Abstract
OBJECTIVE
Bone marrow-derived human mesenchymal stem cells (hMSCs) are capable of localizing to gliomas after systemic delivery and can be used in glioma therapy. However, the mechanism underlying the tropism of hMSCs for gliomas remains unclear. In vitro studies suggest that platelet derived growth factor (PDGF-BB) may mediate this tropism. However, a causal role of PDGF-BB has not been demonstrated in vivo. Therefore, we tested the hypothesis that PDGF-BB mediates the attraction of hMSCs to gliomas in vitro and in vivo.
METHODS & RESULTS
In vitro invasion assays showed that significantly more hMSCs migrated toward glioma cells (U87 or LN229) engineered to secrete high levels of PDGF-BB compared with low-secreting gliomas. Anti-PDGF-BB-neutralizing antibody abrogated this increase in migration. Pretreatment of hMSCs with inhibitory antibodies against PDGF receptor-β also reduced hMSC migration. To demonstrate that PDGF-BB mediates the localization of hMSCs in vivo, hMSCs-Ad-Luc were injected into the carotid artery of mice harboring orthotopic 7 day old U87-PDGF-BB-high secreting or U87-PDGF-BB-low secreting xenografts and analyzed by bioluminescence imaging. Statistically significant increases in hMSCs were seen within PDGF-BB-high xenografts compared with PDGF-BB-low xenografts. To control for PDGF-BB-induced differences in tumor size and vascularity, gfp-labeled hMSCs were injected into the carotid arteries of animals harboring 4-day old PDGF-BB-high secreting xenografts or 7-day old PDGF-BB-low secreting xenografts. At these times tumors had similar size and vessel density. Statistically significant more hMSCs localized to PDGF-BB-high secreting xenografts compared with PDGF-BB-low secreting xenografts. Pretreatment of hMSCs with anti-PDGFR-β-inhibitory antibodies decreased the localization of hMSCs in this intracranial model.
CONCLUSION
PDGF-BB increases the attraction of hMSCs for gliomas in vitro and in vivo, and this tropism is mediated via PDGF-β receptors on hMSCs. These finding can be exploited for advancing hMSC treatment.
Keywords: bioluminescence imaging, glioblastoma multiforme, glioma, human mesenchymal stem cells, platelet derived growth factor, PDGF receptor-β, orthotopic animal models
INTRODUCTION
The survival of patients with malignant gliomas, the most common and aggressive primary adult brain tumor, remains dismal despite maximal conventional therapy (33). This poor outcome relates at least in part to difficulties associated with delivering therapeutic agents to these tumors (15). In this context, recent evidence has suggested that bone marrow-derived mesenchymal stem cells (MSCs) may be effective vehicles for delivering biological therapies to gliomas (17, 24). Compared with other types of stem cells, hMSCs are well-suited for clinical applications, because they are easily isolated from patients, can be expanded in culture, can be genetically manipulated and because autologous transplantation, which obviates immunologic incompatibilities, is possible (2, 4). In an orthotopic animal model of the human disease, we showed that human MSCs (hMSCs) migrate toward gliomas after local delivery and are capable of localizing in gliomas after regional intravascular (intra-carotid) delivery (17). In addition, we showed that hMSCs can be used to deliver antiglioma agents to brain tumors (17). Others have also reported similar findings using rat MSCs in syngeneic rat brain tumor models and have suggested that the MSCs may themselves have intrinsic anti-tumoral properties (18, 35). Indeed, the capacity of hMSCs to localize to gliomas may reflect an intrinsic tropism of these cells for solid tumors in general because hMSCs have been shown to localize to several types of tumors, including breast cancer and melanoma (8, 12, 31, 32).
Although current evidence supports the notion that hMSCS are capable of localizing to gliomas, the mechanism underlying the tropism of bone marrow-derived hMSCs for tumors, in general, and gliomas, in particular, is largely unknown. It is well established that gliomas produce growth factors, cytokines and chemokines (11), and it is reasonable to hypothesize that these secreted proteins may mediate the tropism of hMSCs for gliomas. In support of this hypothesis, we recently reported using in vitro migration assays, that exposure of hMSCs to specific growth factors, particularly platelet-derived growth factor BB (PDGF-BB), epidermal growth factor (EGF), and stroma-derived factor-1α (SDF-1α) enhanced the migration of hMSCs (17). In these studies, PDGF-BB increased the in vitro migration of hMSC to a greater extent than all other growth factors. The secreted form of PDFG-BB is a 24 kD protein (21), which is commonly produced by gliomas (19) and other cancers (28), and which is believed to act on tumor cells via autocrine and paracrine mechanisms (9). Importantly, hMSCs are known to express the PDGF receptors (PDGFR) on their cell surface (16), and thus their function may be regulated by tumor-derived PDGF-BB.
Despite correlative in vitro evidence suggesting that PDGF-BB may mediate the tropism of hMSCs for gliomas (3, 17), there is currently no data showing that PDGF-BB plays a causal role in the localization of hMSCs to gliomas after exogenous delivery, particularly in vivo. Consequently, we undertook both in vitro and in vivo experiments to determine the extent to which PDGF-BB specifically mediates the localization of hMSCs toward human gliomas. For this purpose, the glioma cell lines U87 and LN229 were engineered to express high levels of PDGF-BB. Using these PDGF-BB clones, we demonstrate a direct and specific role of PDGF-BB in enhancing the migration and localization of hMSCs to human gliomas both in vitro and in vivo, and that this tropism of PDGF-BB is mediated via PDGF-β receptors on hMSCs. Besides providing biologically important information about the homing of hMSCs toward gliomas in vivo, these findings can be exploited for improving the specificity of hMSCs for gliomas in the future, or more immediately, for selecting proper patients for successful hMSC-based therapy.
MATERIALS AND METHODS
Cell culture
Human mesenchymal stem cells (hMSCs) were obtained from Lonza (Allendale, NJ, USA) and maintained in α-MEM (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Lonza) and 1 % Penicillin/Streptomycin at 37 °C in a humidified atmosphere containing 5% CO2/95% air. Experiments were performed using hMSCs during passages 3 to 5. Glioma cell lines U87 and LN229 were obtained from the American Type Culture Collection (Manassas, VA). U87 cells were maintained in MEM (Mediatech, Manassas, VA) medium supplemented with 10% fetal bovine serum, non essential amino acid (Hyclone, Logan, UT) and 1 % Penicillin/Streptomycin. LN229 cells were maintained in DMEM/F-12 (Mediatech) supplemented with 10% FBS and 1 % Penicillin/Streptomycin.
Generation and analysis of glioma cell lines with stable expression of PDGF-BB
U87 and LN229 glioma cells were transfected with a plasmid that encodes the human PDGF-B and the Blasticidin S resistance genes (pBLAST49-hPDGF-B, InvivoGen, San Diego, CA) using FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN). Blasticidin-resistant colonies were isolated and expanded. The PDGF-B expression level of each clone was quantified by detecting secreted PDGF-BB protein in conditioned medium using an ELISA for human PDGF-BB (Quantikine®, R&D Systems, Minneapolis, MN).
To compare the in vitro growth of the derived clones, U87 parent cells or the PFGF-high or -low U87 cell lines (5 ×103 ) were cultured in 6 well plates for 0, 3, 5, 7, and 10 days, after which they were trypsinized, and collected. Total viable cells were counted using the Vi-Cell, Cell Viability Analyzer (Beckman Coulter, Inc., Fullerton, CA) and averaged.
In vitro migration assay
U87 or LN229 clones (1 × 106) were cultured in 10 ml of serum-free media for 48 hours and the resulting conditioned-media was collected, centrifuged, filtered, and placed in the lower well of 24 mm tissue culture Transwell plates (8µm pore, Corning Inc., Corning, NY). In other experiments, increasing concentrations of recombinant human PDGF-BB (R&D Systems, Minneapolis, MN) in serum-free media were placed in the lower well. The upper well of the Transwell plates were coated with Matrigel (0.7 mg/mL in MEM-NEAA) and plated with 1.0 × 105 of hMSCs in 1 ml of serum-free media. After 16 or 48 hours incubation (depending on experiment), the migration of hMSCs through the matrigel was determined by fixing the membrane, staining the cells using the Hema3 staining kit (Fisher Diagnostics, Middletown, VA), directly counting the number of migrated cells in 10 high-power fields (x 400), and calculating the average.
For PDGF-BB blocking experiment, conditioned media was incubated with anti-PDGF-BB neutralizing antibody (Sigma Chemical Co., St. Louis, MO) for 3 hours before transfer into the bottom well of Transwell plates. hMSCs were placed in the upper well and assayed as described above.
In experiments assessing inhibition of PDGF receptor-β, before inserting the upper wells into the lower wells, hMSCs were incubated in 10 µg/ml of anti-PDGFR- β antibodies (R&D Systems, Minneapolis, MN) or in 1% serum media with 5% PBS (as control) for 1 hr at 4°C with shaking. hMSCs were washed twice, and then subjected to transwell migration assay for 16 hours.
Animal subjects
Male athymic nude mice (nu/nu) were purchased from the Department of Experimental Radiation Oncology, The University of Texas MD Anderson Cancer Center (Houston, TX). Animal manipulations were performed in the veterinary facilities in accordance with institutional, state, and federal laws and ethics guidelines under an approved protocol. Animals were anesthetized with intra-peritoneal (IP) injection of ketamine (100mg/kg)/xylazine (10mg/kg) during all procedures.
Intracranial xenografting of human glioma cells
Monolayers of U87 cells with high or low expression of PDGF-BB were washed, trypsinized, and resuspended in serum free media at a concentration of 1 ×105 cells/ µl. Cells (5 ×105 or 106) were implanted into the right frontal lobe of nude mouse using a guide-screw system implanted within the skull as described previously (13). To increase uniformity of xenograft take and growth, cells were injected into 10 mice simultaneously using a multiport microinfusion syringe pump at the speed of 30 µl/h or 60µl/h (Harvard Apparatus, Holliston, MA).
Internal carotid artery injection of human mesenchymal stem cells
Monolayer cultures of hMSCs were trypsinized, washed, resuspended in MEM plus 10% FBS at a concentration of 1 ×104 cells/ µl for injection, and placed on ice until injection. On the day of injection, animals were anesthetized, the bifurcation of the right carotid artery was surgically identified using the dissecting microscopic. After ligating the external carotid artery and tying the proximal portion of the common carotid, 1 ×106 hMSCs were injected manually into the distal common carotid artery using 30-guage needle. The artery was then tied just above the injection site. Animals were monitored until recovery.
Brain tissue/tumor preparation
For frozen sections, animals were sacrificed by perfusion through the heart with PBS and then 4% paraformaldehyde (PFA) in PBS. The brains were immediately removed and postfixed in 4% PFA at 4°C overnight. Following dehydration with 30% sucrose in PBS, brain samples were frozen with OCT compound (Sakura Finetek USA Inc., Torrance, CA) at −80°C. For paraffin sections, brain samples were post-fixed in 10% formalin and paraffin embedded. Serial 5-µm coronal sections were obtained. H&E staining or DAPI staining (Vectashield H-1200, Vector Laboratories) staining was performed for visualization of the tumor.
Immunohistochemistry of PDGF-BB
Paraffin sections were deparaffinized in two changes of xylene, and washed in decreasing ethanol concentrations (100% to 95%), followed by sterile deionized water (dH2O) and PBS. The slides were processed for antigen retrieval by microwaving in citrate buffer (0.01 M, pH 6.0) for 10 minutes. After washing in three series of dH2O, endogenous peroxidase was inactivated with 1% H2O2 in methanol, followed by in dH2O and PBS. The slides were incubated overnight with the goat anti-human PDGF-BB antibody (Sigma, St. Louis, MO) at a 1:100 dilution after 1 hour incubation with 5% horse serum (Invitrogen, Carlsbad, CA) in PBS. After rinsed with PBS, the slides were incubated with an avidin-conjugated horse antigoat antibody (Vector Laboratories, Burlingame, CA) at a 1:200 dilution for 1 hour, and then treated with a solution of avidin-biotin-peroxidase complexes (Vectastain ABC kit; Vector Laboratories) for 30 minutes. The DAB substrate kit (Vector Laboratories) was used to develop the stain. Slides were counterstained with hematoxylin.
In vivo quantification of tumor size and vascularity
5 ×105 of U87 cells with high or low secretion of PDGF-BB were implanted into the right frontal lobe of mouse. The mice were sacrificed 4, 7, and 10 days after implantation (N= 3 mice/group/time point). The brain specimens were embedded in paraffin, sectioned every 150 – 200µm, and the 3 sections with the largest cross-sectional areas of tumor were chosen. The area of the tumor was determined by outlining it manually using the Axioskop 40® microscope (Carl Zeiss, Inc., Germany) with ProgRes® digital microscope camera and ProgRes® CapturePro2.5 software (JENOPTIK Laser, Optik, Systeme GmbH). The 3 areas were averaged to provide a final area for a given animal. The mean of the cross-sectional area in each group was used in the evaluation of tumor size.
Xenografts were assessed for vascularity using anti-CD31 antibodies. Briefly, paraffin sections were deparaffinized and hydrated as described above. Heat-induced antigen retrieval was performed by microwaving in Masking solution (Vector Laboratories, Inc, Burlingame, CA) at 100% power for 3 min followed by 20% power for 7 min. Avidin-biotin blocking was performed according to the manufacture’s protocol (Vector Laboratories, Inc), using 5% normal goat serum in PBS with 0.2% Triton-X (blocking solution) for 1 hr at room temperature. Sections were incubated with primary antibodies (PECAM-1 (M-185, Santa Cruz Biotechnology, Inc., Santa Cruz, California) at 1:100 dilution at 4°C overnight. For visualization, the biotinylated goat anti-rabbit secondary antibodies (1:200, Vector Laboratories, Inc, Burlingame, CA) and Texas Red* Avidin D (1:50, Vector) were used. Representative sections from each animal (N=3 animals/group/time point) were analyzed using fluorescent microscopy by counting all vessels with lumens or branches in 10 high power fields (HPF) at magnification of 400x.
Adenoviral vectors and human mesenchymal stem cell transfection
Two methods were used to visualize hMSCs. For bioluminescence assays, hMSCs were transduced with a previously described replication-defective recombinant adenovirus vector containing the cDNA of the firefly luciferase gene and the cDNA of the fiber knob with a the RGD motif (Ad-Luc-RGD) (17). For histological assays, hMSCs were transduced with green fluorescent protein (gfp) using the previously described replication-incompetent Ad5/F35-CMV-GFP vector containing the cDNA of gfp obtained from the Vector Development Laboratory at the Baylor College of Medicine (Houston, TX) (20).
For transfection, 2.5 × 106 hMSCs were plated on 150-cm dish. After 24 hrs, cells were washed with PBS and incubated with Ad-Luc-RGD at a multiplicity of infection (MOI) of 1000 viral particles (vp)/cell in 3 ml serum free media at 37°C with brief agitation every 10 minutes. For Ad-GFP an MOI of 50 plaque forming units (pfu)/cell of in 3 ml serum free media was used. After 1 hr 25 ml of MEM plus 10% FBS was added to the dish. The cells were prepared for the carotid injection as described above.
Bioluminescence Imaging and Quantification
On the day of imaging animals were anesthetized and treated with Luciferin (150mg/kg, IP injection). After 10 minutes, the brains were removed and imaged with 5 minutes of acquisition time using the IVIS Imaging System, 200 Series (Xenogen Corp., Alameda, CA). Bioluminescence color images were overlaid on gray scale photographic images of the brains to allow for localization of the light source using the LIVINGIMAGE V. 2.11 software overlay (Xenogen) and IGOR image analysis software (V. 4.02 A, WaveMetrics Inc., Lake Oswego, OR). Regions of interest (ROI) were manually selected, and signal intensity was expressed in terms of number of photons/cm2/sec.
Histologcal Quantification of hMSCs-gfp in gliomas
Three days later after injection of hMSCs-gfp, mice were sacrificed, brains were removed, fixed, dehydrated, and frozen with OCT compound at − 80 °C. Brains were blocked around the tumor injection site. Five µm sections were obtained every 150 µm from the front to the back of the tumor. In each section the tumor was outlined and the area of the tumor was determined using Image Pro software (Media Cybernetics Inc, Bethesda, MD). The number of gfp-positve cells within the tumor area was counted under the fluorescent microscope (x50) and the number of cells per mm2 of tumor was calculated for each section and averaged among all sections for a given animal.
Statistical Analysis
Statistical differences were assessed by Student`s t-test. Differences were determined to be statistically significant if p < 0.05. The data were represented as mean ± standard error (SE) for at least three replicate determinations for each experiment.
RESULTS
Isolation and characterization of PDGF-B engineered U87 and LN229 glioma cells
To determine the role of tumor-derived PDGF-BB in mediating the tropism of hMSCs for human gliomas, we engineered the glioma cell lines U87 and LN229 to over express PDGF-BB by stable transfection with a plasmid containing PDGF-B cDNA as described in the Materials and Methods. Quantitative ELISA for PDGF-BB was used to determine the amount of PDGF-BB secreted into the medium 48 hrs, 72 hrs and 96 hrs after plating of 106 cells for each clone (data not shown). Based on these results, we expanded two U87 clones (U87-PDGF-B-2 and U87-PDGF-B-7) that expressed high levels of PDGF-BB and two U87 clones that expressed levels of PDGF-BB no different from media (U87-PDGF-B-3 and U87-PDGF-B-4). Likewise, we expanded two LN229 clones (LN229-PDGF-B-9 and LN229-PDGF-B-12) that expressed high levels of PDGF-BB and one that expressed low levels of PDGF-BB (LN229-PDGF-B-8). Figure 1 shows the relative expression of PDGF-BB for each of these clones based on ELISA assays.
Figure 1.
Graphs showing the amount of PDGF-BB secreted into the medium of PDGF-B engineered U87 and LN229 clones. A) After transfection with a plasmid containing the cDNA of PDGF-B, selection, and expansion, 106 U87-PDGFB cells (clones #2, #7, #3 and #4) were plated in serum free media, and after 48 hours conditioned media was collected and analyzed for levels of PDGF-BB by ELISA. As a control, serum free media (media) and untransfected U87 cells were assayed. There was significant difference in the levels of PDGF-BB between the high-secretion clones (#2 and #7) and the low secretion clones (#3 and #4). (p<0.01, *). B) After similar transfection, 106 LN229-PDGF-B cells (clones #8, #9 and #12) were plated and after 48 hrs assayed for PDGF-BB expression. A significant difference between high-secretion (#9 and #12) and the low secretion clone (#8) is evident (p < 0.01, *). Bars are mean +/− SD of triplicate experiments.
Recombinant PDGF-BB or tumor-derived PDGF-BB increase migration of hMSCs: in vitro studies
In order to determine the extent to which PDGF-BB mediates the tropism of hMSCs for gliomas, in vitro matrigel invasion assays were performed. We first assessed the migration of hMSCs toward increasing concentrations of human recombinant PDGF-BB. Specifically, 0.01 – 10 ng/ml of human PDGF-BB was placed in the lower well of Transwell plates. In the upper well, 105 hMSCs were placed on matrigel-coated semiporous membranes (8 µm pores). Migration was assayed after 48 hrs by counting the number of cells on the membrane. Consistent with previous results (17), PDGF-BB was capable of increasing the migration of hMSCs compared with serum free media, and this increase was dose dependent (Figure 2A).
Figure 2.
(A) Graph showing in vitro migration of hMSCs in response to different concentrations of PDGF-BB. hMSCs were plated on matrigel-coated upper wells and exposed to increasing concentrations of PDGF-BB in the lower well. Migration was measured 48 hrs later by counting the number of migrating hMSCs in 10 high-powered fields (HPF). Increased migration of hMSC was seen with 0.1 to 10 ng/ml of PDGF-BB compared with media alone. (B) Graphs showing in vitro invasion of hMSCs in response to conditioned media derived from glioma cell lines engineered to secrete high and low amounts of PDGF-BB. hMSCs were plated on matrigel in the upper wells of transwell plates and exposed to the conditioned media of the indicated U87 cell lines. Invasion was measured 48 hrs later. Increased invasion of hMSC was seen with U87-PDGFB high-secretion clones (#2 and #7) compared with the low-secretion clones (#3 and #4). (C) Graph of hMSCs invasion in response to conditioned media from the indicated LN-229 cell lines. Increased invasion of hMSC was seen with LN229-PDGFB high-secretion clones (#9 and #12) compared with the low-secretion clone (#4). (D) Graph showing effects of PDGF-BB neutralizing antibodies. Conditioned media from U87-PDGF-B-2 cells, along with increasing concentrations of a PDGF-BB neutralizing antibody, was added to the lower well of the transwell experiment described in (B), and after 48 hrs hMSC migration was measured. A dose-dependent inhibition of hMSC migration was observed with the PDGF-BB neutralizing antibody. U87-PDGFB-3 was used as a PDGF-BB-low secreting control. (E) Inhibition of hMSC migration by anti-PDGFR-β antibodies. hMSCs were trypsinized, incubated in 1% serum media with 5% PBS as control (CTR) or with 10µg/ml of anti-PDGFR-β antibodies for 1 hr at 4°C with shaking, twice washed, then placed on matrigel in the upper well. Serum-free media with different concentration of PDGF-BB (5 ng/ml or 10 ng/ml) was placed into the lower wells. hMSC migration was assayed after 16 hrs. hMSC migration was significantly inhibited by pre-treatment of hMSCs with anti-PDGFR- β antibodies. (F) Graph showing inhibition of hMSC migration to conditioned media from U87 PDGF-BB-high secreting clone after pretreatment of hMSC with anti-PDGFR-β antibodies. hMSCs were treated as described in (E) and placed in the upper wells. Conditioned media from U87-PDGFB #2 (high secretion) cells was placed into lower wells and hMSC migration was measured after 16 hrs. hMSC migration was significantly inhibited by treatment of hMSCs with anti-PDGFR-β antibodies. In all graphs bars are mean +/− SD of triplicate experiments (* = p<0.05, ** = p<0.01).
We next assessed the migration of hMSCs toward conditioned media from U87-PDGF-B or LN229-PDGF-B clones that express high or low levels of PDGF-BB. Specifically, 106 U87-PDGFB cells (clones #3, #4, #2, #7) were plated in serum free media and after 48 hrs the conditioned media was collected and placed into the lower well of Transwell plates. In the upper well, 105 hMSCs were placed on the matrigel-coated semiporous membranes. Migration was assayed after 48 hrs as above. Significantly more hMSCs migrated toward the U87-PDGFB high-secreting clones (U87-PDGFB-2 and U87-PDGF-B-7) compared with the low-secreting ones (U87-PDGF-B-3, U87-PDGF-B-4) (Figure 2B). Similar results were obtained for the high- and low-secreting LN229-PDGF-B clones (Figure 2C).
To document that the increase in migration of hMSCs toward U87-PDGF-BB conditioned media was specifically due to the presence of PDGF-BB, transwell migration assays were performed using conditioned media from U87-PDGFB clones that was treated with increasing concentrations of an inhibitory anti-PDGF-BB antibody that neutralizes the activity of PDGF-BB. As shown in Figure 2D, the high level of migration of hMSCs that resulted after exposure to conditioned medium from U87-PDGF-B-2 was significantly attenuated by treatment with the inhibitory anti-PDGF-BB antibody. The inhibition was dose-dependent with 10 µg/ml of antibody resulting in a more effective inhibition of hMSC migration compared with 1 µg/ml of antibody. Taken together, these results indicate that tumor-derived PDGF-BB specifically promotes the migration of hMSCs toward human gliomas in vitro.
In vitro Migration of hMSCs is mediated through PDGF Receptor-β on hMSCs
hMSCs have been shown to express PDGF receptors on their cell surface. To verify that that PDGF-induced hMSC migration was due to interactions of PDGF-BB with PDGF receptors expressed by hMSCs, hMSCs were pretreated with anti-PDGF Receptor-β (PDGFR-β) inhibitory antibodies that block binding of PDGF-BB to PDGFR-β. Specifically, hMSCs were incubated with anti-PDGFR-β antibodies or with medium plus 1% serum (as control) for 1 hr. hMSCs were then placed in the upper wells of Transwell plates and migration was assayed after 16 hrs. In initial experiments, human recombinant PDGF-BB (5 or 10 ng/ml) was placed in the lower wells. Migration of hMSCs treated with anti- PDGFR-β inhibitory antibodies was significantly inhibited after exposure to both concentrations of PDGF-BB (Figure 2E).
In subsequent experiments, hMSCs pretreated with PDGFR-β inhibitory antibodies were exposed to conditioned media from U87-PDGFB-2 (high secretion) which was placed in the lower wells of the transwell plates. The increased migration of hMSCs after exposure to the conditioned media of U87-PDGFB-2 was significantly attenuated by pre-treatment of hMSCs with the anti-PDGFR-β inhibitory antibody (Figure 2F). Together these results indicate that PDGF-BB-induced migration of hMSCs is mediated through PDGFR-β on the surface of hMSCs.
Establishment of intracranial gliomas with high and low PDGF-BB secretion
To better reproduce the clinical and biological scenario, we undertook in vivo experiments to determine the extent to which intravascularly delivered, exogenous hMSCs localize to human gliomas that secrete high and low levels of PDGF-BB. We first verified that the isolated PDGF-secreting clones were able to form tumors in the brains of nude mice. Specifically, 106 tumor cells were implanted into the frontal lobes of nude mice using a guide-screw system (13). Fourteen days after inoculation, the brains were removed, and paraffin sections were analyzed by light microscopy. All U87-PDGF-B clones (#2, #3, #4 and #7) formed intracranial tumors (N=3 animals/clone) (Figure 3). Consequently, the U87-PDGF-B clones were used for further in vivo studies. Immunohistochemical staining using an antibody to PDGF-BB demonstrated strong positive staining in the xenografts of PDGF-BB high-secreting U87 cells (U87-PDGF-B-2), consistent with the in vivo secretion of PDGF-BB, whereas little to no staining was seen in the xenografts of PDGF-BB low-secreting U87 cells (U87-PDGF-B-3) indicating very low levels of PDGF-BB (Figure 3).
Figure 3.
Photomicrograph of mouse brains showing in vivo expression of PDGF-BB in xenografts derived from U87 cells engineered to secrete high and low levels of PDGF-BB. 106 of PDGF-BB high-secreting U87 cells (clone #2) (right panel) or 106 of PDGF-BB low-secreting U87 cells (clone #3) (left panel) were implanted into the right frontal lobes of nude mice and after 14 days, the brains were sectioned and stained with H&E (upper), or by immunohistochemistry (IHC) using an anti-PDGF-BB antibody (lower). High expression level of PDGF-BB was detected in xenografts of PDGF-BB-high secreting U87 xenografts, whereas essentially no staining was observed in the xenografts of PDGF-BB-low secreting U87 xenografts.
Tropism of hMSCs for PDGF-BB secreting glioma cells: intravascular delivery at fixed time point after tumor inoculation
To determine the extent to which tumor-derived PDGF-BB mediates the localization of regionally (intra-carotid) delivered hMSCs in human gliomas, bioluminescence imaging (BLI) of hMSCs was undertaken. BLI is a technology that exploits the emission of visible photons based on the catalysis of luciferin into oxylucifern by luciferase in order to image cells (34). BLI only identifies living cells because dead cells do not emit light. In addition, quantification can be achieved based on the signal intensity measured by the imaging system.
Consequently, hMSCs were transduced with an adenoviral vector containing the firefly luciferase gene (Ad-Luc) producing luciferase-containing hMSCs (hMSCs-Luc) as described in Material and Methods. Xenografts of U87-PDGF-B-2 (high-secreting, N=9) or U87-PDGF-B-3 (low-secreting, N=9) were established in the frontal lobes of nude mice (13). After 7 days, hMSCs- Luc (1× 106 cells in 100 µl MEM plus 10% FBS) were injected into the carotid arteries of these tumor bearing mice. As a control, a group of U87-PDGF-B-2-bearing mice (N=3) received intracarotid injections of 200 µl PBS without cells. Seven days after injection of hMSCs, on the day of imaging, animals were treated with Luciferin (150 mg/kg, IP) and after 10 minutes, animals were imaged with the IVIS Imaging System, 200 Series (Xenogen, CA, acquisition time 4 minutes). Bioluminescence color images were overlaid on gray scale photographic images of the mice to allow for localization of the light source within the animal using the LIVINGIMAGE V. 2.11 software overlay (Xenogen, CA) and IGOR image analysis software (V. 4.02 A, WaveMetrics, OR). To equalize comparisons across animals and between groups, the scale was fixed. Coronal sections of the brains were cut to verify that the signal localized to the tumor implanted in the right frontal lobe.
As shown in figure 4A-C, the light signal detected in the frontal lobes of the animals was qualitatively of greater intensity and size in the nine animals bearing PDGF-BB-2 (high-secreting) clones that were treated with systemic hMSCs-Ad-Luc injections (Figure 4B) compared with the nine animals harboring PDGF-BB-3 clones (low-secreting) (Figure 4A). Light signal was not detected in animals treated with PBS, indicating that light emission resulted from localization of hMSCs with the tumor (Figure 4C). The amount of light signal, which correlates with the number of hMSCs within the tumor, was quantified by measuring the flux of emitted photons within the region of the tumor using the image analysis system. The average signal of hMSCs-Luc in U87-PDGF-B-2 tumors (high-secreting) was significantly greater than that in the U87-PDGF-B-3 tumors (P=0.04) (Figure 4D). Thus, the secretion of PDGF-BB significantly enhanced the localization of hMSCs to the human gliomas xenografts in this model system.
Figure 4.
Photomicrographs of coronal sections of mice brains bearing high PDGF-BB-secreting and low PDGF-BB-secreting U87 xenografts after treatment with intravascularly-delivered hMSCs transduced with Ad-Luc and analyzed by bioluminescence imaged. A) Mice bearing established U87-PDGF-B-3 (low PDGF-BB-secreting) xenografts (N=9) were treated with 106 hMSCs delivered into the carotid artery. Seven days after delivery animals were treated with Luciferin, the brains were removed and imaged as described in Methods. The scale was fixed between animals and groups to allow comparison. Low levels of signal were observed in these low-secreting xenografts. (B) Mice bearing established U87-PDGF-B-2 (high PDGF-BB-secreting) xenografts (N=9) were treated with hMSCs and analyzed identical to the method in (A). Bright signal was detected in all the xenografts derived from high PDGF-secreting clones. (C) As a control U87-PDGF-B-2 (high PDGF-BB-secreting) xenografts were treated with cell free media. As expected, no signal was detected in these xenografts. (D) Graph showing average signal intensity for animals. The level of signal in the right frontal region was measured by outlining this region of interest, as described in Methods. Values are mean +/− SE (*=p<0.05).
Tropism of hMSCs for PDGF-BB secreting glioma cells: intravascular delivery at variable times to control for tumor size and vascularity
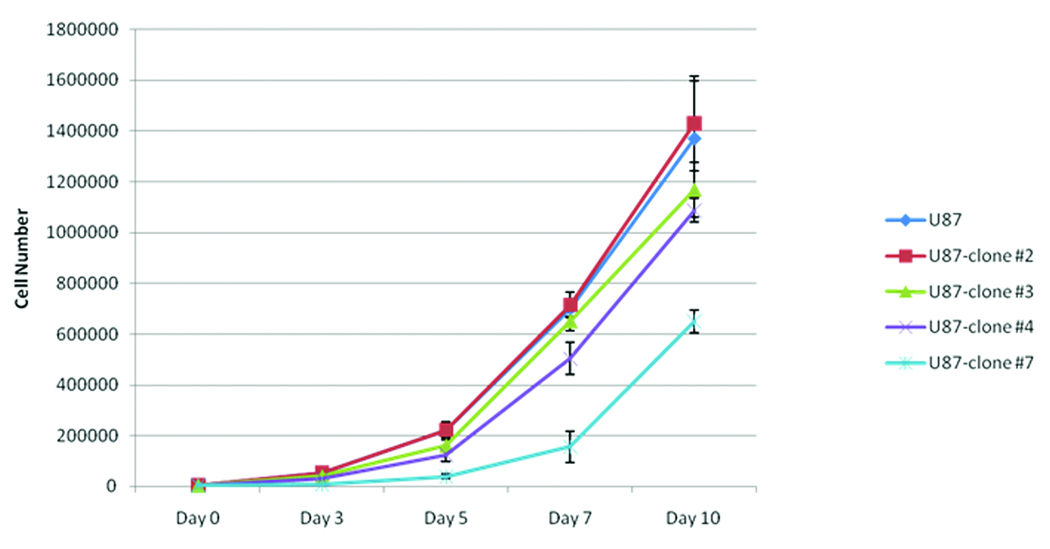
Evaluation of the tumor size in the initial in vivo experiments (see Figure 3) suggested that the xenografts derived from high PDGF-BB secreting clones (PDGF-B-2) was slightly larger than that of the low PDGF-BB secreting clones (PDGF-B-3). Because this size difference may have accounted for the observed differences in the magnitude of the localization of hMSCs to human gliomas, the growth rates of the PDGF-B-2 and PDGF-B-3 clones were compared in order to establish time points for hMSCs injection at which the tumor sizes were equal. Although in vitro growth analyses showed that there was no significant difference in cell proliferation between U87 parent cells, PDGF-BB low-secreting cell lines (clone #3), and PDGF-BB high-secreting U87 cells (clone #2) (Figure 5), in vivo growth analyses indicated that xenografts derived from PDGF-BB-high secreting clones (PDGF-B-2) grew faster than the xenografts derived from the PDGF-BB-low secreting clones (PDGF-B-3) (Figure 6). Specifically, the size of PDGF-BB high-secreting U87 tumors (clone #2, N=3 animals/time point) was significantly larger than that of PDGF-BB low-secreting U87 tumors (clone #3, N=3 animals/time point) when measured at day 7 and day 10 after initial inoculation of 5 ×105 cells into the frontal lobe (p<0.05 and <0.01, respectively, Figure 6B). Due to the different growth rates of the xenografts, the size of U87-PDGF-B-2-derived xenografts (high secreting) at day 4 after implantation was statistically similar to the size of U87-PDGF-B-3 derived xenografts (low secreting) at day 7 after implantation (Figure 6B).
Figure 5.
Graphs showing in vitro growth of U87 high and low secreting PDGF-BB clones. 5 ×103 of U87 parent cells, U87 cells with high (clone #2, 7) or low (clone #3, 4) secretion of PDGF-BB were plated in 6 well plates, then the total cell number of each cell line per well was counted at indicated time points in the triplicate manner, and averaged. The bars are mean +/− SD.
Figure 6.
In vivo growth of PDGF-BB-high and PDGF-BB-low secreting xenografts. (A) Photomicrographs of representative H&E coronal sections through the tumors taken at increasing times after cell implantation. 5 ×105 U87-PDGF-BB-high secreting cells (clone#2, lower panel) or –low secreting cells (clone#3, upper panel) were implanted into the right frontal lobe. Mice were sacrificed 4, 7, or 10 days after implantation (N= 3 mice/group/time point). Tumors were manually outlined. (B). Graph showing quantification of tumor size for xenografts derived from U87-PDGF-BB-high secreting cells (clone#2) or –low secreting cells (clone#3). For each mouse the 3 largest cross-sectional areas of tumor were measured under the microscope with a digital camera and software as described in the Methods, and an average tumor area was determined for each mouse. The bars are the mean +/− standard errors of the average areas of 3 mice/group/time point The size of PDGF-BB-high secreting tumors at day 4 was similar to that of PDGF-BB-low secreting tumors at day 7. Likewise, the size of PDGF-BB-high secreting tumors at day 7 also was similar to that of PDGF-BB-low secreting tumors at day 10. (* = p<0.05, ** = p<0.01).
In addition, because it is assumed that hMSCs reach gliomas via the tumor vasculature, we also compared the density of the blood vessels in the PDGF-B-2-derived xenografts (high secreting) with that of the PDGF-B-3-derived xentografts (low secreting). Xenografts (N= 3 mice/clone/time point) were analyzed by immunostaining with an anti-CD31 antibody, which detects endothelial cells, and the vessel density was quantified by counting the number of vessels per 10 hpf, as described in Materials and Methods. The number of vessels of PDGF-BB high-secreting U87 tumors (clone #2) was greater than that of PDGF-BB low-secreting U87 tumors (clone #3) at day 7 (p<0.05, Figure 7). Similar to tumor size, the vascularity of U87-PDGF-B-2-derived xenografts (high secreting) at day 4 after implantation was similar to the vascularity of U87-PDGF-B-3 derived xenografts (low secreting) at day 7 after implantation (Figure 7).
Figure 7.
In vivo vascularity of PDGF-BB-high and PDGF-BB-low secreting xenografts. (A) Photomicrographs of representative sections through the tumors taken at increasing times after cell implantation. 5 ×105 U87-PDGF-BB-high secreting cells (clone#2, lower panel) or –low secreting cells (clone#3, upper panel) were implanted into the right frontal lobe. Mice were sacrificed 4, 7, or 10 days after implantation (N= 3 mice/group/time point). Sections were stained for endothelial cells using immunofluorescence with anti-CD31 antibodies and counterstained with DAPI. Photomicrographs were taken using fluorescent microscope at magnification of 400x. (B). Graph showing quantification of vessel density for xenografts derived from U87-PDGF-BB-high secreting cells (clone#2, gray bars) or –low secreting cells (clone#3, black bars). The number of CD31-positive vessels with lumen or branching was counted in 10 high power fields. Bars are mean +/− SE of 3 mice/time point/group. The vascularity of PDGF-BB high-secreting U87 tumors at day 4 was similar to that of PDGF-BB low-secreting U87 tumors at day 7. Likewise the vascularity of PDGF-BB high-secreting U87 tumors at day 7 also was similar to that of PDGF-BB low-secreting U87 tumors at day 10.
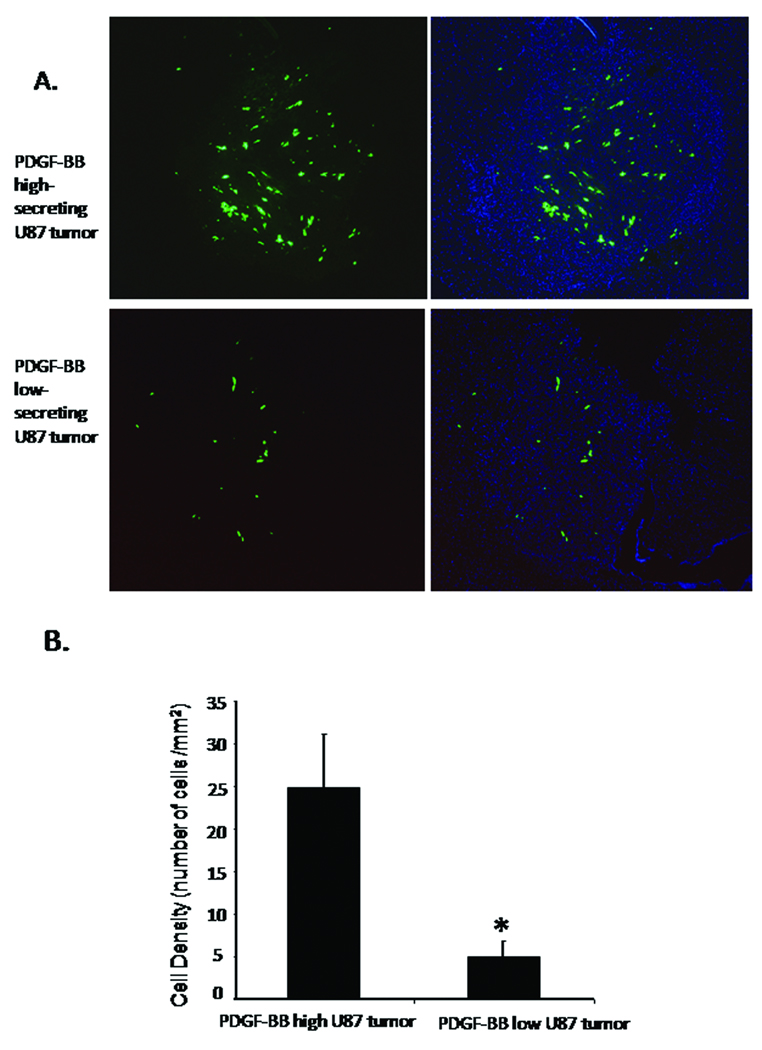
To control for the potential differences in tumor size and vascularity, we repeated the in vivo experiments with the injections of hMSCs administered 4 days after tumor inoculation for the high secreting PDFG-BB clones (PDGF-B-2) and 7 days after tumor inoculation for the low secreting PDFG-BB clones (PDGF-B-3), i.e. when the PDGF-BB-high secreting xenografts and PDGF-BB-low secreting xenografts were of similar size and vascularity. Specifically, hMSCs were transduced with an adenoviral vector containing the cDNA for green fluorescent protein (Ad-gfp) producing gfp-labeled hMSCs (hMSCs-gfp). Xenografts of U87-PDGF-B-2 (high-secretion, N=3) or U87-PDGF-B-3 (low-secreting, N=4) were established in the frontal lobes of nude mice (13). After 4 days, hMSCs-gfp (1× 106 cells in 100 µl MEM plus 10% FBS) were injected into the carotid arteries of mice bearing U87-PDGF-B-2 (high-secretion) xenografts, and after 7 days, hMSCs-gfp (1× 106 cells in 100 µl MEM plus 10% FBS) were injected into the carotid arteries of mice bearing U87-PDGF-B-3 (low-secretion) xenografts. The mice were sacrificed, and the brains removed 3 days after carotid injection. hMSCs were visualized under the fluorescent microscope and quantified as described in the Materials and Methods. The desnity of hMSCs (i.e. hMSCs per unit area) localized within the PDGF-BB-high secreting xenografts was significantly greater than the density of hMSCs that localized to the PDGF-BB-low secreting tumor (24.8 ± 6.3 versus 5.0 ± 1.8 of hMSCs/ mm2, respectively; p<0.05, Figure 8 A and B). Therefore, the increased localization of hMSCs to the PDGF-BB high secreting xenografts was not due to differences in tumor size or vascularity of the xenografts at the time of injection.
Figure 8.
(A) Photomicrographs of coronal sections of mice brains bearing PDGF-BB-high secreting and PDGF-BB-low secreting U87 xenografts after treatment with intravascularly-delivered gfp-labeled hMSCs. Seven days after intracranial implantation of U87-PDGF-B-3 cells (PDGF-BB-low) (N=4) and four days after implantation of U87-PDGF-B-2 cells (PDGFBB-high) (N=3), mice were treated with 106 gfp-labeled hMSCs delivered into the carotid artery. Three days after delivery of hMSCs, animals were sacrificed, the brains were removed and analyzed by fluorescent micrsocopy (magnification 50x). The number of hMSCs in the PDGF-BB-high xenografts (upper panel) was qualitatively greater than in the PDGF-BB-low xenografts. (green: gfp cells; blue: nuclei stained with DAPI). Left panels show GFP images, right panels show merged images. (B) Graph showing average hMSC density (number hMSCs per mm2 tumor) within the PDGF-BB-high and PDGF-BB-low xenografts. hMSC density was determined as described in Methods. The number of migrated hMSCs to PDGF-BB high-secreting U87 tumor was significantly increased compared with PDGF-BB low-secreting U87 tumor. Values are mean +/− SE (* = p<0.05).
Attenuation of hMSCs migration toward PDGF-BB high-secreting gliomas by blockade of PDGF receptor β on hMSCs
To determine the extent to which the PDGF-BB induced localization of hMSCs within intracranial xenografts was due to interactions of PDGF-BB with PDGFR-β expressed by hMSCs, hMSCs-gfp were pretreated for 1 hr with anti-PDGFR-β inhibitory antibodies or with medium plus 1% serum (as control) for 1 hr. These pre-treated hMSCs were then injected into the carotid arteries of mice harboring 4 day old PDGF-B-3 xenografts (PDGF-high secreting clones, N=3 animals/group). The number of hMSCs within U87-PDGF-BB-high secreting xenografts was significantly less after injection of hMSCs that were treated with anti-PDGFR-β antibodies (13.4 ± 1.6 of cells/ mm2 ) compared with injection of serum-treated hMSCs-gfp (23.1 ± 3.8 , p<0.05, Figure 9).
Figure 9.
Decreased hMSC localization in PDGF-BB-high secreting xenografts after treatment of hMSC with anti-PDGFR-β neutralizing antibodies. (A) Mice harboring 4 day old PDGF-BB-high secreting xenografts were injected via the carotid artery with gfp-labeled hMSC that were pre-treated with anti-PDGFR-β antibodies (lower panel, N=3) or with media/PBS as a control (upper panel, N=3). Panel shows representative photographs (magnification 50x) of tumors obtained three days after delivery of hMSCs as viewed under the fluorescent microscope. There is an observable decrease in the number of gfp-labeled hMSCs after inhibition of PDGFR-β. (B) Graph showing quantification of average hMSC density (number hMSCs per mm2 tumor) within the PDGF-BB-high xenografts after injection with hMSCs that were (N=3) or were not (N=3) pretreated with anti-PDGFR-β antibodies. The bars are mean +/− standard errors (* p < 0.05).
DISCUSSION
In this study we provide causal evidence that tumor-derived PDGF-BB mediates the localization of hMSCs to human gliomas both in vitro and in an in vivo intracranial model of the disease. This tropism could be specifically reversed by the addition of a PDGF-BB neutralizing antibody, indicating that PDGF-BB plays a causal role in the localization of hMSCs within gliomas. Additionally, blocking PDGFR-β on hMSCs was also able to abrogate the effects of PDGF-BB both in vitro and in vivo, indicating that the tropism of hMSCs for PDGF-BB is mediated via PDGFR-β on hMSCs.
Although several studies have shown that MSCs have an attraction for gliomas (17, 18, 24, 35), the factors that mediate this tropism have yet to be completely elucidated. It has been hypothesized that growth factors, cytokines, and chemokines produced by the tumors may mediate the attraction of hMSCs for gliomas. Studies have suggested several candidate factors, including epidermal growth factor, stroma-derived factor-1 (SDF-1), interleukin-8, transforming growth factor-β1 (TGF-β1), neurotrophin-3 (NT-3), and vascular endothelial growth factor A (VEGF-A) (1, 6, 27). Our previously published data (17), and more recent data of Cheng et al. (3) have indicated that the growth factor PDGF-BB, which is produced by human gliomas (9, 14, 19, 36), is one of the most potent attractants of hMSCs. However, studies published heretofore have relied exclusively on in vitro migration assays to assess the effects of PDGF-BB and other growth factors on hMSC migration and no study has documented the role of a particular factor in vivo (1, 6, 17, 22, 23, 25, 27, 30). Therefore, to our knowledge, this is the first report providing direct evidence that tumor-derived PDGF-BB is capable of attracting hMSCs in an in vivo intracranial glioma model.
These studies show that PDGF-BB plays a causal role in the tropism of hMSCs for gliomas. First, tumors that produced higher levels of PDGF-BB resulted in a greater migration of hMSCs in Transwell in vitro assays compared with tumors that secreted low levels of PDGF-BB. Second, the migration of hMSCs was significantly reduced when neutralizing antibodies that inhibited PDGF-BB were added to the conditioned media, indicated that the effects of the conditioned media were specifically mediated through secreted PDGF-BB. Lastly, and most importantly, in vivo xenografts that produced higher levels of PDGF-BB resulted in increased attraction of hMSCs after intracarotid injection compared with those that produced low levels based on two independent assays for detecting hMSCs (see below). Therefore, multiple lines of evidence indicate that PDGF-BB is capable of mediating the tropism of hMSCs for human gliomas.
Our results also indicate that the tropic effects of hMSCs for tumor-derived PDGF-BB are mediated through PDGFR-β located on hMSCs. Pretreatment of hMSCs with neutralizing antibodies to PDGFR-β reduced the in vitro migration of hMSCs both to recombinant PDGF-BB and to conditioned media derived from PDGF-BB secreting clones. Most importantly, inhibition of PDGFR-β on hMSCs prior to injection into the carotid artery of mice bearing PDGF-BB-secreting intracranial xenografts resulted in a significant decrease in the number of hMSCs that localized to the tumor. These results are consistent with those of Fielder et al. also reported that blocking of PDGFR-β on “mesenchymal progenitor cells” was capable of inhibiting the PDGF-BB induced hMSC migration albeit only in in vitro studies (5). Likewise, PDGFR has been recently implicated in the migration of retinal pericytes that have properties and markers similar to hMSCs (26). Therefore, the interaction of tumor derived PDGF-BB with PDGFR-β on hMSCs appears to be fundamental to the ability of hMSCs to migrate and, therefore, to localize within gliomas after intravascular delivery.
Because it is a growth factor, PDGF-BB in high levels can increase tumor size. In addition, PDGF-BB has been implicated in stabilizing tumor vasculature (7). Not surprisingly, we showed that the xenografts derived from the PDGF-BB-high secreting cells grew faster than the xenografts from the PDGF-BB-low secreting cells. There were also effects on vessel cell density, although these were less marked. We attempted to control for these variables by injecting hMSCs into the animals at times when the PDGF-BB-high and PDGF-BB-low secreting xenografts were of similar size and vascularity. Our studies indicated that 4-day old PDGF-BB-high secreting xenografts (measured from time cell implantation) were similar in size and vascularity to 7-day old PDGF-BB- low secreting xenografts . Even when hMSCs were injected at these two time points significantly more hMSCs localized to PDGF-BB-high secreting xenografts compared with PDGF-BB-low secreting xenografts, indicating that the effects of PDGF-BB appeared to be independent of tumor size and vascularity. Lastly, because gfp-labeling allowed us to identify individual cells in the tumors, we were able to calculate the density of hMSCs within xenografts , i.e., the number of hMSCs per unit area of tumor. This method allowed us to account for any differences in tumor size between specimens. Therefore, our results indicate that the increased localization of hMSCs in PDGF-BB-high secreting tumors was directly related to the levels of PDGF-BB in the tumor, and not to other aspects of tumor biology.
In order to provide independent methods for quantifying hMSCs in vivo two distinct approaches for detecting hMSCs within gliomas, namely gfp-labeling and BLI, were used in our studies. In a previous report (17), we tracked hMSCs using SP-DiI, a fluorescent vital dye that incorporates into the cell membrane. This vital dye method has been used by others (31), however, the low resolution of this approach makes it difficult to discern individual cells within the tumor and the potential for transfer of this membrane-bound dye to other surrounding cells may over estimate the number of cells within the tumor (data not shown). Although others have shown also that rodent hMSCs localize to gliomas (18, 35), a precise proof that exogenously delivered human MSCs are morphologically intact has not been fully evaluated up to now in human gliomas. The application of gfp-labeled MSCs was valuable because it provided histological proof that the hMSCS which resided within the xenografts were morphologically intact, i.e., histologically whole cells. Equally important, BLI verified that the cells that arrived in the tumor after intravascular delivery were alive and functional because the emmision of a detectable light signal requires production of a catalytically functional luciferase protein in this method (34).
Although both gfp-labelling and BLI demonstrated that the number of hMSCs that localized to the high secreting PDGF-BB U87 clones was statistically significantly greater than the number of hMSCs that localized to the low secreting clones based on objective quantification, there were differences between the two techniques, particularly within the low secreting U87 cells. Indeed, BLI was unable to detect hMSCs in several of the animals harboring PDGF-BB-low secreting xenografts. In contrast, gfp-labelled cells were identified in all animals harboring PDGF-BB-low secreting xenografts, albeit at a lower level than in the high secreting xenografts. These differences are most likely related to the sensitivity of the techniques, rather than the biological behavior of the cells. Because gfp-labelling allows for the detection of individual cells on a single slice of the whole tumor, this technique is much more sensitive than BLI, in which production of a signal is based on the sum of all the cells within the tumor with a threshold of detection limited by the resolution of the camera. In this context, and consistent with our previous result, it should be noted that hMSCs localized even to the PDGF-BB-low secreting xenografts, suggesting that factors other than PDGF-BB may also mediate the attraction of hMSCS to gliomas. Indeed, it is likely that multiple factors are capable of attracting hMSCS to gliomas (1, 6, 17, 22, 27, 30). Obviously, the model system used in this study can be exploited to test these other factors (e.g. EGF, IL-6, SDF-1) individually and in combination. It will be of interest to determine whether the simultaneous secretion of multiple factors produces a synergistic increase in hMSC migration.
Our finding can be exploited for improving the therapeutic efficacy of hMSCs. For example, cell homing may be manipulated by using specific agents or treatments to increase the expression of cell surface receptors (25). More immediately, these studies may provide information for selecting patients whose growth factor expression is most compatible with successful hMSC engraftment. Specifically, the importance of PDGF-BB in attracting hMSCs to gliomas suggests that PDGF-BB production within tumors may be used for selecting patients whose PDGF-BB expression is most compatible with successful hMSC engraftment and thus successful delivery of antiglioma agents during therapeutic applications of hMSCs. In addition, these studies of exogenous hMSCs also may provide insight into endogenous hMSCs. Specifically, these studies of exogenously delivered hMSCs raise the interesting possibility that PDGF-BB may act as a tropic factor for recruiting endogenous hMSCs into gliomas in situ. Indeed, it has been postulated that circulating stem cells may contribute to the biological growth of human tumors, in general, and to gliomas, in particular (8, 17). Although it is currently unclear whether bone marrow-derived hMSCs actually circulate through the blood stream in humans (10), it has been postulated that endogenous hMSCs may contribute to neovascularization by differentiation into pericytes around blood vessels. Interestingly, recent studies indicate that glioma-derived PDGF-BB may be responsible for recruiting peri-endothelial cells to blood vessels (7), or vascular smooth muscle cells that supports tumor angiogenesis (29). Whether endogenous hMSCs contribute to this biological process has not been proven, but the link between PDGF-BB recruitment of hMSCs and the potential for hMSCs to differentiate into pericytes (10) raises the possibility that endogenous hMSCs may contribute to the biological aggressiveness of gliomas by contributing to vascular formation, a hallmark of grade IV gliomas (i.e. glioblastomas). Future studies analyzing the role of PDGF-BB on endogenous hMSCs will be of interest.
CONCLUSION
Taken together, these studies demonstrate that PDGF-BB, which is secreted by human gliomas, is a tropic factor for hMSC both in vitro and in vivo, and that this tropism of PDGF-BB is mediated via PDGF-β receptors on hMSCs. Exogenously delivered hMSCs localize to human gliomas at least in part in response to the presence of PDGF-BB secreted by the tumor. These findings can be exploited to improve the therapeutic efficacy of hMSCs.
Acknowledgment
The authors thank Stephanie Jenkins for her excellent assistance with the preparation of the manuscript.
This work was supported by NIH/NCI grants R01 CA115729 and P50 CA127001, a grant from the National Brain Tumor Foundation, a grant from the MD Anderson Center for Targeted Therapy, and by the Elias Family Fund for Brain Tumor Research, the Gene Pennebaker Brain Cancer Research Fund, and the Brian McCulloch Research Fund (to FFL).
References
- 1.Birnbaum T, Roider J, Schankin CJ, Padovan CS, Schichor C, Goldbrunner R, Straube A. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 3.Cheng P, Gao ZQ, Liu YH, Xue YX. Platelet-derived growth factor BB promotes the migration of bone marrow-derived mesenchymal stem cells towards C6 glioma and up-regulates the expression of intracellular adhesion molecule-1. Neuroscience letters. 2009;451:52–56. doi: 10.1016/j.neulet.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 4.Colter DC, Class R, Di Girolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. Journal of cellular biochemistry. 2004;93:990–998. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- 6.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. Journal of cellular biochemistry. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 7.Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 9.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 10.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 11.Kleihues P, Cavenee WK. Lyon: IARC Press; World Health Organization classification of tumors. Tumors of the nervous system. 2000
- 12.Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M, Marini FC. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 14.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002;62:3729–3735. [PubMed] [Google Scholar]
- 15.Louis DN, Posner J, Jacobs T, Kaplan R. Report of the Brain Tumor Progress Review Group. National Institute of Neurological Disorders and Stroke, National Cancer Institute. 2000 [Google Scholar]
- 16.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 17.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 19.Nister M, Libermann TA, Betsholtz C, Pettersson M, Claesson-Welsh L, Heldin CH, Schlessinger J, Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48:3910–3918. [PubMed] [Google Scholar]
- 20.Olmsted-Davis EA, Gugala Z, Gannon FH, Yotnda P, McAlhany RE, Lindsey RW, Davis AR. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Human gene therapy. 2002;13:1337–1347. doi: 10.1089/104303402760128568. [DOI] [PubMed] [Google Scholar]
- 21.Ostman A, Thyberg J, Westermark B, Heldin CH. PDGF-AA and PDGF-BB biosynthesis: proprotein processing in the Golgi complex and lysosomal degradation of PDGF-BB retained intracellularly. J Cell Biol. 1992;118:509–519. doi: 10.1083/jcb.118.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padovan CS, Jahn K, Birnbaum T, Reich P, Sostak P, Strupp M, Straube A. Expression of neuronal markers in differentiated marrow stromal cells and CD133+ stem-like cells. Cell Transplant. 2003;12:839–848. doi: 10.3727/000000003771000183. [DOI] [PubMed] [Google Scholar]
- 23.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 24.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato H, Kuwashima N, Sakaida T, Hatano M, Dusak JE, Fellows-Mayle WK, Papworth GD, Watkins SC, Gambotto A, Pollack IF, Okada H. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12:757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 26.Scheef EA, Sorenson CM, Sheibani N. Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. American journal of physiology. 2009;296:C724–C734. doi: 10.1152/ajpcell.00409.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schichor C, Birnbaum T, Etminan N, Schnell O, Grau S, Miebach S, Aboody K, Padovan C, Straube A, Tonn JC, Goldbrunner R. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Schiffer CA. Signal transduction inhibition: changing paradigms in cancer care. Semin Oncol. 2001;28:34–39. [PubMed] [Google Scholar]
- 29.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 30.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 31.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 32.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 34.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J, Zhu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol. 2003;36:49–59. doi: 10.5483/bmbrep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]