followed a written manual. Parent groups were conducted by a Ph.D. level psychologist and a registered dietitian. Child groups were conducted by a postdoctoral fellow or graduate student in psychology.
Interventions
Caloric Goals
Breakfast, lunch, dinner and snack were each expected to increase by 250 calories per day, yielding a total increase of 1,000 calories per day by the end of treatment. One type of meal (e.g, breakfast, lunch) was targeted per session and once a meal was addressed in treatment, calorie intake for that meal was expected to remain at the new goal level.
Nutrition Education (NE)
Parent Group
The nutrition intervention was identical in both the behavioral plus nutrition education and NE groups. Parents were provided with information on the caloric content of their child's meals, caloric goals for each meal, and recommendations on how to achieve these goals including cooking methods, calorie boosters, and higher calorie foods. At each session parents were given graphs of their child's actual caloric intake compared to their weekly goal. In the NE group no behavioral child management training was provided.
Child Group
The child group used fun activities to teach about “high energy” foods, provided a practice meal but did not require consumption, and provided the same energy goals as children receiving behavior plus nutrition education but without rewards for meeting goals. Children were given trophies for session attendance.
Behavioral plus Nutrition Education followed the protocol, Behavioral Intervention for Change Around Nutrition and Energy! (Be-In-CHARGE!) developed by Stark and colleagues,12-15 and is available on line at www.oup.com/us/pediatricpsych.
Parent Group
In addition to nutrition education, parents received training in child behavior management strategies.17 Parents were taught to use differential attention for appropriate and inappropriate eating behaviors and to use sticker charts and home-based privileges to reward the children for meeting caloric goals.
Child Group
Children participated in nutrition education and a practice meal. During the practice meal, goal setting and differential attention was used to encourage children to try new foods and meet their caloric goal. Trophies were awarded contingent on meeting the previous week's calorie goals.
Data Collection
The primary outcomes of the study were change in caloric intake and weight pre- to post-treatment. Secondary outcomes were %EER and BMIZ pre- to post-treatment. These four outcomes were also examined 24 months following treatment along with the outcomes of height, HAZ and FEV1 that could only be examined over a longer time period than 9 weeks.
Caloric intake was assessed via daily food monitoring by parents. The average of fourteen days between Sessions 1 and 2 served as pre-treatment and the fourteen days between Sessions 6 and 7 served as post-treatment. Parents kept a 7-day food record before the 3, 6, 12, 18 and 24 months follow-up. Caloric intake was examined as absolute calories per day and as a percentage of the Estimated Energy Requirements (EER). Percent EER was calculated by subtracting the EER for an active child of the same age and gender18 from the individual subject's calorie intake X 100.
Anthropometrics
Weight and height were measured in triplicate following the guidelines established by Cameron 19 by a single trained measurer at each site. To provide a context for growth outcomes weight and height were standardized by converting them to Body Mass Index z-scores for age and gender (BMIZ) and height for age z-scores (HAZ) using the CDC growth curves.20
Parent Satisfaction Questionnaire assessed parents' satisfaction with their child's progress, the impact of the program on child caloric intake and mealtime behaviors, the overall approach used to manage calorie intake and child behavior, the group leader's teaching skills, and whether they would recommend the program to a friend using a 7-point scale (higher numbers indicated greater satisfaction).
Pulmonary function was assessed by forced expiratory volume at 1-second (FEV1) using equations set forth by Wang et al.21 FEV1 was chosen because it is considered the most reliable/valid indicator of lung functioning in children with CF.22, 23
Treatment fidelity was assessed by raters coding 4 videotapes from each of the seven sessions for each intervention. The NE sessions contained 95% of the key nutrition components and 0% of the behavioral components. The behavior plus nutrition sessions contained 97% of the key nutrition components and 93% of the key behavioral components. Interater reliability, calculated as percent agreement on 25% of the tapes, was 97% for the behavioral components and 95% for the nutrition components.
Moderator Variable
Three-day fecal fat studies were performed pre- and post-treatment and at the 12 and 24 month assessments for fat absorption and analyzed by the Mayo Clinic using the van deKamer method.24 The coefficient of fat absorption (CFA) was calculated using the formula: CFA= (dietary fat, grams - stool fat grams)/dietary fat grams x 100.
Statistical Analysis
The demographic variables in Table 1 were compared between the two groups using t-tests for continuous variables and chi-square for categorical variables.
Table 1.
Characteristics for Families in Behavior Plus Nutrition Education And Nutrition Education
| Behavior Plus Nutrition Education (N=33) | Nutrition Education (N=34) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Child Age (years) | 7.5 (2.7) | 7.4 (2.9) |
| Female (%) | 55 | 45 |
| % Caucasian | 100 | 94 |
| Weight for Age %ile | 23 (19.6) | 25 (14) |
| FEV1 (%predicted) | 88 (18) (N=17) | 92 (18) (N=19) |
| % Fat Absorption | 79 (12.5) | 85 (15.4)* |
| Mother's Age (years) | 34.5 (6) | 32.8 (6) |
| Mother's Education (years) | 14 (2) | 13.9 (2) |
| Father's Age (years) | 37.5 (6.5) (N=30) | 36.4 (6.1) (N=31) |
| Father's Education (years) | 14.3 (2.4) (N=31) | 14.5 (2.4) (N=32) |
| Income Before Taxes | ||
| $ 0 – 9,999 | 3% | 6% |
| $10,000 – 19,999 | 9% | 12% |
| $20,000 – 29,999 | 6% | 18% |
| $30,000 – 39,999 | 12% | 9% |
| $40,000 – 49,999 | 18% | 6% |
| >$50,000 | 52% | 50% |
Significantly different, p = .02
Prior to determining if the primary endpoints differed between the two conditions, we assessed for differences in our primary outcomes by site and by therapist. Using linear models on difference scores from pre- to post-treatment for change in calories and weight no significant differences were found due to psychologist (calories: p = 0.80; weight: p = 0.90) or site (calories: p = 0.99; weight: p = 0.84). Sample size precluded analysis of the effects of more complex site and therapist interaction.
The primary analyses were performed using linear modeling in SAS 9.1 (version 9.1; SAS Institute Inc., Cary, North Carolina). The dependent variables were difference scores from pre- to post-treatment for caloric intake, %EER, weight and BMIZ. The independent variable was a fixed effect for group (behavior plus nutrition vs. NE). % CFA was used as a covariate in the weight gain and BMIZ models because the two groups were found to differ on % CFA at pre-treatment (79% in behavioral plus nutrition vs. 85% in NE, p = 0.02). Parent Satisfaction variables were compared with t-tests.
None of the drop-outs received active treatment and only one drop-out from each group had a pre-treatment weight but no further participation, thus percent change could not be calculated. Aside from these early drop-outs there was no missing pre- or post-treatment data for any of the subjects (N = 67) in the study.
Long-Term Outcome across the 24 month Follow-up
Intent-to-treat was followed in the analysis. The two treatments were compared overtime using linear mixed modeling with a repeated measures design and an autoregressive variance-covariance structure in SAS 9.1 The factors in the model were group (random effect), time (3, 6, 12, 18, and 24 months), and the interaction of group with time. The difference scores from pre-treatment for caloric intake, %ERR, weight, BMIZ, height, HAZ and FEV1 across each of the 5 time points were the dependent variables. Missing data from the long-term follow-up assessments was minimal (<10%) for weight, BMIZ, height, HAZ. Approximately 20% of the caloric intake and %EER data was missing from follow-up and 22% of FEV1 data were missing. There were no statistically significant differences in age, gender, or primary or secondary outcome variables at pre-treatment between subjects with and without missing follow-up data. For the variables with >10% missing data the analyses were run for all observed data, data only for those who never missed a visit, and data with three sets of imputed values for missing visits using SOLAS® 3.0 for Missing Data Analysis (Statistical Solutions, Ltd, Saugus, MA). All three analyses were nonsignificant with one exception. One set of imputed values for the FEV1 variable resulted in a significant interaction. However, the observed data and the other two sets of imputed values for this variable were not significant so only non-imputed data are reported.
Results
Study Population
Of 79 enrolled children 39 were assigned to behavior plus nutrition education and 40 to NE. As shown in Figure 1 this represents a recruitment rate of 44% of the eligible children and 48% of families who could be contacted. There were six drop outs in both arms prior to treatment, leaving 67 children for analysis. There were no statistically significant differences between the conditions on demographic variables (p > 0.05) or FEV1 (p > 0.05) however, children in behavior plus nutrition education had lower fat absorption at enrollment than children in NE (p = 0.02) (Table 1).
Pre- to Post-Treatment Efficacy (Table 2)
Table 2.
Pre- to Post-Treatment Means (SD) and Difference Scores (SD) with 95% Confidence Intervals for Caloric Intake, %EER, Weight, and BMIZ score for Children Receiving Behavior Plus Nutrition Education and Nutrition Education.
| Behavior Plus Nutrition Education | Nutrition Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Pre-Treatment (n = 33) | Post-Treatment (n = 33) | Change from Pre-Treatment | Pre-Treatment (n = 34) | Post-Treatment (n = 34) | Change from Pre-Treatment | Difference Between Groups a | p value b |
| Caloric Intake (avg/day) | 1793 (350) | 2665 (553) | 872 (478) | 1826 (476) | 2315 (549) | 489 (314) | 383 (403) | .0002 |
| 703, 1042 | 379, 599 | 186, 580 | ||||||
| %EER | 100 (16) | 148 (30) | 48 (29) | 100 (16) | 127 (25) | 27 (19) | 21 (24) | .0007 |
| 38, 58 | 20, 33 | 9, 33 | ||||||
| Weight (kg) | 21.79 (6.44) | 23.26 (7.10) | 1.47 (1.27) | 22.62 (7.45) | 23.54 (7.78) | 0.92 (1.03) | .55 (1.16) | .01 |
| 1.02, 1.92 | 0.56, 1.28 | -0.02, 1.11 | ||||||
| BMIZ | -0.77 (1.12) | -0.39 (1.08) | 0.38 (0.46) | -0.49 (0.71) | -0.31 (0.81) | 0.18 (0.47) | .20 (.47) | .03 |
| 0.21, 0.54 | 0.02, 0.35 | -0.03, 0.42 | ||||||
a Based on the difference of the change from pre-treatment scores (Behavior Plus Nutrition Education – Nutrition Education).
bBased on linear model on difference score, with a fixed effect for group and % CFA used as a covariate for Weight and BMIZ analyses.
Caloric Intake
Children receiving behavioral plus nutrition education achieved a significantly greater increase in daily caloric intake than children receiving NE, p = 0.0002. At post-treatment, children receiving behavioral plus nutrition education averaged 383 more calories per day than children in NE. In addition, children receiving behavioral plus nutrition education achieved a significantly greater increase in % EER (48%) from pre- to post-treatment than children in NE (27%), p = 0.0007; achieving a %EER of 148% while those in NE achieved 127%.
Weight
Children receiving behavioral plus nutrition education gained significantly more weight, an average of 1.47 kg across the 9 weeks pre- to post-treatment compared to children receiving NE who gained 0.92 kg, p = .01. The change in weight resulted in a significantly greater improvement in BMIZ at post-treatment for children receiving behavioral plus nutrition education (0.38) compared to NE (0.18), p = 0.03.
Parent Satisfaction
Parents in both groups reported high ratings of satisfaction with treatment (>6 on a 7 point scale) with no statistically significant difference on eight of nine dimensions (p > .05). For “approach used to increase child's calorie intake” the behavioral plus nutrition education intervention was rated superior (p = 0.005). However, ratings of both groups were above 6.
Two Year Follow-Up (Table 3)
Table 3.
Two Year Outcomes (Means (SD) and Difference Scores (SD) with 95% Confidence Intervals) on Calorie Intake, %EER, Weight, BMIZ, Height, HAZ and FEV1 for Children Receiving Behavior Plus Nutrition Education and Nutrition Education
| Behavior Plus Nutrition Education | Nutrition Education | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Pre-Treatment | 24 month Follow-up | Change from Pre-Treatmenta | Pre-Treatment | 24 month Follow-up | Change from Pre-Treatmenta | Difference Betweeen Groupsb | |
| Caloric intake (avg/day) | 1793 (350) | 2523 (620) | 721 (522) | 1826 (476) | 2411 (577) | 533 (436) | 188 (482) | |
| (n = 33) | (n = 26) | 510, 932 | (n =34) | (n = 25) | 353, 713 | -83, 459 | ||
| % EER | 100 (16) | 126 (29) | 25 (25) | 100 (16) | 117 (22) | 16 (25) | 9 (25) | |
| (n = 33) | (n = 26) | 12,35 | (n = 34) | (n = 25) | 5, 26 | -5, 23 | ||
| Weight (kg) | 21.79 (6.44) | 28.51 (9.77) | 6.97 (3.60) | 22.62 (7.45) | 29.51 (10.84) | 6.45 (3.67) | 0.52 (3.64) | |
| (n = 33) | (n= 28) | 5.58, 8.37 | (n=34) | (n=31) | 5.10, 7.80 | -1.38, 2.42 | ||
| BMIZ | -0.77 (1.12) | -.56 (0.90) | 0.13 (0.81) | -0.49 (0.71) | -0.71 (0.66) | -0.22 (0.50) | 0.35 (0.67) | |
| (n = 33) | (n = 28) | -0.18, 0.45 | (n = 34) | (n = 31) | -0.40, -0.04 | 0.01, 0.70 | ||
| Height (cm) | 118.93 (14.87) | 131.07 (14.66) | 13.34 (1.93) | 119.35 (16.44) | 133.87 (17.01) | 13.54 (2.93) | -0.20 (2.50) | |
| (n = 33) | (n = 28) | 12.59, 14.08 | (n = 34) | (n = 31) | 12.47, 14.62 | -1.51, 1.10 | ||
| HAZ | -0.95 (0.78) | -0.87 (0.77) | 0.03 (0.30) | -0.74 (0.69) | -0.72 (0.74) | 0.04 (0.32) | -0.01 (0.31) | |
| (n = 33) | (n = 28) | -0.09, 0.15 | (n = 34) | (n = 31) | -0.08, 0.16 | -0.17, 0.16 | ||
| FEV1 | 88 (18) | 87 (18) | 0.16 (22) | 92(18) | 87 (17) | -5 (13) | 5 (18) | |
| (n = 17) | (n = 13) | -13, 14 | (n = 19) | (n=15) | -12, 2 | -8, 19 | ||
Change from pre-treatment value does not equal 24 month follow-up – pre-treatment due to subject attrition at follow-up.
b Based on the difference of the change from pre-treatment scores (Behavior Plus Nutrition Education – Nutrition Education).
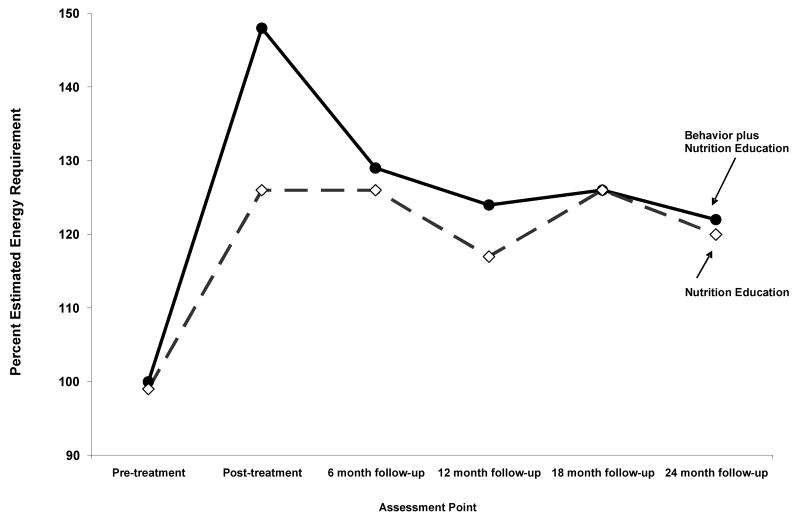
There were no statistically significant group by time interactions across the 5 follow-up assessment points for caloric intake, % EER, weight, BMIZ, height, HAZ or FEV1. As shown in Figure 2, by the 6 month follow-up children receiving behavioral plus nutrition education decreased from 148% EER at post-treatment to 129% EER and remained near 120% EER for the duration of the study. Children in NE remained near 120% EER from post- treatment across all follow-ups.
Figure 2.
The percent estimated energy requirement (EER) for the children in Behavioral plus Nutrition Education (Be-In-CHARGE!) and Nutrition Education at pre-treatment, post-treatment, and the 6, 12, 18 and 24 month follow-up.
Comment
The current study is the first test of a behavioral plus nutrition education intervention to improve caloric intake and weight gain in children with CF compared to an alternative treatment condition, nutrition education alone, with a large enough sample to detect differences on these variables. As hypothesized, the behavioral plus nutrition education intervention resulted in significantly greater increases in calorie intake and weight gain pre- to post-treatment than nutrition education. In addition to being more efficacious, the behavioral plus nutrition education intervention showed consistency on the primary outcomes with previous behavioral studies. The average increase in caloric intake of 872 calories/day in the current study was similar to the calorie increase of 785 to 1,000 per day from our previous studies.13, 15, 25 Similarly, the weight gain seen pre- to post-treatment of 1.47kg, replicates our previous reports of weight gain of 1.42 kg15 to 1.70 kg.14
In contrast to our previous work using a wait-list control,15 children receiving nutrition education increased both their intake and weight over the 9 weeks of treatment Clearly, the structure and delivery of nutrition education provided in the current study is different from standard of care and likely has implications for how to improve the efficacy of standard nutritional care. This is especially worth noting because in the current study both interventions led to a virtually identical EER of approximately 120% across the two year follow-up. Thus, while the behavioral plus nutrition education intervention achieved a greater improvement in calorie intake and weight gain in the short-term, over the longer term both were very successful maintaining the 2002 Consensus guidelines for energy intake3 and intakes 20% higher than pre-treatment. The similar intake between the two treatment groups was likely the mechanism that accounted for the lack of difference in BMIZ across the two year follow-up.
We believe that the unexpected outcome in the nutrition education group on caloric intake and growth during the follow-up period was likely because this condition contained key components that made it similar to certain aspects of the behavioral plus nutrition education intervention and very different from typical nutritional care provided in a fast paced clinical setting. Typical dietary counseling does not occur weekly, specify calorie targets that gradually increase, provide weekly calorie graphs with feedback on how close a child came to their targets, or provide individualized, written suggestions for increasing calories based on usual food and beverage preferences. The structure of the dietary information in the nutrition education intervention was very behavioral, and utilized strategies known to positively impact dietary outcome. Self monitoring, for example, is highly correlated with weight outcome in studies of obesity,26, 27 and providing tailored feedback is very potent in supporting behavior change.28 The current study indicates that we can make significant improvements in the energy and weight outcomes of children with CF by making the important dietary education already available in CF centers more behavioral in its' delivery.29 Making modifications to standard nutritional care such as providing families with individualized energy goals, tailoring based on the child's existing diet, targeting one meal at a time, and having families self monitor their child's dietary intake and presenting these data graphically may reduce the anxiety and uncertainty that parents express about their child's eating9, 11 and improve skills and outcomes.
While the current study did not have a standard of care treatment condition against which the gain in calorie intake of the nutrition education can be compared, there have been numerous studies reporting that the typical intake of children with CF is about 100% of the recommended energy for children and not the 120 to 150% recommended for CF9, 11, 30, 31 and achieved in this study. Thus, the data from this trial suggests that more intensive intervention that is behavior based, even if focused exclusively on nutrition education, could have greater impact than nutrition intervention as it has been reported to be delivered in usual care models.
The exact amount of energy necessary for weight gain in children with CF is not known. In a recent review of the empirical literature the energy intake associated with weight gain in similar age children with CF ranged from 110 to 200% of the energy needs of the children without CF.32 Our pre- to post-treatment data show that achieving an EER of 148% led to a greater weight gain than achieving an EER of 127%. After the intensive treatment phase children in both treatment arms returned to standard care. For the children receiving the behavioral plus nutrition education to have maintained 148% EER they would have had to continually increase their absolute caloric intake over time as they grew bigger and older. Therefore, in addition to making standard nutritional care more behavioral future research should investigate ways to maintain the treatment gain of 148% EER achieved by the behavioral intervention after intensive treatment concludes.
While encouraging the current study has several limitations. Our recruitment rate was only 44% of eligible children, therefore limiting generalization of the treatment effects to those families willing and able to attend weekly treatment. As with any behavioral intervention, it is not possible to keep subjects unaware of the treatment they are receiving or therapists the treatment they are providing. Finally, because the study was conducted prior to widespread adoption of the CONSORT guidelines, aspects such as randomization allocation, sequence, and concealment were not met and may have introduced the type of bias that these procedures are designed to eliminate.
Acknowledgments
Funding/Support: this study was supported by grants R01 DK50092 and D24 DK 059492 from the National Institutes of Health (L.J.S.). Additional support was provided by grant M01 RR 0808 from the National Center for Research Resources of the NIH. We also gratefully acknowledge the Cystic Fibrosis Foundation for use of the CFF Patient Registry.
Footnotes
Clinical Trial Registration Number NCT00006169
Additional Contributions: We thank the many project coordinators and students who contributed to this study as especially to Judy Hollingsworth, RN, MSN, and dietitians Laurie Higgins, MS, RD, LDN, DCE, Terri Schnidler, MS, RD, and Karen Maguiness, MS, RD, CSP.
References
- 1.Konstan MW, Butler SM, Wohl ME, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. Journal of Pediatrics. 2003;142:624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 2.Peterson ML, Jacobs DR, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary functioning in children with cystic fibrosis. Pediatrics. 2003;112:588–592. doi: 10.1542/peds.112.3.588. [DOI] [PubMed] [Google Scholar]
- 3.Borowitz D, Baker RD, Stallings VA. Consensus report on nutrition for pediatric patients with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition. 2002;35:246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey B, Farrell P, Pencharz P. Nutritional assessment and management in cystic fibrosis: Consensus conference. American Journal of Clinical Nutrition. 1992;55:108–116. doi: 10.1093/ajcn/55.1.108. [DOI] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. Patient Registry 2004 Annual Data Report. Bethesda, MD: 2005. [Google Scholar]
- 6.Mackner LM, McGrath AM, Stark LJ. Dietary recommendation to prevent and manage chronic pediatric health conditions: adherence, intervention, and future directions. Journal of Developmental and Behavioral Pediatrics. 2001;22:130–143. doi: 10.1097/00004703-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Quittner AL, DiGirolamo AM, Michel M, Eigen H. The impact of caregiving and role strain on family life: Comparisons between mothers of children with cystic fibrosis and matched controls. Rehabilitation Psychology. 1992b;37:289–304. [Google Scholar]
- 8.Powers SW, Patton SR, Byars KC, et al. Caloric intake and eating behavior in infants and toddlers with cystic fibrosis. Pediatrics. 2002;109(5):1–10. doi: 10.1542/peds.109.5.e75. [DOI] [PubMed] [Google Scholar]
- 9.Stark LJ, Jelalian E, Mulvihill MM, et al. Eating in preschool children with cystic fibrosis and healthy peers: A behavioral analysis. Pediatrics. 1995;95:210–215. [PubMed] [Google Scholar]
- 10.Stark LJ, Jelalian E, Powers SW, et al. Parent and child mealtime behaviors in families of children with cystic fibrosis. Journal of Pediatrics. 2000;136:195–200. doi: 10.1016/s0022-3476(00)70101-6. [DOI] [PubMed] [Google Scholar]
- 11.Stark LJ, Mulvihill MM, Jelalian E, et al. Descriptive analysis of eating behavior in school-age children with cystic fibrosis and healthy control children. Pediatrics. 1997;99:665–671. [PubMed] [Google Scholar]
- 12.Stark LJ, Bowen AM, Tyc VL, Evans S, Passero MA. A behavioral approach to increasing calorie consumption in children with cystic fibrosis. Journal of Pediatric Psychology. 1990;15(3):309–326. doi: 10.1093/jpepsy/15.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Stark LJ, Knapp L, Bowen AM, et al. Increasing calorie consumption in children with cystic fibrosis: Replication with two year follow-up. Journal of Applied Behavior Analysis. 1993;26:435–450. doi: 10.1901/jaba.1993.26-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark LJ, Mulvihill MA, Powers SW, et al. Behavioral intervention to improve calorie intake of children with cystic fibrosis: Treatment vs. wait-list controls. Journal of Pediatric Gastroenterology and Nutrition. 1996;22:240–253. doi: 10.1097/00005176-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Stark LJ, Opipari LC, Spieth L, et al. Contribution of behavior therapy to dietary treatment in cystic fibrosis: A randomized controlled study with two-year follow-up. Behavior Therapy. 2003;34:237–258. [Google Scholar]
- 16.Powers SW, Jones JS, Ferguson KS, Piazza-Waggoner C, Daines C, Acton JD. Randomized clinical trial of behavioral and nutrition treatment to improve energy intake and growth in toddlers and preschoolers with cystic fibrosis. Pediatrics Dec. 2005;116(6):1442–1450. doi: 10.1542/peds.2004-2823. [DOI] [PubMed] [Google Scholar]
- 17.Forehand RL, McMahon RJ. Helping the noncompliant child. New York: The Guilford Press; 1981. [Google Scholar]
- 18.Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein, and Amino Acides. Washington, D.C: National Academy Press; 2002. [Google Scholar]
- 19.Cameron N. The methods of auxological anthropometry. In: Falkner F, Tanner J, editors. Human Growth. Vol. 3. New York: Plenum Press; 1986. pp. 3–43. [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. Adv Data. 314. Jun 8, 2000. CDC: growth charts: United States; pp. 1–27. [PubMed] [Google Scholar]
- 21.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BGJ. Pulmonary functioning between 6 and 18 years of age. Pediatric Pulmonology. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 22.Corey M. Power considerations for studies of lung function in cystic fibrosis. Proc Am Thorac Soc. 2007 Aug 1;4(4):334–337. doi: 10.1513/pats.200611-176HT. [DOI] [PubMed] [Google Scholar]
- 23.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992 Apr 30;326(18):1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 24.Braddock L, Fleisher D, Barbero G. A physical chemical study of the van de Kamer method for fecal fat analysis. Gastroenterology. 1968;55:165–172. [PubMed] [Google Scholar]
- 25.Stark LJ. Behavioral treatment of weight gain in cystic fibrosis. NIH Grant #R01 50092. 1996 [Google Scholar]
- 26.Saelens BE, McGrath AM. Self-monitoring adherence and adolescent weight control efficacy. Children's Health Care. 2003;32:137–152. [Google Scholar]
- 27.Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. The relationship between parent and child self-reported adherence and weight loss. Obes Res Jun. 2005;13(6):1089–1096. doi: 10.1038/oby.2005.127. [DOI] [PubMed] [Google Scholar]
- 28.Ryan P, Lauver DR. The efficacy of tailored interventions. J Nurs Scholarsh. 2002;34(4):331–337. doi: 10.1111/j.1547-5069.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 29.Stark LJ. Can nutrition counselling be more behavioural? Lessons learned from dietary management of cystic fibrosis. Proceedings of the Nutrition Society. 2003;62:793–799. doi: 10.1079/PNS2003294. [DOI] [PubMed] [Google Scholar]
- 30.Anthony H, Bihes J, Phelan P, Paxton S. Relation between dietary intake and nutritional status in cystic fibrosis. Archives of Disease in Childhood. 1998;78:443–447. doi: 10.1136/adc.78.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status and pulmonary functioning in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. Journal of Pediatrics. 2000;137:374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 32.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton HB. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. Journal of the American Dietetic Association. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]