Abstract
Background & Aims
The ErbB4 receptor tyrosine kinase regulates cell growth, survival, and differentiation in several tissues, but its role(s) in the gastrointestinal tract have not been reported. We tested the hypothesis that ErbB4 promotes intestinal cell survival and restitution following injury or inflammation.
Methods
ErbB4 expression in human IBD was determined by immunohistochemistry. Mice were subjected to dextran sulfate sodium (DSS, 3%) colitis or injected with 104 U tumor necrosis factor (TNF), and ErbB4 expression was quantified by immunohistochemistry and Western blot analysis of colon mucosal scrapings. Cultured young adult mouse colon (YAMC) cells were exposed to TNF, then ErbB4 mRNA, protein, and phosphorylation levels were measured. Cells transfected with ErbB4 siRNA, or overexpressing ErbB4, were subjected to wound healing and apoptosis assays.
Results
ErbB4 levels increased in Crohn's colitis and the colon epithelium of mice with DSS colitis or injected with TNF. In YAMC cells, TNF induced ErbB4 mRNA, protein, and phosphorylation; NF-kB activation also stimulated ErbB4 accumulation. ErbB4 siRNA knockdown sensitized YAMC cells to TNF-stimulated apoptosis, while overexpression blocked apoptosis induced by TNF+cycloheximide. Additionally, ErbB4 siRNA decreased YAMC cell wound healing. ErbB4 knockdown attenuated, while overexpression elevated, phosphorylation of the anti-apoptotic kinase Akt in response to TNF. Inhibition of the phosphatidylinositol 3-kinase/Akt signaling cascade reversed the ability of ErbB4 overexpression to protect from cytokine-induced apoptosis.
Conclusions
ErbB4 expression and signaling are key elements for TNF responses in vivo and in cell culture, protecting intestinal epithelial cells from apoptosis in the inflammatory environment, possibly through Akt activation.
Introduction
Regulation of cell proliferation, migration, and apoptosis in the intestinal epithelium by soluble growth factors and their receptors1, 2 is critical for barrier function maintenance and the health of the individual. Signaling through members of the ErbB family of receptor tyrosine kinases, including epidermal growth factor (EGF) receptor (R), represents a potential therapeutic avenue for gastrointestinal inflammatory disorders, as demonstrated by the efficacy of EGF in experimental wound healing assays2 and clinical trials with ulcerative colitis3. However, the influence of individual ErbBs on epithelial responses to injury and inflammation is not yet well understood.
ErbB4 is the most recently described ErbB receptor4, and compared to the other family members little is known about its biology. Responses to ErbB4 ligands or ErbB4 overexpression in cell culture vary widely, ranging from differentiation5, 6 or cell survival7 to migration8, proliferation5, 9 or growth arrest10. These divergent results may be explained by expression of up to four receptor isoforms generated by alternative splicing in the regions coding for the juxtamembrane (JM) and cytoplasmic (CYT) domains. The prototypic ErbB4, JM- a/CYT-1, is subject to a sequential two-step proteolytic cleavage10, giving rise to an 80 kDa intracellular domain fragment (4ICD). 4ICD is a constitutively active tyrosine kinase11 which can translocate to the nucleus and function as a transcriptional regulator12, 13. JM-b forms are resistant to this proteolytic processing, and are expressed only as full-length transmembrane receptors14. In addition to JM variants, CYT-2 isomerization results in the deletion of a C-terminal phosphatidylinositol 3-kinase (PI 3-kinase) binding site8. CYT-1 retains this site and can signal through PI 3-kinase.
ErbB4 is widely expressed in human fetal and adult tissues, including the digestive tract15, but often at low levels, which has made understanding the receptor's physiology in some tissues difficult. As yet no function for ErbB4 has been described in the intestinal epithelium, but the involvement of EGFR and ErbB ligands in recovery from colitis16, 17 suggests potential roles in this response for ErbB4, which can heterodimerize with EGFR and modulate its signaling. In this study we asked whether ErbB4 is involved in intestinal epithelial responses to injury and inflammation, and in particular in response to the proinflammatory cytokine tumor necrosis factor (TNF).
TNF is produced by macrophages and other immune cells as well as epithelial cells, and is expressed at high levels in inflammatory bowel diseases [IBD; reviewed in18]. Many of the cytotoxic effects of the inflammatory environment are mimicked in cell culture by TNF. Epithelial cells express two TNF receptors, the low-affinity TNFR1 and the high-affinity TNFR2, which together trigger an array of cellular outputs. In intestinal epithelial cells, TNF stimulates both cell survival and pro-apoptotic pathways, with the specific cellular context modulating the balance of signals and determining cell fate19. Survival signals induced by TNF include the PI 3-kinase/Akt cascade and nuclear factor (NF)-kB.
Signaling downstream of TNF intersects with EGFR pathways on several levels. TNF stimulates many of the same kinase cascades as EGFR. In contrast, however, TNF can also inhibit phosphorylation and activation of EGFR20, 21. As yet little is known about crosstalk between TNF signaling and other ErbB family members.
In the current study, we asked whether ErbB4 is regulated by inflammation in intestinal epithelial cells. In both a mouse model of acute colitis and mice injected with TNF, we observe increased ErbB4 expression in the colon epithelium; consistent with these results we see elevated ErbB4 levels in human IBD. In cultured young adult mouse colon epithelial (YAMC) cells, TNF exposure induced ErbB4 mRNA, protein, and phosphorylation. Blockade of ErbB4 expression with siRNA inhibited TNF-stimulated Akt phosphorylation and sensitized cells to apoptosis. Conversely, ErbB4 overexpression stimulated Akt phosphorylation and protected from cytokine-induced cell death. Taken together, these data position ErbB4 as a key regulator of the epithelial cell's response to injury and inflammation.
Methods
Apoptosis
Apoptosis was measured by ApopTag terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL; Millipore, Danvers, MA) following the manufacturer's instructions, and by cleaved (active) caspase 3 Western blot analysis. 6h TNF exposure (100 ng/ml) combined with either Wortmannin (100 nM) or cycloheximide (1 µg/ml) served as positive control.
Immunohistochemistry
Immunostaining for ErbB4 was performed using standard techniques22 on 5 µM paraffin-embedded sections with anti-ErbB4 or control IgG at 2 µg/ml in PBS for 2h. Diaminobenzidine substrate was purchased from Vector Labs (Burlingame, CA). Sections were counterstained with methyl green.
Restitution assays
Restitution assays were performed as previously described23. Briefly, fibronectin-plated cultures were subjected to multiple 1.5 mm2 wounds with a drill-mounted silicone tip. Wounds were photographed over time and measured with ImageJ software (NIH, Bethesda, MD).
Constructs
pCDNA3.1-ErbB4 (human JM-a/CYT-1 isoform) expression vector was a gift of Graham Carpenter (Vanderbilt University). Inserts from pCDNA3.1-ErbB4 were polymerase chain reaction (PCR) amplified (primers: 5’-ATGGCGATCGCATGAAGCCGGCGACAGGACTTTG-3’, Sgf I site; 5’-TTGGGCCGGACCGGCCTTACACCACAGTATTCCGGTG-3’, Sfi I site) then cut with Sfi I and Sgf I and ligated into linearized bicistronic LZRS-GFP vector (Albert Reynolds, Vanderbilt). Plasmids were screened by Sfi I/Sgf I digestion. Phoenix packaging cells (Steve Hanks, Vanderbilt) were transiently transfected with LZRS-GFP or LZRS-ErbB4-GFP and monitored for GFP expression. YAMC cells were subjected to 5 rounds of infection with filtered viral supernatant supplemented with 4 µg/ml polybrene. GFP-positive cells were sorted at the Vanderbilt Flow Cytometry Core Lab using a Becton-Dickinson FACSAria; top 20% GFP-expressing cells were maintained as pools.
Mice and experimental colitis
C57Bl/6 mice were subjected to acute colonic injury and colitis with 3% (w/v) DSS (MW 36,000–50,000; MP Biochemicals, Solon, OH) in drinking water for 4d, then fresh water for a 3d recovery period. On days 0, 4, and 7 colons were Swiss-rolled, formalin-fixed, and paraffin-embedded. Colon epithelium from some mice was isolated for lysates by filleting the colon and scraping the mucosal surface with a glass slide. As an additional model of acute inflammation in vivo, mice were injected intraperitoneally with 104 U recombinant TNF. 24h later mice were sacrificed and their colons collected for analysis.
All animal use was approved and monitored by the Vanderbilt Institutional Animal Care and Use Committee.
Human IBD samples
Biopsy slides of intestinal epithelium from pediatric Crohn’s disease cases and normal controls were provided by Dr. Kay Washington in Vanderbilt's Human Tissue Acquisition and Pathology Shared Resource. All slides were fully de-identified before receipt. The study was reviewed by the Vanderbilt Institutional Review Board and determined to not qualify as "human subject" research per §46.102(f)(2).
Statistics and replicates
All data are representative of at least three independent experiments. Statistical significance of differences from controls was assessed by one-way analysis of variance with Tukey post-test. Error bars indicate standard error of means.
Additional methods and materials provided in the Supplementary Data.
Results
Human Crohn's colitis is associated with elevated ErbB4 levels
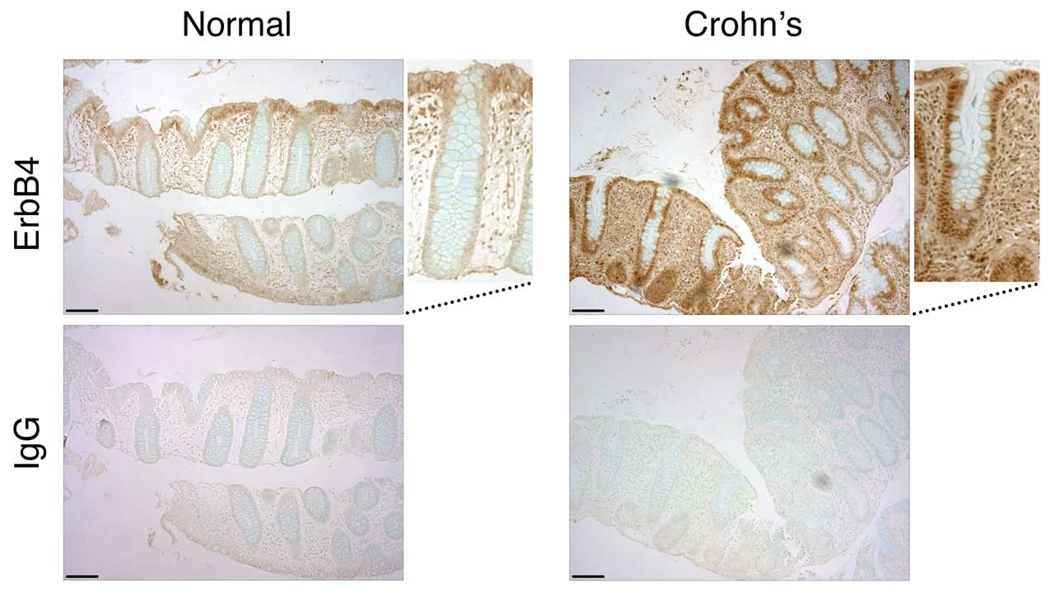
To examine ErbB4 expression and localization during intestinal epithelial inflammation, we performed immunohistochemical analysis on endoscopic biopsies from pediatric patients with Crohn's colitis and normal controls. In 4 of 5 Crohn's cases examined, elevated ErbB4 staining–including increased nuclear detection, indicative of the 80 kDa ICD fragment–was observed as compared with normal biopsies (Fig. 1).
Figure 1. ErbB4 expression increases in human IBD.
Fixed, paraffin-embedded biopsy sections from normal colon and Crohn’s colitis tissue were subjected to immunohistochemical analysis using anti-ErbB4 or Ig control. Bars, 100 µm. Magnified boxes show representative crypts with nuclear staining in the epithelium of the Crohn’s tissue.
ErbB4 expression is elevated during experimental colitis and recovery
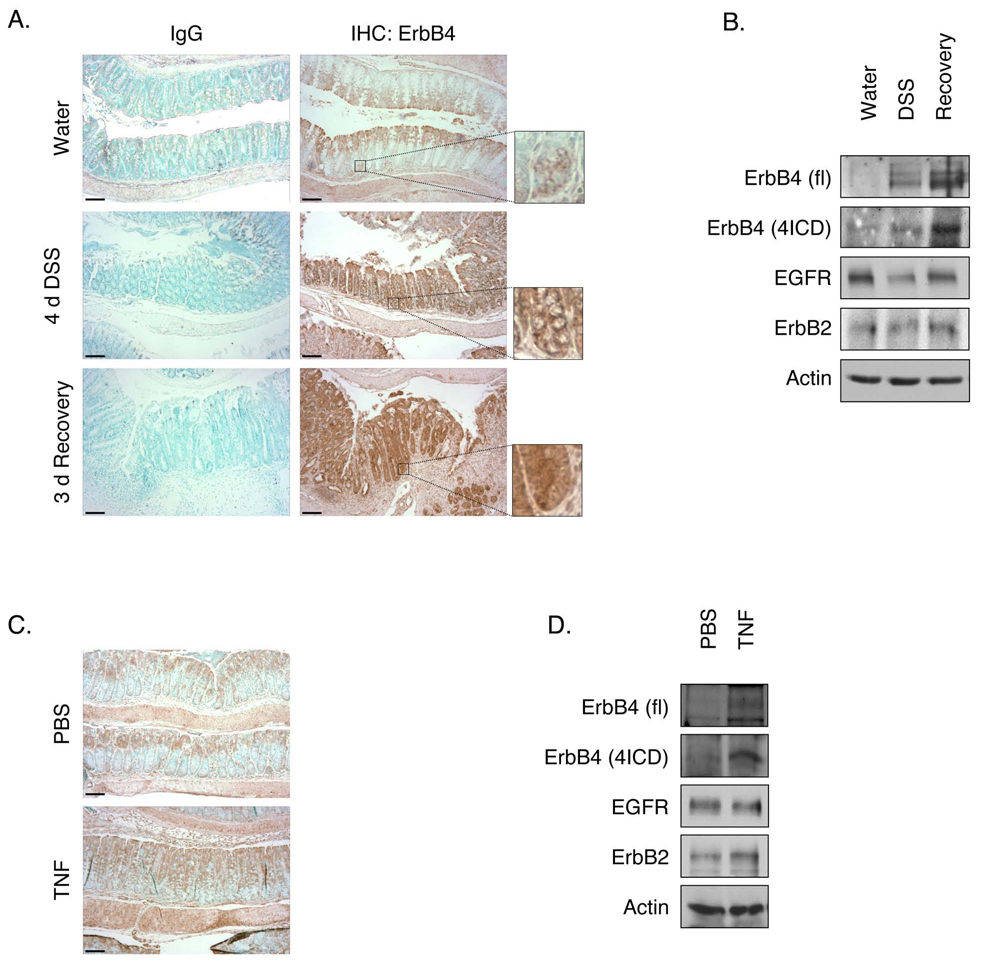
To explore ErbB4's role in an experimentally tractable IBD model, we induced acute colitis in C57BL/6 mice with 3% DSS in drinking water. In the unchallenged colon, ErbB4 is expressed weakly in the differentiated cells of the upper crypt (Fig. 2A), with occasional staining detectable in the cytoplasm and nucleus of cells of the lower crypt (Figs. 2A and 2C, insets). rtPCR analysis of mucosal scrapings detects both JM and both CYT isoforms, with real-time quantitative PCR (RT-qPCR) showing much more robust expression of JM-a versus JM-b but roughly equivalent amounts of CYT-1 and CYT-2 (Supplementary Fig. 1). After 4d exposure to DSS (injury phase), when mice exhibit patchy colitis with inflammation and regions of ulceration (Supplementary Fig. 2), ErbB4 expression was elevated in spared regions of the epithelium (Fig. 2A). During the recovery phase 3d after withdrawal of DSS, at which point the epithelium is remodeling and undergoing hyperproliferative repair, ErbB4 expression was dramatically elevated in the epithelium with expression throughout crypts. Intracellular localization of ErbB4 suggested presence of the cleaved 4ICD form, which was confirmed by Western blot analysis of mucosal lysates (Fig. 2B). Both the full-length and 80 kDa 4ICD ErbB4 levels increased during injury and recovery. RT-qPCR analysis did not detect a significant shift in JM-a/b or CYT-1/2 mRNA ratios during this process (data not shown).
Figure 2. ErbB4 expression increases in experimental colitis.
(A) C57BL/6 mice were given 3% DSS in drinking water for 4d followed by 3d recovery. Fixed colon sections were subjected to immunohistochemical analysis for ErbB4. Inset shows expression at bottom of the crypt. (B) Mucosal lysates were subjected to Western blot analysis for ErbB4. EGFR, ErbB2, and actin were measured as loading controls. (C) Mice were injected with PBS or TNF (104 units); after 24h colons were fixed and stained for ErbB4. (D) Mucosal lysates were subjected to Western blot analysis for ErbB4. fl, full-length ErbB4; 4ICD, 80 kDa intracellular ErbB4; bars, 100 µm.
TNF is elevated in DSS colitis24, and is a key element of IBD pathology18. To test whether ErbB4 expression responds to TNF exposure in the intestine, C57BL/6 mice were injected intraperitoneally with 104 U TNF. 24h after injection, ErbB4 levels in the colon epithelium were increased as detected both by immunohistochemical (Fig. 2C) and Western blot (Fig. 2D) analysis. TNFR1/TNFR2 double-knockout mice did not respond to TNF, but did exhibit a partial increase in ErbB4 following DSS treatment (Supplementary Fig. 3). Thus, while TNF signaling induces ErbB4 there are potentially additional mechanisms which induce the receptor in the context of injury.
TNF stimulates ErbB4 expression in cultured intestinal epithelial cells
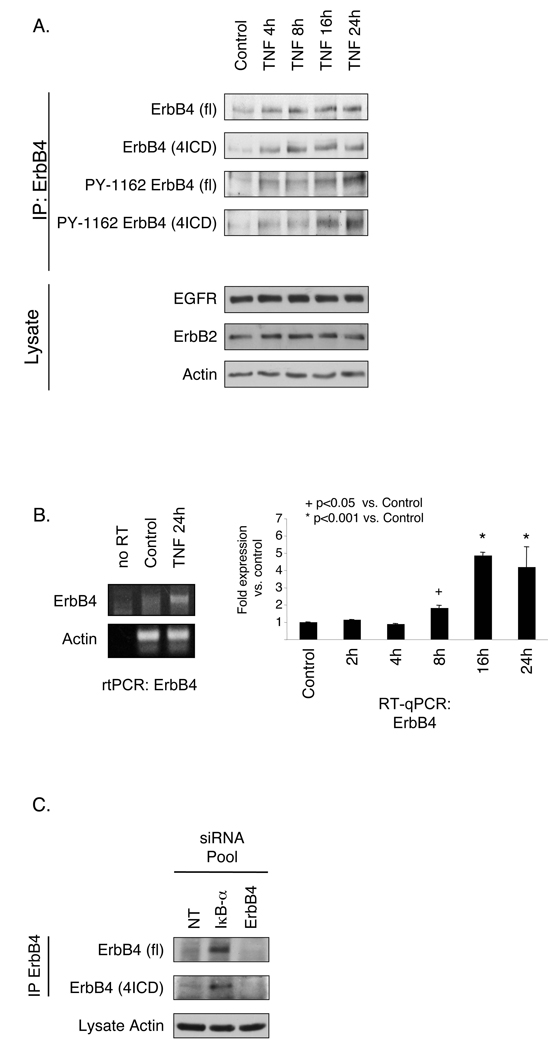
To examine ErbB4 in a cell culture model of inflammation, YAMC colon epithelial cell cultures were exposed to TNF (100 ng/ml) and ErbB4 expression was determined by immunoprecipitation and Western blot, or RT-qPCR. Untreated YAMC cells express low but detectable levels of ErbB4 protein (Fig. 3A) and mRNA (Fig. 3B); 8–24h exposure to TNF stimulated an accumulation of ErbB4 mRNA, full-length receptor, and 4ICD. Blotting with an antibody against phosphorylated ErbB4 indicated that accumulated receptor was phosphorylated and thus presumably active. ErbB4 accumulation appears to be a result of increased receptor synthesis rather than decreased turnover, as TNF did not retard degradation in the presence of the translation blockade (Supplementary Fig. 4). However, increased ErbB4 protein levels were detectable at 4h before the mRNA induction (Figs. 3A&B), suggesting a mechanism which is at least partly due to increased translation. Interestingly, TNF promoted accumulation of ErbB4 in TNFR1−/− and TNFR2−/− as well as wild-type YAMC cells (Supplementary Fig. 5), indicating that either receptor is capable of stimulating ErbB4 expression, similar to published results for TNF-stimulated NF-kB activation25.
Figure 3. TNF stimulates ErbB4 expression in intestinal epithelial cells.
YAMC cells were exposed to 100 ng/ml TNF for indicated times. (A) ErbB4 was immunoprecipitated and immunocomplexes subjected to Western blot analysis for either ErbB4 or phosphorylated (PYY1162) ErbB4. EGFR, ErbB2, and actin in lysates were assessed as loading controls. fl, full-length ErbB4; 4ICD, 80 kDa ErbB4 fragment. (B) Total cellular RNA was subjected to RT-qPCR analysis for ErbB4. (C) YAMC cells were transiently transfected with non-targeting (NT), IkB-α, or ErbB4 siRNA. ErbB4 was immunoprecipitated and assessed by Western blot.
NF-kB activity promotes ErbB4 expression in intestinal epithelial cells
TNF affects epithelial cells through multiple mechanisms, including activation of the NF-kB transcription factor26. To ask whether NF-kB could account for increased ErbB4 expression following TNF exposure, we transfected YAMC cells with siRNA for IkB-α, the inhibitory regulator which sequesters NF-kB in the cytoplasm. IkB-α siRNA enhanced ErbB4 levels (Fig. 3C). Similar results were observed by transfection with a constitutively active p50/p65 NF-kB fusion protein (data not shown). Thus, NF-kB activity, which is stimulated by TNF in these cells [19 and Fig. 7] promotes ErbB4 protein accumulation in YAMC cells.
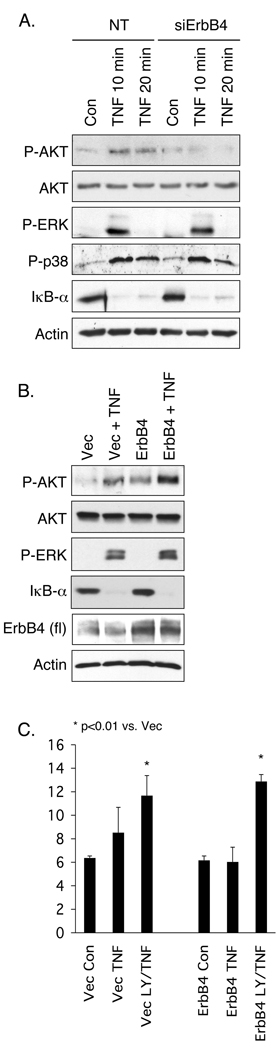
Figure 7. ErbB4 promotes Akt activation in response to TNF.
(A) YAMC cells were transfected with ErbB4 siRNA and treated with 100 ng/ml TNF for indicated times. Signal pathway activation was determined by Western blot analysis. (B) Cells overexpressing vector or ErbB4 were treated with TNF; signaling was determined by Western blot analysis. P, phospho. (C) Vector and ErbB4-expressing cells were exposed to TNF with or without the PI 3-kinase inhibitor LY294002 (5µM) for 6h and apoptosis was measured by TUNEL assay.
ErbB4 deletion decreases intestinal epithelial cell wound closure
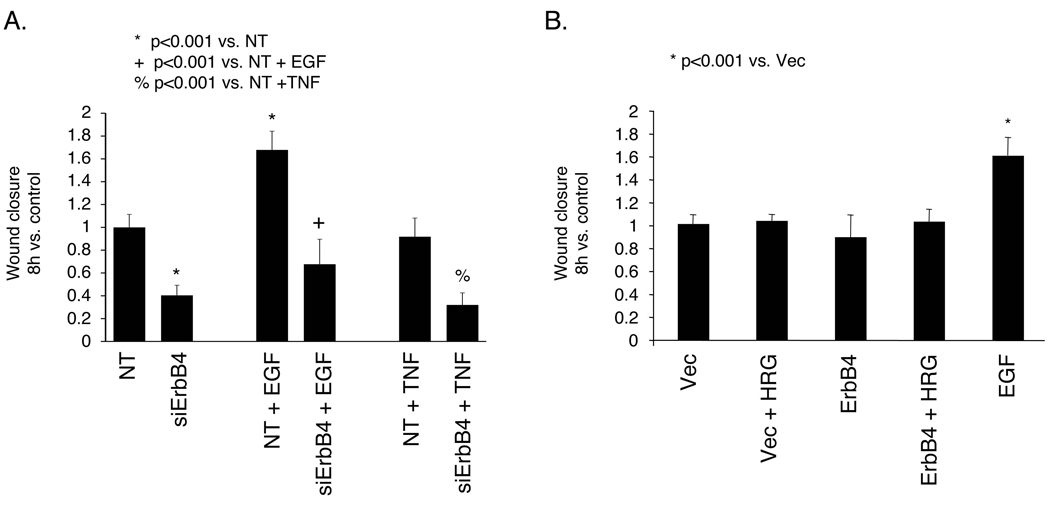
Wound closure is a key mechanism of intestinal recovery from injury. As EGFR promotes epithelial restitution, we tested whether ErbB4 is also involved in this process. YAMC cell monolayers plated on fibronectin were transfected with non-targeting or ErbB4 specific siRNA, which effectively reduced both basal and TNF-stimulated ErbB4 expression in these cells by >80% (Supplementary Fig. 6). Cultures were then wounded with a rotating silicone rod and followed by time-lapse microscopy. ErbB4 siRNA substantially decreased the YAMC cell restitution rate (41% of control; Fig. 4A), and this was reduced further in the presence of 100 ng/ml TNF (32% of control). However, while overall wound closure in the absence of ErbB4 was decreased, EGF (which promotes intestinal epithelial restitution2) was still able to stimulate an increase in cell migration (ErbB4 siRNA + EGF 166% vs. ErbB4 siRNA alone; NT siRNA + EGF 168% vs. NT siRNA alone). Thus, these cells are capable of responding to a migration signal, raising the possibility that ErbB4 siRNA does not directly ablate cell motility but rather affects another cellular parameter which produces a decrease in apparent wound closure as a secondary effect.
Figure 4. ErbB4 deletion inhibits mouse colon epithelial cell monolayer wound healing.
(A) YAMC cells on fibronectin were transfected with non-targeting (NT) or ErbB4 siRNA for 72h and subjected to a wound healing assay. TNF (100 ng/ml) or EGF (10 ng/ml) were included as indicated. (B) YAMC cells were transfected with vector or human ErbB4 expression construct and subjected to a wound closure assay with or without 100 ng/ml HRG.
Consistent with this notion, neither ErbB4 overexpression nor exposure to the ErbB4 ligand HRG (100 ng/ml) stimulated wound closure above basal levels (Fig. 4B). Effects of ErbB4 loss are also unlikely to be a result of altered proliferation, as in this model significant proliferation is not observed over the 8h experimental period under nonpermissive conditions23.
ErbB4 promotes cell survival in the presence of TNF
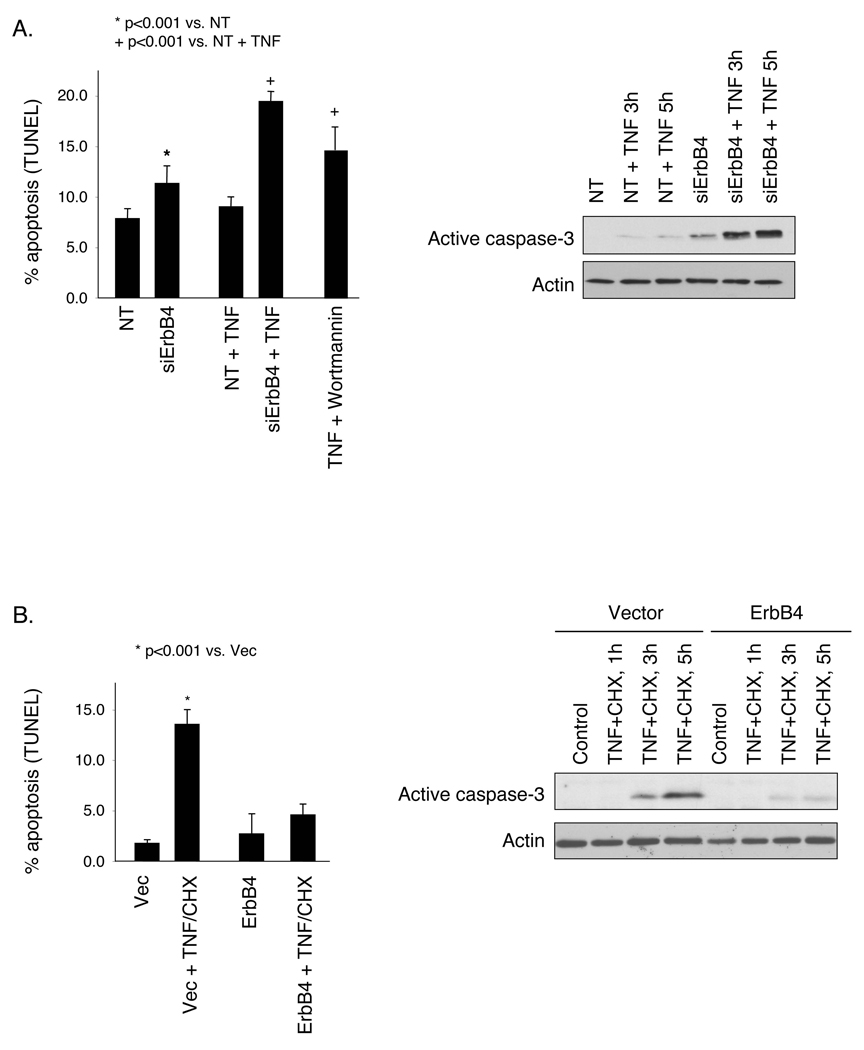
As an increased apoptosis rate could account for a decreased restitution rate, we asked whether loss of ErbB4 affects intestinal cell survival either basally or under stress induced by TNF exposure. YAMC cell cultures were transfected with siRNA pools, incubated with vehicle or 100 ng/ml TNF for 6h, and subjected to TUNEL apoptosis assays. ErbB4 knockdown resulted in a significant increase in both baseline (7% vs. 12%, p < 0.001) and TNF-stimulated (9% vs. 19%, p<0.001) apoptosis (Fig. 5A). Notably, while in control cells TNF did not cause significant cell death, in ErbB4 siRNA transfected cells it showed cell kill rates comparable to TNF+cycloheximide or TNF+Wortmannin positive controls. To control for possible off-target effects of the siRNA, a rescue experiment was performed using expression of human ErbB4, which is only partly affected by mouse ErbB4 siRNA. Re-expression of ErbB4 rescued cells from siRNA-induced apoptosis and loss of wound healing capacity, indicating that these results are specific to ErbB4 knockdown (Supplementary Fig. 6). For biochemical confirmation of apoptosis, lysates from transfected cells exposed to TNF were subjected to Western blot analysis for active caspase 3. TNF induced caspase 3 cleavage by 3h only in ErbB4 siRNA transfected cells (Fig. 5A).
Figure 5. ErbB4 promotes intestinal epithelial survival in the presence of TNF.
(A) YAMC cells were transfected with non-targeting (NT) or ErbB4 siRNA, treated with 100 ng/ml TNF for 6h, and subjected to TUNEL apoptosis assay. Labeled nuclei from >100 cells from at least 3 random fields per condition were counted in each experiment. Western blot analysis for cleaved (active) caspase 3 was performed on lysates of transfected cells exposed to TNF for 3 or 5 h. TNF+Wortmannin, positive control. (B) Vector or ErbB4 overexpressing cells were exposed to TNF+cycloheximide (1 µg/ml) for 5 h and apoptosis was measured by TUNEL assay; caspase 3 activation was assessed by Western blot analysis at 1, 3, and 5 h.
We also asked whether ErbB4 overexpression, such as is observed during experimental colitis (Fig. 2), could support cell survival in a cytotoxic environment. YAMC cells stably transfected with vector or ErbB4 were treated with 100 ng/ml TNF plus 1 µg/ml cycloheximide. As determined by either TUNEL assay or caspase 3 Western blot, TNF+cycloheximide stimulated apoptosis in vector-expressing cells, while overexpression of ErbB4 protected from this cell death (Fig. 5B).
ErbB4 mediates TNF-induced Akt activation in intestinal epithelial cells
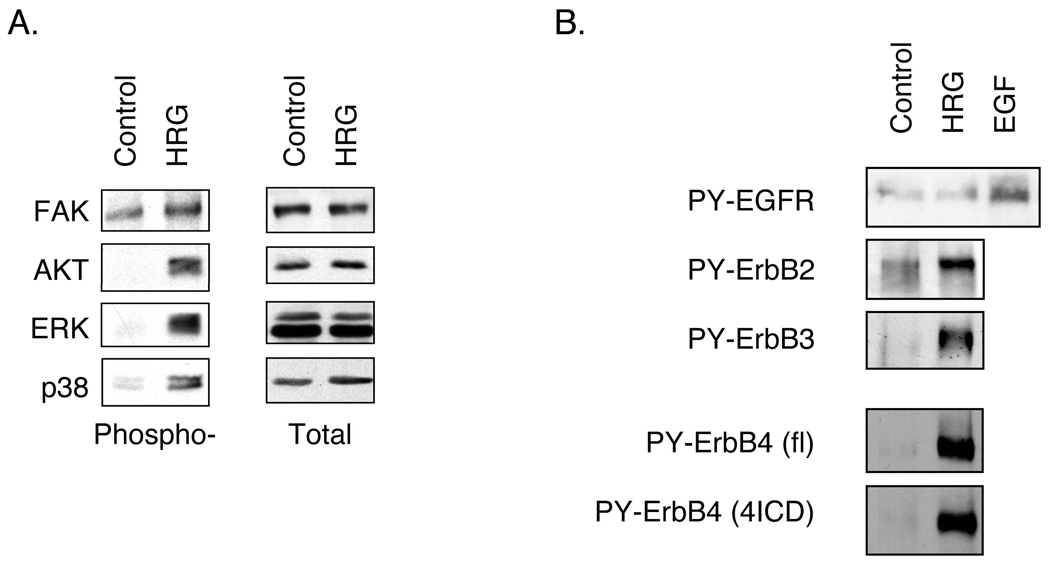
To explore the signal transduction underlying ErbB4 stimulated intestinal epithelial cell survival, YAMC cell cultures were exposed to the ErbB4 ligand HRG (100 ng/ml) and whole cell lysates were probed for phosphorylation of ErbB receptors and downstream signaling modules. HRG induced phosphorylation of both pro- and anti-survival signaling molecules, including Akt, ERK1/2, and p38 (Fig. 6A). However, HRG also activated three ErbBs [ErbB2, -3, and -4 (Fig. 6B)], making it difficult to specifically attribute responses to this ligand to ErbB4. Therefore, we exposed cells transfected with ErbB4 siRNA or overexpressing ErbB4 to TNF and analyzed whole cell lysates for activation of signaling cascades. TNF stimulates Akt, ERK1/2, and p38 phosphorylation as well as IkB-α degradation (and thus NF-kB activation) in YAMC cells (Fig. 7A). Of these, ablation of Akt activation by PI 3-kinase inhibition or blockade of NF-kB activity in the presence of TNF potently stimulate apoptosis (Fig. 5A and data not shown, respectively). ErbB4 siRNA blocked TNF-stimulated Akt phosphorylation but not IkB-α degradation or phosphorylation of p38 or ERK (Fig. 7A). Consistent with this result, ErbB4 overexpression enhanced both basal and TNF-stimulated Akt activation (Fig. 7B and Supplementary Fig. 7) without affecting other downstream signaling cascades tested.
Figure 6. HRG stimulates both pro- and anti-apoptotic signals.
YAMC cells were treated with 100 ng/ml HRG for 10 min. Lysates were subjected to Western blot analysis using the indicated antibodies. PY, phosphotyrosine.
To confirm that PI 3-kinase/Akt signaling in response to TNF is important for ErbB4-mediated cell survival, colon epithelial cells expressing either vector or exogenous ErbB4 were exposed to TNF with or without the PI 3-kinase inhibitor LY294002 (5 µM) for 6h and cell apoptosis was measured by TUNEL assay. At this concentration the inhibitor completely blocked TNF-induced Akt phosphorylation (data not shown). While ErbB4 overexpression protects colon epithelial cells from apoptosis induced by TNF+cycloheximide (Fig. 5B), ErbB4 did not protect against the TNF+LY294002 combination (Fig. 7C), supporting the conclusion that PI 3-kinase/Akt signaling is important for ErbB4-induced cell survival in the presence of TNF.
Discussion
This study describes a novel mechanism for regulation of the ErbB4 receptor tyrosine kinase by inflammatory cytokines. We report that TNF stimulated ErbB4 expression in intestinal epithelial cells both in cell culture and in vivo; receptor levels also increased in human IBD or during injury and recovery in a murine experimental colitis model. siRNA ablation of ErbB4 inhibited TNF-stimulated phosphorylation of the pro-survival Akt kinase and sensitized cells to apoptosis, while ErbB4 overexpression protected cells from TNF+cycloheximide cytotoxicity. Together with the observation that ErbB4 siRNA impairs wound healing of cultured intestinal epithelial cells, these data position ErbB4 as an important participant in the epithelial response to injury and inflammation.
ErbB4 is normally expressed at low levels in unchallenged epithelial tissues. Thus, receptor induction by cytokines represents a potential cytoprotective mechanism under stress conditions. Increased ErbB4 expression is likely to modulate both the amplitude and character of an EGF or HRG-family ligand stimulus, as ErbB receptors form both homo- and heterodimers, and different dimers can promote distinct cellular outputs27, 28. In our studies, basal ErbB4 expression was required for acute TNF-induced Akt signaling (Fig. 7A). ErbB4 overexpression further increased this signal (Fig. 7B), suggesting a model in which the low levels of endogenous ErbB4 are sufficient for initial signaling responses to cytokines, but sustained cell survival requires an accumulation of ErbB4. Interestingly, Akt signaling in mouse colon epithelial cells following TNF exposure appears to be largely acute with only mildly elevated phospho-Akt detectable after several hours, even though PI 3-kinase inhibition has sustained effects on cell survival (Fig. 7C). It is not clear whether the small sustained stimulation of this pathway is necessary for later anti-apoptotic responses, or if in contrast acute activation of this pathway by TNF triggers additional downstream targets which then convey cell survival.
TNF also promotes metalloproteinase-dependent release of ErbB ligands29, 30, specifically through TNF converting enzyme (TACE) activity as shown by Argast and colleagues. ErbB4 can also be cleaved by TACE31, releasing the constitutively active 4ICD fragment11. Thus, TNF-stimulated TACE activity provides a dual mechanism of stimulating cellular tyrosine kinase activity in which ErbB4 expression is induced and full-length receptor rapidly cleaved, while concomitant release of other ErbB ligands activates additional receptors. In this study we observe little to no change in EGFR or ErbB2 expression (Fig. 3), but a few reports have described TNF-induced increases in EGFR levels, primarily in cancer cells32, 33 or fibroblasts34. However, for the most part modulation of other ErbB receptor levels by cytokines in normal epithelial cells has not been reported. It is not clear whether the difference between our study, in which TNF-stimulated changes in ErbB expression were restricted to ErbB4, and other studies is due to tissue type or the difference between cancer cells and our non-transformed cell model. It should be noted that TNF does stimulate EGFR and ErbB2 phosphorylation in colon cells under some conditions35, suggesting that in addition to overall increased tyrosine kinase activity, TNF-stimulated ErbB4 accumulation represents an avenue to alter the pool of heterodimer partners available to EGFR and ErbB2. Such a shift in dimer composition could be critical in specifying the outcome of a cellular stimulus27, 28.
The possibility of an NF-kB-mediated mechanism by which TNF exerts its effects on ErbB4 is raised by the observation that forced NF-kB activation recapitulated TNF-induced ErbB4 expression (Fig. 3). It must be noted that a specific requirement for NF-kB in TNF's effects could not be directly tested as the combination of TNF and NF-kB inhibitor was rapidly cytotoxic, but the available data are consistent with the idea. TNF robustly activates NF-kB in our system [see Fig. 7, IkB-α panel and 19] and NF-kB activation was sufficient to stimulate ErbB4 accumulation. Interestingly, ErbB4 has in turn been implicated in NF-kB activation via NF-kB inducing kinase (NIK). In 293 cells, ErbB4/ErbB2 dimers form complexes with NIK and Grb736, and receptor activation promotes NF-kB activity in a NIK-dependent manner. Taken together with this result, our data suggest a positive feedback loop analogous to a recent report in which Zhu and colleagues showed that ErbB4 4ICD functions as an estrogen receptor transcriptional coregulator in breast cancer cells, while at the same time being a transcriptional target of the estrogen receptor37. It is tempting to speculate that ErbB4's low expression in many adult tissues is part of a regulatory mechanism for induction of an ErbB "reserve" by hormones or cytokines, allowing for rapid accumulation of receptor in stressful conditions.
Pro-survival roles for ErbB4 have been described in a subset of breast cancer cell studies8 and PC12 pheochromocytoma cells7. Our data are in agreement with these reports. However, in some contexts ErbB4 promotes rather than inhibits apoptosis. The Jones laboratory recently showed that nuclear 4ICD stimulates breast cancer cell apoptosis by acting as a BH3-only protein38 and expression of a highly active ErbB4 mutant induced significant cell death in multiple cell lines39. One possible explanation for these divergent results is a biphasic response to ErbB4, in which moderate amounts of receptor support survival but sustained excessive signaling is cytotoxic. In our results, the low endogenous ErbB4 expression in YAMC cells promotes cell survival (Fig. 5A). Our overexpressing cell pools have substantially less ErbB4 than naturally expressing cell models such as T47D (data not shown), and thus may not meet a necessary threshold for ErbB4-mediated cell killing. Alternatively, differing ErbB4 isoform expression, variant expression of other ErbBs, or other specifics of cell context may be key factors in determining whether ErbB4 promotes survival or apoptosis.
TNF primarily induces cleavable ErbB4 in YAMC cells, as demonstrated by a robustly detectable 80 kDa fragment (Fig. 2&Fig 3). Both CYT-1 and CYT-2 forms of the receptor are expressed in intestinal cells (Supplementary Fig. 1). CYT-1 contains the YTPM motif required for PI 3-kinase binding in NIH 3T3 cells8, and thus may be important for the effects observed on Akt activation and cell survival (Fig. 7). On the other hand heterodimerization with ErbB3, which is rich in functional YXXM motifs, could readily result in amplification of Akt activation by CYT-2 ErbB4. The different species of ErbB4 mRNA present in colon epithelial cells may function in different cell physiological pathways. For example, decreased wound healing after ErbB siRNA transfection may reflect increased cell apoptosis and thus a slightly decreased cell density, which is directly reflected in a wound closure assay (data not shown). However given that ErbB4 is required for cell migration and axon guidance in neural development40, a role in epithelial cell adhesion or migration cannot be completely ruled out.
In summary, results presented here identify ErbB4 as a TNF-inducible receptor and position it as a regulator of repair in response to injury and inflammation. In the context of elevated ErbB4 in pediatric Crohn's colitis (Fig. 1), these data have implications for future IBD treatment strategies. It should also be noted that sustained elevation of growth factor receptor activity in the context of injury and inflammation may promote the transition from chronic inflammation to cancer41. Because intestinal cancer is a significant complication for patients with Crohn's Disease or ulcerative colitis42, 43, a thorough understanding of the specific signaling targets and biological function(s) of individual ErbB4 isoforms in both healthy and inflamed epithelia will be key for the development of effective therapeutic avenues targeting this signaling axis.
Supplementary Material
Acknowledgments
This work was supported by NIH grants K01DK077956 (MRF) and R01DK056008 (DBP), a Senior Scientist Award from the Crohn's and Colitis Foundation of America (DBP), and the Vanderbilt Digestive Diseases Research Center (NIH award P30DK058404), including the DDRC's Novel Cell Line Development and Human Tissue Acquisition shared resources.
Footnotes
No conflicts of interest.
References
- 1.Carpenter G, Cohen S. EGF: receptor interactions and the stimulation of cell growth. In: Lefkowitz R, editor. Receptors and Recognition, Series B. Volume 13. London: Chapman and Hall; 1981. pp. 43–46. [Google Scholar]
- 2.Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;117:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- 3.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 4.Plowman GD, Culouscou J-M, Whitney GS, Green JM, Carlton GW, Foy L, Neubauer MG, Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long W, Wagner K-U, Lloyd KCK, Binart N, Shillingford JM, Hennighausen L, Jones FE. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130:5257–5268. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- 6.Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- 7.Erlich S, Goldshmit Y, Lupowitz Z, Pinkas-Kramarski R. ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience. 2001;107:353–362. doi: 10.1016/s0306-4522(01)00350-5. [DOI] [PubMed] [Google Scholar]
- 8.Kainulainen V, Sundvall M, Maatta JA, Santiestevan E, Klagsbrun M, Elenius K. A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J Biol Chem. 2000;275:8641–8649. doi: 10.1074/jbc.275.12.8641. [DOI] [PubMed] [Google Scholar]
- 9.Tang CK, Goldstein DJ, Payne J, Czubayko F, Alimandi M, Wang LM, Pierce JH, Lippman ME. ErbB-4 ribozymes abolish neuregulin-induced mitogenesis. Cancer Res. 1998;58:3415–3422. [PubMed] [Google Scholar]
- 10.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 11.Linggi B, Cheng QC, Rao AR, Carpenter G. The ErbB-4 s80 intracellular domain is a constitutively active tyrosine kinase. Oncogene. 2006;25:160–163. doi: 10.1038/sj.onc.1209003. [DOI] [PubMed] [Google Scholar]
- 12.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxy-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003 doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 13.Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L, Gulino A, Alimandi M. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res. 2004;294:469–479. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998;185:236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Egger B, Procaccino F, Lakshmanan J, Reinshagen M, Hoffmann P, Patel A, Reuben W, Gnanakkan S, Liu L, Barajas L, Eysselein VE. Mice lacking transforming growth factor α have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology. 1997;113:825–832. doi: 10.1016/s0016-5085(97)70177-x. [DOI] [PubMed] [Google Scholar]
- 17.Egger B, Buchler MW, Lakshmanan J, Moore P, Eysselein VE. Mice harboring a defective epidermal growth factor receptor (waved-2) have an increased susceptibility to acute dextran sulfate-induced colitis. Scand J Gastroenterol. 2000;35:1181–1187. doi: 10.1080/003655200750056664. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Fu YX. Tumor necrosis factor family members and inflammatory bowel disease. Immunol Rev. 2005;204:144–155. doi: 10.1111/j.0105-2896.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 19.Yan F, John SK, Polk DB. Kinase suppressor of Ras determines survival of intestinal cells exposed to tumor necrosis factor. Cancer Res. 2001;61:8668–8675. [PubMed] [Google Scholar]
- 20.Kaiser GC, Polk DB. Tumor necrosis factor α regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112:1231–1240. doi: 10.1016/s0016-5085(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 21.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. Embo J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras-1 protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272–1280. doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corredor J, Yan F, Shen CS, Tong W, John SK, Whitehead RH, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Phys Cell Physiol. 2003;284:C953–C961. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- 24.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 25.Thommesen L, Laegreid A. Distinct differences between TNF receptor 1- and TNF receptor 2-mediated activation of NFkappaB. J Biochem Mol Biol. 2005;38:281–289. doi: 10.5483/bmbrep.2005.38.3.281. [DOI] [PubMed] [Google Scholar]
- 26.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegman K, Kronke M. TNF activates NFkB by phosphatidylcholine-specific phospholipase C-induced "acidic" sphinomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Levkowitz G, Tzahar E, Karunagaran D, Lavi S, Ben-Baruch N, Leitner O, Ratzkin BJ, Bacus SS, Yarden Y. An immunological approach reveals biological differences between the two NDF/heregulin receptors, ErbB-3 and ErbB-4. J Biol Chem. 1996;271:7620–7629. [PubMed] [Google Scholar]
- 28.Li Z, Mei Y, Liu X, Zhou M. Neuregulin-1 only induces trans-phosphorylation between ErbB receptor heterodimer partners. Cell Signal. 2007;19:466–471. doi: 10.1016/j.cellsig.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Chen WN, Woodbury RL, Kathmann LE, Opresko LK, Zangar RC, Wiley HS, Thrall BD. Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor alpha. J Biol Chem. 2004;279:18488–18496. doi: 10.1074/jbc.M310874200. [DOI] [PubMed] [Google Scholar]
- 30.Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530–34536. doi: 10.1074/jbc.M405703200. [DOI] [PubMed] [Google Scholar]
- 31.Cheng QC, Tikhomirov O, Zhou W, Carpenter G. Ectodomain cleavage of ErbB-4: Characterization of the cleavage site and m80 fragment. J Biol Chem. 2003 doi: 10.1074/jbc.M302111200. [DOI] [PubMed] [Google Scholar]
- 32.Mujoo K, Donato NJ, Lapushin R, Rosenblum MG, Murray JL. Tumor necrosis factor α and γ–interferon enhancement of anti-epidermal growth factor receptor monoclonal antibody binding to human melanoma cells. J. Immunol. 1993;13:166–174. doi: 10.1097/00002371-199304000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Tumor necrosis factor α induces the expression of transforming growth factor α and the epidermal growth factor receptor in human pancreatic cancer cells. Proc. Natl. Acad. Sci. USA. 1993;90:863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palombella VJ, Yamashiro DJ, Maxfield FR, Decker SJ, Vilcek J. Tumor necrosis factor increases the number of epidermal growth factor receptors on human fibroblasts. J. Biol. Chem. 1987;262:1950–1954. [PubMed] [Google Scholar]
- 35.Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor an ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. PNAS. 2008 doi: 10.1073/pnas.0801463105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D, Xu LG, Chen L, Li L, Zhai Z, Shu HB. NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene. 2003;22:4348–4355. doi: 10.1038/sj.onc.1206532. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Sullivan LL, Nair SS, Williams CC, Pandey AK, Marrero L, Vadlamudi RK, Jones FE. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res. 2006;66:7991–7998. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 38.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 39.Vidal GA, Clark DE, Marrero L, Jones FE. A constitutively active ERBB4/HER4 allele with enhanced transcriptional coactivation and cell-killing activities. Oncogene. 2007;26:462–466. doi: 10.1038/sj.onc.1209794. [DOI] [PubMed] [Google Scholar]
- 40.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 41.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Jess T, Loftus EV, Jr, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, 3rd, Munkholm P, Sandborn WJ. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039–1046. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.