Abstract
Importance of the field:
Cytochrome P450 enzymes comprise a superfamily of heme monooxygenases that are of considerable interest for the: 1) synthesis of novel drugs and drug metabolites, 2) targeted cancer gene therapy, 3) biosensor design, and 4) bioremediation. However, their applications are limited because cytochrome P450, especially mammalian P450 enzymes, show a low turnover rate and stability, and require a complex source of electrons through cytochrome P450 reductase and NADPH.
Areas covered in this review:
In this review, we discuss the recent progress towards the use of P450 enzymes in a variety of above-mentioned applications. We also present alternate and cost-effective ways to perform P450-mediated reaction, especially using peroxides. Furthermore, we expand upon the current progress in P450 engineering approaches describing several recent examples that are utilized to enhance heterologous expression, stability, catalytic efficiency, and utilization of alternate oxidants.
What the reader will gain:
The review will provide a comprehensive knowledge in the design of P450 biocatalysts for potentially practical purposes. Finally, we provide a prospective on the future aspects of P450 engineering and its applications in biotechnology, medicine, and bioremediation.
Take home message:
Because of its wide applications, academic and pharmaceutical researchers, environmental scientists, and health care providers are expected to gain current knowledge and future prospects of the practical use of P450 biocatalysts.
Keywords: biocatalyst, cytochrome P450, directed evolution, drug metabolism, drug toxicity, rpotein engineering
1. Introduction
Cytochrome P450 monooxygenases (CYP) are a superfamily of ubiquitous heme proteins that perform a number of difficult oxidative reactions, such as C-H bond hydroxylation, N-dealkylation, N-hydroxylation, O-dealkylation, S-oxidation, and epoxidation of numerous endogenous and exogenous compounds [reviewed in 1]. They exhibit strict as well as overlapping substrate specificity, in addition to substrate regio- and stereoselectivity and non-Michaelis-Menten kinetics [reviewed in 2]. CYP enzymes are central to the study of toxicology because they are involved in the clearance of a majority of marketed drugs and abused substances, activation of prodrugs, drug-drug and drug-food interactions, and metabolism of carcinogens and other pollutants. Currently, there are over 7000 CYP sequences known, of which 57 are human (http://drnelson.utmem.edu/CytochromeP450.html). Their catalytic versatility, substrate diversity, and atypical kinetics have led to considerable interest in utilizing CYP as biocatalysts in biotechnology, medicine, and bioremediation.
While bacterial CYP are soluble and metabolize only a limited number of natural substrates, such as fatty acids, vitamins, styrene, erythromycin, and terpenes, mammalian CYP are membrane-bound and metabolize an array of substrates, such as steroids, fatty acids, drugs, prodrurgs, carcinogens, pesticides, and herbicides [reviewed in 3]. Bacterial P450BM3 (CYP102) is catalytically self-sufficient, that is, the electron transfer flavin mononucleotide (FMN)/flavin adenine dinucleotide (FAD) reductase domain and the P450 monooxygenase domain are in a single peptide [4]. In contrast, mammalian CYP require additional redox partners, such as cytochrome P450 reductase (CPR) and often cytochrome b5, an expensive cofactor NADPH (nicotinamide adenine dinucleotide phosphate-oxidase), and lipids [1-2]. Furthermore, bacterial CYP show a much higher turnover, expression in E. coli, and coupling efficiency (utilization of NADPH in substrate metabolism vs. formation of superoxide, peroxide, and water molecules) compared to that of their mammalian counterparts [1-2, 5]. These contrasting features of bacterial and mammalian CYP clearly suggest that bacterial CYP are easy to use as biocatalysts, whereas mammalian CYP have much broader applications in pharmaceuticals/biotechnological industries and in green chemistry.
Over the past three decades cytochrome CYP have been reviewed numerous times with an emphasis on CYP's regulation, atypical kinetics, drug metabolism, drug interactions, and structural-function studies [1-3, 6-10]. In 2002, Guengerich wrote the first review on the proposed application of CYP biocatalysts, especially in the synthesis of drugs and other fine chemicals [11]. Thereafter, several reviews reported on proposed applications of bacterial and mammalian CYP [12-17]; e.g., Gillam recently highlighted a need for engineered CYP in such applications [14]. Here we review the recent advancements and future prospects for the use of CYP enzymes as biocatalysts in biotechnology, medicine, and bioremediation, as well as recent success in engineering techniques and examples of engineered CYP for the proposed applications (Figure 1, Tables 1 and 2). To the best of the author's knowledge, this is the first review that combines the potential applications of natural and engineered CYP enzymes.
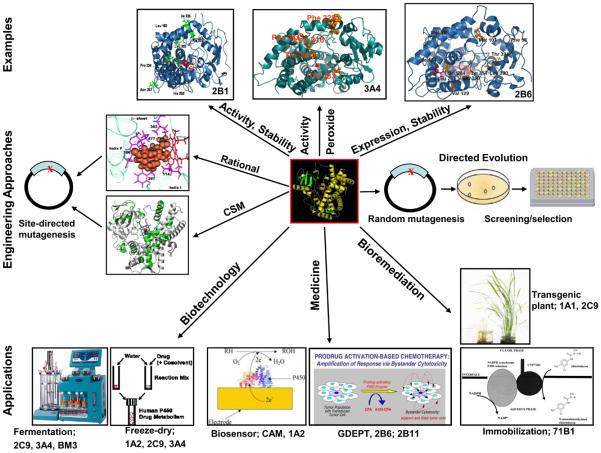
Figure 1.
The figure shows 1) application (bottom panel), 2) approach (middle panel), and 3) examples of engineered P450s for desired characteristics (top panel). The application in: 1) biotechnology includes the synthesis of drug metabolites by using a fermentation process and freeze-dry methods; 2) medicine includes activation of the anti-cancer prodrugs by employing GDEPT and monitoring via biosensors drug levels in blood plasma, and 3) bioremediation of environmental pollutants by applying immobilization methods and transgenic plants. The approaches to engineer P450s are: 1) rational/CSM (knowledge-based) and 2) directed evolution (random). The representative examples of engineered P450 enzymes include: 1) CYP2B1 with enhanced activity and stability, 2) CYP3A4 with enhanced activity and utilization of peroxides, and 3) CYP2B6 with enhanced expression and solubility. The figure was synthesized from the following multiple sources, The figures for freeze-dry method, biosensor design, immobilization, and transgenic plants are reproduced from references 30, 51, 31, and 79, respectively, upon request for permission from the respective sources. The Figure for GDEPT was provided by Dr. David Waxman from Boston University. The figures for directed evolution, rational, CSM, 2B1, 3A4, and 2B6 were reproduced from references 83, 140, 89, 94, 95, and 137, respectively.
Table 1.
Multiple ways to perform cytochrome P450-mediated reactions for practical purposes
| Reaction requirements | Enzyme source | Activity | Cost | Possible applications | CYP |
|---|---|---|---|---|---|
| CYP+CPR+NADPH | Purified | High | High | Synthesis, Bioremediation | 1A2, 2C9, 3A4 |
| CYP+CPR+NADP++ G6P+G6PDH |
Purified | High | Medium | Synthesis, Bioremediation | 1A2, 2C9, 3A4 |
| CYP−CPR+NADPH or NADP++G6P+G6PDH |
Purified/whole cell |
High | Medium | Synthesis, Bioremediation, GDEPT |
1A1, 1A2, 2A6, 2B6, 2B11, 2C18, 3A4, 71B1 |
| CYP+Peroxides | Purified/whole cell |
Low | Low | Synthesis, Bioremediation | 101, 102, 2B1, 2C8, 2C9, 2C18, 2C19, 3A4 |
| CYP+Metal powders | Purified | Low | Low | Synthesis, Bioremediation | 102 |
| CYP+Metal electrode | Purified | Medium | Medium | Synthesis, Biosensor | 101, 102, 1A2, 2B4, 2C9, 2D6, 2E1, 3A4 |
Table 2.
List of important cytochrome P450 enzymes, screening strategies, and possible practical applications
| CYP | Screening strategies | Possible applications in biotechnology, medicine, and bioremediation |
|---|---|---|
| 102 | Fatty acids, alkanes, drugs; H2O2 |
Synthesis of alcohol, indigo and indirubin, and metabolites of propranolol and buspirone; degradation of pollutants, such as PAHs and petroleum components, such as alkanes |
| 101 | Alkanese; H2O2 | Synthesis of alcohols; degradation of petrochemicals, such as alkanes |
| 1A2 | 7-MeQ, 7-MR; NADPH | Synthesis of metabolites of propranolol and warfarin; detoxification of PCBs |
| 1A1/1A2 | Luciferin | Synthesis of metabolites of CYP1A1/1A2's as well as novel drug substrates |
| 2A6 | Indole, 7-MR; NADPH | Syntheiss of indigo compounds |
| 2B1 | 7-EFC; H2O2 | To re-engineer 2B6 or 2B11 for the activation of CPA and IFA |
| 2C8a | C152; CuOOH | Synthesis of metabolites of taxol, chloroquine, and amodiaquine; biomarkers for cancer |
| 2C9a | C152; CuOOH | Synthesis of metabolites of diclofenac, warfarin, and flurbiprofen |
| 2C18a | C152; CuOOH | Activation of CPA and IFA |
| 2C19a | C152; CuOOH | Synthesis of metabolites of diclofenac and warfarin; activation of CPA and IFA |
| 2Cs | Luciferin; NADPH | Synthesis of metabolites of CYP2C's as well as novel drug substrates |
| 3A4 | 7-BQ; CuOOH, NADPH | Synthesis of metabolites of several drugs; activation of IFA |
Directed evolution of these P450s are underway. 2Cs: 2C8, 2C9, 2C18, and 2C19.
2. Applications of Cytochrome P450 Biocatalysts
In recent years there has been an increasing realization of the power of CYP biocatalysts for the industrial synthesis of bulk chemicals, pharmaceuticals, agrochemicals, and food ingredients, especially when high regio- and stereoselective hydroxylation is required [11-13, 17]. In addition, there is an increasing demand for CYP biocatalysts in the detoxification of environmental contaminants [18-20] and gene-directed enzyme prodrug therapy (GDEPT) for cancer treatment [21-23].
2.1. Biotechnology
CYP enzymes have a tremendous potential in the synthesis and discovery of drugs, as well as in drug development. Although several microorganisms have been employed historically for the synthesis of drugs using a hydroxylation reaction, the well-established commercial application of CYP is in the biotransformation of steroids, such as the 11β-hydroxylation of reichstein S to hydrocortisone by Curvularia sp. [24; http://www.schering.de) at a scale of ~100 tons per year. In addition, E. coli-expressed CYP is used to make provastatin from compactin [25], and CYP102 is utilized in the production of epoxyeicosatrienoic acid, leukotoxin B, and eicosanoid epoxides [26]. Many synthetic steps of antibiotics involve bacterial P450s, e.g. synthesis of erythromycin and tetracenomycin requires P450eryF (CYP107) [27]. Similarly, several plant CYP are involved in the biosynthesis of the most widely used chemotherapeutic drug taxol [28]. Achieving a complete taxol synthetic pathway in microorganisms is still challenging. Therefore, through CYP a semi-synthetic approach could be used to produce new taxol analogues with enhanced drug efficacy and potency. In another example, the production of the anti-cancer drug perillyl alcohol from limonene was achieved by CYP153 expressed in Pseudomonas putida, resulting in a 6.8 g/L yield [29]. Thus, the true potential of CYP in the production of drugs or drug intermediates can be further exploited.
The CYP-derived drug metabolites in the liver and other organs/tissues are often biologically active, and understanding their effects is crucial in evaluating a drug's efficacy, toxicity, and pharmacokinetics. Such studies, however, require large quantities of the pure metabolites, which may be difficult to synthesize by chemical methods, especially when regio- and stereoselective hydroxylation is required. An alternate route for the synthesis of drug metabolites is the use of human CYP. When this approach was employed in a 1-L reaction, CYP2C9 co-expressed with CPR produced 110 mg 4′-hydroxydiclofenac with a conversion rate of 93% [30]. However, the limitations in these approaches include poor activity, stability, and expression in E. coli. Recently, an enzyme immobilization approach has been developed with several CYP enzymes to synthesize metabolites [31-33]. The immobilized enzymes exhibited an improved half life. For example, the immobilized CYP102 had a half-life of 29 days at 25°C compared with 2 days for free enzymes [33]. More recently, CYP2C9 was attached to gold electrodes such that the resulting constructs maintained the ability to bind and metabolize substrates in the presence of CPR and NADPH [34]. Similarly, Mie and colleagues showed that CYP3A4, which was immobilized on gold electrodes, can be used for electrochemically driven drug metabolic reactions [35]. They also found that the CYP3A4-mediated reactions are facilitated in the presence of CPR. The activity obtained with the immobilized CYP2C9 and CYP3A4 on gold was similar to that obtained using a standard reaction in addition to exhibiting an increased stability. This opens up the possibility of the construction of bioreactors using biochip technology to synthesize drug metabolites.
In addition to drugs and drug metabolites, several other commercial products, such as dyes and pesticides, could be synthesized by using CYP. Dioxygenases have been used commercially to produce dyes; for example, in the production of indigo from tryptophan [36]. Recently, investigators have shown that CYP can readily form indigo and indirubin in bacterial cultures and transgenic tobacco plants, which points to its important role in the dye industry [37-38]. Another application of dye production by CYP involves horticulture. For example, the production of flowers with unusual colors, e.g., blue roses, is performed by transferring CYP coloration genes from other plants [39]. Accordingly, the introduction of CYP, which oxidizes indole into a variety of colored compounds, into flowering plants, is an alternative method that is proposed in the horticultural industry.
2.2. Medicine
Gene-directed enzyme prodrug therapy (GDEPT) that increases the chemosensitivity of tumor cells by stimulating tumor cell-catalyzed activation of anticancer prodrugs offers a unique opportunity to improve the efficacy of cancer chemotherapeutic agents. In addition, GDEPT may potentially decrease or eliminate the adverse effects from: 1) toxic metabolites arising from side reactions, such as N-dechloroethylation and 2) toxicity of a prodrug at a high dose, if taken orally or intravenously [21, 23]. Over the past decade, Waxman and colleagues have performed elegant studies involving the development of the CYP-based activation of cyclophosphamide (CPA) and ifosfamide (IFA) through GDEPT for cancer treatment [reviewed in 21, 23]. To study the activation of CPA and IFA in tumor cells, they constructed a CYP-IRES-CPR plasmid, which includes CYP, an internal ribosomal entry site (IRES), and CPR. Human CYP2B6 showed a strong cytotoxicity in 9L-gliosarcoma cells with both of the prodrugs CPA and IFA [40-45]. Human CYP2C18 and CYP3A4 showed a strong cytotoxicity with CPA and IFA, respectively, despite their several-fold lower levels of expressed proteins than CYP2B6 in 9L-gliosarcoma cells [42-43]. In addition, low-Km human CYP2C9 and CYP2C19 enzymes are capable of activating both CPA and IFA [46].
Preclinical GDEPT studies with CYP2B6 using CPA demonstrate a substantial improvement in antitumor activity, with a concomitant decrease in systemic toxicity when compared to that found with conventional chemotherapy [47]. Subsequently, results of phase II clinical trials using human CYP2B6 showed a targeted activation of CPA and IFA, leading to the selective death of the tumor cells with a minimal effect on normal cells [48]. Furthermore, canine CYP2B11, a low Km CPA 4-hydroxylase (Km ~100 μM), when compared with a high Km human CYP2B6 (Km ~1 mM), showed enhanced activation of CPA and IFA [49-50]. Furthermore, the activation of CPA in vivo solid tumors that express CYP2B11 was investigated; the findings demonstrated that, when compared with human CYP2B6, canine CYP2B11 showed an increased intratumoral concentration of 4-OH-CPA [51]. These findings provide proof-of-principle for the use of a low Km CYP to augment intratumoral prodrug activation at pharmacologically relevant dosages. The potency of CYP-mediated GDEPT can be further improved by: 1) enhancing the stability and expression of CYP enzymes in tumor cells, 2) increasing the bioactivation (4-hydroxylation) and decreasing the detoxification (N-dechloroethylation) pathways, and 3) finding genetic variants of drug-metabolizing enzymes and drug transporters [21, 23].
Another application of CYP enzymes in medicine is to use them as biosensors to monitor drug levels in blood plasma [52]. Monitoring drug level consistently is critical, because the presence of genetic variants or drug-drug/drug-food interactions lead to an altered drug response and drug toxicity [53]. For example, CYP2C9 and CYP2D6 have several variants with low, high, or no activity, which arise from genetic, environmental, physiological, or pathophysiological factors [54]. Similarly, numerous drugs have been shown to inhibit or activate human CYP enzymes, which alter the metabolism of other drugs, resulting in toxicity [9]. In addition, CYP biosensors can be used to detect food contaminants, such as hazardous carbamate and organophosphate pesticides, in order to improve the food safety [52]. Thus an immobilized CYP enzyme on an electrode, which either binds to drugs or converts them into oxidized products, can act as a biosensor. An electrochemical potential generated upon binding or metabolism can be used to monitor the drug/food contaminant levels.
CYP as a biosensor ranks third after cytochrome c and glucose oxidase [52]. Currently, P450CAM (CYP101), CYP102, CYP1A1, CYP1A2, CYP2B4, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP4A1, CYP11A1, and CYP17A have been used for sensor constructions to monitor drug levels in blood plasma [52, 55-62]. For example, an amperometric drug metabolism biosensor consisting of CYP3A4 encapsulated in a didodecyldimethylammonium bromide vesicular system on a platinum disk electrode was developed for the determination of indinavir, a protease inhibitor antiretroviral drug [55]. Some of the common electrodes used for enzyme immobilization are gold, platinum, tin oxide, glassy carbon, pyrolytic graphite, edge-plane graphite, carbon cloth, etc. However, the use of polymer or a polyelectrolyte enhances the preservation of the native structure, thereby increasing electron transfer between the enzyme and the electrode. Recently, a molecular Lego approach (assembling non-physiological redox modules, generating artificial redox chains, which leads to a new multi-domain construct) has been developed to improve the electrochemical and catalytic properties of CYP102 [63].
2.3. Bioremediation
There are three major classes of ubiquitous global industrial pollutants present in air, water, and soil, namely, polycyclic aromatic hydrocarbons (PAHs); polychlorinated dibenzo-p-dioxins (PCDDs); and polychlorinated biphenyls (PCBs) [64-65]. PAHs are ubiquitous compounds originating from the natural and anthropogenic pyrolysis of organic matter, such as forest fires, fossil fuel consumption, and processes in the oil industry. CYP101, CYP102, CYP1A1, CYP1A2, and CYP1B1 are known to metabolize PAHs [1-3]. CYP1A1 shows high activity toward dibenzo-p-dioxin (DD), and mono-, di-, and tri-chloro-DDs, while the CYP1A1 mutant, F240A, exhibits activity toward 2,3,7,8-tetra-chloro-DD [66-67]. Furthermore, recombinant cells (S. cerevisiae, basidiomycete, Dehalococcoides, and Rhodococcus species), expressing CYP1A1, successfully biodegrade PCDDs [66, 68]. PCBs are metabolized by several CYP enzymes, and the major factors that determine the degree of metabolism are the extent of chlorination and position of chlorine atoms on the biphenyl nucleus [69]. For example, a marked species variation exists in the metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl, and it has been shown that only dog CYP2B11 is capable of metabolizing PCBs [70]. In contrast, CYP1A1 is also known to bioactivate these pollutants into genotoxic and carcinogens leading to high risk for lung cancer [71].
Herbicides provide labor-saving ways of improving crop yield and quality, because weed infestation reduces crop yield and decreases market prices. In addition to chemical methods, many herbicides are removed from the environment by bacterial and plant CYP enzymes, which convert herbicides to less lipophilic and toxic metabolites [72]. Many CYP-dependent oxidations have been reported, including those of chlorotoluron and linuron in maize and wheat. However, molecular information is limited regarding plant CYP related to xenobiotic metabolism. Only some herbicide-metabolizing CYP genes have been cloned and characterized, including CYP73A1 and CYP76B1 from Jerusalem artichoke, CYP71A11 from tobacco, and CYP71A10 from soybean [72].
Detoxification of the contaminants can be performed by immobilizing CYP enzymes on a surface. Recently, plant CYP71B1-reductase fusion protein was immobilized to metabolize chlortoluron [31]. The immobilized CYP71B1 enzyme shows 10-fold higher activity than does the free enzyme for the demethylation of erythromycine, and retained activity for more than 24 h. In addition, creating CYP-expressed transgenic plants has potential for developing herbicide-resistant plants and for reducing the environmental impacts of agrochemicals [73-75]. Recently, several CYP enzymes, such as CYP1A1, CYP2B6, CYP2C9, and CYP2C19, have been introduced in various plants for effective phytoremediation of herbicides [76-81). For example, a rice plant in which the CYP2C9-57R2 gene was introduced showed resistance to sulfonylureas, while the transgenic rice plant CYP2C19-12R1 showed cross-resistance to several herbicides, such as atrazine, norflurazon, metolachlor, and sulfonylureas [76]. A. thaliana expressing xplA (cyclotrimethylenetrinitramine (RDX)-degrading CYP) was grown in RDX-contaminated soil and found to be resistant to RDX phytotoxicity, producing shoot and root biomasses greater than those of wild-type plants [77].
3. Cost-effective Source of Electrons
CYP enzymes require a complicated source of electrons via redox partners and NADPH, and, therefore, it is critical to find alternate strategies to bypass these requirements for practical applications. Table 1 shows various ways to perform CYP-mediated reactions, their cost-effectiveness, practical uses, and examples. The most difficult and expensive strategy is to use NADPH and purified CYP, CPR, and cytochrome b5. The cost of NADPH can be reduced by using an NADPH re-generating system (glucose 6-phosphate (G6P), glucose 6-phosphate dehydrogenase (G6PDH), and NADP+). This strategy can mainly be used for synthesis of specific compounds and bioremediation. The requirement of redox partners can be overcome by expressing CYP and CPR in either the same plasmid (bicistronic expression) or a different one (co-expression) [reviewed in 82]. Several bicistronic CYP-CPR plasmids, such as those from CYP2B6, CYP2B11, CYP2C9, CYP2D6, CYP2C18, and CYP3A4, have been constructed and expressed in E. coli, yeast, insects, and mammalian cells [41-42, 83-86, unpublished observations]. More recently, Purnapatre and colleagues have reviewed methods of production of recombinant CYP enzymes in various expression systems, including E. coli, yeast, insects, and mammalian [85], which offer simple & economical modes of production of target-based anti-cancer drugs. We do not discuss various CYP or CYP-CPR expression systems here, as they are beyond the scope of this review. Bicistronic construct is an absolute requirement in the case of GDEPT [23] and is cost effective in the case of industrial synthesis of chemicals and bioremediation (Table 1).
The most cost-effective and simple method, however, would be to bypass the requirements of CPR, cytochrome b5, and NADPH by using alternate oxidants, such as peroxides (hydrogen peroxide (H2O2), cumene hydroperoxide (CuOOH)), metal powders (Zn, Pt), or metal electrodes (gold, glassy carbon) [reviewed in 19, 52, 87]. These strategies can be used for synthesis, bioremediation, and/or biosensor applications. The use of peroxides and metal powders is the most cost-effective way to synthesize drugs, drug metabolites, or other chemicals, whereas the use of a metal electrode is economical for biosensor design (Table 1). However, not all CYP enzymes show measurable activity with these alternate oxidants, and if they do, the activities are usually very low (>10-fold lower than the NADPH-dependent activity) [19, 87]. The general mechanism by which peroxide supports CYP-mediated substrate oxidation remains unclear, because significant activity requires an appropriate CYP, substrate, and peroxide. Thus, there is an opportunity to engineer CYP enzymes for enhanced utilization of alternate oxidants. Recently, several CYP enzymes were engineered, by rational and directed evolutionary approaches, for such purpose (see section 5).
4. Approaches to Engineer Cytochrome P450
A detailed approach for CYP engineering has been recently reviewed [87-89]. In this section we will briefly describe these approaches, followed by some promising approaches that should be undertaken in the future (Figure 1, Table 2).
4.1. Rational
A rational approach requires prior knowledge of CYP structure-function relationships through chimeragenesis, site-directed mutagenesis, X-ray crystal structure, protein modeling, and solution thermodynamics [10]. Information obtained from the above different sources provides an insight into the functional role of amino acid residue(s), which can be replaced by another residue(s) for enhanced solubility, stability, expression, and activity, as well as for altered substrate specificity and regioselectivity [10]. This method has been employed to engineer several CYP enzymes, which are described in section 5.
4.2. Semi-rational approach
In this approach the relative activity, stability, or expression of CYP enzymes within the same subfamilies, such as CYP2B enzymes (2B1, 2B4, 2B6, and 2B11), are determined. Then, the amino acid sequences between the set of enzymes, which show high or low activity, stability, or expression, are compared to determine the residues that might be responsible for their functional differences [10]. Furthermore, the extent of solvent accessibility of these amino acid residues is determined by using computational analysis based on available X-ray crystal structures or structural models generated through crystal structures. The selected residues that are thought to be responsible for decreased activity, stability, or expression are then replaced by their counterpart residues by site-directed mutagenesis. By using this approach, Kumar and colleagues have recently engineered CYP2B6, which are described in section 5.
4.3. Conserved sequence motif
Conserved sequence motif (CSM) analysis is performed by multiple sequence alignment by employing a linear combination of 237 dimensional physicochemical properties of 20 natural amino acids [90]. Braun's group has developed and applied this approach to extensively identify and investigate CSM in several proteins [91-92]. Recently, CSM approach has been applied to the CYP2 family and identified 20 motifs [93]. A significant correlation was found between the motifs/ residues within the motifs and their known functions from genetic variants and site-directed mutants. This suggests that CSM analysis is a critical approach to identify the functional role of the residues within motifs. Furthermore, functional analysis of CSM 8 in CYP2B4 showed that Arg187, which is extremely conserved within and across the family, is critical for protein stability and binding/metabolizing small ligands. However, in rare examples, CYP1A1 and CYP2E1 contain His at that position, suggesting that these enzymes can be engineered for enhanced stability and ligand selectivity by replacing His by Arg. Thus, this approach could be important to engineer CYP for enhanced expression, stability, and activity as well as altered substrate specificity.
4.4. Directed evolution
Directed evolution does not require prior knowledge of protein structure and function. Rather it is performed by creating variants using error-prone polymerase chain reaction, saturation mutagenesis, or recombination followed by screening and selection variants for desired characteristics, such as enhanced activity, stability, or expression, or for altered substrate specificity [87-89]. Directed evolution has been successfully applied to the design of industrial biocatalysts for enhanced catalytic efficiency, novel activity, and increased stability [94-95]. The main requirement of directed evolution is to develop a high throughput activity screening (HTS) system to screen/select mutants with desired properties (Table 2).
Development of an HTS requires finding a simple, cost-effective, and efficient method to measure enzyme activity directly on a multi-well microplate. Therefore, the use of alternate oxidants, such as peroxides, which bypass the requirements for redox partners, lipids, and NADPH, is highly desirable. Recently, a sensitive and cost-effective activity screening system has been developed for CYP101, CYP102, CYP1A2, CYP2A6, CYP2B1, CYP2C8, CYP2C9, CYP2C18, CYP2C19, and CYP3A4 by screening a number of enzymes, fluorescence substrates, peroxides, and buffer systems [87, 96-102; Table 2; unpublished observations]. The activity screening was further expanded to screen/select CYP mutants for enhanced catalytic tolerance to temperature and organic solvents. This was performed by incubating mutants at T50 and DMSO50 (temperature and DMSO concentration, respectively, at which the enzyme retains 50% activity), and selecting the mutants with higher activity than the wild-type [103-105]. P450 mutants with increased stability can further be screened by monitoring protein unfolding transitions (induced by guanidium-HCl or temperature) by tryptophan fluorescence as described in the literature [106].
Flow cytometry has been recently applied to HTS applications in directed evolution for the analysis of single cells carrying mutant genes/proteins with improved activity [107-109]. The approach requires a sensitive and relatively rapid reaction that uses a fluorogenic substrate in whole-cell suspensions. The individual clones with improved activity are then sorted by fluorescence activated cell sorting techniques, which can sort approximately 2000-3000 cells per second. The authors performed preliminary experiments with CYP2C9 using 7-dimethylamino-4-trifluoromethylcoumarin (C152) as a substrate and peroxides as an oxidant to validate the feasibility of this approach in E. coli. The results, in brief, showed that the cells that produced 7-amino-4-trifluoromethylcoumarin, the product of C152, showed ~2-fold higher fluorescence intensity (unpublished observations). Although the results are promising, this technique requires further optimization. This in vivo approach will potentially replace the conventional in vitro approach, and will significantly improve the cost effectiveness of directed evolution of CYP enzymes.
5. Examples of Engineered Cytochrome P450
5.1. Bacterial cytochrome P450
Because of very high turnover, solubility, and expression in E. coli, bacterial CYP are relatively easy to design as biocatalysts. Among them CYP102 is the most exploited enzyme for this purpose because it contains both CYP and reductase domains in a single polypeptide, is characterized extensively, and has a turnover rate of several thousands. CYP102 was successfully engineered by directed evolution for new and efficient hydroxylation pathways and for enabling the utilization of alternate oxidants, such as H2O2 [96]. CYP102 F87A mutant was created by site-directed mutagenesis, which is capable of hydroxylating lauric acid and myristic acid exclusively at ω-4 rather than at ω-1, ω-2, and ω-3 positions as done by wild-type [110-111]. Subsequently, by using directed evolution, Arnold and other research groups have created several CYP102 mutants that displayed the following properties [110-122]: 1) A >2-fold higher activity towards shorter chain-length fatty acids; 2) Higher activity for indole hydroxylation to indigo and indirubin; 3) Hydroxylation of several other substrates, such as octanoic acid, n-octane, α- and β-ionone, naphthalene, anthracene etc.; 4) Up to a 100-fold increased activity with unnatural alkane substrates, such as benzene, styrene, 1-hexane, and propane; 5) Enhanced activity towards smaller alkanes, especially direct conversion of ethane to ethanol; 6) Strict substrate specificity and enantioselectivity, for example, the 77-9H mutant showed a 52% selectivity for the ω-position of n-octane, and other mutants exhibited a range of oxidation of terminal alkanes to either (R-) or (S-) epoxides; 7) Increased total activities by using air as oxidant in whole-cell bioconversions of propane to propanol under mild conditions; 8) Increased activity towards indole hydroxylation; 9) Generation of reactive metabolites from the drugs clozapine, diclofenac and acetaminophen; 10) Activity towards many non-natural substrates.
In addition, CYP102 was engineered for enhanced utilization of H2O2, thermal stability, and catalytic tolerance to temperature and organic solvents [103-104]. The evolved enzymes enhanced T50 from 43°C to 61°C and displayed enhanced catalytic tolerance up to 10-fold in 2% tetrahydrofuran and 6-fold in 25% dimethylsulfoxide (DMSO). Recently, a novel approach of recombining segments of the CYP102 gene from three different bacterial sources has given an unprecedented opportunity to design CYP102 with diverse characteristics. Upon screening and selection of several recombinant variants, >73% of the chimeric CYP proteins were found to be catalytically active peroxygenases, and some weree more thermostable than the parent proteins [88]. One CYP102 variant showed a >10°C enhanced Tm compared with the wild-type. Accordingly, these engineered CYP enzymes with increased thermostability can be used for industrial biocatalysis.
Achieving CYP-catalyzed oxidation of ethane is a key step in the pathway to CYP-catalyzed methane oxidation, and opens new opportunities for bioconversion of natural gas to fuels and chemicals. Although the rate, total turnover number, and coupling efficiency of ethane hydroxylation are low for practical purposes, it has been shown that continual improvement of CYP102 by further directed evolution will generate a biocatalyst with similar productivity to that obtained with propane (6000 total turnover) [115, 119]. The CYP102 random mutant has been shown to be proficient in the oxidation of indole to indigo, and the catalytic efficiency is similar to that of the human CYP2A6 enzyme [120]. This suggests its potential application in the dye and horticulture industries. More recently, CYP102 was designed to metabolize several important drugs, such as buspirone, dextromethorphan, 3,4-methylenedioxymethylamphetamine (MDMA), and propranolol [116-117, 121, 123]. For example: 1) The evolved CYP102 enzyme showed a 180 turnover in the H2O2 system and could produce >70 mg of propranolol metabolites in a 1-L E. coli culture; 2) The CYP102 mutant 9-10A-F87A (50 nM) converted buspirone to (R)-6-hydroxybuspirone with a 3800 total turnover, 8.9% conversion; 3) Random clones of CYP102 enhanced the activity with dextromethorphan and MDMA by ~100-fold Currently, the CYP102 mutants produce ~100 mg of metabolites per liter of bacterial cultures, which is close to an optimal requirement for industrial production (150-200 mg).
CYP101 primarily catalyzes the hydroxylation of camphor to 5-exo-hydroxycamphor. Using site-directed mutagenesis, CYP101 was engineered to create mutants for efficient oxidation of alkanes, halogenated hexanes, PCBs, PAHs, and unnatural substrates [124-128]. While some variants showed enhanced activity by ≥19-fold compared with the wild-type towards linear alkanes such as pentane, hexane, and heptanes, the F87W/Y96H/T101L/V247L mutant oxidized n-butane to secondary alcohol with a turnover of 750 min−1 [124-125]. The V247L and F87W/Y96F/V247L mutants showed an increased turnover with the highly insoluble pentachlorobenzene without the need for surfactants or organic co-solvents [126]. The F87W and Y96H mutants greatly showed an enhanced activity with PAHs; phenanthrene, fluoranthene, pyrene, and benzo[a]pyrene [127]. Furthermore, CYP101 was engineered with increased activity for the oxidation of diphenylmethane (by Y96F/I395G mutant) and styrene, and ethylbenzene (by Y96F/V247L mutant) [128]. In particular, the Y96F/V247L mutant shows a coupling efficiency of approximately 60% for styrene and ethylbenzene oxidation, with substrate oxidation rates of approximately 100 min−1. More recently, CYP101 was engineered by directed evolution via a step-by-step adaptation to smaller alkanes, from hexane to butane to propane, and finally to ethane [129]. The turnover of ethanol synthesis was 78 min−1.
5.2. Mammalian cytochrome P450
The design of new mammalian CYP biocatalysts is of high priority, because of their extreme substrate diversity and abilities to metabolize numerous xenobiotics. Guengerich and colleagues successfully performed directed evolution of CYP1A2 and CYP2A6 initially by using random mutagenesis of substrate recognition sites and colony-based colorimetric and genotoxicity activity assays, respectively, followed by random mutagenesis of the whole cDNA and in vitro fluorescence-based enzyme assay systems [36-38, 100-101, 130-134]. The engineered CYP1A2 enzymes showed >5-fold enhanced activity with 7-methoxyresorufin (7-MR), 7-ethoxyresorufin (7-ER), phenacetin, and 2-amino-3,5-dimethylimidazo[4,5-f]quinoline (7-MeQ) [100]. Similarly, several CYP2A6 mutants exhibited higher activities towards several indole derivatives [101]. Furthermore, Guengerich's group generated a transgenic tobacco plant harboring engineered CYP2A6, together with an indole synthase (BX1) from maize, which accumulated an indole precursor, indican; a step forward towards development of indigo dyes [38].
Kumar, Halpert, and colleagues have engineered several mammalian CYP2B enzymes for enhanced expression, stability, activity, substrate specificity, and regio-selectivity by using rational and directed evolution approaches [reviewed in 10 and 87]. CYP2B1, CYP2B4, CYP2B6, and CYP2B11 were engineered for enhanced expression and solubility by using a rational approach [135]. CYP2B enzymes were engineered based on pioneering work by Johnson and colleagues, who engineered N-terminal (membrane-bound domain) of CYP2C5 by deleting 21 residues and replacing 5 hydrophobic-to-basic residues [136]. The engineered CYP enzymes (termed dH; N-terminal deleted and C-terminal His-tagged) were soluble and exhibited a 5- to 10-fold higher expression than the wild-type. N-terminal engineering led to a breakthrough in the crystallization of the first mammalian CYP enzyme CYP2C5 [137], followed by several other mammalian CYP, such as CYP2B4, CYP2C5, CYP2C8, CYP2C9, and CYP3A4 [reviewed in 10, 138-139].
Based on known chimeragenesis, protein modeling, and X-ray crystal structures, several CYP2B enzymes were engineered for enhanced activity, stability, and expression as well as for altered regio- and stereoselectivity by site-directed mutagenesis of active-site, substrate access channel, and CSM residues [reviewed in 10, 87, and 142; 89, 140-145; unpublished observations]. Some examples are: 1) I480V of CYP2B1 and L363V of CYP2B11, which exhibited higher, 2′,3,3′,6,6′-hexachlorobiphenyl hydroxylase activity; 2) Residue 209 of F-helix when replaced to Ala in CYP2B1dH, CYP2B4dH, CYP2B11dH showed a 5-, 50-, and 4-fold enhanced catalytic efficiency, respectively, with testosterone; 3) Replacement of seven simultaneous active-site residues of CYP2B1dH to the corresponding CYP2C5dH residues converted progesterone 16α- to 21-hydroxylase activity; 4) Active-site substitution Ile363→Ala in 2B4 showed a 150-fold enhanced catalytic efficiency for testosterone hydroxylation.
More recently, Kumar, Halpert, and colleagues have engineered human CYP2B6dH for enhanced expression and stability by using a semi-rational approach (Figure 1) [140]. The mutants L198M, L264F, and L390P showed a ~3-fold higher expression than did CYP2B6dH. In addition, L264F exhibited an enhanced stability against thermal and chemical denaturation compared to that with CYP2B6dH. Furthermore, CYP2B6dH and CYP2B11dH were engineered by replacing their residues, which are identical in both, to the corresponding residues that are identical in relatively more stable CYP2B1dH and CYP2B4dH. The CYP2B6dH and CYP2B11dH mutants showed increased stability compared to their respective wild-type enzymes (unpublished observations). The engineering of CYP2B6dH led to its structure-function studies, which employed biochemical, biophysical, and X-ray crystallography [140; unpublished observations]. The engineered CYP2B6dH enzymes could be a better candidates for GDEPT, provided these show enhanced expression and stability in tumor cells.
Directed evolution of CYP2B1dH L209A created V183L/F202L/L209A/S334P mutant, which enhanced the utilization of H2O2 for 7-EFC O-deethylation by 6-fold (Figure 1) [98]. Subsequently, the F202L/L209A/S334P mutant was constructed in CYP2B1dH and CYP2B1 full-length backgrounds, which are among the most active P450s for the metabolism of several substrates, such as 7-ethoxy-4-(trifluoromethyl) coumarin (7-EFC), 7-benzyloxyresorufin (7-BR), testosterone, and benzphetamine in the standard NADPH system. Furthermore, L209A/S334P and L209A/V183L mutants showed a >2.5-fold enhanced catalytic efficiency with the anti-cancer prodrugs CPA and IFA for 4-hydroxylation compared with the wild-type [94]. These mutants also showed >2-fold decreased catalytic efficiency for N-dechloroethylation (toxic reaction). The V183L/F202L/L209A/S334P mutant (QM) was further subjected to directed evolution to enhance catalytic tolerance to temperature and DMSO [105]. While the CYP2B1dH QM/L295H mutant exhibited a significantly higher activity than did QM at a broad range of temperatures (35-55°C), the QM/K236I/D257N/L295H mutant displayed a >2-fold higher activity than did QM at nearly the entire range of DMSO concentrations (Figure 1). Furthermore, based on the mutations found in the CYP2B1dH QM, corresponding substitutions were made in CYP2B11dH (the most active enzyme for the activation of CPA and IFA) [141]. Among the mutants created, V183L showed a: 1) 4-fold increased kcat for 7-BR debenzylation, 2) 4.7-fold increased kcat/Km for testosterone 16α-hydroxylation, and 3) 1.7-fold higher kcat/Km for the activation of CPA and IFA, compared with CYP2B11dH. Especially, the CYP2B11dH V183L mutant showed a ~4-fold decreased Km (0.060 mM and 0.030 mM for CPA and IFA, respectively) compared to that with CYP2B11dH [141].
Gillam and colleagues applied a restriction enzyme-mediated DNA shuffling approach within the subfamily to design CYP1A and CYP2C enzymes [19, 146-149]. These engineered CYP enzymes showed distinct and novel activity profiles, including those with drugs. For example, a random sample of 26 CYP2C clones (shuffled from 2C8, 2C9, 2C18, and 2C19) revealed two clones with activity towards luciferin 6′-methyl ether, one towards 6′-deoxyluciferin, and five towards diclofenac 4′-hydroxylation [147]. Of 96 clones screened on solid media, one showed elevated indigo production compared to the parental forms. Similarly, from the clones obtained upon CYP1A (shuffled from CYP1A1 and CYP1A2) different activity profiles were seen with higher specific activity on individual compounds (e.g., clone 22; 9 times the CYP1A1 specific activity toward luciferin 6′-chloroethyl ether); novel activities (e.g., clone 35; activity toward 6′-deoxyluciferin and p-nitrophenol); and broadening of substrate range observed in particular clones (e.g., clone 9; activity toward both selective substrates luciferin 6′-methyl ether and luciferin 6′-chloroethyl ether as well as toward 6′-deoxyluciferin and p-nitrophenol) [148].
CYP2D6 was recently engineered (T309V) by site-directed mutagenesis of the active site residues, Thr-309, which showed a 2- to 4-fold enhanced activity for CuOOH-mediated N-demethylation of 7-methoxy-4-(aminomethyl)-coumarin (MAMC), 3,4-methylenedioxymethylamphetamine (MDMA), and dextromethorphan [150]. CuOOH-supported activity of T309A, however, was enhanced by 70-fold for the oxidation of bufuralol compared with the findings with the wild-type, suggesting its application in the synthesis of bufuralol metabolites. Subsequently, a T309A mutant was created in CYP3A4, which showed the 2- and 4-fold enhanced utilization of CuOOH (kcat/Km,CuOOH) for the oxidation of 7-benzyloxy-4-(trifluoromethyl)coumarin (7-BFC) and 7-benzyloxyquinoline (7-BQ), respectively, compared with the wild-type [99]. Directed evolution of CYP3A4 yielded several mutants (L216W, F228I, F242V, and T433S), which showed a 2- to 4- fold enhanced utilization of CuOOH with 7-BQ compared with the wild-type [99]. More importantly, T433S in the presence of cytochrome b5 in a CuOOH-supported reaction showed an 80% conversion of testosterone to 6β-OH testosterone with a total turn-over of 180 min−1. An effort was made to engineer CYP3A4-CPR for altered enzyme cooperativity for 7-BQ. 7-BQ yields sigmoidal curve with CYP3A4, which become hyperbolic in the presence of 25 μM α-naphthoflavone [99]. Upon directed evolution, we created two CYP3A4 mutants, which exhibited altered enzyme cooperativity with 7-BQ (unpublished observations).
6. Conclusion
In the past decade a need for CYP biocatalysts has emerged because of their use (Figure 1) for: 1) Synthesis of drugs and drug metabolites in the pharmaceutical industry; 2) Synthesis of indigo-based colored compounds in the dye and horticulture industries; 3) Synthesis of agrochemicals and other fine chemicals in the food and chemical industries; 4) P450-based GDEPT for the activation of the anti-cancer prodrugs CPA and IFA; 5) Biosensor design to monitor drug levels on blood plasma in clinics and hazardous components in the food industry; 6) Bioremediation using transgenic plants and immobilized systems. Since the pharmaceutical/biotechnological industries and scientific community have recognized the need for CYP biocatalysts, they are now engaged in designing CYP by rational, semi-rational, CSM, and directed evolution approaches to improve catalytic efficiency, stability, expression, cofactor requirements, and the suitability of CYP-CPR fusion enzymes, as well as regio- and stereoselective hydroxylation of specific substrates (Table 2). In addition, researchers are actively searching for alternate oxidants, such as peroxides, metal powders, and metal electrodes (Table 1), to acquire simple, cost-effective and efficient ways to perform CYP-mediated reactions. Engineering of CYP enzymes by rational, semi-rational, and CSM approaches is limited because of an incomplete understanding of the structure-function relationships of these enzymes. Therefore, directed evolution involving random mutagenesis and DNA shuffling, followed by simple and effective HTS methods, can potentially engineer CYP for novel and enhanced properties (Table 2). In addition, developing HTS by using flow cytometry is an extremely powerful approach for designing CYP. Rational and directed evolution approaches have been successfully applied to engineer bacterial and mammalian CYP enzymes for enhanced activity, stability, and expression in E. coli, as well as for altered substrate specificity and regio- and stereoselectivity, and redox partner requirements. Thus, the future of engineered CYP enzymes in industrial, medical, and environmental applications appears bright.
7. Expert Opinion
7.1. Applications of cytochrome P450 biocatalysts
Approximately 30% of drug candidates are terminated at clinical trials due to drug and its metabolite toxicities in animals and humans [151]. Therefore, “drug metabolites in safety testing” has become an integral part of drug development in the 21st century [152]. This has led to increased demand for drug metabolites to facilitate research to study their possible adverse effects in animals and humans. Thus freeze-dried and immobilized CYP are expected to be used for bulk synthesis of drug metabolites. In addition, a CYP nanobichip has the potential as a bioreactor to generate drug metabolites. Due to differential drug responses as a result of CYP polymorphism and drug interactions, we expect to see a rapid growth in the next five to ten years in the design of CYP biosensors. Application of CYP biosensors can further be expanded into nanotechnology, which would require coupling of CYP enzymes with electrical circuits in an electrode in nonphysiological environments. We predict rapid developments in the practical use of targeted CYP-based GDEPT for cancer treatment, because it has a tremendous potential for reducing adverse effects of chemotherapy. However, although the clinical trials using CYP2B6 are promising, delivery of these genes and prodrugs to targeted cancer cells without affecting the non-malignant cells still poses a serious concern. CYP-cloned transgenic plants that have ability to detoxify soil contaminants, such as atrazine, norflurazon, metolachlor, and sulfonylureas, will not only improve agricultural production, but will also increase unused lands that are polluted by industrial toxic wastes. In addition, the researchers have proposed the use of recombinant CYP in bacterial systems for wastewater treatment. An increasing concern about environmental pollution and global warming reflects a need for green chemistry that uses CYP biocatalysts in the 21st century. However, the use of transgenic plants may be of general concern in public regarding their possible long-term health issues, especially in the European countries.
7.2. Cost-effective approaches to utilize cytochrome P450 biocatalysts
There are significant opportunities for optimizing artificial recombinant fusion proteins by improving the coupling between CYP and reductase domains and exploiting alternative electron transport partners. In most cases the catalytic rates with fusion enzymes are not optimal, which suggest to us that there is a potential for further improvements in creating more effective fusion enzymes. Because fusion enzymes still require the expensive cofactor NADPH, alternative means have been proposed: 1) light-driven NADPH synthesis in chloroplast in the presence of CYP-CPR fusion enzymes [153] and 2) engineering CYP system for increased specificity towards relatively inexpensive NADH [154]. Although in situ NAD(P)H (re)generation may be the most practical source of electrons, recent studies have provided evidence that peroxides and metal powders could be a cheap and effective alternative for in vitro applications, especially in industrial synthesis (Table 1). In the case of biosensor design, metal electrodes immobilized with CYP enzyme are the only source of electrons. Bicistronic expression of CYP and CPR in a retroviral vector has proven to be the most efficient way to activate CPA and IFA in cancer cells. A co-expression system also appears to be the most effective in generating transgenic plants for phytoremediation. Alternatively, CYP enzymes can be engineered to interact with the host cell reductase(s) as well as for their subcellular and tissue distributions.
7.3. Mammalian vs. bacterial cytochrome P450 biocatalysts
It is generally believed that bacterial CYP enzymes, which possess higher turnover, stability, and expression than their mammalian counterparts, are the most suitable candidates for practical purposes; even for the synthesis of non-natural substrates [13]. Furthermore, bacterial CYP biocatalysts can function or can be tailored to function under extreme conditions of temperature, pH, buffer system, or solvent. Indeed, bacterial CYP enzymes have been recently engineered for the metabolism of several non-natural substrates, such as smaller alkanes and drugs. However, it remains to be seen whether bacterial enzymes can be tailored for the regio- or stereoselective synthesis of drugs and drug metabolites of variable size, shape, and geometry. Recent discoveries revealed that, in contrast to the bacterial, mammalian CYP enzymes have large and flexible active sites, which are capable of adapting their conformation based on the size, shape, and geometry of substrates [138-139]. Thus, for regio- and stereoselective chemistry the engineering of mammalian CYP enzymes for enhanced activity for their own array of substrates is easier than engineering bacterial CYP to accommodate the vast majority of substrates of mammalian CYP. In addition, the engineering of mammalian CYP enzymes may be the only way to improve cancer gene therapy using GDEPT and phytoremediation using transgenic plants. Recently, several mammalian CYP enzymes have been engineered, which are expected to find application(s) in drug and chemical synthesis, dye production, horticulture, biosensor, cancer gene therapy, and/or bioremediation.
Acknowledgments
Declaration of Interest
Financial support was provided by NIH Grants ES03619 and GM54995, and Center grant ES06676 (to James R. Halpert), NIH Grants CA49248 and 5 P42 ES07381 (to David J. Waxman), and NIEHS Center pilot grant ES06676 and start-up fund KBE78 (to Santosh Kumar).
References
Papers of special notes have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim Biophys Acta. 2007;1770:314–29. doi: 10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Anzenbacher P, Anzenbacherova F. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 2001;58:737–47. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coon MJ. Cytochrome P450: Nature's most versatile biological catalyst. Ann Rev Pharmacol Toxicol. 2005;45:1–25. doi: 10.1146/annurev.pharmtox.45.120403.100030. [DOI] [PubMed] [Google Scholar]
- 4.Murataliev MB, Klein M, Fulco A, Feyereisen R. Functional interactions in cytochrome P450BM3: flavin semiquinone intermediates, role of NADP(H), and mechanism of electron transfer by the flavoprotein domain. Biochemistry. 1997;36:8401–12. doi: 10.1021/bi970026b. [DOI] [PubMed] [Google Scholar]
- 5.Sligar SG. Coupling of spin, substrate, and redox equilibria in cytochrome P450. Biochemistry. 1976;615:5399–406. doi: 10.1021/bi00669a029. [DOI] [PubMed] [Google Scholar]
- 6.Braeuning A. Regulation of cytochrome P450 expression by Ras- and beta-catenin-dependent signaling. Curr Drug Metab. 2009;10:138–58. doi: 10.2174/138920009787522160. [DOI] [PubMed] [Google Scholar]
- 7.Davydov DR, Halpert JR. Allosteric P450 mechanisms: multiple binding sites, multiple conformers or both? Expert Opin Drug Metab Toxicol. 2008;4:1523–35. doi: 10.1517/17425250802500028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracy TS. Atypical cytochrome P450 kinetics: implications for drug discovery. Drugs R D. 2006;7:349–63. doi: 10.2165/00126839-200607060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Boobis A, Watelet JB, Whomsley R, Benedetti MS, Demoly P, Tipton K. Drug interactions. Drug Metab Rev. 2009;41:486–52. doi: 10.1080/10837450902891550. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YH, Halpert JR. Structure-function analysis of cytochromes P450 2B. Arch Biochem Biophys. 2007;1770:402–12. doi: 10.1016/j.bbagen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11••.Guengerich FP. Cytochrome P450 enzymes in the generation of commercial products. Nat Rev Drug Discov. 2002;1:359–66. doi: 10.1038/nrd792. The first review, which highlights the prospects of the commercial use of cytochromes P450 in the generation of novel drugs, bioremediation, prodrugs activation, and biosensor design. [DOI] [PubMed] [Google Scholar]
- 12.Guengerich FP. Cytochrome P450 oxidations in the generation of reactive electrophiles: epoxidation and related reactions. Arch Biochem Biophys. 2003;409:59–71. doi: 10.1016/s0003-9861(02)00415-0. [DOI] [PubMed] [Google Scholar]
- 13.Urlacher VB, Lutz-Wahl S, Schmid RD. Microbial P450 enzymes in biotechnology. Appl Microbiol Biotechnol. 2004;64:317–25. doi: 10.1007/s00253-003-1514-1. [DOI] [PubMed] [Google Scholar]
- 14•.Gillam EM. Exploring the potential of xenobiotic-metabolising enzymes as biocatalysts: evolving designer catalysts from polyfunctional cytochrome P450 enzymes. Clin Exp Pharmacol Physiol. 2005;32:147–52. doi: 10.1111/j.1440-1681.2005.04165.x. The first review describing the need of engineering of xenobiotic-metabolizing P450 enzymes for the industrial applications. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt R. Cytochromes P450 as versatile biocatalysts. J Biotechnol. 2006;124:128–45. doi: 10.1016/j.jbiotec.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 16.McLean KJ, Girvan HM, Munro AW. Cytochrome P450/redox partner fusion enzymes: biotechnological and toxicological prospects. Expert Opin Drug Metab Toxicol. 2007;3:847–63. doi: 10.1517/17425255.3.6.847. [DOI] [PubMed] [Google Scholar]
- 17.Julsing MK, Cornelissen S, Bühler B, Schmid A. Heme-iron oxygenases: powerful industrial biocatalysts? Curr Opin Chem Biol. 2008;12:177–86. doi: 10.1016/j.cbpa.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 18•.Morant M, Bak S, Møller BL, Werck-Reichhart D. Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol. 2003;14:151–62. doi: 10.1016/s0958-1669(03)00024-7. The first review, which describes the use of the natural plant P450 enzymes in plant defense against insects, pathogens, herbicides, pollutants, and other xenobiotics. [DOI] [PubMed] [Google Scholar]
- 19••.Gillam EM. Engineering cytochrome P450 enzymes. Chem Res Toxicol. 2008;21:220–31. doi: 10.1021/tx7002849. The most recent review on the engineering aspects of P450 enzymes for increased P450 activity, stability, specificity, and redox-partner requirements. [DOI] [PubMed] [Google Scholar]
- 20.Abhilash PC, Jamil S, Singh N. Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol Adv. 2009;27:474–88. doi: 10.1016/j.biotechadv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Waxman DJ. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Curr Pharm Des. 2002;8:1405–16. doi: 10.2174/1381612023394566. [DOI] [PubMed] [Google Scholar]
- 22.Dachs GU, Tupper J, Tozer GM. From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anticancer Drugs. 2005;16:349–59. doi: 10.1097/00001813-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 23••.Roy P, Waxman DJ. Activation of oxazaphosphorines by cytochrome P450: application to gene-directed enzyme prodrug therapy for cancer. Toxicology in Vitro. 2006;20:176–86. doi: 10.1016/j.tiv.2005.06.046. This recent review describes the approaches and feasibility of P450-based gene-directed enzyme prodrug therapy for possible application in targeted cancer therapy. [DOI] [PubMed] [Google Scholar]
- 24.Sonomoto K, Hoq MM, Tanaka A, Fukui S. 11beta-Hydroxylation of Cortexolone (Reichstein Compound S) to Hydrocortisone by Curvularia lunata Entrapped in Photo-Cross-Linked Resin Gels. Appl Environ Microbiol. 1983;45:436–43. doi: 10.1128/aem.45.2.436-443.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii T, Fujii Y, Machida K, Ochiai A, Ito M. Efficient biotransformations using Escherichia coli with tolC acrAB mutations expressing cytochrome P450 genes. Biosci Biotechnol Biochem. 2009;73:805–10. doi: 10.1271/bbb.80627. [DOI] [PubMed] [Google Scholar]
- 26.Falck JR, Reddya YK, Hainesa DC, Reddya KM, Krishnaa UM, Grahama S, Murrya B, Peterson JA. Practical enantiospecific syntheses of 14,15-EET and leukotoxin B (vernolic acid) Tetrahedron Letters. 2001;42:4131–33. [Google Scholar]
- 27.Shafiee A, Hutchinson CR. Macrolide antibiotic biosynthesis: isolation and properties of two forms of 6-deoxyerythronolide B hydroxylase from Saccharopolyspora erythraea (Streptomyces erythreus) Biochemistry. 1987;26:6204–10. doi: 10.1021/bi00393a037. [DOI] [PubMed] [Google Scholar]
- 28.Jennewein S, Rithner CD, Williams RM, Croteau RB. Taxol biosynthesis: taxane 13 alpha-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci (USA) 2001;98:13595–600. doi: 10.1073/pnas.251539398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Beilen JB, Holtackers R, Lüscher D, Bauer U, Witholt B, Duetz WA. Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl Environ Microbiol. 2005;71:1737–44. doi: 10.1128/AEM.71.4.1737-1744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Vail RB, Homann MJ, Hanna I, Zaks A. Preparative synthesis of drug metabolites using human cytochrome P450s 3A4, 2C9, and 1A2 with NADPH-P450 reductase expressed in Escherichia coli. J Ind Microbiol Biotechnol. 2005;32:67–74. doi: 10.1007/s10295-004-0202-1. The first paper proposing the use of the human P450 enzymes for the preparative synthesis of drug metabolites. [DOI] [PubMed] [Google Scholar]
- 31.Lamb SB, Lamb DC, Kelly SL, Stuckey DC. Cytochrome P450 immobilization as a route to bioremediation/biocatalysis. FEBS Lett. 1998;431:343–46. doi: 10.1016/s0014-5793(98)00771-6. [DOI] [PubMed] [Google Scholar]
- 32.Ueda Y, Morigaki K, Tatsu Y, Yumoto N, Imaishi H. Immobilization and activity assay of cytochrome P450 on patterned lipid membranes. Biochem Biophys Res Commun. 2007;355:926–31. doi: 10.1016/j.bbrc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 33•.Nicoli R, Bartolini M, Rudaz S, Andrisano V, Veuthey JL. Development of immobilized enzyme reactors based on human recombinant cytochrome P450 enzymes for phase I drug metabolism studies. J Chromatogr A. 2008;1206:2–10. doi: 10.1016/j.chroma.2008.05.080. This is the most recent review on the development of cytochrome P450-based immobilized enzyme reactors (IMERs) to perform automated on-line phase I drug metabolism studies. [DOI] [PubMed] [Google Scholar]
- 34.Gannett PM, Kabulski J, Perez FA, Liu Z, Lederman D, Locuson CW, Ayscue RR, Thomsen NM, Tracy TS. Preparation, characterization, and substrate metabolism of gold-immobilized cytochrome P450 2C9. J Am Chem Soc. 2006;128:8374–75. doi: 10.1021/ja0608693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mie Y, Suzuki M, Komatsu Y. Electrochemically driven drug metabolism by membranes containing human cytochrome P450. J Am Chem Soc. 2009;131:6646–7. doi: 10.1021/ja809364r. [DOI] [PubMed] [Google Scholar]
- 36.Royo JL, Moreno-Ruiz E, Cebolla A, Santero E. Stable long-term indigo production by overexpression of dioxygenase genes using a chromosomal integrated cascade expression circuit. J Biotechnol. 2005;116:113–24. doi: 10.1016/j.jbiotec.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 37••.Gillam EM, Aguinaldo AM, Notley LM, Kim D, Mundkowski RG, Volkov AA, Arnold FH, Soucek P, DeVoss JJ, Guengerich FP. Formation of indigo by recombinant mammalian cytochrome P450. Biochem Biophys Res Commun. 1999;265:469–72. doi: 10.1006/bbrc.1999.1702. This paper reports a breakthrough observation on the formation of indigo by recombinant cytochrome P450. [DOI] [PubMed] [Google Scholar]
- 38.Warzecha H, Frank A, Peer M, Gillam EM, Guengerich FP, Unger M. Formation of the indigo precursor indican in genetically engineered tobacco plants and cell cultures. Plant Biotechnol J. 2007;5:185–91. doi: 10.1111/j.1467-7652.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 39.Gillam EM, Guengerich FP. Exploiting the versatility of human cytochrome P450 enzymes: the promise of blue roses from biotechnology. IUBMB Life. 2001;52:271–77. doi: 10.1080/152165401317291110. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, Roy P, Waxman DJ. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol. 2000;59:961–72. doi: 10.1016/s0006-2952(99)00410-4. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z, Raychowdhury MK, Waxman DJ. Impact of liver P450 reductase suppression on cyclophosphamide activation, pharmacokinetics and antitumoral activity in a cytochrome P450-based cancer gene therapy model. Cancer Gene Ther. 2000;7:1034–42. doi: 10.1038/sj.cgt.7700200. [DOI] [PubMed] [Google Scholar]
- 42•.Jounaidi Y, Hecht JE, Waxman DJ. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58:4391–401. This paper reports a retroviral transfer of cytochromes P450 genes in 9L-gliosarcoma cells to study the activation of cyclophosphamide and ifosfamide. [PubMed] [Google Scholar]
- 43.Jounaidi Y, Waxman DJ. Use of replication-condition adenovirus as a helper system to enhance delivery of P450 prodrug-activation genes in cancer therapy. Cancer Res. 2004;64:292–303. doi: 10.1158/0008-5472.can-03-1798. [DOI] [PubMed] [Google Scholar]
- 44.Chen CS, Lin JT, Goss KA, He YA, Halpert JR, Waxman DJ. Activation of the anticancer prodrugs cyclophosphamide and ifosfamide: identification of cytochrome P450 2B enzymes and site-specific mutants with improved enzyme kinetics. Mol Pharmacol. 2004;65:1278–85. doi: 10.1124/mol.65.5.1278. [DOI] [PubMed] [Google Scholar]
- 45.Chen CS, Jounaidi Y, Waxman DJ. Enantioselective metabolism and cytotoxicity of R-ifosfamide and S-ifosfamide by tumor cell-expressed cytochromes P450. Drug Metab Dispos. 2005;33:1261–67. doi: 10.1124/dmd.105.004788. [DOI] [PubMed] [Google Scholar]
- 46.Chang TK, Yu L, Goldstein JA, Waxman DJ. Identification of the polymorphically expressed CYP2C19 and the wild-type CYP2C9-ILE359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics. 1997;7:211–21. doi: 10.1097/00008571-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Jounaidi Y, Waxman DJ. Frequent, moderate-dose cyclophosphamide administration improves the efficacy of cytochrome P-450/cytochrome P450 reductase-based cancer gene therapy. Cancer Res. 2001;61:4437–44. [PubMed] [Google Scholar]
- 48••.Hunt S. Technology evaluation: MetXia-P450. Oxford Biomedica. Curr Opin Mol Ther. 2001;3:595–98. This report shows the success of phase II clinical trial of CYP2B6 and suggests its potential in P450-based gene-directed enzyme prodrug therapy. [PubMed] [Google Scholar]
- 49.Jounaidi Y, Chen CS, Veal GJ, Waxman DJ. Enhanced antitumor activity of P450 prodrug-based gene therapy using the low Km cyclophosphamide 4-hydroxylase P450 2B11. Mol Cancer Therap. 2006;5:541–55. doi: 10.1158/1535-7163.MCT-05-0321. [DOI] [PubMed] [Google Scholar]
- 50.Ma Y, Waxman DJ. Collaboration between hepatic and intratumoral prodrug activation in a P450 prodrug-activation gene therapy model for cancer treatment. Mol Cancer Ther. 2007;6:2879–90. doi: 10.1158/1535-7163.MCT-07-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Chen CS, Jounaidi Y, Su T, Waxman DJ. Enhancement of intratumoral cyclophosphamide pharmacokinetics and antitumor activity in a P450 2B11-based cancer gene therapy model. Cancer Gene Ther. 2007;14:935–44. doi: 10.1038/sj.cgt.7701092. This report shows the importance of a low-Km enzyme for an enhanced activation of cyclophosphamide in tumor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Bistolas N, Wollenberger U, Jung C, Scheller FW. Cytochrome P450 biosensors-a review. Biosens Bioelectron. 2005;20:2408–23. doi: 10.1016/j.bios.2004.11.023. A comprehensive review, which describes the utilization of various isoforms of P450 in the construction of biosensors for their applications in environmental monitoring, pharmaceutical industry, and clinical practices. [DOI] [PubMed] [Google Scholar]
- 53.Bozina N, Bradamante V, Lovrić M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh Hig Rada Toksikol. 2009;60:217–42. doi: 10.2478/10004-1254-60-2009-1885. [DOI] [PubMed] [Google Scholar]
- 54.Alonso-Lomillo MA, Yardimci C, Domínguez-Renedo O, Arcos-Martínez MJ. CYP450 2B4 covalently attached to carbon and gold screen printed electrodes by diazonium salt and thiols monolayers. Anal Chim Acta. 2009;633:51–56. doi: 10.1016/j.aca.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 55•.Ignaszak A, Hendricks N, Waryo T, Songa E, Jahed N, Ngece R, Al-Ahmed A, Kgarebe B, Baker P. Iwuoha EI Novel therapeutic biosensor for indinavir-a protease inhibitor antiretroviral drug. J Pharm Biomed Anal. 2009;49:498–501. doi: 10.1016/j.jpba.2008.10.025. The most recent paper on the practical application of CYP3A4 biosensor for monitoring indinavir level in blood plasma. [DOI] [PubMed] [Google Scholar]
- 56.Ferrero VE, Andolfi L, Di Nardo G, Sadeghi SJ, Fantuzzi A, Cannistraro S, Gilardi G. Protein and electrode engineering for the covalent immobilization of P450 BMP on gold. Anal Chem. 2008;80:8438–46. doi: 10.1021/ac8011413. [DOI] [PubMed] [Google Scholar]
- 57.Carrara S, Shumyantseva VV, Archakov AI, Samorì B. Screen-printed electrodes based on carbon nanotubes and cytochrome P450scc for highly sensitive cholesterol biosensors. Biosens Bioelectron. 2008;24:148–50. doi: 10.1016/j.bios.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Alonso-Lomillo MA, Gonzalo-Ruiz J, Domínguez-Renedo O, Muñoz FJ, Arcos-Martínez MJ. CYP450 biosensors based on gold chips for antiepileptic drugs determination. Biosens Bioelectron. 2008;23:1733–37. doi: 10.1016/j.bios.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 59.Liu S, Peng L, Yang X, Wu Y, He L. Electrochemistry of cytochrome P450 enzyme on nanoparticle-containing membrane-coated electrode and its applications for drug sensing. Anal Biochem. 2008;375:209–16. doi: 10.1016/j.ab.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Iwuoha E, Ngece R, Klink M, Baker P. Amperometric responses of CYP2D6 drug metabolism nanobiosensor for sertraline: a selective serotonin reuptake inhibitor. IET Nanobiotechnol. 2007;1:62–7. doi: 10.1049/iet-nbt:20070005. [DOI] [PubMed] [Google Scholar]
- 61.Shumyantseva VV, Bulko TV, Archakov AI. Electrochemical reduction of cytochrome P450 as an approach to the construction of biosensors and bioreactors. J Inorg Biochem. 2005;99:1051–63. doi: 10.1016/j.jinorgbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Paternolli C, Antonini M, Ghisellini P, Nicolini C. Recombinant cytochrome p450 immobilization for biosensor applications. Langmuir. 2004;20:11706–12. doi: 10.1021/la048081q. [DOI] [PubMed] [Google Scholar]
- 63.Fantuzzi A, Meharenna YT, Briscoe PB, Sassone C, Borgia B, Gilardi G. Improving catalytic properties of P450BM3 haem domain electrodes by molecular Lego. Chem Commun (Camb) 2006;28:1289–91. doi: 10.1039/b517472d. [DOI] [PubMed] [Google Scholar]
- 64.Vehlow J, Bergfeldt B, Hunsinger H. PCDD/F and related compounds in solid residues from municipal solid waste incineration--a literature review. Waste Manag Res. 2006;24:404–20. doi: 10.1177/0734242X06066321. [DOI] [PubMed] [Google Scholar]
- 65.Ishaq R, Näf C, Zebühr Y, Broman D, Järnberg U. PCBs, PCNs, PCDD/Fs, PAHs and Cl-PAHs in air and water particulate samples--patterns and variations. Chemosphere. 2003;50:1131–50. doi: 10.1016/s0045-6535(02)00701-4. [DOI] [PubMed] [Google Scholar]
- 66.Sakaki T, Shinkyo R, Takita T, Ohta M, Inouye K. Biodegradation of polychlorinated dibenzo-p-dioxins by recombinant yeast expressing rat CYP1A subfamily. Arch Biochem Biophys. 2002;401:91–98. doi: 10.1016/S0003-9861(02)00036-X. [DOI] [PubMed] [Google Scholar]
- 67.Shinkyo R, Sakaki T, Takita T, Ohta M, Inouye K. Generation of 2,3,7,8-TCDD-metabolizing enzyme by modifying rat CYP1A1 through site-directed mutagenesis. Biochem Biophys Res Commun. 2003;308:511–7. doi: 10.1016/s0006-291x(03)01439-6. [DOI] [PubMed] [Google Scholar]
- 68.Shinkyo R, Kamakura M, Ikushiro S, Inouye K, Sakaki T. Biodegradation of dioxins by recombinant Escherichia coli expressing rat CYP1A1 or its mutant. Appl Microbiol Biotechnol. 2006;72:584–90. doi: 10.1007/s00253-005-0286-1. [DOI] [PubMed] [Google Scholar]
- 69.Preston BD, Allen JR. 2,2′,5,5′-Tetrachlorobiphenyl: isolation and identification of metabolites generated by rat liver microsomes. Drug Metab Dispos. 198;08:197–203. [PubMed] [Google Scholar]
- 70.Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2′,3,3′,6,6′-hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem Res Toxicol. 1999;12:690–9. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- 71.Billet S, Abbas I, Le Goff J, Verdin A, André V, Lafargue PE, Hachimi A, Cazier F, Sichel F, Shirali P, Garçon G. Genotoxic potential of Polycyclic Aromatic Hydrocarbons-coated onto airborne Particulate Matter (PM 2.5) in human lung epithelial A549 cells. Cancer Lett. 2008;270:144–55. doi: 10.1016/j.canlet.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 72.Morant M, Bak S, Møller BL, Werck-Reichhart D. Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol. 2003;14:151–62. doi: 10.1016/s0958-1669(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 73.Van Aken B. Transgenic plants for enhanced phytoremediation of toxic explosives. Curr Opin Biotechnol. 2009;20:231–36. doi: 10.1016/j.copbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Abhilash PC, Jamil S, Singh N. Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol Adv. 2009;27:474–88. doi: 10.1016/j.biotechadv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 75••.Kawahigashi H. Transgenic plants for phytoremediation of herbicides. Curr Opin Biotechnol. 2009;20:225–30. doi: 10.1016/j.copbio.2009.01.010. Ref. 73-75 are the most recent and comprehensive reviews on the application of P450-generated transgenic plants for phytoremediation. [DOI] [PubMed] [Google Scholar]
- 76.Inui H, Ohkawa H. Herbicide resistance in transgenic plants with mammalian P450 monooxygenase genes. Pest Manag Sci. 2005;61:286–91. doi: 10.1002/ps.1012. [DOI] [PubMed] [Google Scholar]
- 77••.Rylott EL, Jackson RG, Edwards J, Womack GL, Seth-Smith HMB, Rathbone DA, Strand SE, Bruce NC. An explosive-degrading cytochrome P450 activity and its targeted application for the phytoremediation of RDX. Nat Biotechnol. 2006;24:216–19. doi: 10.1038/nbt1184. The first example of the practical use of P450-generated transgenic plant for the detoxification of RDX, which improved the health of the plants. [DOI] [PubMed] [Google Scholar]
- 78.Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y. Phytoremediation of the herbicides atrazine and metolachlor by transgenic rice plants expressing human CYP1A1, CYP2B6, and CYP2C19. J Agric Food Chem. 2006;54:2985–91. doi: 10.1021/jf052610u. [DOI] [PubMed] [Google Scholar]
- 79.Kawahigashia H, Hirosea S, Ohkawab H, Ohkawa Y. Herbicide resistance of transgenic rice plants expressing human CYP1A1. Biotechnol Adv. 2007;25:75–84. doi: 10.1016/j.biotechadv.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Kawahigashi H, Hirose S, Ohkawa H, Ohkawa Y. Transgenic rice plants expressing human P450 genes involved in xenobiotic metabolism for phytoremediation. J Mol Microbiol Biotechnol. 2008;15:212–19. doi: 10.1159/000121332. [DOI] [PubMed] [Google Scholar]
- 81.James CA, Xin G, Doty SL, Strand SC. Degradation of low molecular weight volatile organic compounds by plants genetically modified with mammalian cytochrome P450 2E1. Environ Sci Technol. 2008;42:289–93. doi: 10.1021/es071197z. [DOI] [PubMed] [Google Scholar]
- 82•.Munro AW, Girvan HM, McLean KJ. Cytochrome P450-redox partner fusion enzymes. Biochim Biophys Acta. 2007;1770:345–59. doi: 10.1016/j.bbagen.2006.08.018. This review focuses on the nature and diversity of CYP/redox partner fusion enzymes and their biocatalytic potential. [DOI] [PubMed] [Google Scholar]
- 83.Cheng J, Wan DF, Gu JR, Gong Y, Yang SL, Hao DC, Yang L. Establishment of a yeast system that stably expresses human cytochrome P450 reductase: application for the study of drug metabolism of cytochrome P450s in vitro. Protein Expr Purif. 2006;47:467–76. doi: 10.1016/j.pep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Peters FT, Dragan CA, Kauffels A, Schwaninger AE, Zapp J, Bureik M, Maurer HH. Biotechnological synthesis of the designer drug metabolite 4′-hydroxymethyl-alpha-pyrrolidinohexanophenone in fission yeast heterologously expressing human cytochrome P450 2D6--a versatile alternative to multistep chemical synthesis. J Anal Toxicol. 2009;33:190–7. doi: 10.1093/jat/33.4.190. [DOI] [PubMed] [Google Scholar]
- 85••.Purnapatre K, Khattar SK, Saini KS. Cytochrome P450s in the development of target-based anticancer drugs. Cancer Lett. 2008;259:1–15. doi: 10.1016/j.canlet.2007.10.024. This review is focused on over-expression of human CYP in bacteria, yeast, insect and mammalian cells, followed by their purification on an industrial scale to facilitate identification of novel anticancer drugs. [DOI] [PubMed] [Google Scholar]
- 86.Parikh A, Gillam EM, Guengerich FP. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat Biotechnol. 1997;15:784–8. doi: 10.1038/nbt0897-784. [DOI] [PubMed] [Google Scholar]
- 87••.Kumar S, Halpert JR. Use of directed evolution of mammalian cytochromes P450 for investigating the molecular basis of enzyme function and generating novel biocatalysts. Biochem Biophys Res Commun. 2006;338:456–64. doi: 10.1016/j.bbrc.2005.08.080. The first and comprehensive review on various directed evolution strategies and its application in designing mammalian P450 enzymes to generate novel biocatalysts. [DOI] [PubMed] [Google Scholar]
- 88•.Otey CR, Landwehr M, Endelman JB, Hiraga K, Bloom JD, Arnold FH. Structure-guided recombination creates an artificial family of cytochromes P450. PLoS Biol. 2006;4:e112. doi: 10.1371/journal.pbio.0040112. The first report on the generation of artificial bacterial P450 enzymes using structure-guided recombination of DNA shuffling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Rosic NN, Huang W, Johnston WA, DeVoss JJ, Gillam EM. Extending the diversity of cytochrome P450 enzymes by DNA family shuffling. Gene. 2007;395:40–8. doi: 10.1016/j.gene.2007.01.031. This review article demonstrates the use of DNA shuffling for generating artificial mammalian P450 enzymes. [DOI] [PubMed] [Google Scholar]
- 90.Ivanciuc O, Oezguen N, Mathura VS, Schein CH, Xu Y, Braun W. Using property based sequence motifs and 3D modeling to determine structure and functional regions of proteins. Curr Med Chem. 2004;11:583–93. doi: 10.2174/0929867043455819. [DOI] [PubMed] [Google Scholar]
- 91.Ivanciuc O, Braun W. Robust quantitative modeling of peptide binding affinities for MHC molecules using physical-chemical descriptors. Protein Pept Lett. 2007;14:903–16. doi: 10.2174/092986607782110257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Negi SS, Braun W. Statistical analysis of physical-chemical properties and prediction of protein-protein interfaces. J Mol Model. 2007;13:1157–67. doi: 10.1007/s00894-007-0237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Oezguen N, Kumar S, Hindupur A, Braun W, Muralidhara BK, Halpert JR. Identification and analysis of conserved motifs in cytochrome P450 family 2: Functional role of a motif 187RFDYKD192 in P450 2B enzymes. J Biol Chem. 2008;283:21808–16. doi: 10.1074/jbc.M708582200. The first report on a conserved sequence motif approach to investigate the functional role of non-active site residues in P450s, which suggests the use of this approach in P450 engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuen CM, Liu DR. Dissecting protein structure and function using directed evolution. Nature Methods. 2007;4:995–7. doi: 10.1038/nmeth1207-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Woycechowsky KJ, Vamvaca K, Hilvert D. Novel enzymes through design and evolution. Adv Enzymol Relat Areas Mol Biol. 2007;75:241–94. doi: 10.1002/9780471224464.ch4. [DOI] [PubMed] [Google Scholar]
- 96.Joo H, Lin Z, Arnold FH. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature. 1999;399:670–2. doi: 10.1038/21395. [DOI] [PubMed] [Google Scholar]
- 97.Schwaneberg U, Otey C, Cirino PC, Farinas E, Arnold FH. Cost-effective whole-cell assay for laboratory evolution of hydroxylases in Escherichia coli. J Biomol Screen. 2001;6:111–7. doi: 10.1177/108705710100600207. [DOI] [PubMed] [Google Scholar]
- 98••.Kumar S, Chen CS, Waxman DJ, Halpert JR. Directed evolution of mammalian cytochrome P450 2B1: mutations outside of the active site enhance the metabolism of several substrates, including the anticancer prodrugs cyclophosphamide and ifosfamide. J Biol Chem. 2005;280:9569–75. doi: 10.1074/jbc.M500158200. The first report on the engineering of mammalian P450 enzyme for enhanced activity using directed evolution of the whole cDNA. [DOI] [PubMed] [Google Scholar]
- 99.Kumar S, Liu H, Halpert JR. Engineering of cytochrome P450 3A4 for enhanced peroxide-mediated substarte oxidation using directed evolution and site-directed mutagenesis. Drug Metab Dispos. 2006;34:1958–65. doi: 10.1124/dmd.106.012054. [DOI] [PubMed] [Google Scholar]
- 100.Kim D, Guengerich FP. Enhancement of 7-methoxyresorufin O-demethylation activity of human cytochrome P450 1A2 by molecular breeding. Arch Biochem Biophys. 2004;432:102–8. doi: 10.1016/j.abb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Kim D, Guengerich FP. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J Biol Chem. 2005;280:40319–27. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
- 102.Kumar S. Identification of a novel laser dye substrate of mammalian cytochromes P450: Application in rapid kinetic analysis, inhibitor screening, and directed evolution. J Biolmol Screen. 2007;12:677–82. doi: 10.1177/1087057107301496. [DOI] [PubMed] [Google Scholar]
- 103•.Salazar O, Cirino PC, Arnold FH. Thermostabilization of a cytochrome P450 peroxygenase. ChemBioChem. 2003;4:891–3. doi: 10.1002/cbic.200300660. The first report on the engineering of bacterial P450BM3 for enhanced thermostability using directed evolution. [DOI] [PubMed] [Google Scholar]
- 104.Wong TS, Arnold FH, Schwaneberg U. Laboratory evolution of cytochrome P450BM3 monooxygenase for organic cosolvents. Biotechnol Bioeng. 2004;85:351–8. doi: 10.1002/bit.10896. [DOI] [PubMed] [Google Scholar]
- 105•.Kumar S, Sun L, Liu H, Muralidhara BK, Halpert JR. Engineering mammalian cytochrome P450 2B1 by directed evolution for enhanced catalytic tolerance to temperature and dimethyl sulfoxide. Protein Eng Des Select. 2006;19:547–54. doi: 10.1093/protein/gzl042. The first report on the engineering of mammalian P450 for enhanced thermostability and tolerance to organic solvent using directed evolution. [DOI] [PubMed] [Google Scholar]
- 106.Aucamp JP, Cosme AM, Lye GJ, Dalby PA. High-throughput measurement of protein stability in microtiter plates. Biotechnol Bioeng. 2005;89:599–607. doi: 10.1002/bit.20397. [DOI] [PubMed] [Google Scholar]
- 107•.Bergquist PL, Hardiman EM, Ferrari BC, Winsley T. Applications of flow cytometry in environmental microbiology and biotechnology. Extremophiles. 2009;13:389–401. doi: 10.1007/s00792-009-0236-4. The most recent review on the use of novel technology flow cytometry for in vivo high throughput activity screening for directed evolution. [DOI] [PubMed] [Google Scholar]
- 108.Bershtein S, Tawfik DS. Advances in laboratory evolution in enzymes. Cur Opin Chem Biol. 2008;12:151–8. doi: 10.1016/j.cbpa.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 109.Gupta RD, Tawfik DS. Directed enzyme evolution via small and effective neutral drift libraries. Nat Meth. 2008;5:939–42. doi: 10.1038/nmeth.1262. [DOI] [PubMed] [Google Scholar]
- 110.Farinas ET, Schwaneberg U, Glieder A, Arnond FH. Directed evolution of a cytochrome P450 mooxygenase for alkane oxidation. Adv Synth Catal. 2001;343:601–6. [Google Scholar]
- 111.Glieder A, Farinas ET, Arnold FH. Laboratory evolution of a soluble, self-sufficient, highly active alkane hydroxylase. Nat Biotechnol. 2002;20:1135–9. doi: 10.1038/nbt744. [DOI] [PubMed] [Google Scholar]
- 112.Cirino PC, Arnold FH. A self-sufficient peroxide-driven hydroxylation biocatalyst. Angew Chem Int Ed. 2003;42:3299–301. doi: 10.1002/anie.200351434. [DOI] [PubMed] [Google Scholar]
- 113.Peters MW, Meinhold P, Glieder A, Arnold FH. Regio- and enantioselective alkane hydroxylation with engineered cytochromes P450BM3. J Am Chem Soc. 2003;125:13442–50. doi: 10.1021/ja0303790. [DOI] [PubMed] [Google Scholar]
- 114.Bloom JD, Labthavikul ST, Otey CR, Arnold FH. Protein stability promotes evolvability. Proc Natl Acad Sci USA. 2006;103:5869–74. doi: 10.1073/pnas.0510098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115•.Meinhold P, Peters MW, Chen MMY, Takahashi K, Arnold FH. Direct conversion of ethane to ethanol by engineered cytochrome P450BM3. ChemBioChem. 2005;6:1765–68. doi: 10.1002/cbic.200500261. This paper reports the use of engineered P450BM3 in efficient synthesis of ethanol. [DOI] [PubMed] [Google Scholar]
- 116••.Otey CR, Bandara G, Lalonde J, Takahashi K, Arnold FH. Preparation of human metabolites of propranolol using laboratory-evolved bacterial cytochromes P450. Biotechnol Bioeng. 2005;93:494–9. doi: 10.1002/bit.20744. The first report on the engineering of P450BM3 for an efficient synthesis of drug metabolites. [DOI] [PubMed] [Google Scholar]
- 117.Landwehr M, Hochrein L, Otey CR, Kasrayan A, Bäckvall JE, Arnold FH. Enantioselective alpha-hydroxylation of 2-arylacetic acid derivatives and buspirone catalyzed by engineered cytochrome P450BM3. J Am Chem Soc. 2006;128:6058–9. doi: 10.1021/ja061261x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kubo T, Peters MW, Meinhold P, Arnold FH. Enantioselective epoxidation of terminal alkenes to (R)- and (S)-epoxides by engineered cytochromes P450BM3. Chemistry. 2006;12:1216–20. doi: 10.1002/chem.200500584. [DOI] [PubMed] [Google Scholar]
- 119.Fasan R, Chen MM, Crook NC, Arnold FH. Engineered alkane-hydroxylating cytochrome P450BM3 exhibiting native-like catalytic properties. Angew Chem Int Ed Engl. 2007;46:8414–8. doi: 10.1002/anie.200702616. [DOI] [PubMed] [Google Scholar]
- 120•.Li HM, Mei LH, Urlacher VB, Schmid RD. Cytochrome P450BM3 evolved by random and saturation mutagenesis as an effective indole-hydroxylating catalyst. Appl Biochem Biotechnol. 2008;144:27–36. doi: 10.1007/s12010-007-8002-5. This report shows the production of indigo by laboratory-evolved bacterial P450BM3 at the rate similar to that of mammalian CYP2A6. [DOI] [PubMed] [Google Scholar]
- 121•.Damsten MC, van Vugt-Lussenburg BMA, Zeldenthuis T, de Vlieger JBS, Commandeur JNM, Vermeulen NPE. Application of drug metabolizing mutants of cytochrome P450BM3 (CYP102A1) as biocatalysts for the generation of reactive metabolites. Chemico-Biol Interac. 2008;171:96–107. doi: 10.1016/j.cbi.2007.09.007. The most recent report on the engineering of P450BM3 for enhanced activity towards the production of several drug metabolites, such as clozapine, diclofenac, and acetaminophen. [DOI] [PubMed] [Google Scholar]
- 122.Whitehouse CJ, Bell SG, Yang W, Yorke JA, Blanford CF, Strong AJ, Morse EJ, Bartlam M, Rao Z, Wong LL. A highly active single-mutation variant of P450BM3 (CYP102A1) Chembiochem. 2009;10:1654–6. doi: 10.1002/cbic.200900279. [DOI] [PubMed] [Google Scholar]
- 123.Whitehouse CJ, Bell SG, Tufton HG, Kenny RJ, Ogilvie LC, Wong LL. Evolved CYP102A1 (P450BM3) variants oxidise a range of non-natural substrates and offer new selectivity options. Chem Commun (Camb) 2008;28:966–8. doi: 10.1039/b718124h. [DOI] [PubMed] [Google Scholar]
- 124.Walsh ME, Kyritsis P, Eady NA, Hill HA, Wong LL. Catalytic reductive dehalogenation of hexachloroethane by molecular variants of cytochrome P450CAM (CYP101) Eur J Biochem. 2000;67:5815–20. doi: 10.1046/j.1432-1327.2000.01666.x. [DOI] [PubMed] [Google Scholar]
- 125.Bell SG, Stevenson JA, Boyd HD, Campbell S, Riddle AD, Orton EL, Wong LL. Butane and propane oxidation by engineered cytochrome P450CAM. Chem Commun (Camb) 2002;7:490–1. doi: 10.1039/b110957j. [DOI] [PubMed] [Google Scholar]
- 126.Jones JP, O'Hare EJ, Wong LL. Oxidation of polychlorinated benzenes by genetically engineered CYP101 (cytochrome P450CAM) Eur J Biochem. 2001;268:1460–7. doi: 10.1046/j.1432-1327.2001.02018.x. [DOI] [PubMed] [Google Scholar]
- 127.Harford-Cross CF, Carmichael AB, Allan FK, England PA, Rouch DA, Wong LL. Protein engineering of cytochrome P450CAM (CYP101) for the oxidation of polycyclic aromatic hydrocarbons. Protein Eng. 2000;13:121–8. doi: 10.1093/protein/13.2.121. [DOI] [PubMed] [Google Scholar]
- 128.Bell SG, Harford-Cross CF, Wong LL. Engineering the CYP101 system for in vivo oxidation of unnatural substrates. Protein Eng. 2001;14:797–802. doi: 10.1093/protein/14.10.797. [DOI] [PubMed] [Google Scholar]
- 129•.Xu F, Bell SG, Lednik J, Insley A, Rao Z, Wong LL. The heme monooxygenase cytochrome P450CAM can be engineered to oxidize ethane to ethanol. Angew Chem Int Ed Engl. 2005;44:4029–32. doi: 10.1002/anie.200462630. This report shows the engineering of P450CAM by directed evolution using a step-by-step adaptation to smaller alkanes; from hexane to butane to propane to ethane. [DOI] [PubMed] [Google Scholar]
- 130••.Nakamura K, Martin MV, Guengerich FP. Random mutagenesis of human cytochrome P450 2A6 and screening with indole oxidation products. Arch Biochem Biophys. 2001;395:25–31. doi: 10.1006/abbi.2001.2569. This is the first report on random mutagenesis of mammalian CYP2A6 for increased production of a variety of indole compounds. [DOI] [PubMed] [Google Scholar]
- 131•.Yun CH, Miller MP, Guengerich FH. Rate-determining steps in phenacetin oxidations by human cytochrome P450 1A2 and selected mutants. Biochemistry. 2000;39:11319–29. doi: 10.1021/bi000869u. The first example of random mutagenesis of mammalian P450 enzymes at substrate recognition sites to engineer them for enhanced activity. [DOI] [PubMed] [Google Scholar]
- 132.Kim D, Guengerich FP. Selection of human cytochrome P450 1A2 mutants with enhanced catalytic activity for heterocyclic amine N-hydroxylation. Biochemistry. 2004;43:981–8. doi: 10.1021/bi035593f. [DOI] [PubMed] [Google Scholar]
- 133.Wu Z, Guengerich FP. Expansion of substrate specificity of cytochrome P450 2A6 by random and site-directed mutagenesis. J Biol Chem. 2005;280:41090–100. doi: 10.1074/jbc.M508182200. [DOI] [PubMed] [Google Scholar]
- 134.Zhang ZG, Liu Y, Guengerich FP, Matse JH, Chen J, Wu ZL. Identification of amino acid residues involved in 4-chloroindole 3-hydroxylation by cytochrome P450 2A6 using screening of random libraries. J Biotechnol. 2009;139:12–8. doi: 10.1016/j.jbiotec.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scott EE, Spatzenegger M, Halpert JR. A truncation of 2B subfamily cytochromes P450 yields increased expression levels, increased solubility, and decreased aggregation while retaining function. Arch Biochem Biophys. 2001;395:57–68. doi: 10.1006/abbi.2001.2574. [DOI] [PubMed] [Google Scholar]
- 136••.Cosme J, Johnson EF. Engineering microsomal cytochrome P450 2C5 to be a soluble, monomeric enzyme. Mutations that alter aggregation, phospholipid dependence of catalysis, and membrane binding. J Biol Chem. 2000;275:2545–53. doi: 10.1074/jbc.275.4.2545. The first report on the engineering of microsomal P450s for enhanced expression and solubility. [DOI] [PubMed] [Google Scholar]
- 137.Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell. 2000;5:121–31. doi: 10.1016/s1097-2765(00)80408-6. [DOI] [PubMed] [Google Scholar]
- 138.Johnson EF, Stout CD. Structural diversity of human xenobiotic-metabolizing cytochrome P450 monooxygenases. Biochem Biophys Res Commun. 2005;338:331–6. doi: 10.1016/j.bbrc.2005.08.190. [DOI] [PubMed] [Google Scholar]
- 139.Scott EE, Halpert JR. Structures of cytochrome P450 3A4. Trends Biochem Sci. 2005;30:5–7. doi: 10.1016/j.tibs.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 140•.Kumar S, Zhao Y, Sun L, Halpert JR, Muralidhara BK. Engineering human cytochrome P450 2B6 for enhanced expression and stability: Importance of Leu264→Phe substitution. Mol Pharmacol. 2007;72:1191–9. doi: 10.1124/mol.107.039693. The first paper describing the engineering of human CYP2B6 for enhanced expression and stability using semi-rational approach. [DOI] [PubMed] [Google Scholar]
- 141.Sun L, Chen CS, Waxman DJ, Liu H, Halpert JR, Kumar S. Re-engineering cytochrome P450 2B11dH for enhanced metabolism of several substrates including the anti-cancer prodrugs cyclophosphamide and ifosfamide. Arch Biochem Biophys. 2007;458:167–74. doi: 10.1016/j.abb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Domanski TL, Halpert JR. Analysis of mammalian cytochrome P450 structure and function by site-directed mutagenesis. Curr Drug Metab. 2001;2:117–37. doi: 10.2174/1389200013338612. [DOI] [PubMed] [Google Scholar]
- 143.Kumar S, Scott EE, Liu H, Halpert JR. A rational approach to Re-engineer cytochrome P450 2B1 regioselectivity based on the crystal structure of cytochrome P450 2C5. J Biol Chem. 2003;278:17178–84. doi: 10.1074/jbc.M212515200. [DOI] [PubMed] [Google Scholar]
- 144.Scott EE, He YQ, Halpert JR. Substrate routes to the buried active site may vary among cytochromes P450: mutagenesis of the F-G region in P450 2B1. Chem Res Toxicol. 2002;15:1407–13. doi: 10.1021/tx020057u. [DOI] [PubMed] [Google Scholar]
- 145.Hernandez CE, Kumar S, Liu H, Halpert JR. Investigation of the role of cytochrome P450 2B4 active site residues in substrate metabolism based on crystal structures of the ligand-bound enzyme. Arch Biochem Biophys. 2006;455:61–7. doi: 10.1016/j.abb.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gillam EM. Extending the capabilities of nature's most versatile catalysts: directed evolution of mammalian xenobiotic-metabolizing P450s. Arch Biochem Biophys. 2007;464:176–86. doi: 10.1016/j.abb.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 147.Huang W, Johnston WA, Hayes MA, De Voss JJ, Gillam EM. A shuffled CYP2C library with a high degree of structural integrity and functional versatility. Arch Biochem Biophys. 2007;467:193–205. doi: 10.1016/j.abb.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 148.Johnston WA, Huang W, De Voss JJ, Hayes MA, Gillam EM. A shuffled CYP1A library shows both structural integrity and functional diversity. Drug Metab Dispos. 2007;35:2177–85. doi: 10.1124/dmd.107.017939. [DOI] [PubMed] [Google Scholar]
- 149.Johnston WA, Huang W, De Voss JJ, Hayes MA, Gillam EM. Quantitative whole-cell cytochrome P450 measurement suitable for high-throughput application. J Biomol Screen. 2008;13:135–41. doi: 10.1177/1087057107312780. [DOI] [PubMed] [Google Scholar]
- 150.Keizers PH, Schraven LH, de Graaf C, Hidestrand M, Ingelman-Sundberg M, van Dijk BR, Vermeulen NP, Commandeur JN. Role of the conserved threonine 309 in mechanism of oxidation by cytochrome P450 2D6. Biochem Biophys Res Commun. 2005;338:1065–74. doi: 10.1016/j.bbrc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 151.Guengerich FP. Cytochrome P450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 152.Baillie TA. Metabolism and Toxicity of Drugs: Two Decades of Progress in Industrial Drug Metabolism. 2008;21:129–37. doi: 10.1021/tx7002273. [DOI] [PubMed] [Google Scholar]
- 153.Hara M, Iazvovskaia S, Ohkawa H, Asada Y, Miyake J. Immobilization of P450 monooxygenase and chloroplast for use in light-driven bioreactors. J Biosci Bioeng. 1999;87:793–7. doi: 10.1016/s1389-1723(99)80155-8. [DOI] [PubMed] [Google Scholar]
- 154.Döhr O, Paine MJI, Friedberg T, Roberts GCK, Wolf CR. Engineering of a functional human NADH-dependent cytochrome P450 system. Proc Natl Acad Sci USA. 2001;98:81–6. doi: 10.1073/pnas.98.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]