Abstract
To improve the thromboresistance of a titanium alloy (TiAl6V4) surface which is currently utilized in several ventricular assist devices (VADs), a plasma-induced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine (MPC) was carried out and poly(MPC) (PMPC) chains were covalently attached onto a TiAl6V4 surface by a plasma induced technique. Cleaned TiAl6V4 surfaces were pretreated with H2O-vapor-plasma and silanated with 3-methacryloylpropyltrimethoxysilane (MPS). Next, a plasma-induced graft polymerization with MPC was performed after the surfaces were pretreated with Ar plasma. Surface compositions were verified by X-ray photoelectron spectroscopy (XPS). In vitro blood biocompatibility was evaluated by contacting the modified surfaces with ovine blood under continuous mixing. Bulk phase platelet activation was quantified by flow cytometric analysis, and surfaces were observed with scanning electron microscopy after blood contact. XPS data demonstrated successful modification of the TiAl6V4 surfaces with PMPC as evidenced by increased N and P on modified surfaces. Platelet deposition was markedly reduced on the PMPC grafted surfaces and platelet activation in blood that contacted the PMPC-grafted samples was significantly reduced relative to the unmodified TiAl6V4 and polystyrene control surfaces. Durability studies under continuously mixed water suggested no change in surface modification over a one month period. This modification strategy shows promise for further investigation as a means to reduce the thromboembolic risk associated with the metallic blood-contacting surfaces of VADs and other cardiovascular devices under development.
Keywords: titanium alloy, surface modification, phospholipid polymer, blood compatibility, ventricular assist device (VAD)
1. Introduction
Suboptimal blood compatibility in many cardiovascular devices puts patients at increased risk for thromboembolism, often necessitating the use of chronic anti-coagulation and its accompanying increased risk for bleeding. The composition of the biomaterial surface, the nature of the blood flowing across the device surfaces and the bias of the patient’s blood toward hemostatic reactions all combine to define thrombotic and thromboembolic risk. Thus much work has focused on utilizing computational fluid dynamics to improve flow characteristics over biomaterial surfaces in circulatory support devices [1] and similarly there has been great interest in developing chemical modifications for blood contacting surfaces [2].
Our recent interest has been in developing a rotary blood pump for pediatric applications where aggressive anticoagulation may be problematic and biocompatibility is thus of primary concern. For design and machinability considerations the titanium alloy TiAl6V4 makes up the blood contacting surfaces of this pump as well as several other rotary blood pumps in clinical use and in pre-clinical development [1,3–9]. Although titanium and its alloys have exhibited generally acceptable biocompatibility in a variety of settings, its surface modification remains of interest for blood contact since platelet deposition still can occur in vitro and thrombosis and thromboembolism can occur in these devices in vivo [10–12].
Biomaterial surface modification by plasma based techniques has frequently been applied due to the high efficiency of this approach for a variety of substrates and geometry [13]. Active functional groups can be introduced by utilizing a specific atmosphere such as water, ammonia or argon (Ar) which allows further modification by covalent attachment of other polymers or biomolecules on the substrate [14–17]. Plasma induced graft polymerization after Ar plasma pretreatment is attractive for its high efficiency and others have previously reported on the effects of plasma treatment time, plasma power and storage time on surface modifications with this method [18, 19]. Polytetrafluoroethylene, silicon, and stainless steel surfaces have successfully been modified by Ar plasma induced grafting copolymerization with a poly (ethylene glycol) containing macromonomer to improve their surface blood compatibility [20–22].
Synthetic phospholipid polymers (phosphorylcholine (PC) group-bearing polymers) have been extensively studied due to their excellent biological and blood compatibility [23]. One of the most representative phospholipid polymers, 2-methacryloyloxyethylphosphorylcholine (MPC) polymer has received considerable interest for medical applications [24–26]. Many researchers have shown previously that surface modification with the MPC polymers or the introduction of PC groups by blending, coating and graft modification techniques are effective in improving blood compatibility by resisting non-specific protein adsorption and platelet activation and adhesion on polymer biomaterials surfaces [27–31]. The MPC polymers have also been applied to some metallic biomaterial surfaces including ventricular assist devices (VADs) and demonstrated apparent improvements in blood compatibility [32–35]. However, to date most of the surface modification techniques with the MPC polymers involve physical adsorption onto metallic surfaces. We recently reported on the MPC polymer covalent immobilization approach for TiAl6V4 that showed promise in improving blood biocompatibility [36]. While effective, concerns with this technique were the lability of the amide bond linking the copolymer to the surface and the extent of surface coverage that might be achieved.
Our objective in this study was to introduce a new, potentially more robust method of polymerizing MPC off of a modified TiAl6V4 surface using a plasma-induced graft technique that might offer durability under high shear and for extended blood contacting periods. The modified TiAl6V4 surface was characterized in terms of its surface composition and acute platelet deposition and activation after contact with ovine blood in vitro.
2. Materials and Methods
2.1 Materials
A titanium alloy (TiAl6V4) sheet was obtained from California Metal & Supply Inc., Gardena, CA. MPC was obtained from NOF Corp. (Tokyo, Japan), which was synthesized as previously described [24]. 3-Methacryloylpropyltrimethoxysilane (MPS, Aldrich, USA) was used as a silane coupling agent. Heparin (Pharmacia & Upjohn Co., Ann Arbor, MI) was used for blood anticoagulation.
2.2 Surface pretreatment and silanization of a TiAl6V4
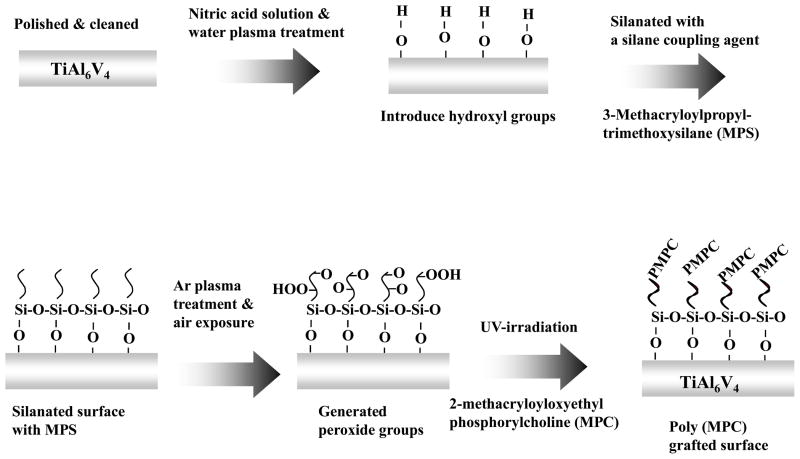
The TiAl6V4 sheet was polished with 3.0, 1.0, 0.25, and 0.1 micron diamond pastes in sequence (Electron Microscopy Sciences, Washington, PA) and cleaned ultrasonically 3 times for 5 min each with ethanol and acetone. The surfaces were then passivated with a 35% nitric acid solution for 1 h and rinsed with distilled water. Next, the TiAl6V4 surfaces were pretreated under H2O plasma with radio frequency glow discharge (RFGD, MARCH GCM250, March Instrument Inc, CA). The RFGD power applied was 25 W at a frequency of 13.65 MHz. The titanium surface was subjected to RFGD for 5 min supplying H2O vapor at a vacuum pressure of 0.4 torr. The H2O plasma pre-treated TiAl6V4 surfaces were silanated by immersion in an MPS solution for 3 h in a 90°C oil bath. The MPS solution consisted of 2% MPS in ethanol that was hydrolyzed by adding water and stirring for 1 h. The pH of the MPS solution was adjusted to approximately 3–4 by adding 0.1M HCl. The silanated samples were dried at 110°C for 1 h, then rinsed repeatedly with ethanol and water, and stirred in deionized water for 1 h to remove adsorbed MPS. Samples treated in this manner were referred to as Ti-MPS (Figure 1)
Figure 1.
Scheme of surface functionalization and modification of a TiAl6V4 surface with PMPC.
2.3 Plasma-induced surface graft modification with MPC
The silanated TiAl6V4 samples were treated with Ar plasma by using RFGD (25 W, 20 sec, 0.6 torr), and then the surface was exposed to the atmosphere for 10 min to create surface peroxide groups. The TiAl6V4 sample was then immersed in MPC solution (0.5 mM) which was placed in a transparent polystyrene round-bottom tube (BD Bioscience, San Jose, CA) The monomer solution was passed through argon gas for 1 min and 0.005 mM riboflavin (Sigma-Aldrich, St. Louis, MO) was added to eliminate any oxygen [14]. Then, graft modification onto the TiAl6V4 surface was carried out under a high intensity UV lamp for 24 h. This decomposed the surface peroxide groups to free radicals and poly(MPC) (PMPC) was grafted from the surface. The modified samples were rinsed three times with ethanol and water and stirred in deionized water for 24 h to remove physically adsorbed PMPC. Samples treated in this manner were referred to as Ti-MPS-PMPC (Figure 1).
2.4 Surface characterization
The surface composition of the titanium samples was analyzed by X-ray photoelectron spectroscopy (XPS) using a Surface Science Instruments S-probe spectrometer and a take-off angle of photoelectron was 55°. The Service Physics ESCAVB Graphics Viewer program was used to determine peak area, calculate the elemental compositions from peak areas, and peak fit the high resolution spectra. The surface composition on a given sample was averaged from three composition spots for each sample. The mean value for three different samples was determined.
The static contact angle of water on the surfaces of unmodified and modified titanium samples was measured at room temperature using a contact angle goniometer (VCA optima, AST Product Inc., Billerica, MA) by placing 1 μL of double distilled water on the surfaces. The droplet was imaged using a video camera coupled to a light microscope, and the contact angle was determined on the screen of a monitor employing imaging software. Five measurements were made on each sample to obtain the contact angle of the sample. The contact angle was also measured weekly in several of the modified samples that underwent continuous stirring under deionized water for one month to test the long term stability of the surface modification. XPS was also performed on the surfaces after one month of water contact.
2.5 Blood collection and blood contact test
Whole ovine blood was collected by jugular venipuncture directly into a syringe containing heparin (3.0 or 6.0 U/mL for 1 and 2.5 h blood contacting experiments respectively) using an 18 gauge 1 ½″ needle, after discarding the first 3 mL. NIH guidelines for the care and use of laboratory animals were observed. Modified titanium and unmodified samples were placed into Vacutainer® blood collection tubes without additives (BD Biosciences, Franklin Lakes, NJ) filled with heparinized ovine blood and incubated for a specified time at 37°C on a hematology mixer (Fisher Scientific, Pittsburgh, PA).
2.6 Observation and quantification of platelet deposition and activation
The TiAl6V4 surfaces were observed by scanning electron microscopy (SEM; JSM-6330F, JEOL USA, Inc., Peabody, MA) after 2.5 h contact with heparinized blood (6.0 U/mL). The TiAl6V4 surfaces were also observed with epi-fluorescence microscopy (ZEISS, Carl Zeiss, Inc. Thornwood, NY) after contact for 1 h with heparinized blood (3.0 U/mL) that was treated with quinacrine dihydrochloride (10 μM final concentration, Sigma) to fluorescently label the platelets. The number of platelets for each sample was also estimated by a lactate dehydrogenase (LDH) assay [37] with an LDH Cytotoxicity Detection Kit (Takara Bio, Japan). In this assay, the surfaces were rinsed thoroughly with 50 mL PBS following 2.5 h contact with blood (6.0 U/mL heparin) and then immersed in 1 mL of 2% Triton X-100 solution (Sigma) for 20 min to lyse deposited platelets. Calibration of spectrophotometer absorbance results to platelet numbers was accomplished using a calibration curve generated from known dilutions of ovine platelet rich plasma in the lysing solution.
The percentage of activated ovine platelets in the bulk phase of the blood during surface contact was determined using flow cytometric quantification of Annexin V binding as recently described [38]. Blood samples (10 μL) were taken during test surface contact experiments described above for LDH measurement of platelet deposition after 2 h. Activation levels from 5 independent samples were averaged for each surface type.
2.7 Statistical analyses
Data are presented as means with standard deviation. Statistical significance between sample groups was determined using ANOVA followed by post-hoc Newman-Keuls testing and accepted at p<0.05.
3. Results
The high resolution spectra from XPS are shown in Figure 2. The C1s data was calibrated to the hydrocarbon peak (C-C/C-H) at 285.0 eV. The MPS modified TiAl6V4 surface (Ti-MPS) showed an increase in the peak at 286.6 eV and 288.5 eV which is likely due to C-O and O-C=O type species. The PMPC modified TiAl6V4 surface showed a further increase in the peak at 286.6 eV by the addition of the C-N type species that are attributed to the MPC units. Furthermore, the Ti-MPS-PMPC also has a peak at 290.5 eV which is likely due to peroxide OOC=O type species that is attributed to the Ar plasma treatment. The surface atomic compositions of TiAl6V4 samples are shown in Table 1. The oxygen composition of the pre-treated TiAl6V4 surfaces (Ti-H2O plasma) rose significantly in comparison with the Ti which was not pre-treated (p<0.05). The MPS modified surface showed an increase in Si which was attributed to the presence of MPS (p<0.05). Furthermore, the XPS data from Ti-MPS-PMPC provide evidence for the successful modification with PMPC by reflecting increased nitrogen (N) and phosphorus (P) (p<0.05).
Figure 2.
XPS high resolution (C1s) spectra of the modified and unmodified TiAl6V4 samples.
Table 1.
Atomic percentage by X-ray photoelectron spectroscopy
| C | O | Ti | Al | Si | N | P | |
|---|---|---|---|---|---|---|---|
| TiAl6V4 (Ti) | 42.0 (±8.0) | 41.1 (±5.2) | 9.5 (±1.1) | 4.3 (±3.1) | 1.0 (±1.0) | 1.0 (±0.5) | 0.1(±0.2) |
| Ti (H2O plasma) | 24.1 (±6.6) | 50.8 (±0.4) | 10.5 (±3.3) | 2.3 (±1.1) | 1.3 (±1.4) | 0.8 (±0.3) | 0.0 (±0.0) |
| Ti-MPS | 49.4 (±8.9) | 34.3 (±5.4) | 1.3 (±1.3) | 1.2 (±1.1) | 13.9 (±4.8) | 0.2 (±0.4) | 0.0 (±0.0) |
| Ti-MPS-PMPC | 42.8 (±12.6) | 40.0 (±7.8) | 1.4 (±1.6) | 0.0 (±0.0) | 14.1 (±3.8) | 1.8 (±0.3)* | 1.0 (±0.2)* |
p<0.05 vs. other surfaces
N=7, ± standard deviation for Ti
N=3, ± standard deviation for Ti (H2O plasma)
N=5, ± standard deviation for Ti-MPS
N=4, ± standard deviation for Ti-MPS-PMPC
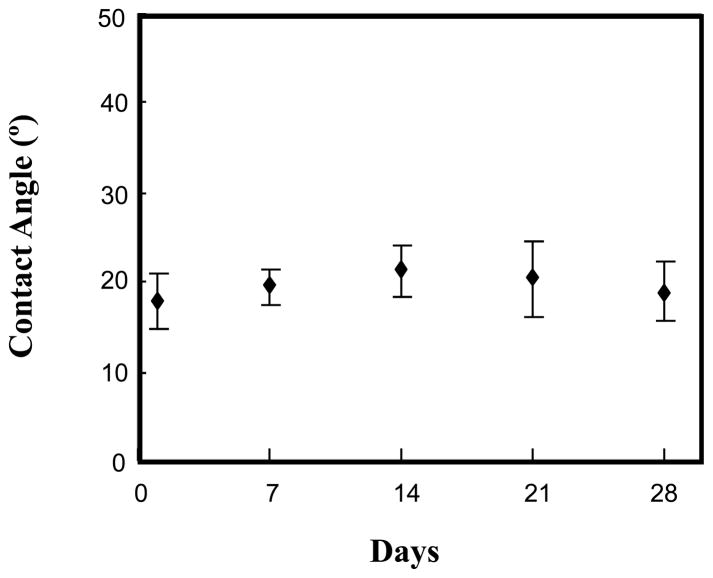
The surface tension on the modified and unmodified titanium samples was shown in Table 2. The contact angle on TiAl6V4 surfaces was decreased from 58 ± 6° to 35 ± 4° after H2O plasma treatment. The Ti-MPS increased in surface tension to 88 ± 6° due to modification with hydrophobic MPS on the TiAl6V4 surface. However, the surface tension of the PMPC grafted surfaces decreased substantially (Ti-MPS-PMPC = 18 ± 4°) by modification with the hydrophilic PMPC on the surface in comparison with all of the other surfaces (p<0.05). The surface contact angle on the PMPC grafted surface (Ti-MPS-PMPC) measured every 7 days during a one month period of mixing with water did not significantly change (Figure 3). Additionally, the surface composition of several of the modified samples was also measured after mixing for 1 month in water. The XPS analysis results also showed no significant difference in the surface composition of phosphorus before (P: 1.1 ± 0.1%) and after (P: 1.0 ± 0.1%) the water contacting experiment.
Table 2.
Surface tension on the unmodified and modified titanium samples
| TiAl6V4 (Ti) | Ti (H2O plasma) | Ti-MPS | Ti-MPS-PMPC | |
|---|---|---|---|---|
| Contact angle (°) | 58.3 (±6.2) | 34.7 (±4.4) | 87.6 (±6.4) | 18.1 (±4.0)* |
p<0.05 vs. other surfaces
n=3, ± standard deviation
Figure 3.
Contact angle measurements of the PMPC modified TiAl6V4 surface (Ti-MPS-PMPC) after continuous mixing under deionized water.
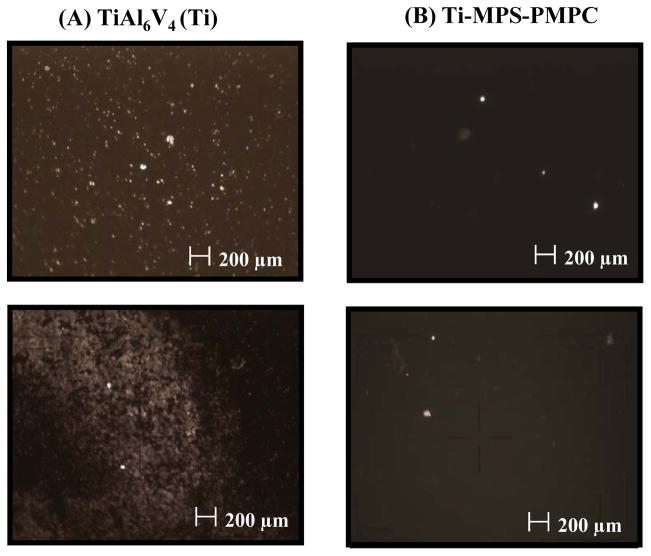
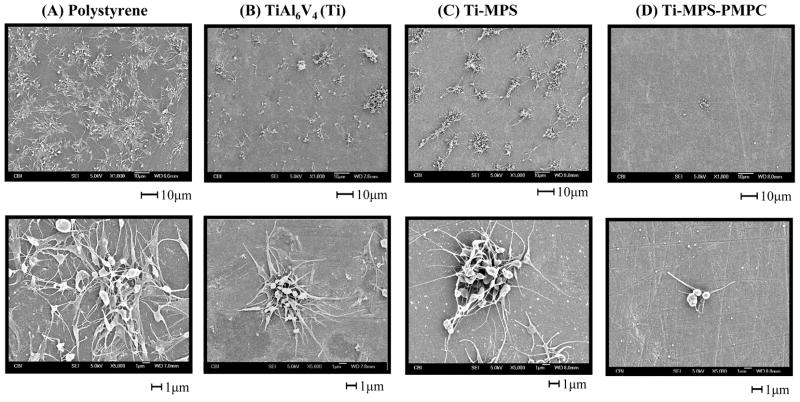
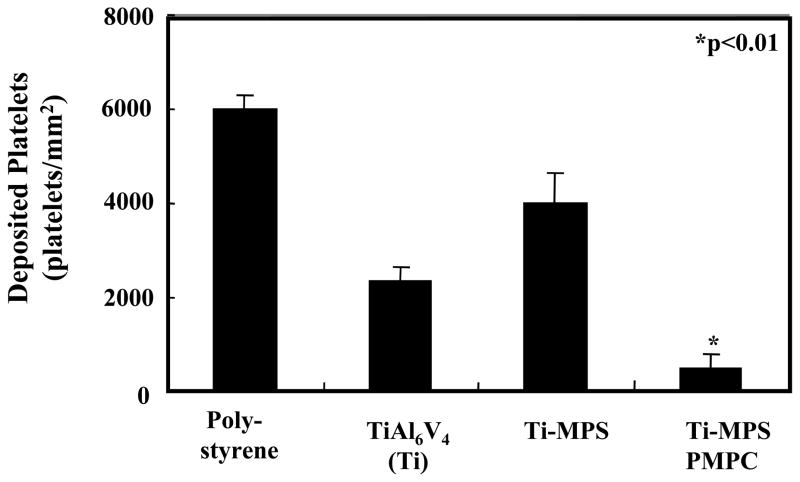
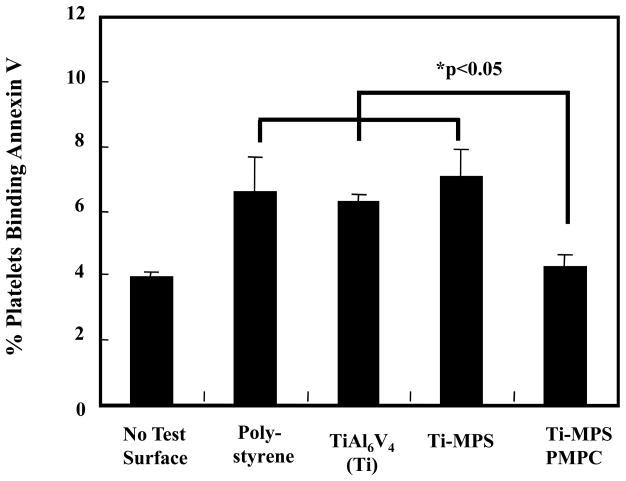
The modified and unmodified TiAl6V4 surfaces after contact with anticoagulated ovine blood for 1 h at 37°C were observed with an epi-fluorescence microscope (Figure 4). Fluorescent platelets are seen to be adhered and aggregated on the surface. The unmodified titanium had relatively high numbers of deposited platelets, whereas the PMPC modified surfaces (Ti-MPS-PMPC) showed few adherent platelets. Platelet adhesion and morphology was also observed with SEM after contact with ovine blood for 2 h at 37°C. The SEM images on the surfaces of the positive control polystyrene, unmodified and modified titanium samples are seen in Figure 5. The polystyrene control surface (Figure 5A) supported heavy platelet deposition with most of the deposited platelets exhibiting extended pseudopodia. The unmodified titanium surface (Figure 5B) had a moderate amount of deposited platelets on its surface, with the number of platelets appearing to be less than for the polystyrene surface. The silanated titanium surface (Ti-MPS, Figure 5C) also showed a moderate amount of deposited platelets, and these platelets exhibited extended pseudopodia. The quantity of adhered platelets on the Ti-MPS surface appeared to be slightly more than for the unmodified titanium (Figure 5B). Platelet deposition was decreased dramatically on the Ti-MPS-PMPC surfaces, and the platelets that were adhered generally retained their discoid morphology with some pseudopodia extension (Figure 5D). It is worth noting that it was difficult to identify adhered platelets on the Ti-MPS-PMPC surface. The number of deposited platelets as quantified by the lactate dehydrogenase (LDH) assay after blood contact (Figure 6) was significantly less for Ti-MPS-PMPC surfaces than for all of the other surfaces (p<0.01). Flow cytometric quantification of bulk phase platelet activation using the Annexin V assay (Figure 7) was also significantly lower in blood contacting Ti-MPS-PMPC surfaces than for unmodified titanium and polystyrene samples (p<0.05).
Figure 4.
Fluorescent micrograph images of unmodified and modified TiAl6V4 samples after contact with minimally anticoagulated (3.0 U/mL heparin) ovine blood for 60 min at 37°C.
Figure 5.
SEM micrographs of polystyrene, unmodified and modified TiAl6V4 samples after contact with ovine blood (heparin 6U/mL) for 2.5 h at 37°C. (A) polystyrene (B) Ti (C) Ti-MPS (D) Ti-MPS-PMPC.
Figure 6.
Platelet deposition onto surfaces after contact with ovine blood for 2.5 h as determined by lactate dehydrogenase (LDH) assay (n=3).
Figure 7.
Quantification of activated platelets in the bulk phase of ovine blood after surface contact under continuous rocking. No test surface indicates blood from a rocked tube into which no test surface was placed. Platelet activation was quantified by flow cytometric measurement of Annexin V binding onto platelets (n=3).
4. Discussion
Improvement of surface blood compatibility is of keen interest to the cardiovascular device community given the morbidity and mortality associated with device-related thrombotic complications and with the anticoagulation applied to minimize these risks. In the case of VADs, biocompatibility concerns are a major reason why these devices are underutilized in heart failure patients [3, 39–41]. PC groups and PC group-bearing polymer modified surfaces have previously demonstrated marked improvement in blood compatibility when compared to unmodified surfaces [24–31]. Moreover studies involving self-assembled monolayers (SAM) with well defined and highly ordered and oriented PC groups have shown that this type of surface is one of the most promising for anti-fouling since it strongly resists non-specific protein adsorption and cell adhesion [42–44]. There are several successful studies where a PC group-bearing was physically adsorbed onto the metallic surfaces in vascular stent and VAD applications [9,33,34]. In the VAD studies the blood contacting surface containing physically adsorbed MPC polymer was shown to be superior in terms of blood compatibility when compared to the same device with a blood contacting surface modified with a diamond like carbon coating [9,34]. Despite the improved hemocompatibility in these studies, there was concern about the long term stability of the surface PMPC. Furthermore, in the report of Kihara et al, the physically adsorbed MPC polymer was demonstrated to elute over time during VAD operation [34].
In spite of widespread examination of grafting PC groups onto polymer or silica surfaces, there are few reports where a PC group-bearing polymer has been grafted onto metallic surfaces, and even fewer that have subsequently evaluated the hemocompatibility of the resulting surfaces [36, 45, 46]. In this study, we showed that a TiAl6V4 surface can be successfully modified with the MPC moiety by a plasma-induced grafting method after the TiAl6V4 surface was silanized, and then Ar plasma treated. Our XPS results demonstrated successful modification of the TiAl6V4 surface with PMPC and the surface showed significant decreases in platelet deposition and activation in comparison with unmodified surfaces. The higher platelet deposition observed on the control Ti-MPS surface relative to the Ti surface could generally be attributed to an increase in surface hydrophobicity as reflected by the higher contact angle of this surface relative to the unmodified Ti alloy. Such an increase in hydrophobicity may serve as a driving force for increased protein adsorption and in this process result in more fibrinogen adsorption in a state that would support platelet adhesion on the surface.
For the PMPC-grafted surface the contact angle and XPS results after a month of mixed water contact were not significantly different from our initial surface tension and XPS evaluation signifying the stability of the grafted PMPC and its sustained hydrophilicity. We previously reported on reduced thrombogenicity after immobilizing an MPC polymer poly(MPC-co-methacryl acid) (PMA) onto titanium surfaces. This surface modification strategy involved a condensation reaction between the carboxyl groups of the PMA and amino groups which were introduced on the titanium surface by a silane coupling agent [34]. However despite its covalent attachment, the long term stability of the PMA immobilized surface is a concern. The cause for concern is that over time the amide bonds between PMA and the titanium surface may be hydrolyzed under continuous blood contact. The PMPC grafting in this report is not linked to the titanium surface via an amide bond, potentially providing an advantage over the PMA immobilized surface in terms of longer term stability under similar circumstances. Another advantage over the PMA immobilization strategy is that the PMPC grafting technique uses Ar plasma treatment which could be applied to other metallic surfaces, and also on many medically-relevant polymeric surfaces including polytetrafluoroethylene (PTFE).
The PMPC grafting method of this report might allow for more control of PMPC surface coverage and offer improved uniformity of surface PC groups when compared with the previous PMA immobilization method [34]. Immobilization of the long-chain PMA copolymer onto the titanium surface might have caused steric hindrance to further copolymer addition and this may have led to low and non-uniform areas of PMA coverage on the surface. Since the PMPC grafting method employs the addition of MPC rather than the long chain PMA copolymer there is less concern about steric hindrance. Less steric hindrance might lead to higher coverage of the MPC unit on the modified surface provided the surface has sufficient radical formation sites. However it is worth noting that in this grafting method it may be difficult to prepare a surface with 100% MPC unit coverage. This method also has some limitation in terms of the level of control achievable with respect to chain length of the modified PMPC. To obtain better control of the PMPC chain length on the surface, an alternative surface modification method such as surface initiated atom transfer radical polymerization could be considered. If a method were developed to prepare a covalently attached PMPC chains self assembled monolayer on a TiAl6V4 surface, it would ensure high coverage and uniformity of the PC group and might be even more advantageous in the manufacture of biocompatible blood contacting device surfaces.
In preliminary studies, we chose MPS as a pre-modification agent since it possesses a double bond in its structure and could provide a radical formation site in UV-initiated graft polymerization of MPC. This approach alone, without a UV catalyst, had minimal success (data not shown), so Ar plasma was employed to modify the Ti-MPS surface with the hypothesis that Ar plasma treatment would provide a greater number of radical formation sites, as well as peroxide, on the surface than with UV irradiation alone [18]. The application of both an Ar plasma pre-treatment followed by UV irradiation in the presence of a catalyst such as benzophenone [30, 46] on the Ti-MPS surface might have led to even further surface grafting, however this was not investigated.
The graft modification approach using Ar plasma treatment with the PMPC on TiAl6V4 described in this study might be improved with additional study into ways to further control the surface coverage of the grafted PMPC polymer and therefore maximize the anti-thrombogenic properties of the surface. Several factors that should be considered to improve the surface modification process include control of the water plasma in the MPS silanization process, treatment time under Ar plasma and RFGD power settings. The latter two factors are important in this modification technique since they affect the amount of peroxide groups that are able to become radical formation sites by the decomposition of the peroxide groups [12]. In this study, the Ar plasma treatment time was set to 20 sec under 25W of plasma power at a pressure of 0.6 Torr. Several other Ar treatment time points were evaluated between 0 and 120 sec in our preliminary studies. However, the PMPC grafted samples from the 20 sec Ar plasma treatment showed the lowest surface contact angle (18.2° ±4.1) and were used in our subsequent studies. The amount of peroxide concentration generated on the TiAl6V4 surfaces after the 20 sec Ar plasma treatment was 3.4(±1.6) ×10−8 mol/cm2, which was assessed with the plasma treated titanium samples in this study by a peroxide determination method using a 2,2-diphenyl-1-pricylhydrazyl (DPPH) solution [18, 19]. However, further study may be necessary to better understand the relationship between the amount of generated peroxide groups and PMPC grafting rate under various Ar plasma treatment conditions in order to establish the best conditions and maximize PMPC incorporation onto the TiAl6V4 surface.
The MPS modified surface was prepared under hydrolytic condition which allows the methoxy groups in MPS to change to hydroxyl groups and activates the reaction between TiOH and MPS as well as MPS self interaction, thus producing bulk deposition on the surface. Furthermore, the surface roughness might be increased after PMPC grafting, because, the PMPC grafted layer might not be uniform [46]. However the surface roughness or thickness on the MPS and PMPC grafted surface could not measured. We polished the raw titanium alloy sheet by hand with diamond pastes of 3, 1, 0.25 and 0.1 μm size, thus the roughness of the original titanium surface was of a scale that additional texture added from the surface modification was not likely to be detectable. We chose to use this surface polishing technique since this is the process used industrially to prepare the titanium alloy surfaces before use in blood pump assembly. The surface roughness of the unmodified TiAl6V4 sample and bulk layer deposition of MPS may have contributed to deviations observed in the atomic composition (Table 1) and contact angle (Table 2) data on the unmodified and modified surfaces. Further study is necessary to measure the surface roughness and thickness of the grafting layer depending on the reaction conditions with a highly polished TiAl6V4 sample. Fluorescence microscopy observation after staining the PMPC [46], atomic force microscopy and ellipsometry analysis data could be helpful to measure the thickness and roughness of PMPC grafting layer.
In this study, UV irradiation was used to decompose the generated peroxide group and initiate the graft polymerization of MPC. However, the peroxide decomposition also occurs by heating [12, 13] and polymerization of MPC might be carried out at 70°C without UV irradiation if UV irradiation is not appropriate due to complex device geometry. Another limitation to the study is that the blood biocompatibility tests were performed using an in vitro system with ovine blood and under flow conditions that, although mixed, do not replicate any specific application. A next step might be to apply this modification to the interior surfaces of a rotary VAD and assess platelet deposition onto these surfaces in a mock circulatory loop under appropriately high shear blood flow. Comparison studies with other types of modified surfaces such as poly(ethylene glycol)-based or other types of zwitterionic moieties might be of interest to compare the efficacy of this plasma induced grafting technique.
5. Conclusions
TiAl6V4 surfaces were successfully modified with PMPC chains by a plasma-induced grafting technique following surface silanization and plasma treatment. The PMPC modified TiAl6V4 surfaces showed significantly decreased platelet deposition and bulk phase platelet activation in vitro relative to the unmodified Ti samples. This PMPC grafting on TiAl6V4 surfaces shows promise for further investigation as a means to reduce the thromboembolic risk associated with the blood-contacting surfaces of cardiovascular devices. In the setting of a rotary blood pump design, such a coating may allow reduction in anticoagulation levels. Further pre-clinical evaluation is warranted to investigate this potential.
Acknowledgments
This research was supported by NIH contract # HHSN268200448192C and the NSF Engineering Research Center for Revolutionizing Metallic Biomaterials (Award Number: 0812348). Mr. Johnson was supported by a United Negro College Fund MERCK Graduate Science Research Dissertation Fellowship. Mr. Woolley was supported by an NIH training grant # T32-HL076124.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borovetz HS, Badylak S, Boston JR, Johnson C, Kormos R, Kameneva MV, Simaan M, Snyder TA, Tsukui H, Wagner WR, Woolley J, Antaki J, Diao C, Vandenberghe S, Keller B, Morell V, Wearden P, Webber S, Gardiner J, Li CM, Paden D, Paden B, Snyder S, Wu J, Bearnson G, Hawkins JA, Jacobs G, Kirk J, Khanwilkar P, Kouretas PC, Long J, Shaddy RE. Cell Transplant. 2006;15(Suppl 1 Review):69. doi: 10.3727/000000006783982304. [DOI] [PubMed] [Google Scholar]
- 2.Sin DC, Kei HL, Miao X. Expert Review of Medical Devices. 2009;6:51. doi: 10.1586/17434440.6.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Wagner WR, Borovetz HS, Griffith BP. Implantable Cardiac Assist Devices. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials Science: An Introduction to Materials in Medicine. San Diego, CA: Elsevier Academic Press; 2004. p. 494. [Google Scholar]
- 4.McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Ann Thora Surg. 1999;67:1233. doi: 10.1016/s0003-4975(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 5.Snyder TA, Watach MJ, Litwak KN, Wagner WR. Ann Thorac Surg. 2002;73:1933. doi: 10.1016/s0003-4975(02)03549-x. [DOI] [PubMed] [Google Scholar]
- 6.Frazier OH, Myers TJ, Gregoric ID, Khan T, Delgado TR, Croitoru M, Miller K, Jarvik R, Westaby S. Circulation. 2002;105:2855. doi: 10.1161/01.cir.0000018167.47314.af. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DJ. Circulation. 2003;108(II):272. doi: 10.1161/01.cir.0000087387.02218.7e. [DOI] [PubMed] [Google Scholar]
- 8.Pitsis AA, Visouli AN, Vassilikos V, Ninios VN, Sfirakis PD, Mezilis NE, Dardas PS, Filippatos GS, Bougioukas GI, Kremastinos DT, Long JW. Hellenic J Cardiol. 2006;47:368. [PubMed] [Google Scholar]
- 9.Snyder TA, Tsukui H, Kihara S, Akimoto T, Litwak KN, Kameneva MV, Yamazaki K, Wagner WR. J Biomed Mater Res. 2007;81A:85. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Chu PK, Ding C. Mater Sci & Eng R. 2004;47:49. [Google Scholar]
- 11.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos RL, Naftel DC, Kirklin JK, Taylor DO. Third annual report 2005. J Heart Lung Transplant. 2005;24:1182. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Jahanyar J, Noon GP, Koerner MM, Youker KA, Malaisrie SC, Ngo UQ, Amione GT, Loebe M. J Heart Lung Transplant. 2007;26:200. doi: 10.1016/j.healun.2006.11.602. [DOI] [PubMed] [Google Scholar]
- 13.Chu PK, Chen JY, Wang LP, Huang N. Mater Sci& Eng R. 2002;36:143. [Google Scholar]
- 14.Kang ET, Tan KL, Kato K, Uyama Y, Ikada Y. Macromolecules. 1996;29:6872. [Google Scholar]
- 15.Xiao SJ, Textor M, Spencer ND. Langmuir. 1998;14:5507. [Google Scholar]
- 16.Yu ZJ, Kang ET, Neoh KG, Tan KL. Surf Coat Tech. 2001;138:48. [Google Scholar]
- 17.Chevallier P, Castonguay M, Turgeon S, Dubrulle N, Mantovani D, McBreen PH, Wittmann JC, Laroche G. J Phys Chem B. 2001;105:12490. [Google Scholar]
- 18.Suzuki M, Kishida A, Iwata H, Ikata Y. Macromolecules. 1986;19:1804. [Google Scholar]
- 19.Huang CY, Lu WL, Feng YC. Surface Surf Coat Tech. 2003;167:1. [Google Scholar]
- 20.Zhang F, Kang ET, Neoh KG, Wang P, Tan KL. Biomaterials. 2001;22:1541. doi: 10.1016/s0142-9612(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 21.Zou XP, Kang ET, Neoh KG. Surf Coat Tech. 2002;149:119. [Google Scholar]
- 22.Zou XP, Kang ET, Neoh KG. Plasmas and Polymers. 2002;7:151. [Google Scholar]
- 23.Ishihara K. Trend in Polym Sci. 1997;5:401. [Google Scholar]
- 24.Ishihara K, Ueda T, Nakabayashi N. Polym J. 1990;22:355. [Google Scholar]
- 25.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. J Biomed Mater Res. 1998;39:323. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Lewis AL. Colloids Surf B: Biointerfaces. 2000;18:261. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 27.Yoneyama T, Sugihara K, Ishihara K, Iwasaki Y, Nakabayashi N. Biomaterials. 2002;23:1455. doi: 10.1016/s0142-9612(01)00268-x. [DOI] [PubMed] [Google Scholar]
- 28.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. J Membr Sci. 2005;249:133. [Google Scholar]
- 29.Ye SH, Watanabe J, Iwasaki Y, Takai M, Ishihara K. Biomaterials. 2006;27:1955. doi: 10.1016/j.biomaterials.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Goda T, Konno T, Takai M, Moro T, Ishihara K. Biomaterials. 2006;27:5151. doi: 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Hong Y, Ye SH, Nieponice A, Soletti L, Vorp DA, Wagner WR. Biomaterials. 2009;30:2457. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whelan DM, Giessen WJ, Krabbendam SC, Vliet EA, Verdouw PD, Serruys PW, Beusekom HMM. Heart. 2000;83:338. doi: 10.1136/heart.83.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli M, Sommariva L, Prati F, Zerboni S, Politi A, Bonattie R, Mameli S, Butti E, Pagano A, Ferrari G. Catheter Cardiovasc Interv. 2001;53:182. doi: 10.1002/ccd.1145. [DOI] [PubMed] [Google Scholar]
- 34.Kihara S, Yamazaki K, Litwak P, Kameneva MV, Ushiyama H, Tokuno T, Borzelleca DC, Umezu M, Tomioka J, Tagusari O, Akimoto T, Koyanagi H, Kurosawa H, Kormos RL, Griffith BP. Artif Organs. 2003;27:188. doi: 10.1046/j.1525-1594.2003.t01-2-06993.x. [DOI] [PubMed] [Google Scholar]
- 35.Choi J, Konno T, Matsuno R, Takai M, Ishihara K. Colloids Surf B: Biointerfaces. 2008;67:216. doi: 10.1016/j.colsurfb.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Ye SH, Johnson CA, Jr, Woolley WR, Snyder TA, Gamble LJ, Wagner WR. J Biomed Mater Res A. 2008 Aug 5; doi: 10.1002/jbm.a.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamada Y, Kulik EA, Ikada Y. Biomaterials. 1995;16:259. doi: 10.1016/0142-9612(95)92126-q. [DOI] [PubMed] [Google Scholar]
- 38.Johnson CA, Jr, Snyder TA, Woolley JR, Wagner WR. Artif Organs. 2008;32(2):136. doi: 10.1111/j.1525-1594.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson LO, Loebe M, Noon GP. ASAIO J. 2003;49:518. doi: 10.1097/01.mat.0000085672.42122.49. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds M, Delgado RM., III Curr Opin Cardiol. 2003;18:199. doi: 10.1097/00001573-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Mussivand T. Artif Organs. 2008;32(1):1. doi: 10.1111/j.1525-1594.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 42.Holmlin RE, Chen X, Chapman RG, Takayama S, Whitesides GM. Langmuir. 2001;17:2841. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 43.Tegoulia VA, Rao W, Kalambur AT, Rabolt JF, Cooper SL. Langmuir. 2001;17:4396. [Google Scholar]
- 44.Chung YC, Chiu YH, Wu YW, Tao YT. Biomaterials. 2005;26:2313. doi: 10.1016/j.biomaterials.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki Y. N Saito Colloids Surf B: Biointerfaces. 2003;32:77. [Google Scholar]
- 46.Kyomoto M, Iwasaki Y, Moro T, Konno T, Miyaji F, Kawaguchi H, Takatori Y, Nakamura K, Ishihara K. Biomaterials. 2007;28:3121. doi: 10.1016/j.biomaterials.2007.03.010. [DOI] [PubMed] [Google Scholar]