Abstract
Objectives
Develop a clinical confocal microlaparoscope for imaging ovary epithelium in vivo with the long term objective of diagnosing cancer in vivo.
Study Design
The first confocal microlaparoscope was developed and used to image the ovaries of twenty-one patients in vivo using fluorescein sodium and acridine orange as the fluorescent contrast agents.
Results
The device was tested in vivo and demonstrated to be safe and function as designed. Real-time cellular visualization of ovary epithelium was demonstrated.
Conclusions
The confocal microlaparoscope represents a new type of in vivo imaging device. With its ability to image cellular details in real time, it has the potential to aid in the early diagnosis of cancer. Initially, the device may be used to locate unusual regions for guided biopsies. In the long term, the device may be able to supplant traditional biopsies and allow the surgeon to identify early stage ovarian cancer.
Keywords: confocal microendoscopy, fluorescence confocal imaging, optical biopsy, ovarian cancer
1 Introduction
In this paper, we present the first known use of a confocal microlaparoscope in humans. This new type of laparoscope allows surgeons to obtain nondestructive optical biopsies of tissue in real-time. We present results from a pilot study consisting of twenty-one patients. The device was used to image ovaries in vivo prior to laparoscopic oophorectomy.
The American Cancer Society estimates that over 230,000 new cases of ovarian cancer were diagnosed in 2007 while more than 141,000 women died from the disease worldwide.1 Over eighty percent of all cases are diagnosed during the later stages (Stage III or IV) of the disease when treatment is expensive and generally unsuccessful.2 If diagnosis occurs when the cancer is localized to the ovary (Stage I), the survival rate increases to ninety-two percent. The overall prevalence of ovarian cancer is relatively low (1.7 percent lifetime risk). However, the lifetime risk for the disease increases two-fold if the individual has one first or second-degree relative with the disease. With two first-degree relatives the risk increases by twenty-five times (a forty percent lifetime risk).
For the subgroups of women at increased risk, few options exist to allow early detection of the disease. An NIH 1994 consensus stated that there is no single acceptable screening test for ovarian cancer and no evidence that combining the available screening tests—CA125, transvaginal ultrasound, and pelvic exam—has an acceptable sensitivity and specificity.3
Several novel techniques are being developed that could provide clinicians with diagnostic tools to detect early changes in ovarian tissue. Techniques, such as confocal microscopy4–12, two photon microscopy14–15, optical spectroscopy16–21, and optical coherence tomography22–28 are able to assess tissue morphology and/or biochemical composition. In vivo use of these methods has the potential to provide real-time diagnostic information allowing clinicians to assess the subtle changes that occur early in the disease process. These techniques may increase the sensitivity and specificity of a diagnosis, especially when used as a means for targeting traditional biopsies to regions that can be identified as abnormal at the cellular level.
Confocal microendoscopy, an emerging in vivo fluorescence imaging technique, can resolve individual cells and subcellular features. The confocal microendoscope is able to image thin sections of thick samples. This optical sectioning property enables high-resolution microscopic imaging of thick biological samples at moderate depths (typically up to one-hundred to two-hundred microns below the tissue surface).
In vivo confocal imaging has the potential to aid clinicians during screening and/or surgical procedures. Commercial confocal microendoscope systems have recently been developed for visualization of colon and esophagus.5 Results have been encouraging in these areas and additional applications for this technology are under investigation. The purpose of this paper is to describe the first clinical confocal microlaparoscope system. In the following sections we describe the new device and present the results of a pilot study.
2 Materials and methods
We have previously reported on a the development of a multi-spectral fluorescence confocal microendoscope.11,29–32 To evaluate whether such a device could be used to image ovaries in vivo, a clinical confocal microlaparoscope system was developed.33–37 To create a viable clinical system, several criteria had to be met. First, the microlaparoscope system had to be compact and mobile so that it could be quickly moved into the surgical suite. The microlaparoscope also had to be compatible with standard trocars, be sterilizable, have the ability to deliver small localized volumes of contrast agents, and have the ability to focus at selected depths. Finally, the device had to be comfortable and easy to use during surgery. A confocal microlaparoscope system was constructed meeting these criteria.
To view a real-time cellular image using the confocal microlaparoscope, the surgeon places the rigid probe through a five millimeter trocar port and contacts the epithelial surface of the organ. Pressing a button on the microlaparoscope handle delivers a controlled volume of fluorescent contrast agent to the field of view. Instantly, a live video of the cells appears on screen. As the probe tip is moved across the surface of the organ, the epithelium can be interrogated in real-time. Pressing another button on the handle saves videos and still frames. By default, the system is configured to image the epithelial layer of cells. However, controls located on the handle allow the surgeon to view deeper cell layers. After use, the microlaparoscope can be disconnected from the system, cleaned, and ETO sterilized for reuse.
In the following paragraphs we describe the confocal microlaparoscope system, discuss the fluorescent contrast agents that were used during imaging, and describe the imaging protocol used to test the device.
2.1 Confocal microlaparoscope system
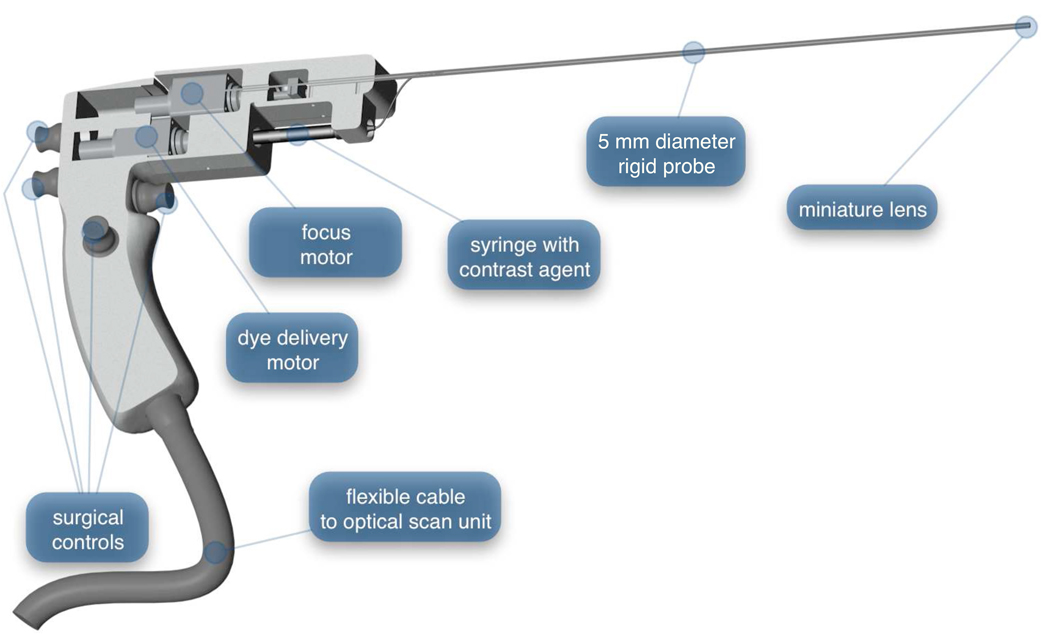
The confocal microlaparoscope system consists of a microlaparoscope connected to a mobile cart containing a confocal optical scan unit. The microlaparoscope (shown in Figure 1) has a 35 cm rigid probe extending from its handle. The handle contains controls that allow the surgeon to adjust the focus, deliver contrast agents, save still images, and record videos. A flexible cable connects the microlaparoscope to the mobile cart.
Figure 1.
The confocal microlaparoscope. Four push button controls located on the handle allow the surgeon to adjust the focus, deliver contrast agents, save still images, and record videos. Inside the handle, two tiny motors control focus and dye delivery.
The tip of the microlaparoscope’s probe contains a miniature 3 mm diameter objective lens that provides a 450 um field-of-view and a 3 um lateral resolution. The miniature objective lens images the tissue plane onto a flexible coherent fiber-optic imaging bundle (Sumitomo Electric Industries, White Plains, NY). The fiber bundle runs through a six meter long cable that connects to the optical scan unit, located on the mobile cart. To adjust the imaging depth in the tissue, the focus motor inside the microlaparoscope’s handle changes the spacing between the fiber bundle and the objective lens.
To deliver contrast agent to the imaging site, a syringe with fluorescent dye is placed into a spring-loaded port in the handle of the device. The second motor in the microlaparoscope handle acts as a syringe pump that forces the contrast agent through a tiny fluid delivery line in the rigid probe and onto the tissue. The system is able to deliver dye volumes with 50 nL precision.
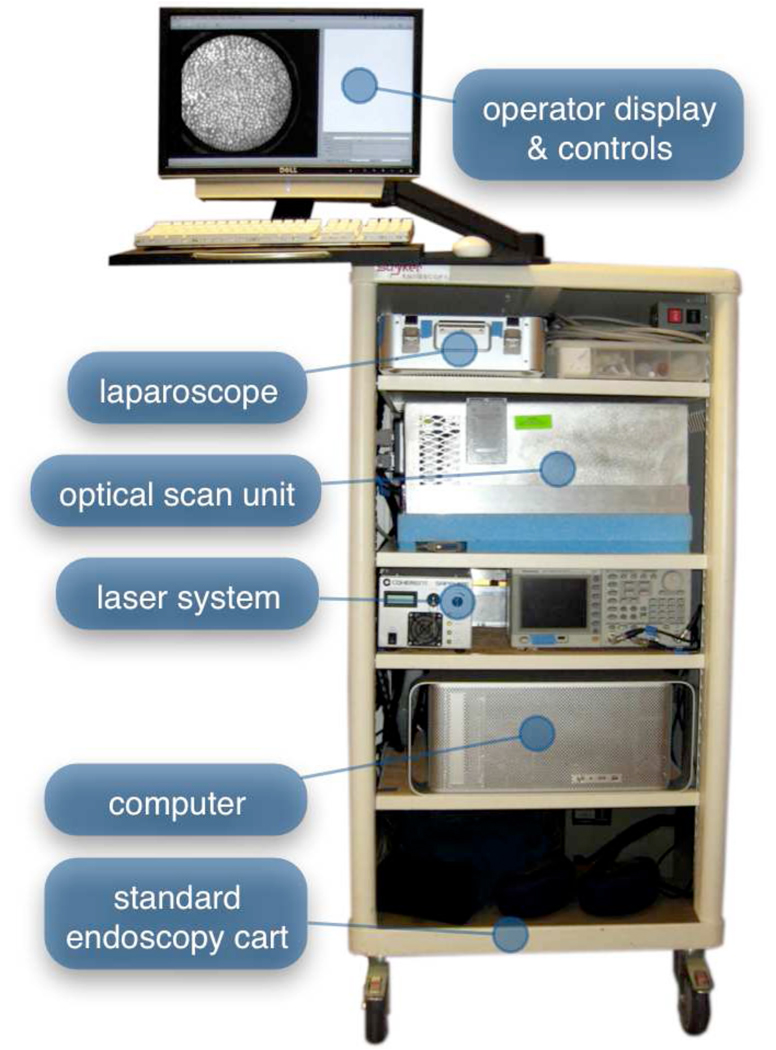
The mobile cart, shown in Figure 2, contains the optical scan unit, laser, computer, and primary operator console. Live images are visible from the surgical field on a secondary display. The entire mobile unit can be moved into a surgical suite and set up to image within a few minutes.
Figure 2.
The mobile cart. Shelves on the cart, from top to bottom, contain the sterilized microlaparoscope, optical scan unit, laser power supply and system electronics, and computer. The top of the cart holds the operator controls and the primary display.
To collect images, the microlaparoscope illuminates the tissue with laser light. The laser illumination excites the locally delivered contrast agent. Fluorescent signal emitted in the tissue is then collected by the miniature objective lens. The lens images the fluorescent signal onto the distal face of the coherent fiber optic bundle. This signal is then relayed back to the optical scan unit. The optical scan unit is a high-speed confocal microscope. It contains the laser, scan mirrors, optics, confocal aperture, and detector. Detailed technical descriptions of the miniature objective lens, the optical scan unit, and the confocal microlaparoscope system are discussed elsewhere. 29–32
2.2 Fluorescent Contrast Agents
Imaging with the microlaparoscope requires the application of an exogenous fluorescent contrast agent. Fluorescein sodium is a dye approved for use in humans for retinal angiography and was selected as the contrast agent for initial testing of the confocal microlaparoscope. The initial clinical study was conducted using approximately 1 to 3 uL of one percent fluorescein sodium per imaging site. This volume is sufficient for staining a 5 mm diameter area on the tissue surface. The localized application of fluorescein sodium also marks the imaging site for correlated biopsies.
Topically applied fluorescein sodium binds rather non-specifically to proteins in tissue. This results in low contrast images that have limited diagnostic value for detecting ovarian cancer. The ovaries were also imaged using acridine orange (AO) following extraction from the patient. AO is a nuclear stain and an excellent fluorophore for visualizing cellular distributions. Its diagnostic potential has been demonstrated previously in the context of detecting ovarian cancer.38
Sites on the ovary that were imaged with fluorescein sodium during the in vivo procedure were identified using a UV flashlight. At nearby locations, approximately 1 to 3 uL of 330 uMole/L AO was applied to the surface of the excised ovary and imaged with the microlaparoscope. These sites were biopsied and processed with standard histopathologic procedures using H&E staining.
Towards the end of the pilot study AO was used in vivo to validate that the same contrast observed ex vivo could be obtained in vivo. For in vivo testing, 1 uL of 330 uMole/L AO was used to image the ovaries using a protocol that prevented any other organ from coming into contact with the dye. This was accomplished by imaging the ovary inside an endobag. FDA approval to use AO in this context was granted under IND 102603.
2.3 Confocal Imaging Procedure
The pilot study involved twenty-one patients imaged over a one year time period. All procedures in the study were approved by the Institutional Review Board at the University of Arizona. Participants in the study were recruited at the University Medical Center, Tucson, Arizona. Subjects were eligible to participate in the study if they were at least eighteen years of age and not pregnant. All subjects were from the cohort of patients undergoing laparoscopic oophorectomy or open surgery. No financial compensation was offered and there was no diagnostic benefit to the patient for participation in the study.
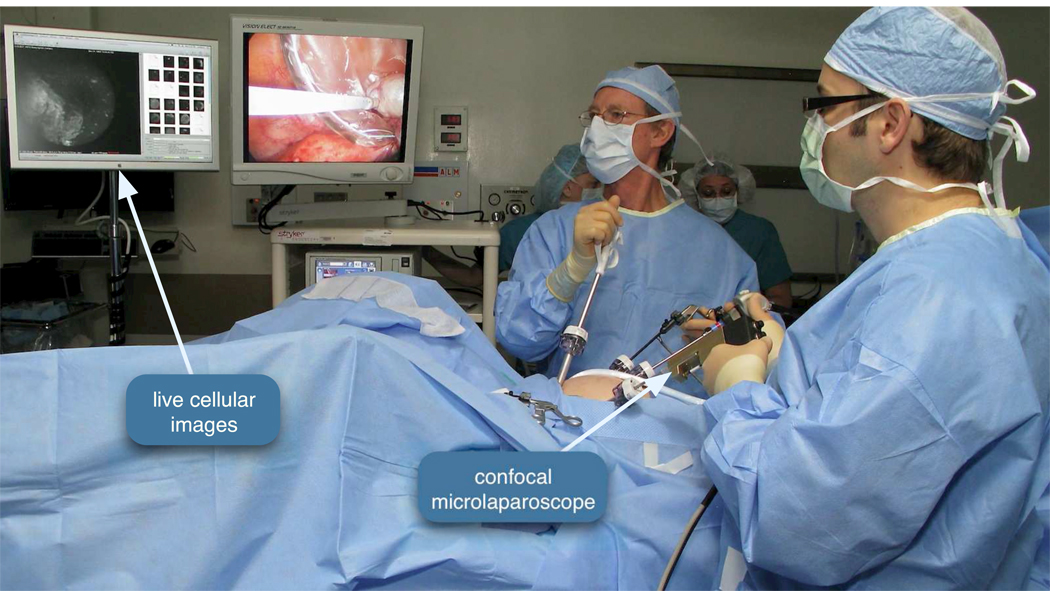
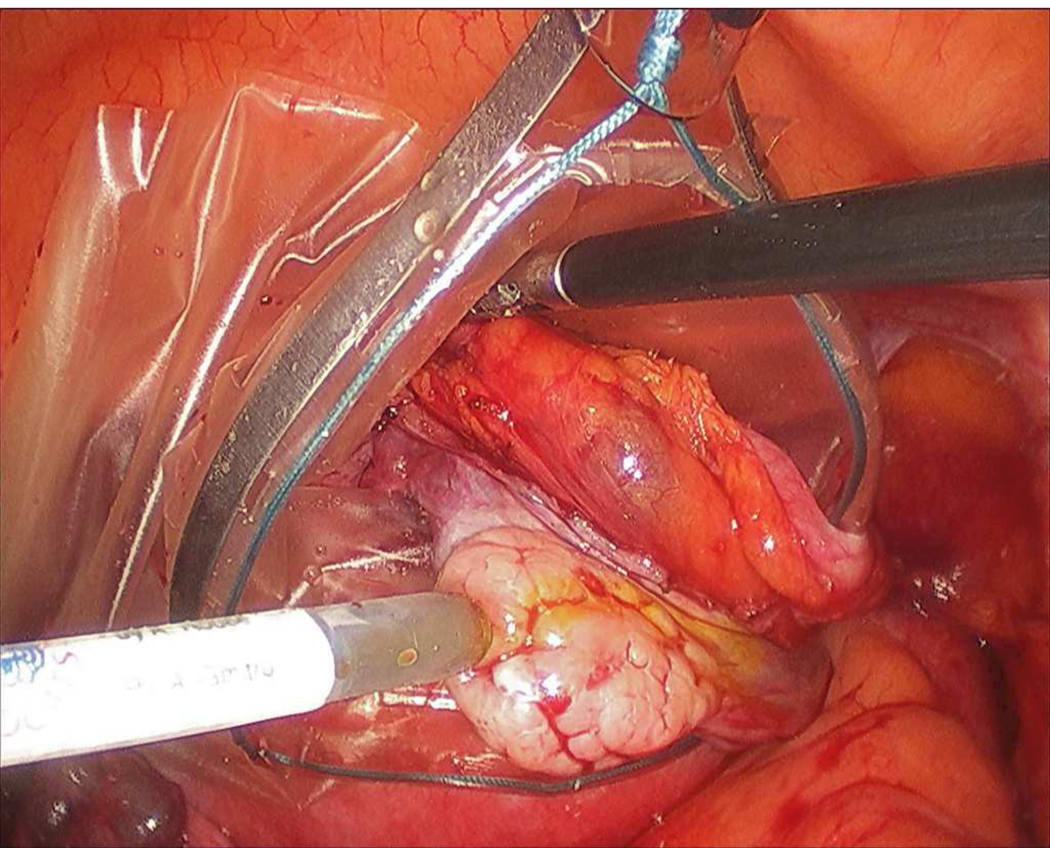
Standard surgical procedures for clinical oophorectomy were followed with the addition of microlaparoscope imaging prior to ovary removal. Figure 3 shows the microlaparoscope in use during a clinical procedure. All patients were placed under general endotracheal anesthesia and abdominal cavities were insufflated with carbon-dioxide to raise the abdominal wall. The ovary was partially resected and placed inside an endobag to prevent the patient from being exposed to the contrast agent (Figure 4). The microlaparoscope was inserted through a 5 mm trocar. Conventional laparoscopic image guidance was used to position the microlaparoscope tip into contact with the ovary. Several locations on the surface of the ovary were interrogated using the confocal microlaparoscope. In all patients, the extra imaging with the microlaparoscope was completed in under ten minutes.
Figure 3.
Confocal microlaparoscope imaging the epithelial surface of an ovary in vivo. The patient’s left ovary has been located using a wide field laparoscope (second display from the left). The surgeon on the right (holding the device in his left hand) has inserted the microlaparoscope through a five millimeter trocar port and placed the tip in contact with the ovary. Live cellular images are viewed on the left most display.
Figure 4.
In vivo imaging of the ovary. Under standard wide field laparoscope visualization, the ovary is located and placed an endobag to prevent other organs from receiving the fluorescent contrast agent. The microlaparoscope (coming in from the lower left) is brought into contract with the ovary, contrast agent is locally applied, and real-time cellular imaging commences.
Once the microlaparoscope imaging was complete, the ovaries were resected, sealed inside the endobag, and removed as part of the standard surgical procedure. Immediately following removal, the ovary was imaged ex vivo with the microlaparoscope using AO as the contrast agent. The ovaries were sent to histopathology for clinical diagnosis.
3 Results
Twenty patients were imaged in vivo using fluorescein sodium followed by ex vivo imaging with AO. One patient was imaged with AO in vivo. For the group of patients imaged with fluorescein sodium, seventeen had normal ovaries when evaluated by histopathology and three of the patients were found to have ovarian cancer. For this pilot study the confocal microlaparoscope images were obtained to show clinical feasibility but not used to make a diagnosis. No surgical complications occurred in any of the patients imaged with the microlaparoscope.
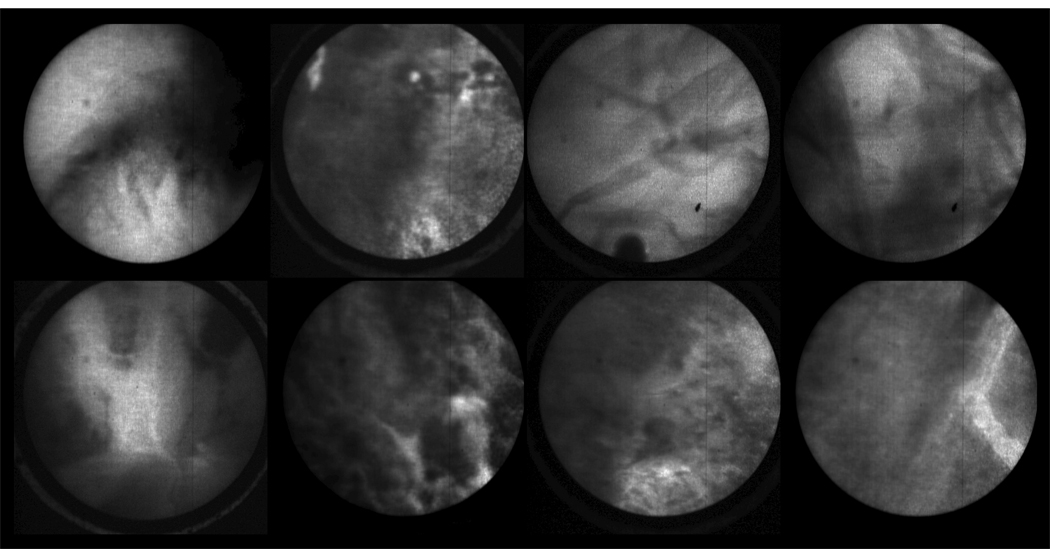
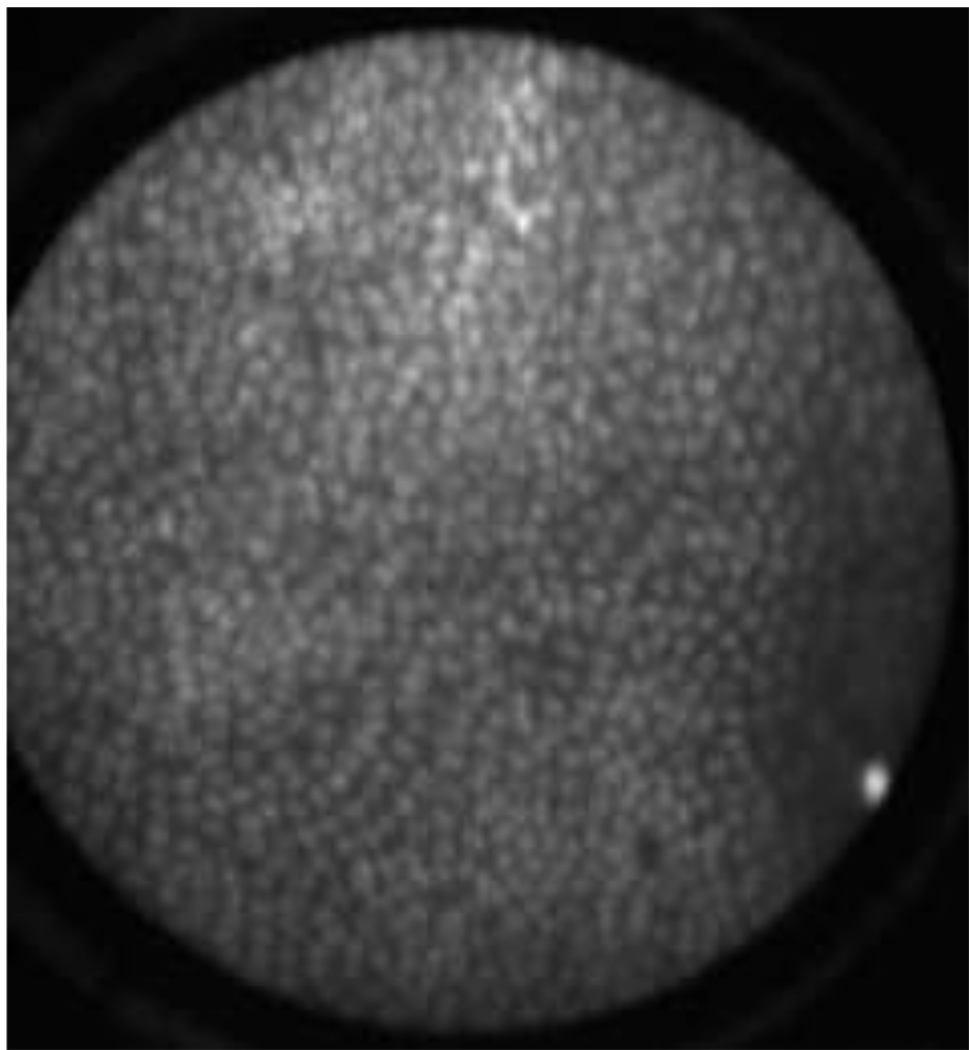
In vivo images in Figure 5 and an in vivo video in Figure 6 are from ovary stained with fluorescein sodium. As expected, these images have low contrast because fluorescein sodium binds non-specifically to proteins. Much of the observed contrast can be attributed to the distribution of proteins on the tissue surface and uneven pooling of the dye. Although the contrast was limited, we were able to demonstrate that the focus system and dye delivery system performed properly.
Figure 5.
In vivo confocal microlaparoscope images of the human ovary using topically applied fluorescein sodium as the contrast agent. (Circular field of view is 450 um.)
Figure 6.
(VIDEO) In vivo confocal microlaparoscope video of the human ovary using topically applied fluorescein sodium as the contrast agent. The pathology report diagnosis was serous cystadenoma. (Circular field of view is 450 um.)
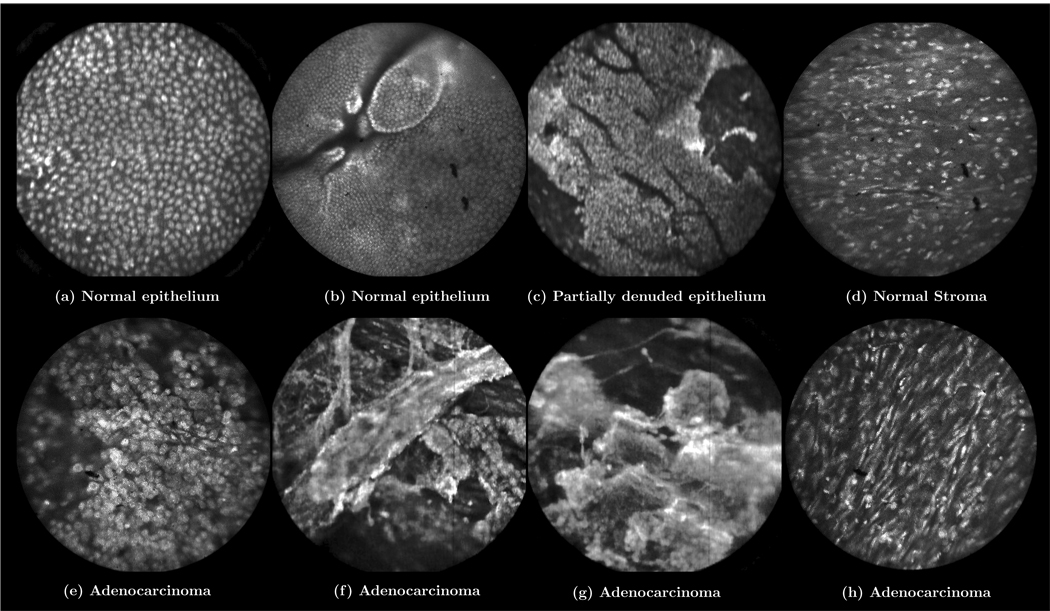
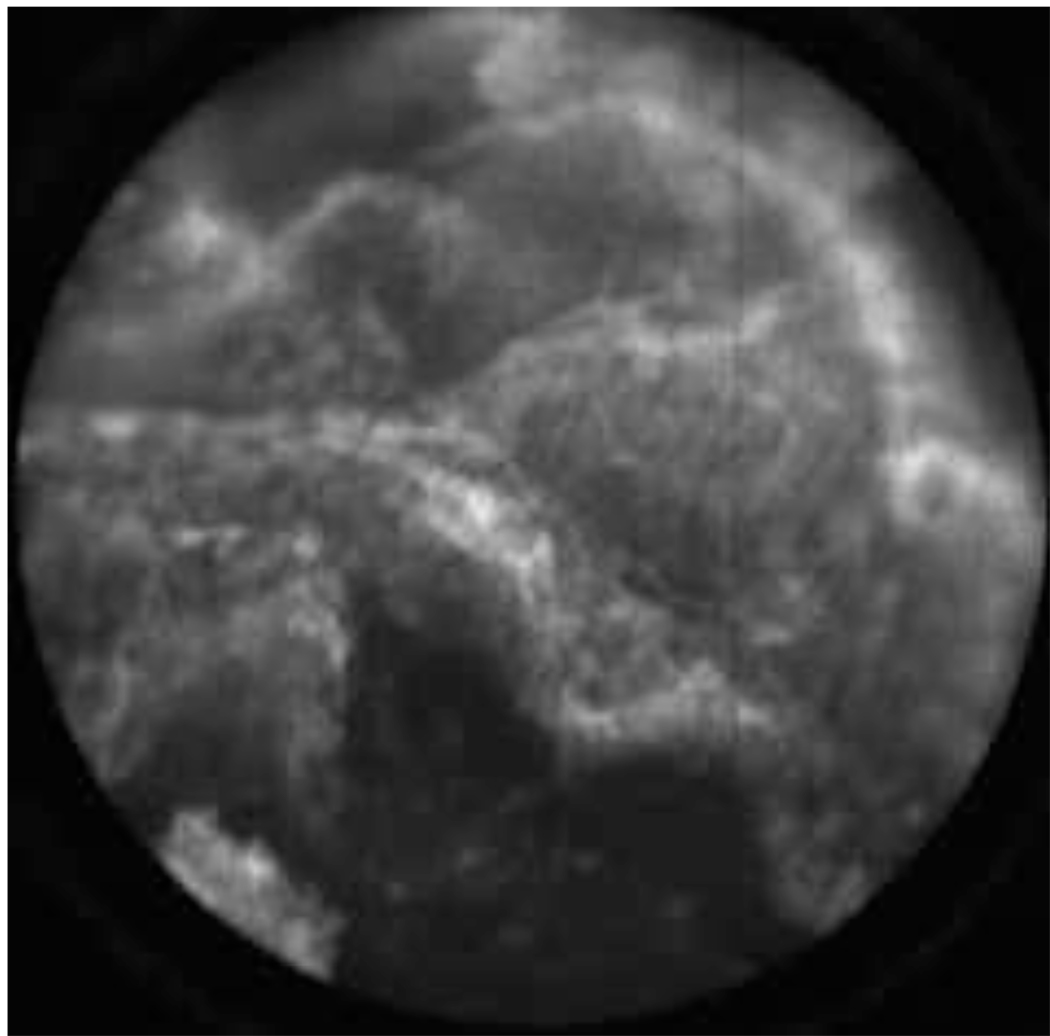
Ex vivo images and videos of ovarian tissue stained with AO are shown in Figure 7–Figure 9. These images and videos were collected on ex vivo tissue immediately following removal of the ovary. The results show normal ovarian epithelium in Figure 7(a,b) and Figure 8, partially denuded epithelium in Figure 7(c), normal ovarian stroma in Figure 7(d), and adenocarcinoma of the ovary in Figure 7(e–h) and Figure 9. The bright regions in these images correspond to uptake of AO in the cell nuclei. Normal ovarian epithelium typically has an appearance such as that shown in Figure 7(a). The ovary shown in Figure 7(b) has smaller nuclei and micro-structural tissue features visible on the surface, which may be associated with a cyst that was observed in pathology. Figure 7(f) and Figure 7(g) were obtained from the same ovary with pathology reporting serous adenocarcinoma and a tumor measuring in excess of 5 mm.
Figure 7.
Ex vivo images of ovarian tissue stained with acridine orange. Sub-captions indicate pathology diagnosis. (Circular field of view is 450 um.)
Figure 9.
(VIDEO) Ex vivo video of abnormal appearing ovary epithelium tissue stained with acridine orange. The pathology report diagnosis was serous cystadenoma. (Circular field of view is 450 um.)
Figure 8.
(VIDEO) Ex vivo video of normal appearing ovary epithelium tissue stained with acridine orange. (Circular field of view is 450 um.)
The results suggest that the confocal microlaparoscope has sufficient resolution to differentiate cellular structures and to diagnose cancer. The images show a uniform nuclear distribution for normal tissues and a heterogeneous nuclear distribution for cancerous tissue. The quality of the ex vivo images obtained in this study is similar to, if not better than, the image quality observed during another ex vivo AO imaging study using a previous version of the confocal microlaparoscope that was able to identify ovarian cancer with a 98 percent sensitivity and 90 percent specificity employing computer-aided diagnosis. 38
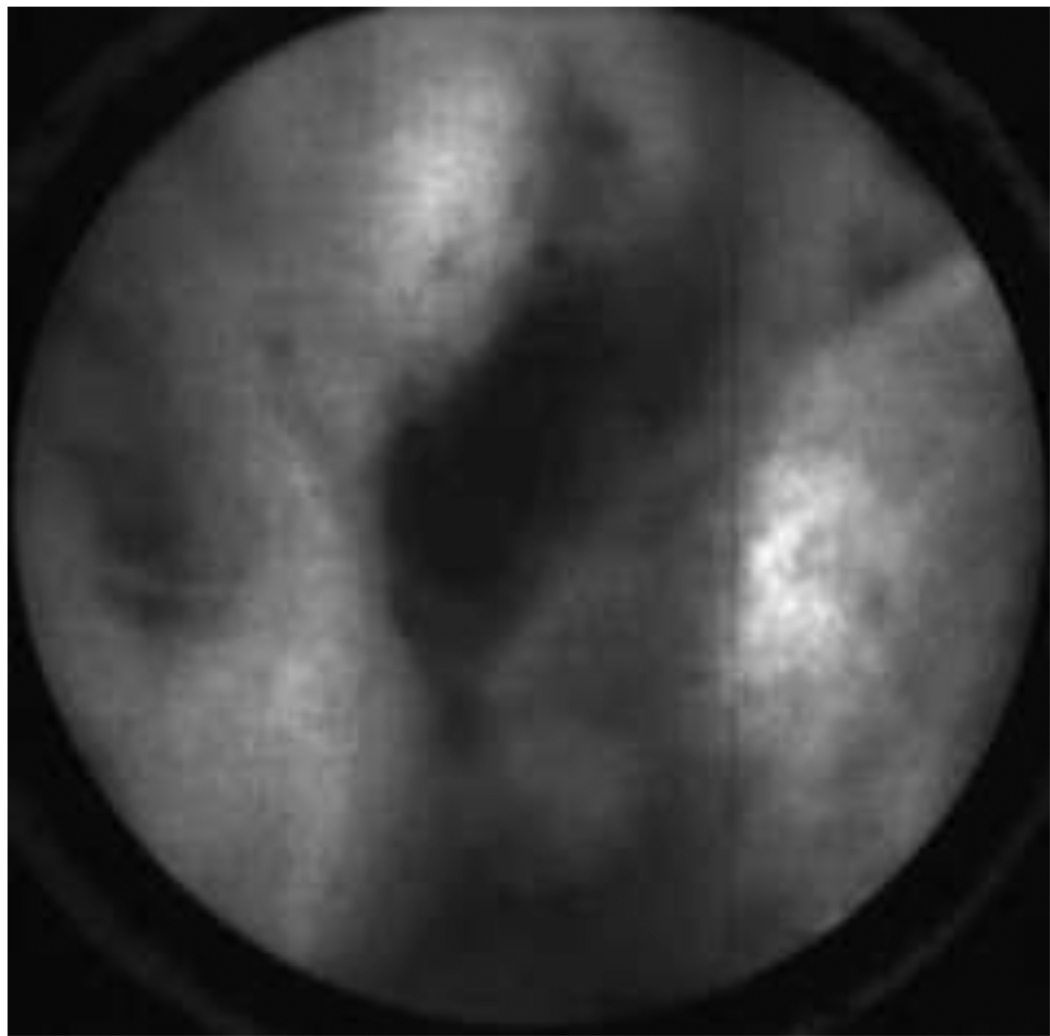
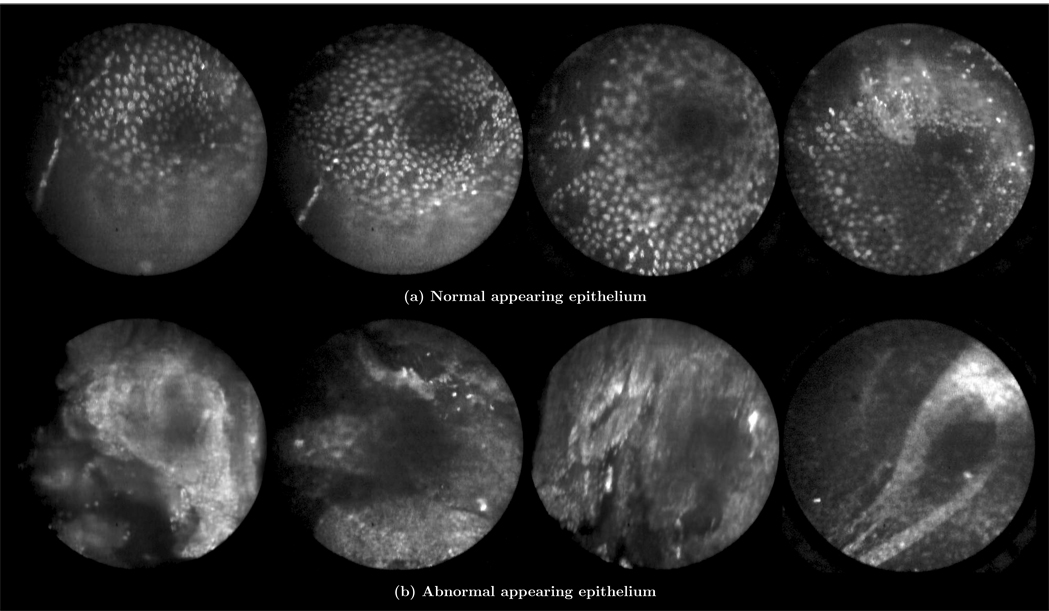
Figure 10 shows results from the patient imaged in vivo using AO. The pathology report diagnosis was serous cystadenoma. The confocal laparoscope’s ability to locally identify normal and abnormal regions is highlighted by the two kinds of images obtained in Figure 10. The group of results in Figure 10(a) have the same appearance as normal epithelium as observed ex vivo in Figure 7(a). The four images in Figure 10(b) appear abnormal and are believed to have been taken in the region that contained the cystadenoma. The dark region visible in the upper right hand quadrant of the images suggests that the probe may not have been in complete contact with the tissue. Some of the images also appear to show denuding of the epithelium, which may be a result of the partial-resection and placement of the ovary in the endobag before imaging. We expect that with further system optimization and more physician practice using the microlaparoscope, the same quality images seen with AO ex vivo will be attainable in vivo.
Figure 10.
In vivo images of ovarian tissue stained with acridine orange from the same patient. The top row of images (a) depict epithelial cells that have a normal appearance. The bottom row of images (b) depict epithelial cells that have an abnormal appearance. The pathology report diagnosed the ovaries with serous cystadenoma. (Circular field of view is 450 um.)
4 Comment
The pilot study showed that the confocal microlaparoscope can be successfully used in the surgical setting for in vivo laparoscopic imaging of the ovary. All aspects of the imaging procedure including setup of the instrument, positioning of the microlaparoscope, delivery of dye, instrument focus, and data collection worked flawlessly without complication. Images obtained in vivo with topically applied fluorescein sodium demonstrate that the device functions as designed and that the imaging procedure is safe. Due to the low contrast of topically applied fluorescein sodium, it is uncertain if it can provide sufficient contrast for diagnosis of ovarian pathology. Ex vivo images obtained with AO show excellent contrast that highlights cellular morphology. Initial results from in vivo use of AO provide preliminary evidence that the same contrast can be obtained in vivo.
In the context of this study, AO was used solely to validate that in vivo and ex vivo contrast was comparable. Although AO has been used clinically on humans in a therapeutic context for the treatment of synovial sarcomas39, its long term safety when used for diagnostic imaging has not yet been established. The dye has not been used extensively in clinical application due to concerns about cytotoxicity and mutagenicity; however acriflavine, a related compound, is currently being used for diagnostic imaging of the colon in Germany.40 In terms of optical biopsy, cytotoxicity is not a major concern since tissue extraction biopsy, the current approach, is completely destructive to the interrogated tissue. Mutagenicity, on the other hand, is a concern. We have recently completed a pilot study in our laboratory evaluating the safety of AO and SYTO 16 in a mouse model.41 The results showed no increase in death rate or cancer incidence in animals treated with a high doses of dye injected into the peritoneal cavity. However, the power of the study was not sufficient to establish safety. Further study is needed before such a dye could be used clinically in a situation where removal of the ovary is not a predetermined outcome.
The development of targeted fluorescent contrast agents with specificity for the molecular signature of ovarian cancer is an active area of research. Combined with the confocal microlaparoscope, these agents may provide the ability to pick up early precancerous changes in the epithelium before the actual onset of disease.
While the confocal microlaparoscope provides excellent in vivo images of cells near surface layer of tissue, its imaging depth is ultimately limited to a few hundred microns. It is believed that most ovarian cancers originate in the epithelial layer; however, epithelial cells can line the inner surface of inclusion cysts located well below the surface. Such deep lying structures are not amenable to confocal microlaparoscope imaging, but their presence may be detected by other lower resolution imaging modalities such as ultrasound or optical coherence tomography. The most effective diagnostic tool may be a multi-modality imaging system that combines the capabilities of in vivo confocal microscopy with an additional imaging method.
The microlaparoscope represents a new type of in vivo diagnostic imaging device. With its ability to image cellular details in real time, it has the potential to aid in the early diagnosis of cancer. Rather than biopsying tissue, sending samples for analysis, and waiting for tissue processing, the microlaparoscope system instantly displays live cellular images at thirty frames per second. Nondestructive optical biopsy enables more extensive interrogation than traditional methods allow. Initially, the device may be used to locate unusual regions for guided biopsies. In the long term, the device may be able to supplant traditional biopsies altogether and allow the surgeon to identify early stage ovarian cancer.
With the success of this pilot study, we will now move forward to quantitatively evaluate the sensitivity and specificity of the microlaparoscope when used to detect ovarian cancer. We will also continue to evaluate potential contrast agents that are safe for in vivo use and provide diagnostically useful contrast.
Supplementary Material
Acknowledgements
This work was supported by the Arizona Disease Control Research Commission grant number 971 and NIH grants CA 94287 and CA 115780. The second author made significant contributions to this work. The authors would like to thank Dr. Molly Brewer for insight and ideas on adapting the confocal microendoscope for laparoscopic imaging of the ovary. Additional support was provided by Kathy Schmidt who prepared samples and assisted in consenting patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human Experiments: The University of Arizona Human Subjects board approved the protocols used and described in this paper for testing the device on humans. Approval was granted for project number BIO-06-147 on December 22, 2006 and project number 06-0742-04 on October 8, 2008. FDA IND approval number 102603 was obtained to use Acridine Orange in vivo.
Conflicts of interest: None.
Presentation information: A portion of the results contained in this paper were presented at SPIE’s Photonics West BIOS January 25, 2009, San Jose, California Conference (proceedings number 7172-17).
Condensation
The first clinical confocal laparoscope capable of performing real-time optical biopsies was developed. The device was used to image the ovaries in 21 patients.
References
- 1.Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global cancer facts & figures. 2007 http://www.cancer.org/downloads/STT/Global_Cancer_Facts_and_Figures_2007_rev.pdf.
- 2.National Cancer Institute. NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. 2006 January; http://www.cancer.gov/newscenter/pressreleases/IPchemotherapyrelease.
- 3.Kramer BS, Gohagan J, Prorok PC. NIH consensus 1994: screening. Gynecologic Oncology. 1994;55(3):20–21. doi: 10.1006/gyno.1994.1335. [DOI] [PubMed] [Google Scholar]
- 4.Wang T, Mandella M, Contag C, Kino G, et al. Dual-axis confocal microscope for high-resolution in vivo imaging. Optics Letters. 2003;28(6):414–416. doi: 10.1364/ol.28.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polglase A, McLaren W, Skinner S, Kiesslich R, Neurath M, Delaney P. A fluorescence confocal endomicroscope for in vivo microscopy of the upper-and the lower-GI tract. Gastrointestinal Endoscopy. 2005;62(5):686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Boudoux C, Yun S, Oh W, White W, Iftimia N, Shishkov M, Bouma B, Tearney G. Rapid wavelength-swept spectrally encoded confocal microscopy. Optics Express. 2005;20(13):8214–8221. doi: 10.1364/opex.13.008214. [DOI] [PubMed] [Google Scholar]
- 7.Nakao M, Yoshida S, Tanaka S, Takemura Y, Oka S, Yoshihara M, Chayama K. Optical biopsy of early gastroesophageal cancer by catheter-based reflectance-type laser-scanning confocal microscopy. J Biomed Opt. 2008 Sep–Oct;13(5) doi: 10.1117/1.2983674. 054043. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Raighne A, McCabe E, Dunbar L, Scharf T. Confocal microscopy using variable-focal-length microlenses and an optical fiber bundle. Applied Optics. 2005;44(28):5928–5936. doi: 10.1364/ao.44.005928. [DOI] [PubMed] [Google Scholar]
- 9.Shin H, Pierce M, Lee D, Ra H, Solgaard O, Richards-Kortum R. Fiber-optic confocal microscope using a MEMS scanner and miniature objective lens. Optics Express. 2007;15(15):9113–9122. doi: 10.1364/oe.15.009113. [DOI] [PubMed] [Google Scholar]
- 10.Lin C, Webb R. Fiber-coupled multiplexed confocal microscope. 10/830,905. uS Patent App. 2004 Apr 22; doi: 10.1364/ol.25.000954. [DOI] [PubMed]
- 11.Gmitro A, Aziz D. Confocal microscopy through a fiber-optic imaging bundle. OPTICS LETTERS. 1993;18:565–565. doi: 10.1364/ol.18.000565. [DOI] [PubMed] [Google Scholar]
- 12.Jean F, Bourg-Heckly G, Viellerobe B. Fibered confocal spectroscopy and multicolor imaging system for in vivo fluorescence analysis. Optics Express. 2007;15(7):4008–4017. doi: 10.1364/oe.15.004008. [DOI] [PubMed] [Google Scholar]
- 13.Knittel J, Schnieder L, Buess G, Messerschmidt B, Possner T. Endoscope-compatible confocal microscope using a gradient index-lens system. Optics Communications. 2001;188(5–6):267–273. [Google Scholar]
- 14.Konig K, Ehlers A, Riemann I, Schenkl S, Buckle R, Kaatz M. Clinical two-photon microendoscopy. Microsc Res Tech. 2007 May;70(5):398–402. doi: 10.1002/jemt.20445. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo S, Chen J, Luo T, Jiang X, Xie S, Chen R. Two-layered multiphoton microscopic imaging of cervical tissue. Lasers Med Sci. 2008 doi: 10.1007/s10103-008-0570-2. [DOI] [PubMed] [Google Scholar]
- 16.Richards-Kortum R, Ramanujam N, Mahadevan-Jansen A, Follen M, Utzinger U. Method for probabilistically classifying tissue in vitro and in vivo using fluorescence spectroscopy. 7,236,815. uS Patent. 2007 Jun 26;
- 17.Utzinger U, Richards-Kortum R. Fiber optic probes for biomedical optical spectroscopy. Journal of Biomedical Optics. 2003;8:121. doi: 10.1117/1.1528207. [DOI] [PubMed] [Google Scholar]
- 18.Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Diagnosing breast cancer by using raman spectroscopy. Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12371–12376. doi: 10.1073/pnas.0501390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motz JT, Gandhi SJ, Scepanovic OR, Haka AS, Kramer JR, Dasari RR, Feld MS. Real-time raman system for in vivo disease diagnosis. J Biomed Opt. 2005 May–Jun;10(3) doi: 10.1117/1.1920247. 031113. [DOI] [PubMed] [Google Scholar]
- 20.Freeberg JA, Benedet JL, MacAulay C, West LA, Follen M. The performance of fluorescence and reflectance spectroscopy for the in vivo diagnosis of cervical neoplasia; point probe versus multispectral approaches. Gynecol Oncol. 2007 Oct;107(1 Suppl 1):S248–S255. doi: 10.1016/j.ygyno.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Mayes P, Dicker D, Liu Y, El-Deiry W. Noninvasive vascular imaging in fluorescent tumors using multispectral unmixing. Biotechniques. 2008 Oct;45(4):459–460. doi: 10.2144/000112946. [DOI] [PubMed] [Google Scholar]
- 22.Tumlinson A, Barton J, Považay B, Sattman H, Unterhuber A, Leitgeb R, Drexler W. Endoscope-tip interferometer for ultrahigh resolution frequency domain optical coherence tomography in mouse colon. Optics Express. 2006;14(5):1878–1887. doi: 10.1364/oe.14.001878. [DOI] [PubMed] [Google Scholar]
- 23.Tearney G, Boppart S, Bouma B, Brezinski M, Weissman N, Southern J, Fujimoto J. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. SPIE MILESTONE SERIES MS. 1998;147:656–658. [Google Scholar]
- 24.Wilder-Smith P, Jung W-G, Brenner M, Osann K, Beydoun H, Messadi D, Chen Z. In vivo optical coherence tomography for the diagnosis of oral malignancy. Lasers Surg Med. 2004;35(4):269–275. doi: 10.1002/lsm.20098. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZG, Lee C, Waltzer W, Yuan ZJ, Wu ZL, Xie HK, Pan YT. Optical coherence tomography for noninvasive diagnosis of epithelial cancers. Conf Proc IEEE Eng Med Biol Soc. 2006;1:129–132. doi: 10.1109/IEMBS.2006.259452. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Aguirre AD, Hsiung P-L, Desai S, Herz PR, Pedrosa M, Huang Q, Figueiredo M, Huang S-W, Koski A, Schmitt JM, Fujimoto JG, Mashimo H. Ultrahigh resolution optical coherence tomography of barrett’s esophagus: preliminary descriptive clinical study correlating images with histology. Endoscopy. 2007 Jul;39(7):599–605. doi: 10.1055/s-2007-966648. [DOI] [PubMed] [Google Scholar]
- 27.Lam S, Standish B, Baldwin C, McWilliams A, leRiche J, Gazdar A, Vitkin AI, Yang V, Ikeda N, MacAulay C. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res. 2008 Apr 1;14(7):2006–2011. doi: 10.1158/1078-0432.CCR-07-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Testoni P-A, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J Gastroenterol. 2008 Nov 14;14(42):6444–6452. doi: 10.3748/wjg.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouse AR, Kano A, Udovich JA, Kroto SM, Gmitro AF. Design and demonstration of a miniature catheter for a confocal microendoscope. Applied Optics. 2004;43(31):5763–5771. doi: 10.1364/ao.43.005763. [DOI] [PubMed] [Google Scholar]
- 30.Rouse AR, Gmitro AF. Multispectral imaging with a confocal microendoscope. Optics Letters. 25(23) doi: 10.1364/ol.25.001708. [DOI] [PubMed] [Google Scholar]
- 31.Sabharwal YS, Rouse AR, Donaldson L, Hopkins MF, Gmitro AF. Slit-scanning confocal microendoscope for high-resolution in vivo imaging. Applied Optics. 1999;38(34):7133–7144. doi: 10.1364/ao.38.007133. [DOI] [PubMed] [Google Scholar]
- 32.Tanbakuchi AA, Rouse AR, Udovich JA, Hatch KD, Gmitro AF. A clinical confocal microlaparoscope for real-time in vivo optical biopsies. submitted to the Journal of Biomedical Optics. 2009 February; doi: 10.1117/1.3207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanbakuchi A, Rouse A, Hatch K, Sampliner R, Udovich J, Gmitro A. Clinical evaluation of a confocal microendoscope system for imaging the ovary. In: Tearney, Guillermo J, Wang, Thomas D, editors. Endoscopic Microscopy III; Proceedings of the SPIE; 2008. pp. 685103–685103. [Google Scholar]
- 34.Makhlouf H, Tanbakuchi AA, Rouse AR, Gmitro AF. Design of a multi-spectral channel for in-vivo confocal microscopy. Vol. 6432. SPIE; 2007. p. 643206. URL http://link.aip.org/link/?PSI/6432/643206/1. [Google Scholar]
- 35.Udovich JA, Rouse AR, Tanbakuchi A, Brewer MA, Sampliner R, Gmitro AF. Confocal micro-endoscope for use in a clinical setting. Vol. 6432. SPIE; 2007. p. 64320H. URL http://link.aip.org/link/?PSI/6432/64320H/1. [Google Scholar]
- 36.Tanbakuchi AA, Rouse AR, Udovich JA, Gmitro AF. Surgical imaging catheter for confocal microendoscopy with advanced contrast delivery and focus systems. Vol. 6082. SPIE; 2006. p. 608202. URL http://link.aip.org/link/?PSI/6082/608202/1. [Google Scholar]
- 37.Rouse AR, Tanbakuchi AA, Udovich JA, Gmitro AF. Design of an in vivo multi-spectral confocal microendoscope for clinical trials. Vol. 6082. SPIE; 2006. p. 608205. URL http://link.aip.org/link/?PSI/6082/608205/1. [Google Scholar]
- 38.Srivastava S, Rodr_guez J, Rouse A, Brewer M, Gmitro A. Computer-aided identification of ovarian cancer in confocal microendoscope images. Journal of Biomedical Optics. 2008;13 doi: 10.1117/1.2907167. 024021. [DOI] [PubMed] [Google Scholar]
- 39.Kusuzaki K, Murata H, Matsubara T, Miyazaki S, Shintani K, Seto M, Matsumine A, Hosoi H, Sugimoto T, Uchida A. Clinical outcome of a novel photodynamic therapy technique using acridine orange for synovial sarcomas. Photochem Photobiol. 2005;81(3):705–709. doi: 10.1562/2004-06-27-RA-218. [DOI] [PubMed] [Google Scholar]
- 40.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127(3):706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 41.Udovich JA, Besselsen DG, Gmitro AF. Assessment of acridine orange and SYTO 16 for in vivo imaging of the peritoneal tissues in mice. Journal of Microscopy. doi: 10.1111/j.1365-2818.2009.03153.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.