Abstract
Despite two decades of research since R. Smithells and colleagues began exploring its benefits, the mechanisms through which folic acid supplementation supports neural tube closure and early embryonic development are still unclear. The greatest progress toward a molecular genetic understanding of folate effects on neural tube defect (NTD) pathogenesis has come from animal models. The numbers of NTD-associated mouse mutants accumulated and studied over the past decade have illuminated the complexity of both genetic factors contributing to NTDs and also NTD-gene interactions with folate metabolism. This article discusses insights gained from mouse models into how folate supplementation impacts neurulation. A case is made for renewed efforts to systematically screen the folate responsiveness of the scores of NTD-associated mouse mutations now identified. Designed after Crooked tail, supplementation studies of additional mouse mutants could build the molecular network maps that will ultimately enable tailoring of therapeutic regimens to individual families.
Keywords: Folic acid, neural tube defects, folate metabolic pathway, mouse models, nucleotide biosynthesis, methylation
INTRODUCTION
This special issue honors the estimable contribution of Richard Smithells and colleagues for recognizing as early as 1976 that deficiencies of folic acid, a simple water-soluble vitamin, might indicate increased risk of neural tube defects (NTDs) including anencephaly and spina bifida (Smithells and others, 1976). His subsequent landmark study showed that periconceptual folate supplementation could reduce the recurrence of neurulation defects, comparing control and supplemented cohorts of approximately 240 women each (Smithells and others, 1983). Larger therapeutic trials in the UK and eastern Europe established folate supplementation as the most effective birth defect prevention method ever identified, capable of reducing NTD occurrence rates by 70% or more (Czeizel and Dudas, 1992; MRC, 1991; Seller and Nevin, 1984). These and later supportive studies led to the current recommendation by the Center for Disease Control and the World Health Organization that all women of child bearing age receive folate supplementation of 0.4 mg daily and those women who have had a child with an NTD should receive 4 mg daily in the periconceptual period. Indeed, after voluntary folate supplementation rates indicated relative public inattention to this important measure, the US mandated fortification of food stuffs such as cereals to provide an additional 0.1 mg daily folate intake in the average US diet, implemented starting in 1996. A reduction in US NTD rates of 20–30% has been attributed to this fortification over the subsequent decade (Canfield and others, 2005; Honein and others, 2001; Pitkin, 2007; Wald and Oakley, 2007).
Surprisingly, more than 20 years after recognition that folic acid supplementation can reduce the occurrence of spina bifida and anencephaly, the mechanism of this rescue and why some families benefit while others do not is still largely unknown. The lack of present clarity is in no small measure due to the complexity of the interactions of genetics, metabolic status and environment that must converge to result in an NTD. Advances in this intriguing field have led to an appreciation of the intricacies of these interactions. Examining NTD mechanisms and their intersection with folate metabolism in mouse models, linked with investigation of human genetics and epidemiology, will likely unravel patterns of these gene-environment interactions. This level of understanding would certainly herald improved strategies for NTD prevention in individual families, including new options for the 30% or more who will not benefit from folate supplementation.
Here, we discuss insights from mouse models demonstrated to be protected from NTD by folate supplementation and highlight questions that these studies raise.
MOUSE MODELS as an INROAD to FOLATE ACTION in NTDs and PREVENTION
Studies in mice provide numerous advantages as an adjunct to clinical investigation. The availability of inbred mouse strains and their shorter life cycles makes it possible to reduce the complexities of genetic background, environmental influences, and limited observations per family that are inherent in human studies. Experimental mouse models are more amenable to pharmacological manipulation of metabolism and detailed investigation of pathological outcomes in various tissues and fetuses. They also enable gene manipulation to perturb systems and probe gene-environment interactions. Animal studies have proven challenging, as no single mouse model appears to encompass the features of clinical experience. Nevertheless, a number of insights into folate-dependent influences on neurulation have been mined from mouse models. Evolving technical capabilities provide new options for the analysis of complex gene-environment interactions that underlie folate supplementation effects.
Folate Deficiency is not Enough (or may be too much)
Several studies in mice suggest that folate deficiency alone can impair implantation and early embryogenesis, but it is insufficient in isolation to produce NTDs. Heid and colleagues compared the reproductive tracts of female Swiss-Webster mice 12 days postcoitum (dpc) that had been maintained on low-folate diets ranging from 45 nmol/kg chow to 2266 nmol/kg chow (Heid and others, 1992). They found that diets containing less than 181 nmol/kg prevented implantation and diets containing 221–453 nmol/kg folate produced 100% resorption rates. Dietary folate levels producing 75% resorptions did not produce NTDs in viable embryos. A decade later, Burgoon and colleagues examined ICR mice maintained on defined folate diets containing succinyl sulfathiazole, an antibiotic used to reduce gut flora to remove sources of absorbable folate not present in the diet (Burgoon and others, 2002). Those few implantations that were viable past dpc 11–12 did not display NTDs.
Nevertheless, genetic disruption of folate intracellular transport in folate binding protein 1 (Folbp1, also known as folate receptor 1, Folr1) or reduced folate carrier 1 (RFC1) knockout mice (C57BL6 and SWV backgrounds, respectively) results in NTDs and this strongly argues that impairment of intracellular folate metabolism is deleterious to neurulation (Gelineau-van Waes and others, 2008; Piedrahita and others, 1999). These nullizygous embryos die peri-implantation unless dams are heavily supplemented with folic acid. A detailed study of the RFC1 deficient embryos from dams receiving 50 mg/kg/day folic acid indicate the critical importance of folate transport in chorioallantoic fusion, hematopoesis and the development of neural tube, limbs, lungs, heart, and skin (Gelineau-van Waes and others, 2008).
These are prime examples of numerous investigations recently reviewed elsewhere (Beaudin and Stover, 2007; Blom and others, 2006; Harris and Juriloff, 2007). The ultimate message of these studies in mice is that the folate metabolic pathway is crucial for implantation and many aspects of embryonic development, and severe impairment of the pathway is incompatible with survival beyond early embryogenesis. However, intracellular folate utilization is an important aspect of neurulation. Therefore, it is likely that lesser alterations in the molecular pathways supported by folic acid metabolism would result in viable fetuses with defects more restricted to neural tube and its derivatives. In other words, a compilation of partial loss- or gain-of-function mutations must conspire to result in an NTD. This contention certainly parallels clinical experience. While this complexity has led to a degree of pessimism over how well studies in mice can emulate the human condition, one must also be heartened that the degree of complexity demonstrated in the mouse is up to the challenge of providing clinically relevant information. The field has only to be clever enough to take advantage of the opportunity.
Mouse Mutants and NTD Prevention by Folate Supplementation
Perhaps the most under utilized opportunity for investigation of folate actions in NTD prevention is the examination of folate supplementation in mice bearing a genetic predisposition to neurulation defects. Well over 190 naturally occurring and engineered mouse mutations are known to produce exencephaly (the mouse equivalent of anencephaly in humans), spina bifida or both, and over 155 of the responsible genes have been identified (Harris and Juriloff, 2007). Yet only a small handful has been tested for the ability of added folate to reduce the occurrence of NTDs in these mice and still fewer have been rigorously evaluated (Table 1). Studies in several mouse mutants, among them Crooked tail, suggest that screening of folate supplementation in genetic NTD models should be revisited to more broadly apply experimental paradigms that have proven informative.
Table 1.
Mouse NTD mutants tested for responsiveness to Folate
| Folate Responsive NTD Mutant Mice | ||
|---|---|---|
| Gene | Folate dose/route | Reference |
| Cart1 null | 3 mg/kg i.p., 0.5–9.5 dpc | (Zhao and others, 1996) |
| Cited2 null | 3 mg/kg i.p. 0–14 dpc | (Barbera and others, 2002) |
| Cd; Lrp6 gain of function | 10 mg/kg chow Diet | (Carter and others, 2005) |
| Folr1 null | 25 mg/kg/d gavage | (Piedrahita and others, 1999) |
| RFC1 reduced folate carrier null | 50 mg/kg sc./d 0–18 dpc | (Gelineau-van Waes and others, 2008) |
| Sp2H; Pax3 Loss of function | 200 ug/ml in culture | (Fleming and Copp, 1998) |
| Sp; Pax3 | 200 mg/kg chow Diet | (Wlodarczyk and others, 2006) |
| Folate Unresponsive NTD Mutant Mice | ||
|---|---|---|
| Gene | Folate dose/route | Reference |
| Axd mutant | 33 mg/kg i.p, 8–10 dpc | (Essien and Wannberg, 1993) |
| Fkbp8 null | 40 mg/kg Gavage 0–18.5 dpc | (Wong and others, 2008) |
| ct; Grhl3 loss of function | 60 mg/kg folinic a. i.p. @ 9 dpc | (Seller, 1994) |
| Grhl3 null | as per Seller, 1994 | (Ting and others, 2003) |
| SELH/Bc strain | 10 mg/kg chow Diet | (Harris and Juriloff, 2005) |
| Efna5 null | Data not shown | (Holmberg and others, 2000) |
| Map3k4 null | 25 mg/kg i.p./d, 6.5–9.5 dpc | (Chi and others, 2005) |
| Nog | 10 mg/kg i.p./d, 7.5–9.5 dpc | (Stottmann and others, 2006) |
| Ski null | 10 mg/kg chow Diet | (Ernest and others, 2006) |
i.p., intraperitoneal; sc., subcutaneous; d, day; dpc, days postcoitum
Crooked tail (Cd)
This naturally occurring mutation arose first in the A/J strain as a semidominant allele on mouse chromosome 6 (Lane, 1972; Morgan, 1954). Heterozygous Cd mice display a crooked tail while homozygous mice express phenotypes including early embryonic lethality, exencephaly or viable neonates that are runted with more severe skeletal deformity confined to tail and thoracolumbar spinal column. These skeletal deformities are characteristic of a condition recognized as the Wiebel-Rippen syndrome, placing Cd in a group of mouse mutations including Malformed vertebrae, pudgy (mutated in delta-like 3Dll3, (Kusumi and others, 1998)), Rachiterata, Rib fusions, Rib-vertebrae and Fused (mutated in Axin (Zeng and others, 1997)) (Theiler, 1988). More recently, this group was expanded to include Ringelschwanz (Lrp6 hypomorph) and doubleridge (Dkk1 hypomorph) (Kokubu and others, 2004; MacDonald and others, 2004).
Mapping of Cd to a 0.2 cM region of chromosome 6 enabled the assessment of prenatal dietary folate supplementation effects on birth defect rates in this line, which proved to parallel human anencephaly responses in a number of respects (Carter and others, 1999). First, NTDs and other defects in Cd embryos were incompletely penetrant, as exencephaly occurred in roughly 30% of Cd/Cd embryos, and heterozygous offspring demonstrated variable rates of the dominant crooked tail phenotype, depending on the strain background carrying the Cd allele (Carter and others, 1999). This was consistent with the clinical experience that recurrence risks in families with one NTD affected offspring almost never approach the Mendelian distribution of an autosomal recessive trait (Elwood and others, 1992). Second, NTD rates in Cd/Cd embryos displayed a gender bias, affecting more females by 3 to 1, similar to the gender bias observed in human studies (Elwood and others, 1992). Third, folate supplementation reduced NTD incidence by as much as 55%, lowering rates in females to equal those of males. Importantly, the rescue effect occurred even though Cd dams were not inherently folate deficient, since baseline and supplemented red blood cell folate levels were indistinguishable from wild type mice.
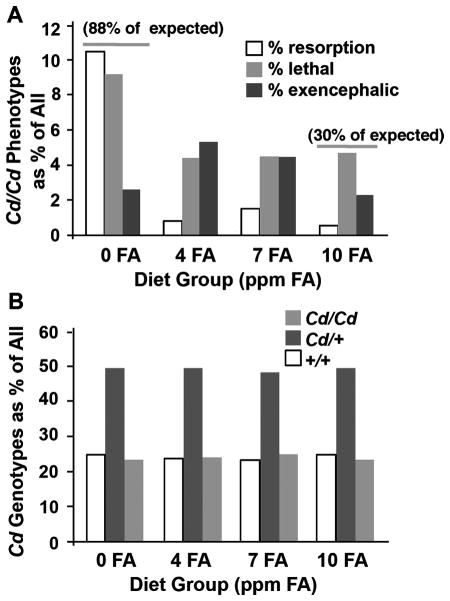
Among the controversies aired in the clinical literature, is the question of how added folate may reduce the incidence of birth defects--that is, could folate reduce the apparent incidence of defects by hastening fetal loss (Klein, 1996). Dietary folic acid supplementation of Cd mice, at near-physiological doses, affected a true rescue of NTDs (Fig. 1) (Carter and others, 1999). Not only was the NTD incidence lowered by folate supplementation, but also embryo viability was increased. Importantly, when all observations were scored and genotyped, a Mendelian distribution was obtained (1:2:1; +/+:Cd/+:Cd/Cd), indicating that pre-implantation loss of mutant embryos was not induced by folate supplementation (Fig. 1B).
Figure 1.
Dietary folic acid supplementation produces a true rescue of Cd/Cd embryos. (A) Distribution of embryonic phenotypes as a percentage of all embryos scored at E12.5 from dams maintained on diets containing defined amounts of folic acid (FA) (parts per million, ppm = mg/kg chow). On a 0 ppm FA diet, homozygous phenotypes accounted for 22%, nearly all of the expected 25% for a Mendelian distribution of an autosomal recessive mutation. However on a 10 ppm FA diet, 7.5% displayed embryopathy (30% of the expected 25%). Compared to embryos on a 4 ppm FA diet, the exencephaly encountered on a 10 ppm FA diet was reduced more than half. (B) Cd/Cd embryos are not lost prior to implantation. The genotype distribution observed for E12.5 embryos maintained on defined FA diets showed the expected Mendelian pattern of 1:2:1 for a heterozygous parental cross. Data from Carter et al., 1999
Positional cloning identified Cd as a missense mutation, substituting an aspartate amino acid in place of a highly conserved glycine in the second extracellular propeller domain of Lrp6 (Carter and others, 2005). A member of the low density lipoprotein receptor (LDLR) family, Lrp6, like its close cousin Lrp5, is a single-pass transmembrane protein and an obligate co-receptor with Frizzled that is required for canonical Wnt signaling (He and others, 2004; Logan and Nusse, 2004; Schweizer and Varmus, 2003). This connection of NTD with Lrp6 is of particular interest, since unlike other Wnt pathway genes associated with neurulation, Lrp5/6 are thought to be synonymous with the canonical pathway. Other Wnt-related mutations associated with NTD have been shown to impair neurulation through the non-canonical Wnt pathway and planar cell polarity (PCP) signaling. These include Frizzled 3 and 6 (Wang and others, 2006b), and Disheveled 2 (Wang and others, 2006a), which genetically interact with the loop tail gene, Vangl 2. Those NTD-associated Wnt pathway gene mutations that have yet to be mechanistically studied are known to have multiple roles, both in canonical and non-canonical Wnt pathways. The Lrp6Cd mutation indicates that either canonical Wnt signaling also has a role in neural tube closure or Lrp6 function also has an as yet unrecognized impact on the non-canonical Wnt pathway and this will be an important issue to resolve.
The Lrp6Cd allele provides a first link between folate metabolic status and a major developmental signaling pathway. However, the mechanism of folate rescue from NTD and other birth defects in Cd mice is not yet known. Array data analysis of folate gene expression and metabolite levels in tissues obtained from wild type and mutant Cd mice indicate an intracellular folate utilization defect due to the mutation (Ernest and others, 2006). One significant step toward mechanistic understanding will be the determination of folate effects on other Lrp6 mutants. A gene trap mutation inactivating Lrp6 is associated in null embryos with severe defects of limbs, truncation of the caudal body axis and NTDs including exencephaly and spina bifida (Pinson and others, 2000). In contrast, the Ringelschwanz mouse that carries a hypomorphic point mutation in Lrp6 displays lumbosacral bone abnormalities and spina bifida, but apparently not exencephaly (Kokubu and others, 2004). If NTDs in other Lrp6 mutants (Lrp6 nulls or Ringelschwanz mice) are also sensitive to folate status, then the effect of the vitamin must be due to a direct interaction of folate metabolism with Lrp6 function and not to an idiosyncratic interaction with the Cd mutation. Similarly, establishing whether NTDs associated with the Disheveled 1/2 (Hamblet and others, 2002) mouse lines respond to folate supplementation would help to determine whether the folate metabolic pathway impacts Lrp5/6 function specifically or Wnt signaling more globally, downstream of the receptor. Regardless of whether the folate interaction is unique to Cd or relates to Lrp6 action, it will be important to investigate how the Cd mutation leads to impaired intracellular folate utilization.
Axial defects (Axd)
NTD-prone mutants found to be unresponsive to folic acid may also be highly informative. Spina bifida occurs in some 30% of Axd/+ mice and 100% of homozygotes. While folic acid or B12 supplementation did not affect occurrence rates, high doses of methionine given on dpc 8&9 reduced NTD rates 40–47% (Essien and Wannberg, 1993). An essential amino acid, methionine is required in the folate metabolic pathway for the production of the body’s principal methyl donor, S-adenosyl methionine. The lack of response in Axd to folate, yet significant reduction in its NTD rate by methionine, implies the presence of a metabolic block in this mutant line that can be circumvented by high doses of methionine. Thus, there are likely other agents feeding into folate/homocysteine metabolism that will be effective in preventing NTDs, depending on the individual mutation and genetic background of the individual.
Possible Mechanisms Affecting Folate-dependent NTD Prevention
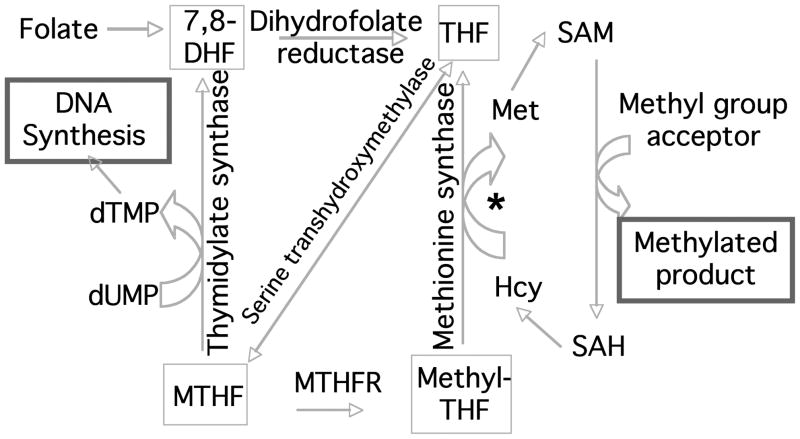
The folate metabolic pathway encompasses a multi-faceted series of reactions that regulate one carbon metabolism (OCM) through the formation and utilization of formate and tetrahydrofolate (THF), enzymatically converting THF to different oxidation states from formyl-to methylated versions (Beaudin and Stover, 2007; Stover, 2004). The two major arms of the pathway lead to nucleotide biosynthesis through thymidylate and to methylation reactions through the methyl-donor S-adenosyl methionine (Fig. 2). It is likely that the success of folic acid supplementation in the face of the complex genetic underpinnings of NTDs is due to the diversity of pathways to which folate contributes. Connections between NTDs and both arms of the folate metabolic pathway have been uncovered with the aid of mouse mutant models.
Figure 2.
Summary of folate-homocysteine metabolism. The primary outputs of the pathway are to 1. support proliferation and DNA repair by the synthesis of nucleotides thymidine via thymidylate synthase (and purines from 10-Formyl THF); 2. support methylation of DNA, RNA, proteins and lipids through the major methyl donor, S-adenosyl methionine (SAM). In this part of the cycle, Hcy is converted to Met by methionine synthase, gaining a methyl group from methyl-THF. * In some tissues, Hcy can also be converted to Met from betaine through conversion of choline to betaine and transfer of a methyl group catalyzed by betaine-homocysteine methyltransferase. DHF=dihydrofolate, THF=tetrahydrofolate, MTHF=methyltetrahydrofolate, Hcy=homocysteine, Met=methionine, SAH=S-adenosyl homocysteine, dUMP=deoxyuridine monophosphate, dTMP=deoxythymidine monophosphate, MTHFR=methyltetrahydrofolate reductase.
Nucleotide Biosynthesis
A principal arm of the folate metabolic pathway contributes to purine and thymidine biosynthesis. Reduction in nucleotide availability can impact DNA replication and repair and decrease mitotic rates during critical points in morphogenesis. (Keller-Peck and Mullen, 1997). Particularly important is the increased incorporation of Uracil into DNA in face of thymidylate deficiency, impacting DNA replication and repair, thereby reducing proliferation and promoting genomic instability (Andersen and others, 2005; Courtemanche and others, 2004; Mashiyama and others, 2004).
Pax3/Splotch
Mutations in the Pax3 transcription factor lead to deficits in neural crest-derived cells in heterozygous mice, giving them the white patches of the ‘splotch’ coat color, and to either exencephaly or spina bifida in homozygous Splotch (Sp/Sp) mice (Epstein and others, 1993; Vogan and others, 1993). The NTD phenotypes can be rescued by folate supplementation in culture and in utero (Fleming and Copp, 1998; Wlodarczyk and others, 2006). Using the deoxyuridine (dU) suppression assay, the ability of Sp/Sp embryos to synthesize thymidylate was shown to be impaired (Fleming and Copp, 1998). In addition, either folic acid or thymidine added to culture media could prevent NTD and relieve the metabolic block in homozygous embryos, but addition of methionine exacerbated the metabolic defect, inducing NTD in 50% of heterozygous Sp/+ embryos (Fleming and Copp, 1998). Thus, rescue of SpPax3 mutants from NTDs is associated with that segment of the folate/homocysteine metabolic pathway that supports nucleotide biosynthesis, while in contrast, tipping the balance toward the methylation arm of the pathway exacerbates embryopathy.
Connection of Pax3 loss of function with a folate metabolism defect that impacts nucleotide biosynthesis suggests that the rescue of NTD in this case may relate to folate effects on cell proliferation. Indeed, proliferation defects have been observed in Sp mutant embryos (Keller-Peck and Mullen, 1997). A recent examination of the Sp2H mutant line has shown that folate deficiency increases NTD rates in mutant, but not in wild type, embryos (Burren and others, 2008). This is associated with reduced growth rates in the mutants, especially in female Sp2H embryos. At the same time, no changes in global DNA methylation were found in that study, leading those investigators to hypothesize that the cellular defect in Sp2H mice is at least in part compensated for by FA supplementation that restores a folate metabolic pathway block, normalizes nucleotide biosynthesis and supports intrauterine growth (Burren and others, 2008). Nevertheless, this study does not rule out the possibility of methylation effects on specific key genes that would not be detected in this experimental design.
Studies of Splotch mutants support the contention that there is a critical balance between the nucleotide biosynthesis and the methionine biosynthesis/methylation arms of OCM that must be maintained to promote successful neural tube closure (Beaudin and Stover, 2007; Stover, 2006). There is some evidence that these insights from mouse models can be translated into clinical relevance. Impaired thymidylate biosynthesis has recently been reported in a subset of human NTD-affected embryos, assessed using a dU suppression assay to screen primary fibroblast cells derived from human fetuses affected by NTD (Dunlevy and others, 2007). It will be of considerable interest whether dU suppression will prove abnormal in fibroblasts derived from mothers of those affected fetuses with thymidylate biosynthesis abnormalities.
An intriguing question with regard to folate rescue of NTD is whether a flaw or at least partial impairment within the folate metabolic pathway is necessary in order for FA supplementation to have a protective effect on neurulation. For example, might it be possible for FA to rescue neurulation by virtue of enhancing cell proliferation, in effect giving mutant embryos a “boost” without directly addressing the underlying genetic functional defect? While certainly possible, this has yet to be demonstrated. The observation that folate deficiency had no impact on intrauterine growth rate or neurulation in wild type embryos (Burren and others, 2008) suggests that added FA would have an impact via this mechanism only if a cell division defect were part of the neurulation failure. On the other hand, clearly not all NTD mutants expressing a proliferation defect will respond to folate. For example, the ct mutant suffers NTD attributed to reduced proliferation in the hindgut, notochord and caudal tailbud, but ct is insensitive to FA supplementation (van Straaten and Copp, 2001). At this early stage, the limited data available from NTD mutants like SpPax3, CdLrp6 and ctGrhl3 seems to support that neurulation sensitivity to supplementation requires some inefficiency of folate intracellular uptake or utilization in the embryo, or possibly a maternal component of OCM homeostasis.
Methylation Cycle
There are multiple indications that methylation—especially of DNA or histones—is a robust contributor to neurulation. Knockout in the mouse of the DNA methyltransferase, Dnmt3b, produces NTDs and distortions of the neural tube (Okano and others, 1999). In fact, no fewer than eight mutations in genes involved in DNA methylation or in regulating chromatin structure produce neurulation defects, including Cerc2, Dnmt3b, Dnmt3l, Gtf2i, Hdac4, Sirt1, Smarca4 and Smarcc1 (Cheng and others, 2003; Harris and Juriloff, 2007; Okano and others, 1999; Vega and others, 2004). Variant alleles of methylenetetrahydrofolate reductase (MTHFR) have been associated with increased NTD risk in some but not all clinical populations studied (Boyles and others, 2005). This enzyme is a hub in the pathway and diverts OCM toward methylation reactions at the expense of purine and thymidine biosynthesis. The MTHFR 677 T/T genotype has been associated with a global reduction in DNA methylation (Castro and others, 2004; Friso and others, 2002). Experiments in chick and mouse embryos indicate that exposure to homocysteine or inhibitors of the methylation cycle delayed neural tube closure in a dose-dependent manner (Afman and others, 2005; Dunlevy and others, 2006). There remains some controversy in the literature as to whether folic acid supplementation results in hyper- or hypomethylation of DNA globally, whether the most important effects for neurulation result from the methylation status of specific target genes, or whether the methylation of histone proteins to influence chromatin structure is the key event (Waterland, 2006). Certainly it would be of interest, in the eight or more mutants affecting DNA, chromatin or histone methylation, to determine whether their NTDs are prevented by folate supplementation. However, this may not be relevant if the ability to respond to folate by increasing DNA/histone methylation is substantially precluded by the mutation. The more pertinent question is whether the expression of methylation-sensitive genes contributing to NTDs will be favorably modulated by folate supplementation.
Although much attention has focused on the role of DNA/chromatin modification in NTDs, it must be recognized that methylation is an important modulator of other molecules including RNA, proteins, lipids and even neurotransmitters. Indeed, neurulation defects were induced in wild type rat embryos when cultured in reduced levels of methionine (Coelho and Klein, 1990). Methionine deficiency was associated with a failure of the neural folds to turn medially, suggesting a deficit in ‘microfilaments’ and diminished cytoskeletal contractility. The authors’ hypothesis was supported by measured reductions in microfilament-associated methylated amino acids (Coelho and Klein, 1990). It is likely that one or more of the several recognized possible folate-related mechanisms contribute to NTD prevention in a particular genetic context.
A sobering insight regarding folate supplementation is that its effects on methylation can result in sustained alteration of gene expression, as demonstrated by the AVY mouse (Dolinoy and others, 2007; Dolinoy and others, 2006; Waterland, 2006; Wolff and others, 1998). Coat color in this inbred mouse line is determined by the agouti locus and hypomethylation of CpGs in its transcription unit shifts the adult coat toward yellow. Maternal supplementation with methyl donor precursors folate, choline and betaine leads to hypermethylation of the AVY locus and shifts coat color from yellow to pseudo-agouti (Wolff and others, 1998). Intrauterine exposure to bisphenol A (BPA) leads to hypomethylation at the AVY locus and this is prevented by either folate supplementation or treatment with the phytoestrogen, genistein. Importantly, these effects on CpG methylation at the AVY locus created a permanent alteration in the individual epigenome. Moreover, varying the methyl donor sources folate, methionine and choline in a post-weaning diet can permanently influence the imprinting of insulin like growth factor 2 (Igf2) (Waterland and others, 2006). Thus, effects of folate metabolism can be felt long after periods of supplementation, underscoring the importance of understanding the range of possible effects of folate, the dose dependence of those effects and likely outcome for an individual genotype.
CONCLUDING REMARKS
The examples provided by Cd, Axd, Folb1, RFC1, Pax3 and several other mutations in mice suggest that, with knowledge of the maternal-fetal genetic background, it is within our reach to target particular aspects of the folate metabolic pathway and so optimize NTD prevention for families using individually tailored supplement regimens. The gene expression and metabolite ‘signatures’ indicating NTD risk and likely effective prevention for a particular profile will be best identified in mouse models, followed by searches for parallels in human subjects. It may indeed now be time to revisit metabolic studies in mice to take advantage of the over 155 genetic mutants in hand, with more NTD-associated mutations being identified every day.
In such metabolic studies, it will be important to determine for a particular NTD-associated gene, not only whether the rate of NTDs is reduced by folate supplementation, but also by what mechanism. That is, for a particular genetic profile, does folic acid affect a true rescue and improve embryo viability or enhance embryonic lethality? Does folate alter proliferation in the neural tube or in surrounding mesenchyme? Does it alter convergence-extension during neurulation or is there another agent that does a better job preventing NTDs due to defects in planar cell polarity (PCP) pathways? What does folate supplementation do to the gene expression pattern of that particular allele and genetic background? Does folate impact post-translational regulation of protein function during neurulation? How much supplementation is enough—where is the boundary defining too much of a good thing? Indeed, there may be a level at which folate supplementation becomes counterproductive. For example, dietary supplementation of Sp mice with very high levels of folate (200 mg/kg chow, or 20 times higher folate content than in other dietary supplementation studies) resulted in a 30% reduction in NTDs in homozygous embryos, but also a six-fold increase in the number of resorptions (Wlodarczyk and others, 2006). However, the level of supplementation required is likely to vary according to the mutation involved. Certainly, very high folate levels are required to ameliorate the Folbp1 or RFC1 knockouts (Gelineau-van Waes and others, 2008). Only when we have these more complete datasets will therapeutically useful parallels be obtainable in clinical populations.
Over decades of study in animal models and in humans, it is now clear that folate deficiency has significant deleterious effects on embryonic and fetal development. There is also compelling evidence that folate supplementation can, in the right genetic setting, exert a decided rescue from neurulation failure and other serious birth defects. In addition, supplementation can bring measurable benefits even in the absence of folate deficiency. However, more must be understood regarding the intracellular folate levels that will be optimal for a particular genetic mutation and background, in order to avoid unintended consequences and achieve better individual therapeutic advantage over population-directed strategies for supplementation (Pitkin, 2007; Smith and others, 2008; Stover, 2004; Stover and Garza, 2002).
More than 20 years have elapsed since Richard Smithells and colleagues blazed the trail for folic acid supplementation to prevent NTDs, and significant reductions in incidence have been achieved. Nevertheless many questions remain and a new era is emerging in which focus will shift away from populations at risk for NTDs to individual couples. The field has made breathtaking advances over the last two decades, though much remains to be understood. With accurate assessment of the relative contributions from specific genes, expression patterns of genetic networks, and the role of epigenetic and environmental factors, individually tailored strategies for NTD prevention will become feasible. It all began with the connection between a small molecule and a devastating malformation. To even think that vitamin supplementation could reduce the occurrence of birth defects was a bold stroke, for which much is owed to Dr. Smithells.
Acknowledgments
Supported by NRSA NS059562 to JDG and the WCMC-THMRI initiative to MER
References
- Afman LA, Blom HJ, Drittij MJ, Brouns MR, van Straaten HW. Inhibition of transmethylation disturbs neurulation in chick embryos. Brain Res Dev Brain Res. 2005;158(1–2):59–65. doi: 10.1016/j.devbrainres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen S, Heine T, Sneve R, Konig I, Krokan HE, Epe B, Nilsen H. Incorporation of dUMP into DNA is a major source of spontaneous DNA damage, while excision of uracil is not required for cytotoxicity of fluoropyrimidines in mouse embryonic fibroblasts. Carcinogenesis. 2005;26(3):547–555. doi: 10.1093/carcin/bgh347. [DOI] [PubMed] [Google Scholar]
- Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11(3):283–293. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- Beaudin AE, Stover PJ. Folate-mediated one-carbon metabolism and neural tube defects: balancing genome synthesis and gene expression. Birth Defects Res C Embryo Today. 2007;81(3):183–203. doi: 10.1002/bdrc.20100. [DOI] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nat Rev Neurosci. 2006;7(9):724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles AL, Hammock P, Speer MC. Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135(1):9–23. doi: 10.1002/ajmg.c.30048. [DOI] [PubMed] [Google Scholar]
- Burgoon JM, Selhub J, Nadeau M, Sadler TW. Investigation of the effects of folate deficiency on embryonic development through the establishment of a folate deficient mouse model. Teratology. 2002;65(5):219–227. doi: 10.1002/tera.10040. [DOI] [PubMed] [Google Scholar]
- Burren KA, Savery D, Massa V, Kok RM, Scott JM, Blom HJ, Copp AJ, Greene ND. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Collins JS, Botto LD, Williams LJ, Mai CT, Kirby RS, Pearson K, Devine O, Mulinare J. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defects Res A Clin Mol Teratol. 2005;73(10):679–689. doi: 10.1002/bdra.20210. [DOI] [PubMed] [Google Scholar]
- Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, Campagne F, Weinstein H, Ross ME. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci U S A. 2005;102(36):12843–12848. doi: 10.1073/pnas.0501963102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, Ulrich S, Oofuji Y, Williams DA, Ross ME. Crooked tail (Cd) models human folate-responsive neural tube defects. Hum Mol Genet. 1999;8(12):2199–2204. doi: 10.1093/hmg/8.12.2199. [DOI] [PubMed] [Google Scholar]
- Castro R, Rivera I, Ravasco P, Camilo ME, Jakobs C, Blom HJ, de Almeida IT. 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C-->T and 1298A-->C mutations are associated with DNA hypomethylation. J Med Genet. 2004;41(6):454–458. doi: 10.1136/jmg.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Sarkisian MR, Rakic P, Flavell RA. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc Natl Acad Sci U S A. 2005;102(10):3846–3851. doi: 10.1073/pnas.0500026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CN, Klein NW. Methionine and neural tube closure in cultured rat embryos: morphological and biochemical analyses. Teratology. 1990;42(4):437–451. doi: 10.1002/tera.1420420412. [DOI] [PubMed] [Google Scholar]
- Courtemanche C, Elson-Schwab I, Mashiyama ST, Kerry N, Ames BN. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004;173(5):3186–3192. doi: 10.4049/jimmunol.173.5.3186. [DOI] [PubMed] [Google Scholar]
- Czeizel A, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlevy LP, Burren KA, Mills K, Chitty LS, Copp AJ, Greene ND. Integrity of the methylation cycle is essential for mammalian neural tube closure. Birth Defects Res A Clin Mol Teratol. 2006;76(7):544–552. doi: 10.1002/bdra.20286. [DOI] [PubMed] [Google Scholar]
- Dunlevy LP, Chitty LS, Burren KA, Doudney K, Stojilkovic-Mikic T, Stanier P, Scott R, Copp AJ, Greene ND. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain. 2007;130(Pt 4):1043–1049. doi: 10.1093/brain/awm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood JM, Little J, Elwood JH. Epidemiology and Control of Neural Tube Defects. Oxford: Oxford University Press; 1992. [Google Scholar]
- Epstein DJ, Vogan KJ, Trasler DG, Gros P. A mutation within intron 3 of the Pax-3 gene produces aberrantly spliced mRNA transcripts in the splotch (Sp) mouse mutant. Proc Natl Acad Sci USA. 1993;90:532–536. doi: 10.1073/pnas.90.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S, Carter M, Shao H, Hosack A, Lerner N, Colmenares C, Rosenblatt DS, Pao YH, Ross ME, Nadeau JH. Parallel changes in metabolite and expression profiles in crooked-tail mutant and folate-reduced wild-type mice. Hum Mol Genet. 2006;15(23):3387–3393. doi: 10.1093/hmg/ddl415. [DOI] [PubMed] [Google Scholar]
- Essien FB, Wannberg SL. Methionine but not folinic acid or vitamin B-12 alters the frequency of neural tube defects in Axd mutant mice. J Nutr. 1993;123(1):27–34. doi: 10.1093/jn/123.1.27. [DOI] [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280(5372):2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, Selhub J. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99(8):5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Heller S, Bauer LK, Wilberding J, Maddox JR, Aleman F, Rosenquist TH, Finnell RH. Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation. Birth Defects Res A Clin Mol Teratol. 2008;82(7):494–507. doi: 10.1002/bdra.20453. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129(24):5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Maternal diet alters exencephaly frequency in SELH/Bc strain mouse embryos. Birth Defects Res A Clin Mol Teratol. 2005;73(8):532–540. doi: 10.1002/bdra.20170. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79(3):187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Heid MK, Bills ND, Hinrichs SH, Clifford AJ. Folate deficiency alone does not produce neural tube defects in mice. J Nutr. 1992;122(4):888–894. doi: 10.1093/jn/122.4.888. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408(6809):203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. Jama. 2001;285(23):2981–2986. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Mullen RJ. Altered cell proliferation in the spinal cord of mouse neural tube mutants curly tail and Pax3 splotch-delayed. Brain Res Dev Brain Res. 1997;102(2):177–188. doi: 10.1016/s0165-3806(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Klein NW. Caution advised before rushing to start folic acid supplements. Teratology. 1996;53(6):331. doi: 10.1002/(SICI)1096-9926(199606)53:6<331::AID-TERA1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, Wahl MB, Galceran J, Grosschedl R, Ozono K, Imai K. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. 2004;131(21):5469–5480. doi: 10.1242/dev.01405. [DOI] [PubMed] [Google Scholar]
- Kusumi K, Sun ES, Kerrebrock AW, Bronson RT, Chi DC, Bulotsky MS, Spencer JB, Birren BW, Frankel WN, Lander ES. The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat Genet. 1998;19(3):274–278. doi: 10.1038/961. [DOI] [PubMed] [Google Scholar]
- Lane P. Linkage of Hd, Mi, and Cd. Mouse News Lett. 1972;47:37. [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131(11):2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- Mashiyama ST, Courtemanche C, Elson-Schwab I, Crott J, Lee BL, Ong CN, Fenech M, Ames BN. Uracil in DNA, determined by an improved assay, is increased when deoxynucleosides are added to folate-deficient cultured human lymphocytes. Anal Biochem. 2004;330(1):58–69. doi: 10.1016/j.ab.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Morgan W. A new crooked tail mutation involving distinctive pleiotropism. J Genet. 1954;52:354–373. [Google Scholar]
- MRCV Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23(2):228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85(1):285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4(1):4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seller M. In: Bock G, Marsh J, editors. Vitamins, folic acid and the cause and prevention of neural tube defects; Neural Tube Defects Ciba Foundation Symposium; Chichester: John Wiley & Sons; 1994. pp. 161–179. [DOI] [PubMed] [Google Scholar]
- Seller MJ, Nevin NC. Periconceptional vitamin supplementation and the prevention of neural tube defects in south-east England and Northern Ireland. J Med Genet. 1984;21(5):325–330. doi: 10.1136/jmg.21.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87(3):517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Nevin NC, Seller MJ, Sheppard S, Harris R, Read AP, Fielding DW, Walker S, Schorah CJ, Wild J. Further experience of vitamin supplementation for prevention of neural tube defect recurrences. Lancet. 1983;1(8332):1027–1031. doi: 10.1016/s0140-6736(83)92654-5. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin dificiencies and neural tube defects. Arch Dis Child. 1976;51(12):944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev Biol. 2006;295(2):647–663. doi: 10.1016/j.ydbio.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev. 2004;62(6 Pt 2):S3–12. doi: 10.1111/j.1753-4887.2004.tb00070.x. discussion S13. [DOI] [PubMed] [Google Scholar]
- Stover PJ. Influence of human genetic variation on nutritional requirements. Am J Clin Nutr. 2006;83(2):436S–442S. doi: 10.1093/ajcn/83.2.436S. [DOI] [PubMed] [Google Scholar]
- Stover PJ, Garza C. Bringing individuality to public health recommendations. J Nutr. 2002;132(8 Suppl):2476S–2480S. doi: 10.1093/jn/132.8.2476S. [DOI] [PubMed] [Google Scholar]
- Theiler K. Vertebral malformations. Adv Anat Embryol Cell Biol. 1988;112:1–99. doi: 10.1007/978-3-642-73775-6. [DOI] [PubMed] [Google Scholar]
- Ting SB, Wilanowski T, Auden A, Hall M, Voss AK, Thomas T, Parekh V, Cunningham JM, Jane SM. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat Med. 2003;9(12):1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Copp AJ. Curly tail: a 50-year history of the mouse spina bifida model. Anat Embryol (Berl) 2001;203(4):225–237. doi: 10.1007/s004290100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Vogan KJ, Epstein DJ, Trasler DG, Gros P. The splotch-delayed (Spd) mouse mutant carries a point mutation within the paired box of the Pax-3 gene. Genomics. 1993;17(2):364–369. doi: 10.1006/geno.1993.1333. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Oakley GP. Should folic acid fortification be mandatory? Yes. Bmj. 2007;334(7606):1252. doi: 10.1136/bmj.39232.493252.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006a;133(9):1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006b;26(8):2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA. Assessing the effects of high methionine intake on DNA methylation. J Nutr. 2006;136(6 Suppl):1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. 2006;15(5):705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk BJ, Tang LS, Triplett A, Aleman F, Finnell RH. Spontaneous neural tube defects in splotch mice supplemented with selected micronutrients. Toxicol Appl Pharmacol. 2006;213(1):55–63. doi: 10.1016/j.taap.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. Faseb J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- Wong RL, Wlodarczyk BJ, Min KS, Scott ML, Kartiko S, Yu W, Merriweather MY, Vogel P, Zambrowicz BP, Finnell RH. Mouse Fkbp8 activity is required to inhibit cell death and establish dorso-ventral patterning in the posterior neural tube. Hum Mol Genet. 2008;17(4):587–601. doi: 10.1093/hmg/ddm333. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90(1):181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13(3):275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]