Abstract
Background
HIV-1 and HSV-2 are responsible for two intersecting epidemics in which the disease caused by one virus facilitates transmission and pathogenesis by the other. Therefore, suppression of one virus infection will affect the other. Acyclovir (ACV), a common antiherpetic drug, was shown to directly suppress both viruses in co-infected tissues. However, both antiviral activities of ACV are dependent on phosphorylation by the nucleoside kinase activity of co-infecting herpesviruses.
Methods
We developed monophosphorylated ACV with the phosphate group masked by lipophilic groups to allow efficient cellular uptake (ACV ProTides) and investigated their antiviral potential.
Results
ACV ProTides suppressed both viruses with EC50 values in the sub-micromolar range in ex vivo lymphoid and cervicovaginal human tissues and between 3–12 μM in CD4+ T cells. ACV ProTides retained activity against ACV-resistant HSV-2.
Conclusions
ACV ProTides represent a new class of antivirals that suppress both viruses by directly and independently blocking the key replicative enzymes of HIV-1 and HSV-2. Further optimization of such compounds may lead to double-targeted antivirals able to prevent viral transmission and to treat the two synergistic diseases caused by HIV-1 and HSV-2. The ACV ProTides represent the first example of acyclic nucleosides being active against HIV.
Keywords: acyclovir ProTides, HIV-1, HSV-2, reverse transcriptase, tonsils
INTRODUCTION
HIV-1 infection is commonly associated with infection with other (co)pathogens that greatly affect HIV transmission and HIV disease progression [1–4]. HSV-2, one of the most common HIV-1 copathogens, establishes with HIV-1 a vicious circle in which each virus facilitates the replication, shedding, and acquisition of the other [2, 5, 6]. Epidemiological studies have demonstrated that HIV-1 and HSV-2 are responsible for two epidemics that, by overlapping in risk populations, reinforce the spread of both HIV-1 disease and genital herpes [1]. In particular, HSV-2 may have contributed substantially to the spreading of HIV-1 in Africa, where genital herpes has been estimated to account for approximately 30% of HIV infections [7, 8].
We recently discovered that the antiherpetic drug acyclovir (ACV), a well known inhibitor of herpesviral DNA polymerase [9], is also an HIV-1 reverse transcriptase inhibitor after being intracellularly converted to its 5′-triphosphate derivative. We demonstrated that ACV inhibits HIV in various human tissues coinfected with herpesviruses (HHVs) [10]. However, the anti-HIV activity of ACV is limited by its dependence on ACV phosphorylation by coinfecting HHV-encoded kinases.
Here, we report on ACV monophosphate-based prodrugs, ACV ProTides, whose anti-HIV activities are independent of HHV coinfection. We demonstrate that ACV ProTides inhibit replication of HSV-2 and HIV-1 in T cell lines. Also, ACV ProTides suppress these viruses in various human tissues ex vivo infected by either of these viruses alone or in combination. Moreover, ACV ProTides suppressed HSV-2 variants that are resistant to ACV because of their lack of thymidine kinase activity, demonstrating the effective intracellular delivery of the free ACV monophosphate.
Thus, the ACV ProTides reported here constitute a new class of antivirals that uniquely combine two distinct anti-HIV strategies: direct suppression of HIV RT and indirect effect through inhibition of HSV-2, a virus known to facilitate HIV infection. Importantly, they represent the first acyclic nucleosides to inhibit HIV RT, probing new structural space in this key viral enzyme and drug target.
METHODS
Cell and human tissue culture
Human T-lymphocyte MT-4 and CEM cell lines (American Type Culture Collection (Manassas, VA)) were cultured in RPMI-1640 medium with 10% fetal bovine serum. PBMC from healthy donors were stimulated with PHA at 2 μg/ml (Sigma, Bornem, Belgium) for 3 days at 37°C and then seeded at 0.5 × 106 cells per well into a 48-well plate in cell culture medium (RPMI 1640) containing 10% FCS and IL-2 (25 U/ml, R&D Systems Europe, Abingdon, UK). Tonsillar tissues from routine surgery were obtained from the Children’s National Medical Center, Washington, DC. Cervicovaginal tissues were obtained from routine hysterectomy through the National Disease Research Interchange (Philadelphia, PA) according to IRB-approved protocols. Tissues were dissected into 2-mm3 blocks and placed onto collagen sponge gels at the air-liquid interface and cultured as described earlier [11].
ProTides synthesis
Viral infection and antiviral assays
Tissue blocks were inoculated with X4LAI.04 or R5BaL HIV-1 (Rush University Virology Quality Assurance Laboratory, Chicago, IL) as described [11, 13]. 105 MT-4 cells were inoculated with 10μL (0.7ng/mL of p24) of X4LAI.04 and cultured for 3 days. HIV replication was evaluated by p24gag antigen release in MT-4 cell culture medium or in pooled medium bathing 27 tissue blocks using a bead-based assay [10]. CEM cells (4.5 × 105 cells/mL) were suspended in fresh culture medium and infected with HIV-1 at 100 CCID50 per ml of cell suspension and viral replication was evaluated by giant cell formation 4 to 5 days later. PBMCs were infected with HIV-1 isolates of different clades kindly provided by Dr. L. Lathey (BBI Biotech Research Laboratories, Inc., Gaithersburg, MD) (final dose of 250pg p24 or p27/ml). For HIV-2 p27 Ag detection the INNOTEST from Innogenetics (Temse, Belgium) was used.
HSV-2 strain G (ATCC) or a clinical ACV-resistant HSV-2 isolate [14] was inoculated in tonsils (27 tissue blocks) in five microliters (1.4 TCID50). Replication was evaluated by real-time PCR [10].
Antiviral activities of ACV ProTides were investigated in cell lines and human tissues ex vivo. MT4 cells were pre-incubated with ACV (Bedford Laboratories, Bedford, OH), ACV-MP, or ACV-TP (Moravek Biochemicals and Radiochemicals, CA) at 100μM overnight suspended in fresh medium containing various concentrations of compounds and infected with HIV-1. To test the anti HIV activity of ACV ProTides, HIV-1 infected MT-4, CEM cells, PBMC or human tissue blocks were incubated with test compounds and viral replication in treated and untreated cells was evaluated. EC50 presented are means ± SEM of compound’s concentration required to suppress viral replication by 50%. The EC50 was calculated by fitting the data points to a sigmoidal dose-response curve using GraphPad Prism (Version 4.0).
Viability assays
Viability assays were performed in the MT-4 cell cultures with the Nucleocounter automated cell counting system (Chemometec, Denmark). Number of total and dead cells in cultures untreated and treated with ACV ProTides was enumerated using a propidium iodide-based assay according to the manufacturer protocol. Data were collected and analyzed using Nucleoview software (Chemometec, Denmark).
Flow-cytometry
To assess ACV ProTides’ cytotoxicity in human tonsillar tissues after 12 days of culture, cells isolated from tissue blocks treated or not with ACV ProTide were stained for surface markers. Cells were stained with combinations of the following fluorescence-labeled monoclonal antibodies: anti-CD3-QD655, anti-CD4-QD605, anti-CD8-PacificBlue, anti-CD45RA-PE-Cy7, anti-CCR7-APC-Cy7, and anti-Ki67-PE antibodies (Caltag laboratories, Burlingame, CA). Detection and enumeration of HIV-1 infected cells were performed by intracellular staining using anti-p24-PE (Beckman Coulter, Miami, FL). Data were acquired and analyzed as described [10]. Cell depletion was quantified using Trucount™ beads (Becton Dickinson) for volumetric control and cell numbers were normalized by tissue-block weights.
RESULTS
Treatment of HIV-1 infected cell lines with phosphorylated ACV
As an initial attempt to overcome ACV dependency on HHV-mediated phosphorylation, we evaluated the ability of already phosphorylated ACV (monophosphorylated ACV-MP or triphosphorylated ACV-TP) to inhibit HIV-1 replication. Treatment of HIV-1LAI.04-infected MT-4 cell cultures with ACV-MP or ACV-TP suppressed HIV replication, albeit with low potency: EC50s for ACV-MP and ACV-TP were respectively 100 ± 11 and 58 ± 2.6μM, while EC50 for ACV itself was more than 250μM. Likewise, ACV did not efficiently suppress viral replication in HIV-1IIIB- or HIV-2ROD-infected CEM cells (data not shown). To improve the potency of phosphorylated forms of ACV in inhibiting HIV-1 replication, we designed phosphorylated prodrugs of ACV where the phosphate moiety is protected by lipophilic groups (ACV Pro-Tides).
ACV ProTides efficiently inhibit HIV-1 in CD4+ T cell lines
We designed a variety of ProTides based on the structure of ACV-MP and investigated their anti HIV-1 activities. In these compounds, the phosphate group is attached to the ACV core and is protected from cleavage by extracellular enzymes by lipophilic aryl and aminoacyl ester groups [15] (Fig. 1). For the current work, we chose four ACV ProTides, designated as Cf2648, Cf2649, Cf2676 and Cf2681 (Table 1). All tested ACV ProTides efficiently inhibited dose-dependently HIV-1 replication in MT-4 and CEM cell cultures. Their EC50s varied from 1.7 to 12 μM (Table 2).
Figure 1. Generic structure of ACV ProTides.
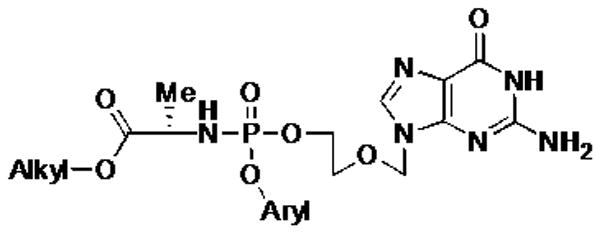
ACV ProTides consist of monophosphorylated ACV and of aryloxy and aminoacyl ester groups linked to the phosphate moiety.
Table 1.
ACV ProTides variants
| Compound | Aryl | Ester | Amino acid | ClogP |
|---|---|---|---|---|
| Cf2648 | Ph | Bn | L-Ala | 0.89 |
| Cf2649 | 1-Nap | Me | L-Ala | 0.35 |
| Cf2676 | 1-Nap | Et | L-Ala | 0.88 |
| Cf2681 | 1-Nap | isoProp | L-Ala | 1.19 |
The aryl groups represent a 1-naphthyl or phenyl while the aminoacyl ester group could be a benzyl or alkyl (Me, Et, iPr) group. The amino acid in the four ProTides used in this study was L-alanine. Compounds were synthesized as described in supplemental data. The ClogP is a measure of hydrophilicity: ClogP = log (coctanol/cwater) where coctanol and cwater are concentrations of a compound portioned in the two-phase system
Table 2.
Inhibitory activity of ACV ProTides against HIV-1 in CD4+ T cell cultures.
| Compound | EC50 (μM)a (MT-4) | EC50 (μM)a (CEM) | CC50 (μM)b (MT-4) | CC50 (μM)b (CEM) | IC50 (μM)c (MT-4) | IC50 (μM)c (CEM) | SId |
|---|---|---|---|---|---|---|---|
| ACV | > 250 | > 250 | > 150 | > 150 | > 150 | > 250 | NA* |
| Cf2648 | 5.7 ± 1.6 | 6.4 ± 5.8 | > 150 | > 150 | 34 ± 11 | 33 ± 7.1 | > 26 |
| Cf2649 | 4.7 ± 2.1 | 10 ± 7.9 | > 150 | > 150 | 19 ± 3.2 | 57 ± 45 | > 32 |
| Cf2676 | 1.7 ± 0.8 | 12 ± 9.8 | > 150 | > 150 | 12 ± 5.3 | 32 ± 7.8 | > 88 |
| Cf2681 | 5.4 | 6.2 ± 5.4 | > 150 | > 150 | 73 | 36 ± 15 | > 28 |
EC50, effective concentration that inhibits 50% of HIV-1 replication.
CC50, concentration that reduce the viability of the MT-4 cells by 50%.
IC50, concentration that inhibit MT-4 or CEM cell growth by 50%.
SI, selective index or ratio of CC50 (MT-4)/EC50.
Values were calculated for ACV, Cf2648, Cf2649, Cf2676 and Cf2681 from respectively 3, 5, 3, 3 and 2 independent experiments.
Not Applicable
The concentrations of ProTides that killed 50% of the MT-4 cells (CC50) were greater than 150 μM (Table 2). Nonetheless, ProTides Cf2676, Cf2649, Cf2648 and Cf2681 reduced cell proliferation by 50% (IC50) at the concentrations of 12, 19, 34, and 73μM respectively. To study the reversibility of the cytostatic effect, we treated cell cultures with ProTide Cf2648 at 50μM or 150μM for 3 days and then washed the drug away. Three days after Cf2648 removal, the number of cells in these cultures had increased 3-fold, an increase similar to that in non-treated cultures (2.5 fold). Thus, ACV ProTides suppressed HIV-1 replication at concentrations that do not significantly decrease cell viability while at higher concentrations they reversibly slow down cell growth.
ACV ProTides inhibit HIV-1 replication in PBMCs and in human tissues
As shown in Table 3, in PBMC ACV ProTides Cf2648 and Cf2649 efficiently inhibited HIV-1 variants of subtypes A, B, C, D and A/E, including R5 and X4 variants. Furthermore, we investigated the anti-HIV ProTide activity in an in vivo-like system of human lymphoid and cervicovaginal tissue explants. This system retains tissue cytoarchitecture and supports HIV replication without exogenous activation [11, 13, 16, 17]. Blocks of human tonsillar tissue were treated with ProTides overnight and infected with a prototypical X4 HIV-1 (HIV-1LAI.04). ACV ProTides were present during the entire culture period. The absolute HIV replication level in tissues from different donors varied from 3.1 to 15.8 ng/mL. The EC50 for ACV ProTides Cf2648 and Cf2649 were respectively 1 ± 0.4μM (n=4) and 0.14 ± 0.03μM (n=5) (Fig. 2A). Next, we compared the numbers of HIV-infected cells in tissues treated and non-treated with ProTide Cf2649. On day 12 post infection, using flow-cytometry, we evaluated the fraction of p24+CD8− T cells in ProTide-treated and control tissues (Fig. 2B). This fraction includes CD4+ cells that downregulated CD4 because of HIV infection [18]. On average, 3.16 ± 1.63% of CD8− T cells were HIV-infected in ProTide-treated cultures whereas in control donor-matched culture these cells constituted 11.71 ± 3.87% of total CD8− T cells (n=4; P<0.05)
Table 3.
Inhibitory activity of ACV ProTides against HIV-1 strains and clinical isolates in PBMC cultures.
| EC50a (μM) | CC50b (μM) | SIc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NL4.3 | BaL | UG275 | US 3 | ETH 2220 | UG 270 | ID12 | |||
| X4 | R5 | R5 (clade A) | R5 (clade B) | R5 (clade C) | X4 (clade D) | R5 (clade A/E) | |||
| Cf 2648 | 13 ± 3.8 | 6.6 ± 1.4 | 25 ± 10 | 28 ± 12 | 19 ± 14 | 55 ± 40 | 16 ± 15 | 319 | 14 |
| Cf 2649 | 11 ± 3.1 | 5.2 ± 2.4 | 16 ± 11 | 4.9 ± 6.1 | 6.8 ± 3.9 | 10 ± 12 | 53 ± 4.4 | 352 | 23 |
50% Effective concentration (μM) required to inhibit p24 production in virus-infected PBMC.
50% Cytostatic concentration (μM) as measured by the MTS viability staining method.
SI, selectivity index or ratio of CC50/EC50. For the EC50, the average EC50 values for all virus strains together were taken (i.e. 23.2 μM for Cf2648 and 15.3 μM for Cf2649).
Figure 2. ACV ProTides inhibit HIV-1 replication in human lymphoid tissues.
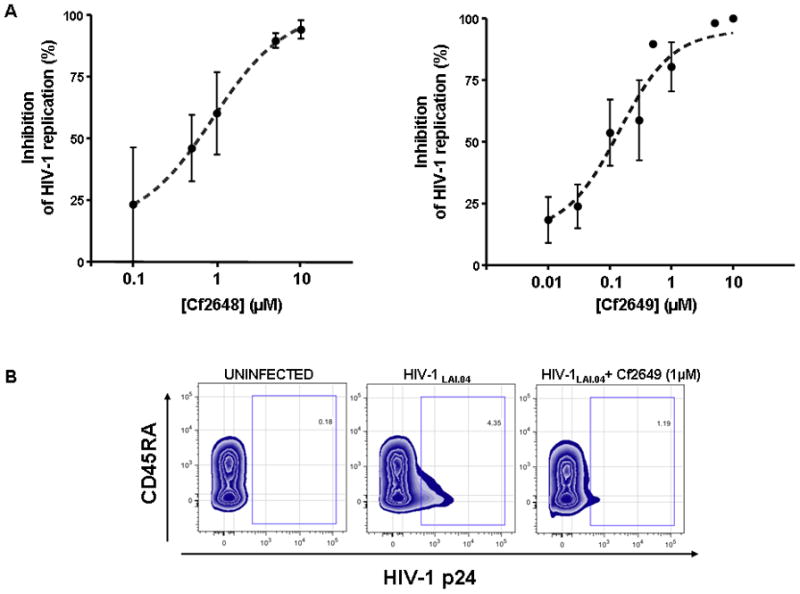
Human tonsillar tissues (27 blocks of tissue from each of n donors for each experimental condition) were infected with HIV-1LAI.04 and treated with ACV ProTide Cf2648 or Cf2649 at various concentrations. Anti-HIV-1 activities of ProTides were evaluated by comparing viral replication in drug-treated and untreated donor-matched tissues.
A. Presented is HIV inhibition at different concentrations of ProTides as defined by the following formula: Inhibition = (1 − RProTide/Rcontr)×100% where RProTide and Rcontr are the amounts of p24 accumulated in the medium over the 12 day-culture period in ProTide-treated and donor-matched untreated cultures, respectively. The 50% effective concentration for each ACV ProTide (EC50) was estimated. EC50 for Cf2648 and Cf2649 are respectively 1 ± 0.4μM and 0.14 ± 0.03μM. Presented are means ± SEM of the results with tissues from four to five donors.
B. Presented are flow-cytometry density plots of HIV-1LAI.04-infected CD8− T cells at day 12 after HIV-1 infection of a representative experiment. Isolated cells from donor-matched uninfected, HIV-infected, and HIV-infected ACV ProTide Cf2649 treated tissues were stained for CD3, CD4, CD8 and CD45RA surface markers as well as for intracellular p24.
Also, we used flow-cytometry to evaluate the effect of ACV ProTides on tissue cell viability. For this purpose, we isolated cells from tissue blocks treated with 1 μM Cf2649 (a concentration that completely suppresses HIV replication in this system) and from donor-matched untreated tissue and stained them for CD3, CD4, CD8, CD45RA, and CCR7. Since HIV-1 preferentially replicates in memory T cells, we evaluated cellular depletion in all three main subsets of memory CD4+ T cells, central (TCM: CD45RA−CCR7+), effector (TEM: CD45RA−CCR7−), and terminally differentiated effector (TEMRA: CD45RA+CCR7−) as well as in naïve (CD45RA+CCR7+) CD4+ T cells. On day 12 post-infection, the amounts of total CD4+ T cells, naïve, TCM, TEM, and TEMRA CD4+ T cells in Cf2649-treated tissues were similar to those in untreated tissues (107 ± 5%, 98 ± 2%, 107 ± 3%, 111 ± 9%, and 97 ± 6% of untreated matched tissue controls, respectively; n=2). Similarly, Cf2681 did not affect the numbers of these cells in treated tissues. Likewise, the fractions of CD8+ T cells and their subsets (naïve, TCM, TEM, and TEMRA) in tissues treated with ACV ProTides Cf2649 or Cf2681 were similar to those in untreated control tissues (data not shown).
Next, we investigated the effect of ACV ProTides on HIV replication in cervicovaginal tissue. Although the absolute HIV replication level in tissues from different donors varied from 1 to 5 ng/mL, 1 μM ACV ProTide Cf2649 consistently suppressed replication of HIV-1BAL on average by 75.8 ± 10.4% (n=4, P<0.01) relative to donor-matched tissues infected with HIV-1BAL alone (Fig. 3A).
Figure 3. ACV ProTides suppress HIV-1 replication in human cervicovaginal tissues and in HSV-2-coinfected tonsillar tissues.
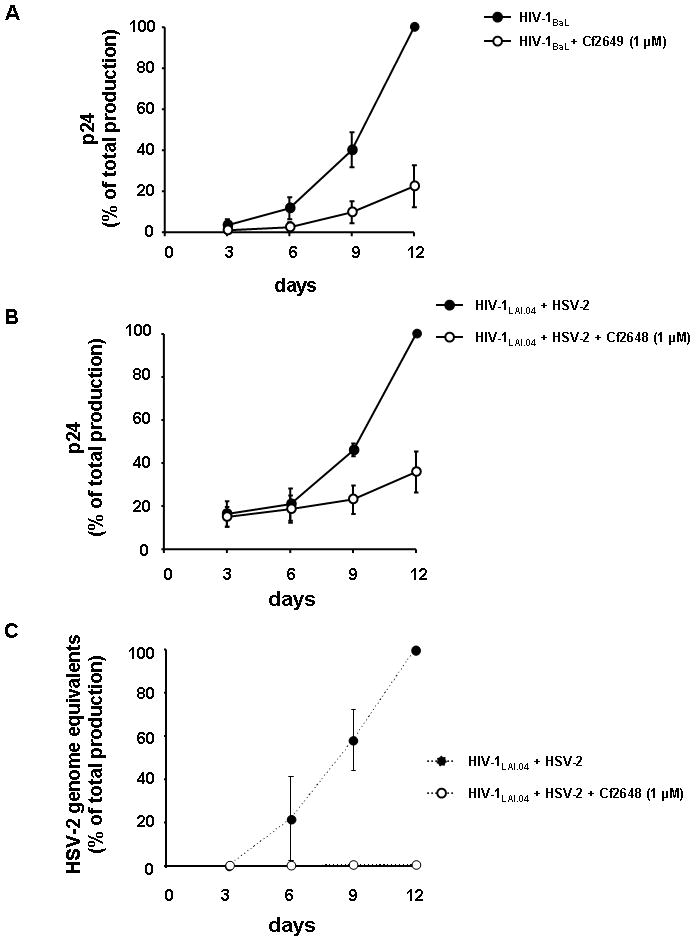
A. ACV ProTides suppress HIV-1 replication in human cervicovaginal tissues. Blocks of human cervicovaginal tissue were infected with HIV-1BaL and treated or not (control) with the ACV ProTide Cf2649 for 12 days at the concentration of 1μM. Each measurement represented the amount of HIV-1 accumulated in culture medium over a 3-day period and presented as percentage of the maximal p24 concentration in untreated control tissues to account for the variation in absolute replication levels in tissues from different donors. Presented in the cumulative curve are means ± SEM of the results with tissues from four donors.
B. Blocks of human tonsillar tissue were coinfected with HIV-1LAI.04, and HSV-2 (G). Tissues were treated or not with the ACV ProTide Cf2648 at 1μM. Replication of HIV-1 LAI was evaluated by p24gag core antigen release in pooled medium bathing 27 tissue blocks using a bead-based assay. Presented are means ± SEM of percent of maximal cumulative p24 production.
C. Blocks of human tonsillar tissue were coinfected with HIV-1LAI.04, and HSV-2 (G). Tissues were treated or not with the ACV ProTide Cf2648 at 1μM. Replication of HSV-2 was monitored by real time PCR for viral DNA accumulated in the culture media. Presented are means ± SEM of maximal of cumulative production of genome equivalent concentration.
Also, we investigated whether ACV ProTides affect HIV replication after the compounds had been removed. Tonsillar tissue blocks were pre-treated with Cf2649 1μM for 1 day, then the drug was removed and HIV-1LAI.04 was inoculated. On day 12, HIV-1 replication was still inhibited by 69.3 ± 2.3% (n=3; P<0.05). In other experimental conditions, blocks of cervicovaginal tissues were treated overnight with Cf2648 (1μM) and infected with HIV-1BaL. For one set of tissue blocks, Cf2648 was replenished at each medium change, while in another set it was removed on day 3 post-infection and not replenished. In the first case, HIV replication was inhibited by 100% (n=2) while in the second case, HIV-1 replication was suppressed by 57.1 ± 16.0% (n=4, P<0.05). If the concentration of Cf2648 was increased to 5μM, 3-day incubation followed by drug removal resulted in 90.3 ± 9.2% (n=3, P<0.05) inhibition of HIV replication on day 12 post-infection.
Finally, in a complementary set of experiments (n=3), tonsillar tissue blocks were first infected with HIV-1LAI.04 and then treated with ACV ProTide Cf2649 (1μM) at different time-points between day 0 and day 6 post-infection. HIV-1 replication was inhibited by 96.9 ± 3% if the treatment started simultaneously with infection; 94.4 ± 5.5% if the treatment started 2 days post-infection; 90.2 ± 9.8% if the treatment started 4 days post-infection and by 54.1 ± 13.5% if the treatment started 6 days post-infection. Only a 6-day delay of ACV ProTide treatment resulted in lower HIV-1 inhibition, although it was statistically not significant (P=0.055).
ACV ProTides inhibit HSV-2 and HIV-1 replication in coinfected human tissues
We investigated the ability of ACV ProTides to suppress HSV-2 in singly infected human lymphoid tissues and in tissues coinfected with HIV-1. We infected blocks of human tonsillar tissues with HSV-2 (strain G) and treated them with 1μM ACV ProTide Cf2648, which was maintained throughout the entire culture period. At day 12 post infection, HSV-2 replication was suppressed by 99.6 ± 0.3% (n=4). ACV ProTide Cf2648 was also active in suppressing an HSV-2 variant that does not encode for a functional thymidine kinase and thus is resistant to ACV [14]: replication of this HSV-2 variant was inhibited by 94.9 ± 5.1% (n=2), while ACV showed no inhibition at 10μM.
In HIV-1/HSV-2 coinfected human lymphoid tissue, the ACV ProTides retain their inhibitory activity against both viruses: 1μM Cf2648 inhibited the replication of HIV by 81.1 ± 5.9% (n=5, P<0.01) (Fig. 3B) and that of HSV-2 by 99.6 ± 0.2% (n= 5, P<0.01) (Fig. 3C). ACV ProTides treatment reduced the average production of p24gag in culture medium from 13.2 ± 4.6 to 3.6 ± 1.6 ng/mL in HIV-1/HSV-2 coinfected tissues. Thus, ACV ProTides efficiently suppress replication of HIV-1 and its common copathogen HSV-2 in coinfected human tissues.
DISCUSSION
Drugs directly suppressing HIV play a major role in containing AIDS epidemics. However, it is becoming clear that other viruses that infect the human body prior to or after HIV infection play a significant role in HIV transmission, pathogenesis and disease. Therefore, the development of drugs that target not only HIV directly but also affect HIV by suppressing coinfecting viruses may be a valid strategy for HIV therapy. Here, we describe such a drug that suppresses both HIV-1 and HSV-2, one of the viruses that is commonly associated with HIV-1 infection and which facilitates HIV-1 transmission and pathogenesis.
ACV was designed to suppress HHVs, in particular HSV-2 [9]. Its potency and specificity against HSV-2 and some other HHVs are based primarily on the unique ability of HHV nucleoside kinases to phosphorylate ACV and thus enable its eventual conversion into the 5′-triphosphate form, which is an HHV DNA chain terminator. Non-herpetic viruses, including HIV, lack such nucleoside kinase activity, and therefore ACV is inactive against them. However, recently we found that phosphorylated ACV is capable of terminating not only the elongation of HHV DNA but also that of HIV-1 DNA [10]. This occurs in tissues coinfected with HSV-2 (or with other HHVs capable of phosphorylating ACV) and HIV-1, but not in HHV-free systems [10]. However, ACV would be inactive against HIV-1 in tissues infected with HSV variants with nonfunctional TK (ACV-resistant HSV), and in tissues in which HHVs are absent, not replicating, or which phosphorylate ACV inefficiently. Thus, the anti-HIV activity of ACV heavily depends on HHV nucleoside kinases.
Here, we describe masked phosphorylated ACV derivatives (ACV ProTides) that suppress HIV-1 replication independently of HHV nucleoside kinases. The ProTide strategy has been earlier successfully used in designing an abacavir-based drug against hepatitis B virus [19], d4T and L-C-d4A-based agents against HIV-1 [20–22], and a 4′-azidonucleoside-based drug against hepatitis C virus [23] as well as in several cancer studies [24, 25].
Here, we used the ProTide strategy to deliver intracellularly phosphorylated ACV. Inside the cells the ProTides undergo ester hydrolysis followed by P-N cleavage resulting in a release of free ACV-MP that is further converted into ACV-TP, which terminates HIV-1 DNA elongation. In contrast to other NRTIs such as tenofovir and adefovir, which are “stable” acyclic purine nucleoside phosphonate analogues, phosphorylated ACV is the first known non-phosphonate acyclic nucleoside analogue that inhibits HIV-1 RT.
We evaluated ACV ProTide activity in two experimental models of HIV infection: single cell cultures (T-cell lines or PBMC) and ex vivo human lymphoid and cervicovaginal tissues in which the gross cytoarchitecture and local microenvironment are preserved and where anti-HIV drugs have already been evaluated [17, 26]. In these tissues 1 μM ACV ProTides was sufficient to inhibit HIV-1 replication by 75 to 100% and the EC50s were as low as 0.14 to 1μM. In cell lines (MT-4 and CEM), ACV ProTides suppressed HIV-1 replication with EC50 between 1.7 and 12μM while they ranged between 4.9 and 55μM in PBMC. The lower potency of ACV ProTides in PBMC and cell lines compared to tissues may be related to the level of cell activation and proliferation. Immortalized cell lines and PHA-stimulated PBMC have high concentrations of intracellular pyrimidine and purine nucleotides, in particular of dGTP [27], which competes with ACV-TP for DNA chain incorporation by HIV-1 RT. However, since the critical events of HIV pathogenesis in vivo occur in tissues, the system of human tissues ex vivo that supports HIV production without exogenous activation or stimulation appears to be more adequate.
To suppress HIV-1 replication in tissues, ACV Pro-Tides do not need to be present permanently: despite removal of the drugs several days post infection, inhibition of HIV-1 replication was sustained. It is conceivable that the block of the initial infection or the maintenance of effective intracellular drug concentrations even after its removal from the culture medium could be responsible for sustained HIV inhibition.
HIV replication was significantly inhibited either by brief exposure of ACV ProTide followed by its removal, or if the treatment was delayed. Thus, on the time-scale of our experiments, ACV ProTide can be applied before or after HIV infection, or removed during the course of infection and still significantly suppress HIV-1 replication in human tissues ex vivo. Moreover, ACV ProTides appeared to be inhibitory to a variety of HIV-1 isolates, irrespective of viral subtype and coreceptor tropism.
Importantly, none of the tested ACV ProTides killed cells in tissues or cell lines. Flow-cytometry of tissue cells treated with ACV ProTides even at a concentration 10 times higher than the EC50 did not reveal depletion of CD4+ and CD8+ T cells, or of their subsets, naïve TCM, TEM, and TEMRA. Neither did we observe cell death in cell line cultures with the ACV ProTides at a concentration as high as 150μM. However, it cannot be excluded that prolonged application of these compounds in vivo may reveal mitochondrial poisoning that often takes several months to materialize in the case of the most toxic NRTIs [28]. In vitro, the tested compounds although being non-toxic were cytostatic but at concentrations clearly higher than their EC50. Furthermore, the cytostatic effect was reversible since after removal of the compound, cells proliferated to the same level than in untreated control. The cytostatic effect of the ACV Pro Tides was not observed in ex vivo tissues, since presumably cell proliferation was negligible in this system [29].
While ACV ProTides inhibit HIV, they also retain their antiherpetic activity. In coinfected lymphoid and cervicovaginal human tissues, ACV ProTides efficiently inhibited both viruses. Furthermore, they suppressed an ACV-resistant strain of HSV-2 both in cell lines [12] and in ex vivo human tissues. These findings clearly demonstrate that the activity of ACV Pro-Tides is entirely independent of the virus-induced thymidine kinase.
The dual inhibitory activity of ProTides is important, since such drugs may interrupt a vicious circle of mutual facilitation of HSV-2/HIV infections. In particular, HSV-2 infection results in an increased risk of HIV-1 acquisition and transmission because of HSV-induced disruption of genital mucosa, recruitment of activated cells, and increase of HIV-1 genital and plasma loads [30–33]. In turn, HIV-1 infection increases the frequency of HSV-2 reactivations and its mucosal shedding [34].
Strategies to control HSV-2 in HIV-1 prevention and care are now being tested in several clinical trials. As of now, two targeted clinical trials failed to demonstrate a protective effect of HSV-2 suppression on HIV-1 acquisition [35, 36]. However these trials were marked by low drug dosage, as well as by low adherence and behavioral disinhibition [37–40]. On the other hand, in HSV-2-positive individuals who were already infected with HIV-1, suppression of HSV-2 with ACV or its prodrug valacyclovir resulted in HIV-1 load decrease in plasma, genital, rectal, and seminal compartments [41–46]. Thus, the pronounced antiherpetic activity of ACV ProTides [12], in combination with their anti-HIV activity, seems to be an advantage of these compounds that can be used in the framework of these new strategies.
In conclusion, the anti-HIV activity of ACV ProTides, in conjunction with their potent anti-herpetic activity, makes them prototypes of new dual-targeted antivirals. Such drugs allow the suppression of both HIV and HSV-2, a copathogen that significantly enhances HIV-1 sexual transmission and acquisition. These antivirals, structurally based on ACV and intracellularly delivered by the ProTide strategy, may become valuable components of future anti-HIV drug cocktails.
Acknowledgments
Funding statement
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, and by the K.U. Leuven (GOA no. 05/19).
We thank Dr. Dana Ashley Hill and the staff of the Department of Pathology of Children’s National Medical Center for their generous assistance in obtaining human tonsillar tissues. The technical assistance of Mrs. Leen Ingels and Mrs. Sandra Claes was greatly appreciated.
Footnotes
Conflict of interest statement
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med. 2004;12:104–7. [PubMed] [Google Scholar]
- 2.Corey L. Synergistic copathogens--HIV-1 and HSV-2. N Engl J Med. 2007;356:854–6. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 3.Wenner M. Virology: the battle within. Nature. 2008;451:388–9. doi: 10.1038/451388a. [DOI] [PubMed] [Google Scholar]
- 4.Lisco A, Vanpouille C, Margolis L. Coinfecting viruses as determinants of HIV disease. Curr HIV/AIDS Rep. 2009;6:5–12. doi: 10.1007/s11904-009-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8:490–7. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 6.Delany-Moretlwe S, Lingappa JR, Celum C. New Insights on Interactions Between HIV-1 and HSV-2. Curr Infect Dis Rep. 2009;11:135–42. doi: 10.1007/s11908-009-0020-8. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 Serostatus on Acquisition of HIV by Young Men: Results of a Longitudinal Study in Orange Farm, South Africa. J Infect Dis. 2009 doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elion GB. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983;12 (Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- 10.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grivel JC, Margolis L. Use of human tissue explants to study human infectious agents. Nat Protoc. 2009;4:256–69. doi: 10.1038/nprot.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuigan C, Derudas M, Bugert JJ, Andrei G, Snoeck R, Balzarini J. Successful kinase bypass with new acyclovir phosphoramidate prodrugs. Bioorg Med Chem Lett. 2008;18:4364–7. doi: 10.1016/j.bmcl.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 13.Grivel JC, Elliott J, Lisco A, et al. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. Aids. 2007;21:1263–72. doi: 10.1097/QAD.0b013e3281864667. [DOI] [PubMed] [Google Scholar]
- 14.Snoeck R, Andrei G, De Clercq E. Chemotherapy of varicella zoster virus infections. Int J Antimicrob Agents. 1994;4:211–26. doi: 10.1016/0924-8579(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 15.Cahard D, McGuigan C, Balzarini J. Aryloxy phosphoramidate triesters as pro-tides. Mini Rev Med Chem. 2004;4:371–81. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 16.Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher PS, Elliott J, Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. Aids. 2006;20:1237–1245. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson I, Grivel JC, Chen SS, et al. Differential pathogenesis of primary CCR5-using human immunodeficiency virus type 1 isolates in ex vivo human lymphoid tissue. J Virol. 2005;79:11151–60. doi: 10.1128/JVI.79.17.11151-11160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuigan C, Harris SA, Daluge SM, et al. Application of phosphoramidate pronucleotide technology to abacavir leads to a significant enhancement of antiviral potency. J Med Chem. 2005;48:3504–15. doi: 10.1021/jm0491400. [DOI] [PubMed] [Google Scholar]
- 20.McGuigan C, Hassan-Abdallah A, Srinivasan S, et al. Application of phosphoramidate ProTide technology significantly improves antiviral potency of carbocyclic adenosine derivatives. J Med Chem. 2006;49:7215–26. doi: 10.1021/jm060776w. [DOI] [PubMed] [Google Scholar]
- 21.Balzarini J, Karlsson A, Aquaro S, et al. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc Natl Acad Sci U S A. 1996;93:7295–9. doi: 10.1073/pnas.93.14.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuigan C, Cahard D, Sheeka HM, De Clercq E, Balzarini J. Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J Med Chem. 1996;39:1748–53. doi: 10.1021/jm950605j. [DOI] [PubMed] [Google Scholar]
- 23.Perrone P, Luoni GM, Kelleher MR, et al. Application of the phosphoramidate ProTide approach to 4′-azidouridine confers sub-micromolar potency versus hepatitis C virus on an inactive nucleoside. J Med Chem. 2007;50:1840–9. doi: 10.1021/jm0613370. [DOI] [PubMed] [Google Scholar]
- 24.Lackey DB, Groziak MP, Sergeeva M, et al. Enzyme-catalyzed therapeutic agent (ECTA) design: activation of the antitumor ECTA compound NB1011 by thymidylate synthase. Biochem Pharmacol. 2001;61:179–89. doi: 10.1016/s0006-2952(00)00542-6. [DOI] [PubMed] [Google Scholar]
- 25.McGuigan C, Thiery JC, Daverio F, Jiang WG, Davies G, Mason M. Anti-cancer ProTides: tuning the activity of BVDU phosphoramidates related to thymectacin. Bioorg Med Chem. 2005;13:3219–27. doi: 10.1016/j.bmc.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Frank I, Williams V, et al. Blockade of Attachment and Fusion Receptors Inhibits HIV-1 Infection of Human Cervical Tissue. J Exp Med. 2004;199:1065–75. doi: 10.1084/jem.20022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daxecker H, Raab M, Cichna M, Markl P, Muller MM. Determination of the effects of mycophenolic acid on the nucleotide pool of human peripheral blood mononuclear cells in vitro by high-performance liquid chromatography. Clin Chim Acta. 2001;310:81–7. doi: 10.1016/s0009-8981(01)00526-5. [DOI] [PubMed] [Google Scholar]
- 28.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995;1:417–22. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 29.Malkevich N, Womack C, Pandya P, Grivel JC, Fauci AS, Margolis L. Human immunodeficiency virus type 1 (HIV-1) non-B subtypes are similar to HIV-1 subtype B in that coreceptor specificity is a determinant of cytopathicity in human lymphoid tissue infected ex vivo. J Virol. 2001;75:10520–2. doi: 10.1128/JVI.75.21.10520-10522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. Journal of Infectious Diseases. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 31.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 32.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. Journal of Infectious Diseases. 1997;176:766–70. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 33.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–6. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 34.Posavad CM, Wald A, Kuntz S, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004;190:693–6. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 35.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. [see comment] New England Journal of Medicine. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. [see comment] Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson CP. Effect of aciclovir on HIV-1 acquisition in HSV-2-positive patients. Lancet. 2008;372:1298. doi: 10.1016/S0140-6736(08)61543-3. author reply 1298–9. [DOI] [PubMed] [Google Scholar]
- 38.Lisco A, Vanpouille C. HSV-2 suppression and the incidence of HIV.[comment] New England Journal of Medicine. 2008;359:535. doi: 10.1056/NEJMc081075. author reply 535. [DOI] [PubMed] [Google Scholar]
- 39.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–63. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stirratt MJ, Gordon CM. Adherence to biomedical HIV prevention methods: considerations drawn from HIV treatment adherence research. Curr HIV/AIDS Rep. 2008;5:186–92. doi: 10.1007/s11904-008-0027-z. [DOI] [PubMed] [Google Scholar]
- 41.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 42.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 44.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. Aids. 2009;23:461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuckerman RA. HSV suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1, HSV-2 seropositive men: A randomized, double-blind, placebo-controlled, crossover trial. Journal of Infectious Disease. 2007 doi: 10.1086/522523. in press. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. Aids. 2009;23:479–83. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]


