Abstract
Pathogenesis of Bacillus anthracis is associated with the production of lethal toxin (LT), which activates the murine Nalp1b/Nlrp1b inflammasome and induces caspase-1-dependent pyroptotic death in macrophages and dendritic cells. Here, we investigated the effect of allelic variation of Nlrp1b on the outcome of LT challenge and infection by B. anthracis spores. Nlrp1b allelic variation did not alter the kinetics or pathology of end stage disease induced by purified LT, suggesting that, in contrast to previous reports, macrophage lysis does not contribute directly to LT-mediated pathology. However, animals expressing LT-sensitive alleles of Nlrp1b showed an early inflammatory response to LT and increased resistance to infection by B. anthracis. Data presented here support a model whereby LT-mediated activation of Nlrp1b and subsequent lysis of macrophages is not a mechanism used by B. anthracis to promote virulence but rather a protective host-mediated innate immune response.
INTRODUCTION
Bacillus anthracis is the pathogenic bacterium responsible for the acute disease anthrax. Virulence of B. anthracis is mediated in large part via the production of a protein exotoxin called lethal toxin (LT). Indeed, purified LT induces many symptoms associated with fulminant anthrax including vascular collapse and death (1–3). LT is a bipartite toxin in which the binding subunit, protective antigen (PA), attaches to anthrax toxin receptors and subsequently delivers the catalytic moiety, lethal factor (LF), into the host cell cytosol. Once intracellular, LF functions as a zinc-dependent metalloproteinase, cleaving the N-termini of mitogen-activated protein kinase (MAPK) kinases (MKKs) and thereby disrupting cell signaling through the ERK1/2, JNK and p38 pathways (3). As a result, LT cripples the host innate immune system by blocking cytokine production from numerous cell types, inhibiting chemotaxis of neutrophils, and inducing apoptosis in activated macrophages (3). At high concentrations, similar to those found late in infection, LT induces cytokine-independent shock and death in animals that is associated with vascular collapse (1, 2, 4).
Interestingly, LT induces rapid cell lysis in macrophages and dendritic cells (DCs) derived from a subset of inbred mouse and rat strains (3, 5). This finding led to the model that the cytokine burst resulting from LT-induced macrophage lysis contributes to pathology associated with this toxin (6, 7). Such a model is attractive as rapid release of proinflammatory cytokines concomitant with macrophage lysis could, in theory, exacerbate the vascular damage associated with anthrax and LT-mediated pathology (3). Furthermore, macrophages play an important role in limiting B. anthracis infection (8–10), and their rapid destruction by LT would be predicted to result in increased bacterial fitness. However, this model is at odds with the observation that animals resistant to purified LT are sensitive to challenge by B. anthracis spores and vice versa (11). A similar inverse relationship exists in inbred mouse strains whereby many strains whose macrophages lyse in response to LT display increased resistance to infection by B. anthracis (12). Therefore, contrary to one model, LT-mediated lysis of macrophages appears to be associated with protection against infection by B. anthracis.
A single gene, Nlrp1b, controls macrophage and DC sensitivity to LT (3, 13), and when heterologously expressed with caspase-1 in human fibroblasts, confers susceptibility to LT in these cells (14). Nlrp1b is a member of the nucleotide binding domain – leucine rich repeat (NB-LRR) family of proteins found in plants, called R proteins, and animals, termed NLR proteins (6, 13). Plant R proteins function in host immunity by recognizing pathogens and/or danger signals and initiating a hypersensitive response (HR) that can function locally through induction of cell death or distally through production and release of antimicrobial products and signaling molecules. Localized cell death induced by R proteins represents a mechanism to limit bacterial infection and can be triggered by a number of upstream stimuli including the presence of bacterial proteases in the host cytosol (6, 15). We reasoned that a similar HR may also occur in B. anthracis-exposed animals and could explain why macrophage susceptibility to LT varies inversely with susceptibility to spore challenge as described above. Therefore, we sought to determine how Nlrp1b influences outcome to LT and spore challenge.
MATERIALS AND METHODS
Mouse Maintenance and Breeding
All mice were cared for in accordance with the University of California Animal Research Committee and the USAMRIID Animal Care and Use Committee. C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice expressing a 129S1/SvImJ(129S1)-derived LTS allele of Nlrp1b on a LT-resistant (LTR) B6 background (B6Nlrp1b(129S1)), backcrossed to B6 for seven generations, were obtained from Drs. E. Boyden and W. Dietrich (Harvard Medical School). Heterozygous B6Nlrp1b(129S1) were intercrossed or crossed with B6, and transgene-positive offspring were identified by PCR genotyping as previously described (13).
Toxin Preparation and Challenge
PA was expressed in E. coli and purified as previously described (16), followed by Sephacryl S-200 (GE Healthcare) size exclusion chromatography. LF was obtained from Dr. J. Mogridge (University of Toronto). A dose of 5 µg PA and 2.5 µg LF, diluted in pharmaceutical grade saline, per g body weight was injected i.p. Alternatively, PA and LF were purified from B. anthracis strain BH450 (17). LF produced from strain BH450 displayed 3-fold lower activity (18), and consequently a dose of 15 µg PA and 7.5 µg LF per g body weight was used to achieve a similar mortality rate. Endotoxin was removed from all toxin preparations as described (16). Walking ataxia was scored as follows: Mild - reduced exploratory behavior or rearing on hindlimbs, a slower and/or less steady gait, but free ambulation throughout the cage; Moderate - preferred sedentary state, but the mouse was able to generate a slow, unsteady (e.g. wobbly) gait usually for < 7 sec before resting; and Severe - typically in a stationary state, but upon stimulation the mouse could generate a few unstable steps (e.g. severe wobble and/or tremor) before stopping. Body temperatures were measured following LT injection using a rectal thermometer. Baseline temperatures were determined prior to LT injection and no differences were observed between animal groups (not shown). For cytokine analysis, blood was collected via cardiac puncture and allowed to coagulate. Sera was collected and stored at −80 °C. Cytokines were detected using the Millipore Milliplex™ MAP Mouse Cytokine Kit per manufacturer’s instructions.
Spore Challenge and Cellular Analysis
B6Nlrp1b(129S1) and non-transgenic littermate/cagemate mice were injected i.p. with ~2.5 × 107 unencapsulated, toxigenic Sterne strain (7702) or 4 × 102 Ames strain spores per mouse and monitored daily for 14 days. For cellular analysis, mice were infected i.p. with ~1.6 × 107 Sterne spores and euthanized at 4, 28, 52, 76, and 135 h post infection. Uninfected mice were used to determine baseline cell populations in the peritoneal cavity of each strain. Peritoneal exudates were harvested by injecting 7 mL of sterile HBSS and 3 mL of air into the peritoneal cavity, followed by extraction. Samples were stained with fluorescently conjugated antibodies to surface markers Mac1/Cd11b (Invitrogen) and Ly6G (BD Pharmingen) and analyzed by flow cytometry. Due to the cross-reactivity of the anti-Mac1 antibody, polymorphonuclear neutrophils (PMNs) were defined as Ly6G+ and Ly6G+/Mac1+. Monocytes were defined as Mac1+/Ly6G−. The average % of each cell type per mouse was then converted to total cell number by multiplying with the mean hemocytometer count for each mouse group. Similar values were obtained by histochemical and microscopic analyses (data not shown).
RESULTS
Nlrp1b-mediated response to LT
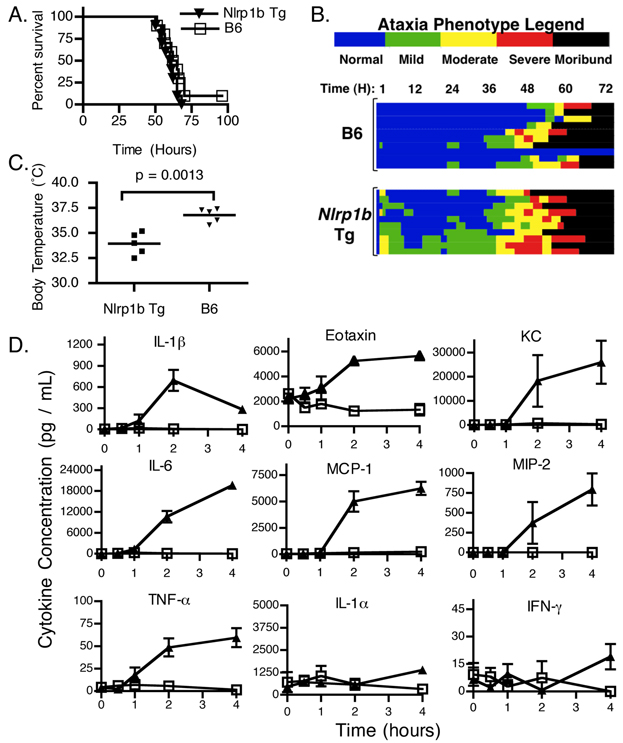
To determine whether the presence of a LTS allele of Nlrp1b controls whole animal susceptibility to purified LT, we challenged B6Nlrp1b(129S1) mice with LT via i.p. injection (13). Surprisingly, B6Nlrp1b(129S1) mice displayed a time to a moribund state similar to non-transgenic littermate controls following LT challenge (Fig. 1A), indicating that the expression of a LTS allele of Nlrp1b does not contribute to whole animal susceptibility to LT. Histopathological analysis also revealed no differences at the end stage of disease (data not shown), consistent with earlier reports (1). However, a previously undescribed rapid and transitory response was observed following LT challenge, which was characterized by ataxia (Fig. 1B), bloat, dilated vessels on pinnea, loose/watery feces, labored abdominal breathing and/or mild hypothermia (Fig. 1C). This distinctive response was designated as the ‘Early Response Phenotype’ (ERP) as some animals presented as early as 30 min post LT administration, and the remaining animals typically presented by 1 – 2 h. Wild type B6 and littermate control (not shown) animals displayed no significant ERP following LT challenge (Fig 1B, 1C). Surprisingly, B6Nlrp1b(129S1) mice recovered to seemingly normal behavior following the ERP before succumbing to LT in a manner similar to control animals (Fig. 1B).
FIGURE 1.
Influence of Nlrp1b on the response in mice to LT. A. B6Nlrp1b(129S1) transgenic mice (Nlrp1b Tg)(n=10) expressing a LTS allele of Nlrp1b or transgene-negative control animals (B6)(n=11) were challenged with 5 µg PA + 2.5 µg LF per g body weight via i.p. injection. Animals were closely monitored for the first 4 – 6 h following LT injection and then every 3 h for 5 d and euthanized upon reaching a moribund state. B. Heat map representing ataxia severity of the animals shown in (A). Each horizontal line represents an individual animal from time of LT injection (left) until the end of the experiment (right). Ataxia severity is indicated by color. Data are representative of 7 independent experiments. C. Body temperature of B6Nlrp1b(129S1) (n=5), or non-transgenic littermate control mice (n=5) was measured following i.p. injection of 15 µg PA + 7.5 µg LF per g body weight. Temperature was monitored hourly and lowest temperature observed during first 5 h post toxin injection is plotted. D. B6Nlrp1b(129S1) transgenic mice (closed triangles) or transgene-negative control animals (open squares) were challenged with LT as in (C). Uninjected animals served as t = 0 controls. Animals were sacrificed at 0.5, 1, 2, and 4 h post toxin injection and serum cytokines levels were measured. Data represent the average values of 5 animals +/− SD.
The pathology, timing, and clinical presentations associated with the ERP are consistent with an inflammatory response, the rate of macrophage lysis ex vivo and the previously reported cytokine response in LTS strains of mice (1, 2). We therefore tested whether expression of a LTS allele of Nlrp1b is sufficient to induce a proinflammatory cytokine response to LT. Activation of Nlrp1b results in formation of a caspase-1-containing inflammasome and subsequent proteolytic maturation of IL-1β (13, 19). As expected, IL-1β increased rapidly after LT administration (Fig 1D). In addition, several proinflammatory cytokines not directly activated by caspase-1 also increased (Fig. 1D)(1, 2). In contrast to previous findings with LTS strains of mice (1, 2), there was a mild increase in TNF-α in B6Nlrp1b(129S1) mice (Fig. 1D). No changes were observed in either IL-1α or IFNγ. Endotoxin contamination of PA or LF was not responsible for cytokine induction as no response was detected following injection of a 2x dose of individual toxin components (data not shown). Further, B6 animals showed no ERP or cytokine response to LT (Fig 1D), indicating that these responses are a result of Nlrp1b detection of LF activity rather than LPS contamination. Therefore, expression of a LTS allele of Nlrp1b in LTR B6 mice is sufficient to induce a proinflammatory cytokine response to LT in mice.
LT-sensitive Nlrp1b alleles provide protection against B. anthracis infection
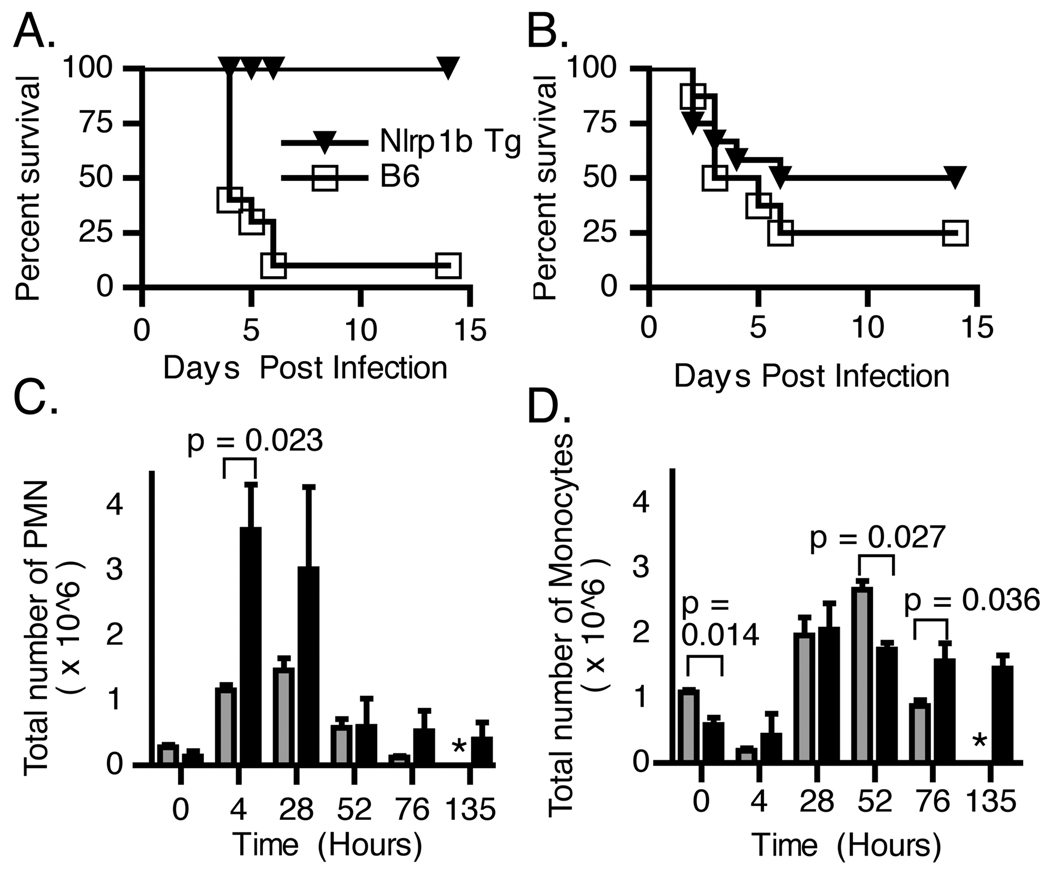
To test the role of Nlrp1b in an infection model, B6Nlrp1b(129S1) mice and transgene-negative littermate control animals were challenged with the unencapsulated, toxigenic B. anthracis Sterne strain. Within six days, 8/9 control animals succumbed to infection, while all B6Nlrp1b(129S1) mice survived for the duration of the experiment (Fig. 2A). To test the role of a LTS Nlrp1b allele in response to a fully virulent B. anthracis infection, B6Nlrp1b(129S1) mice were challenged with B. anthracis Ames strain. Although B6Nlrp1b(129S1) mice displayed a trend towards protection, the data were not statistically significant (Fig. 2B). The latter finding is not surprising given that virulence associated with the Ames strain is governed primarily by the presence of a poly-d-glutamic acid capsule rather than LT in the mouse model (20).
FIGURE 2.
LTS allele of Nlrp1b provides protection from B. anthracis spore challenge. A. B6Nlrp1b(129S1) transgenic mice (n=11) or transgene negative control animals (n=9) were challenged i.p. with 2.5 × 107 spores of B. anthracis Sterne strain 7702 (p<0.0001, log rank test of Kaplan Meier survival curves). B. B6Nlrp1b(129S1) transgenic mice (n=12) or transgene negative control animals (n=8) were challenged i.p. with 4 × 102 spores of B. anthracis Ames strain (p=0.3847, log rank survival curve). C,D. B6Nlrp1b(129S1) transgenic mice (black bars) or transgene-negative control animals (grays bar) were challenged i.p. with 1.6 × 107 spores of B. anthracis Sterne strain 7702. Animals were euthanized at the indicated timepoints and the number of PMNs (C) and monocytes (D) in the peritoneal cavity were determined as described in the Methods section. Data represent mean values (n=2 at 135 h timepoint and n=3 at all other timepoints) +/− SD. Asterisk indicates no B6 survivors at the 135 h timepoint.
To determine the cellular mediators contributing to Nlrp1b-mediated resistance to infection, peritoneal exudates were collected and analyzed at various timepoints following spore challenge. Both strains responded with an increase in the number of Ly6G+ PMNs (Fig. 2C). However, the levels of PMNs were higher in B6Nlrp1b(129S1) mice at early timepoints following spore challenge compared to non-transgenic littermate control animals. This influx of PMNs was followed by an increased number of Ly6G−/Mac1+ monocytes in both strains (Fig. 2D) that were maintained in B6Nlrp1b(129S1) but not control mice.
DISCUSSION
Based on LT and spore challenge data from different animal species, Lincoln et al. hypothesized that animals resistant to infection by B. anthracis were susceptible to challenge by its toxin, and that the inverse was true for infection-susceptible species (11). Using inbred and recombinant strains of mice, Welkos and colleagues substantiated this proposed inverse correlation between the sensitivity of animals to challenge with purified LT and with B. anthracis spores and explored the genetic basis for this phenomenon (12, 21, 22). Specifically, mice whose macrophages rapidly lyse in response to LT were more resistant to spore challenge than mice whose macrophages were LTR (12, 13, 23). Further, mice resistant to spore challenge had increased rates of PMN infiltration at early timepoints and sustained higher monocyte numbers at the site of B. anthracis infections (22). Here we report that allelic variation at Nlrp1b accounts for these previously observed phenomena, thereby providing molecular insight into host defense against anthrax.
B. anthracis triggers activation of TLRs and NOD2 in human and mouse macrophages, resulting in production of TNF-α through a MAPK signaling pathway (24). However, the presence of LT blocks this response by cleaving and inactivating MKK proteins (24). LTS alleles of Nlrp1b counteract this immunosuppressive effect by triggering a rapid proinflammatory programmed cell death. Interestingly, IL-1β is released upon LT-mediated macrophage lysis (19). IL-1β is a proinflammatory cytokine that recruits PMNs and monocytes, cell types that are predicted to resolve infection (9, 10, 25). Although Nlrp1b inflammasome activation in response to LT is detrimental to the toxin-exposed macrophage, our data demonstrate that Nlrp1b activation is ultimately beneficial for the host by inducing inflammation (e.g., enhanced cytokine production and PMN infiltration) at the site of LT production. Of note, a similar mechanism has been described in plants where R proteins recognize bacterial virulence factors in the host cell cytosol and induce localized cell death to limit infection. Importantly, the finding that the Nlrp1b-mediated inflammatory response is protective against B. anthracis infection is consistent with previous data that mice deficient in caspase-1, IL-1β, or IL-1R display increased sensitivity to anthrax (25, 26). Therefore, we propose that Nlrp1b-mediated cell death provides a selective advantage to the host rather than pathogen.
ACKNOWLEDGEMENTS
The authors thank Drs. E. Boyden and W. Dietrich for providing B6Nlrp1b(129S1) mice, Dr. G. Lawson for histopathological analyses, Alyssa Leiva, Sylvia Trevino and Sonela Schlottmann for their technical assistance, and Diana Fisher for statistical assistance.
Footnotes
This research was supported by the UCLA Microbial Pathogenesis Training Grant #2-T32-AI-07323 (JKT), NIH grant AI077791 (KAB and SML) and the Joint Science and Technology Office for Chemical and Biological Defense (JSTO-CBD)/Defense Threat Reduction Agency project 1.1A0010-07-RDB (CKC, AJ, and SLW)
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
REFERENCES
- 1.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-{alpha}-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moayeri M, Martinez NW, Wiggins J, Young HA, Leppla SH. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect Immun. 2004;72:4439–4447. doi: 10.1128/IAI.72.8.4439-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks DJ, Ward SC, Bradley KA. New insights into the functions of anthrax toxin. Expert Rev Mol Med. 2006;8:1–18. doi: 10.1017/S1462399406010714. [DOI] [PubMed] [Google Scholar]
- 4.Culley NC, Pinson DM, Chakrabarty A, Mayo MS, LeVine SM. Pathophysiological manifestations in mice exposed to anthrax lethal toxin. Infect Immun. 2005;73:7006–7010. doi: 10.1128/IAI.73.10.7006-7010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nye SH, Wittenburg AL, Evans DL, O'Connor J A, Roman RJ, Jacob HJ. Rat survival to anthrax lethal toxin is likely controlled by a single gene. Pharmacogenomics J. 2007 doi: 10.1038/sj.tpj.6500448. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna PC, Acosta D, Collier RJ. On the role of macrophages in anthrax. Proc Natl Acad Sci U S A. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cote CK, Dimezzo TL, Banks DJ, France B, Bradley KA, Welkos SL. Early interactions between fully virulent Bacillus anthracis and macrophages that influence the balance between spore clearance and development of a lethal infection. Microbes Infect. 2008;10:613–619. doi: 10.1016/j.micinf.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Cote CK, Rea KM, Norris SL, van Rooijen N, Welkos SL. The use of a model of in vivo macrophage depletion to study the role of macrophages during infection with Bacillus anthracis spores. Microb Pathog. 2004;37:169–175. doi: 10.1016/j.micpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect Immun. 2006;74:469–480. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lincoln RE, Walker JS, Klein F, Rosenwald AJ, Jones WI., Jr Value of field data for extrapolation in anthrax. Fed Proc. 1967;26:1558–1562. [PubMed] [Google Scholar]
- 12.Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 14.Liao KC, Mogridge J. Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin. Infect Immun. 2009;77:4455–4462. doi: 10.1128/IAI.00276-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science. 2003;301:1230–1233. doi: 10.1126/science.1085671. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado-Arocho FJ, Bradley KA. Anthrax edema toxin induces maturation of dendritic cells and enhances chemotaxis towards macrophage inflammatory protein 3beta. Infect Immun. 2009;77:2036–2042. doi: 10.1128/IAI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif. 2000;18:293–302. doi: 10.1006/prep.2000.1208. [DOI] [PubMed] [Google Scholar]
- 18.Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS One. 2008;3:e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordoba-Rodriguez R, Fang H, Lankford CS, Frucht DM. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J Biol Chem. 2004;279:20563–20566. doi: 10.1074/jbc.C300539200. [DOI] [PubMed] [Google Scholar]
- 20.Welkos SL, Vietri NJ, Gibbs PH. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence. Microb Pathog. 1993;14:381–388. doi: 10.1006/mpat.1993.1037. [DOI] [PubMed] [Google Scholar]
- 21.Welkos SL, Friedlander AM. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb Pathog. 1988;4:53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- 22.Welkos SL, Trotter RW, Becker DM, Nelson GO. Resistance to the Sterne strain of B. anthracis: phagocytic cell responses of resistant and susceptible mice. Microb Pathog. 1989;7:15–35. doi: 10.1016/0882-4010(89)90108-3. [DOI] [PubMed] [Google Scholar]
- 23.Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 24.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008;38:1574–1584. doi: 10.1002/eji.200838141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalns J, Scruggs J, Millenbaugh N, Vivekananda J, Shealy D, Eggers J, Kiel J. TNF receptor 1, IL-1 receptor, and iNOS genetic knockout mice are not protected from anthrax infection. Biochem Biophys Res Commun. 2002;292:41–44. doi: 10.1006/bbrc.2002.6626. [DOI] [PubMed] [Google Scholar]