Abstract
Objectives
We hypothesized that fetal LPS exposures to the chorioamnion, lung or gut would induce distinct systemic inflammatory responses.
Study Design
Groups of 5–7 time-mated ewes were used to surgically isolate the fetal respiratory and the gastrointestinal systems from the amniotic compartment. Outcomes were assessed at 124d gestational age, 2d and 7 d after LPS (10 mg, E.coli 055:B5) or saline infusions into the fetal airways or amniotic fluid.
Results
LPS induced systemic inflammatory changes in all groups in the blood, lung, liver, and thymic lymphocytes. Changes in lymphocytes in the posterior mediastinal lymph node draining lung and gut, occurred only after direct contact of LPS with the fetal lung or gut.
Conclusions
Fetal systemic inflammatory responses occurred after chorioamnion, lung or gut exposures to LPS. The organ responses differed based on route of the fetal exposure.
Keywords: fetal inflammation, innate immunity, maturation, chorioamnionitis, antigen exposure
INTRODUCTION
Preterm birth at less than 32 week gestation is frequently associated with a chronic, often clinically silent chorioamnionitis 1. The inflammatory exposures result in fetal risks ranging from no apparent effect to sepsis and fetal death 2. Some preterm newborns have a fetal systemic inflammatory response syndrome (FIRS) diagnosed histologically by fetal inflammatory cells in the cord or by elevated interleukin (IL)-6 levels in cord blood 2, 3. The nature of the fetal response to infection/inflammation remains poorly described and is likely quite variable depending on organism, duration of exposure, and host responses 4, 5. We have used chorioamnionitis induced with LPS in fetal sheep to evaluate the responses of the fetal lung to inflammation 6. This chorioamnionitis also causes a modest systemic inflammatory response 7. We demonstrated that direct lung contact with the pro-inflammatory mediators lipopolysaccharide (LPS) or IL-1 were required for the lung inflammation, injury, and maturation responses and that chorioamnionitis induced by subchoronic LPS infusion did not induce lung maturation 8–10. Because so little is known about how a fetus detects and responds to chorioamnionitis, we have surgically manipulated the fetal sheep to allow us to selectively expose the fetus to LPS via the lung, the chorioamnion, or the chorioamnion and gut 11. We then asked if the targeted fetal exposures would induce lung and systemic inflammatory responses and alter cell populations that modulate immune responses in the blood, thymus and posterior mediastinal lymph node that drain the lung and gut.
METHODS
Animals
Time-mated ewes with singletons were randomized to surgically isolate the respiratory and the gastrointestinal systems from the amniotic sac (table 1) 9. Ewes were sedated with intramuscular ketamine (10 mg/kg body wt) and xylazine (0.5 mg/kg, Troy Laboratories Pty Ltd., Smithfield, Australia) and anesthetized with halothane in oxygen. The fetal head was delivered through a uterine incision, and the fetal trachea of each fetus was attached to 2 L plastic bag (Baxter Healthcare Corp., Deerfield, IL) with a short length of silicone rubber tubing (Silastic, Dow Corning, Midland, MI). A fine catheter was passed through the tracheostomy site, positioned in the distal trachea and connected to an Alzet osmotic pump (Alzet, Inc., Chicago, IL) that delivers 2 ml over 24h. The pump contained 1 mg LPS in saline (E.coli LPS, serotype 055:B5, Sigma Chemical Co., St. Louis, MO) or saline. A second Alzet pump containing either saline or 10 mg LPS was tied to an extremity for the intra-amniotic exposures. The LPS dose was chosen based on a study demonstrating that an intratracheal dose of 1 mg LPS causes lung maturation 9, and a 10 mg intraamniotic dose of LPS causes chorioamnionitis 11. The inflammatory responses in the lungs were similar after the two LPS exposure routes. In some animals, the esophagus was ligated with sutures 11. We randomized fetuses to exposure to LPS infusion into the amniotic fluid, with or without esophageal ligation and to LPS or saline infusion into the trachea. The controls received intra-amniotic and intratracheal saline. The tracheal conduit to the bag to collect fetal lung fluid separated the lungs from the amniotic fluid in all fetuses. Fetal sheep swallow about half the amniotic fluid daily 12, and esophageal ligation prevented distal gut exposure to LPS. The surgeries were performed 2d or 7 d prior to preterm delivery at 124d gestation (term is 150d). The ewes tolerated the surgery well and were healthy until delivery of the fetuses at 124d gestation. Each pregnant ewe was heavily sedated and given spinal anesthesia for delivery of the fetus. Amniotic fluid samples were collected and the fetus was killed with intravascular pentobarbital. A cord blood sample was obtained for a white blood cell count.
TABLE 1.
Animals Studied and LPS in Amniotic Fluid
| Group/Exposure | Fetal Exposures | N | Birth Wt. kg | LPS in Amniotic Fluid (EU/ml×103) |
|---|---|---|---|---|
| Surgical Controls | IA + IT Saline | 5 | 2.4±0.1 | 0.004±0.001 |
| Lung - 2d | IA Saline + IT LPS | 5 | 2.4±0.1 | 0.06±0.02 |
| Lung - 7d | IA Saline + IT LPS | 6 | 2.3±0.1 | 0.03±0.02 |
| Amniotic Fluid + Gut - 2d | IA LPS + IT Saline | 5 | 2.4±0.1 | 4.8±1.2 |
| Amniotic Fluid + Gut - 7d | IA LPS + IT Saline | 3 | 2.3±0.1 | 0.33±0.13 |
| Amniotic Fluid - 2d | IA LPS + IT Saline + Eso Lig | 5 | 2.5±0.1 | 16.4±3.9 |
| Amniotic Fluid - 7d | IA LPS + IT Saline + Eso Lig | 5 | 2.4±0.1 | 0.25±0.06 |
IA - Intra-amniotic Infusion; IT - Intratracheal Infusion; Eso Lig. - Esophageal Ligation
Processing of Lung
The thorax was opened and the lungs were inflated to 40 cm H2O airway pressure to measure a maximal lung volume 7. An alveolar wash of the left lung with 0.9% NaCl was repeated 3 times 7. Bronchoalveolar lavage fluid was centrifuged at 500 ×g for 10 min and the cell pellet resuspended in PBS 7. Total cells were stained with tryptan blue and counted. Differential cell counts were performed on cytospin preparations after staining with Diff-Quick (Scientific Products, McGaw Park, IN).
Lymphocyte Assays
The thymus and posterior mediastinal lymph node (PMLN) were isolated, weighed, and a cell suspension was made for FACS analysis 7, 13. Cells were kept on ice and in the dark. After counting of the cells, separate aliquots were incubated with sheep specific monoclonal antibodies against CD4, CD8, CD25, or for gamma delta T lymphocytes 7. All antibodies, isotype controls, and corresponding secondary antibodies were obtained from VMRD, Pullman, WA.
Cytokine mRNA
Pro-inflammatory cytokine mRNAs for interleukin IL-1, IL-6, and IL-8 were measured using RNA extracted from homogenized lung tissue 14. With ovine specific riboprobes the amount of mRNAs were quantified and mRNA for ribosomal protein L32 was the internal control 14. Results from the control group were standardized to 1 and are expressed as fold increases.
Serum Amyloid A as an Acute Phase Reactant
Serum amyloid A (SAA) is an acute phase reactant that is produced in both hepatic and extra hepatic tissues and is a sensitive marker of inflammation 15. SAA mRNA was quantified by RNAse protection assay with an ovine specific riboprobe. The SAA riboprobe protected a SAA fragment, which encompassed portions of the 5' untranslated region and the coding sequence of SAA mRNA, including a conserved region present among all SAA isoforms (1 to 143 represents amino acids of the translated SAA protein) 15.
LPS Quantification
LPS bioactivity was quantified with Limulus amebocyte lysate assays (Biowhitaker, Walkersville, MD) as previously described 16. In brief, each amniotic fluid sample was diluted with PBS until the sample yielded a value on the linear part of the curve.
LPS tolerance
Monocytes from the lung were isolated as described previously 17, 18. In brief, following vascular perfusion of the right lower lobe with Hank’s Balanced Salt Solution (HBSS) to remove blood, the lobe was minced thoroughly into fine pieces. Following passage through a 100 µm mesh filter, the resulting cell suspension was centrifuged twice to recover the cells. The cells were then layered over a 2-step Percoll (Sigma-Aldrich, USA) gradient (1.085 g/ml and 1.046 g/ml) and centrifuged at 400×g for 40 min at 4°C. Monocytic cells were recovered from the interface between the two Percoll densities and the cell concentration adjusted to 2.5 × 106 cells/ml. After incubation at 37°C for 2 h, non-adherent cells were removed by washing. Monocytes were cultured overnight in the presence of LPS (100 ng/mL) 19. Tumor necrosis factor (TNF)-alpha was measured in the supernatant of the cell media with a commercial ELISA (Endogen, IL) 20.
Data Analysis
Results are given as means ± SEM. Comparisons between the groups were done by ANOVA with Student-Newman test as post-hoc analysis. Comparisons between groups were with 2 tailed t tests. Significance was accepted at p<0.05.
RESULTS
Animals and LPS in Amniotic Fluid
The treatment groups and birth weights of the animals are given in Table 1. All animals survived the surgery and were apparently healthy at delivery with no metabolic acidosis (data not shown). LPS in amniotic fluid was detected in high levels 2d after the intra-amniotic LPS and was low following intratracheal LPS and at 7 d.
Lung Effects
Lung maturation increased with the intratracheal exposure to LPS as indicated by large increases in lung gas volumes and in the amount of saturated phosphatidylcholine recovered in the bronchoalveolar lavages (Table 2). There was no indication of lung maturation in the other groups. Similarly, inflammatory cells expressed as the sum of neutrophils and monocytes/macrophages increased in the BAL at 2 and 7 d only in the group exposed to intratracheal LPS. Pro-inflammatory cytokine mRNA for interleukin (IL)-1β, IL-6, and IL-8 was increased at 2 d only with intra-tracheal LPS. These results demonstrate that lung inflammation/maturation requires contact of the pro-inflammatory agonist with the fetal lung 9. Chorioamnionitis alone does not cause fetal lung inflammation or maturation.
TABLE 2.
Lung Responses to Compartmental LPS Exposures
| Measurements | Surgical Controls (IA + IT Saline) |
Lung (IA Saline + IT LPS) |
Amniotic Fluid + Gut (IA LPS + IT Saline) |
Amniotic Fluid (IA LPS + IT Saline + Eso Lig) |
|---|---|---|---|---|
| Lung Gas Volume at 7d (ml/kg at 40 cmH2O pressure) |
5.7±0.6 | 28.8±3.8* | 7.9±1.5 | 8.7±1.3 |
| Saturated phosphatidylcholine at 7d (µmol/kg) |
0.06±0.02 | 2.4±0.8* | 0.10±0.05 | 0.31±0.10 |
| Inflammatory cells in BAL - 2d (×106/kg) |
6.6±4.5 | 129±41* | 4.0±0.6 | 5.6±2.7 |
| Inflammatory Cells in BAL - 7d (×106/kg) |
6.6±4.5 | 141±27* | 6.1±0.1 | 19.6±5.5 |
| Cytokine mRNA in lung at 2d (fold increase over control) |
||||
| IL-1β | 1.0±0.1 | 5.6±2.0* | 1.3±0.1 | 1.0+0.1 |
| IL-6 | 1.0±0.1 | 2.3±0.5* | 1.1+0.1 | 0.9±0.1 |
| IL-8 | 1.0±0.1 | 11.3+3.5* | 1.3±0.2 | 1.2±0.2 |
p<0.05 vs. surgical control
IA - Intra-amniotic Infusion; IT - Intratracheal Infusion; Eso Lig. - Esophageal Ligation; BAL – bronchoalveolar lavage
Systemic Inflammation
Neutrophil and monocyte numbers in cord blood 2 d after exposure were similar to the control group (Fig. 1). However, neutrophils increased with LPS exposure in all three LPS groups 7 d after exposure. Therefore, fetal neutrophils can be induced by LPS exposure to the lung or amniotic fluid. Only an amniotic plus gut exposure increased cord blood monocytes at 7 d, suggesting that gut exposure contributes to the increase in fetal blood monocytes.
Figure 1.
Neutrophils and monocytes in cord blood. A. The number of neutrophils in cord blood increased following LPS exposure in all groups relative to controls after 7 days but not after 2 days. B. The number of monocytes in cord blood increased in the 7 days group after IA LPS and IA saline but not in the other groups (* p<0.05 versus control). IA- intraamniotic, IT-intratracheal, Eso. Lig- esophageal ligation.
The mRNA for acute phase reactant gene SAA increased in the fetal liver at 2 d following all LPS exposures but not in the control group that underwent the same surgical procedures (Fig. 2). SAA mRNA levels had returned to control levels by 7 d (data not shown). This finding indicates a similar liver inflammatory response after contact of fetal gut, lung or membranes with LPS. Therefore, systemic acute phase and inflammatory responses can be induced by the exposure of the fetal lung or chorioamnion by LPS.
Figure 2.
mRNA for the acute phase reactant serum amyloid A (SAA) in the fetal liver at 2 d. LPS given by the three different exposure routes increased SAA mRNA relative to the control group (p<0.05). IA- intraamniotic, IT-intratracheal, Eso. Lig-esophageal ligation.
Immune Modulation
Thymus
We evaluated intrathoracic thymus weight and lymphocyte subpopulations in the thymus as markers of short-term immune cell responses to the LPS exposures. There were no consistent changes in thymus to body weight ratios with the LPS exposures (Table 3). The percentages of CD4 positive lymphocytes increased after 2 d with the lung LPS exposure and at 7 d in all LPS groups. Similar increases in the percentages of CD8 lymphocytes occurred at 2 and 7 d in all groups (Table 3). CD4/CD25 double positive lymphocytes increased at 7 d in all exposure groups (Fig. 3). Gamma delta T lymphocytes also increased for all LPS exposure groups with significant differences from controls in all groups at 7 d (Fig. 3). Therefore, a large thymic response occurred independent of route of exposure, and exclusion of the lung or gut did not prevent a thymic response to chorioamnionitis.
TABLE 3.
| Groups and Exposures | ||||
|---|---|---|---|---|
| Surgical Controls (IA + IT Saline) |
Lung (IA Saline + IT LPS) |
Amniotic Fluid + Gut (IA LPS + IT Saline) |
Amniotic Fluid (IA LPS + IT Saline + Eso Lig) |
|
| Fetal Thymus | ||||
| Weight/body weight (g/kg) - 2d | 1.38±0.09 | 1.19+0.21 | 1.27±0.14 | 1.61±0.20 |
| 7d | - | 1.39±0.12 | 1.02±0.19 | 1.44±0.19 |
| CD4 Cells (%) - 2d | 22.3±10.3 | 39.7±12.5* | 26.9±12.6 | 23.6±10.7 |
| 7d | - | 46.5±13.3* | 49.6±9.8* | 53.7±12.2* |
| CD8 Cells (%) - 2d | 14.5±10.7 | 29.3±12.5* | 29.5±12.4* | 30.9±16.1* |
| 7d | - | 40.5±20.2* | 39.4±10.3* | 53.9±16.6* |
|
Fetal Posterior Mediastinal Lymph Node |
||||
| Weight/body weight (g/kg) - 2d | 0.19±0.05 | 0.31±0.04* | 0.22±.034 | 0.16±0.01 |
| 7d | - | 0.33±0.02* | 0.33±0.04* | 0.26±0.04 |
| CD4 Cells (%) - 2d | 12.1±4.6 | 27.4±7.9* | 26.9±12.6* | 37.2±12.9* |
| 7d | - | 29.6±8.4* | 49.6±9.8* | 48.6±17.2* |
| CD8 Cells (%) - 2d | 9.4±3.2 | 32.7±16.8* | 25.5±11.4* | 31.5±15.2* |
| 7d | - | 44.8±19.3* | 44.7±21.2* | 39.6±18.2* |
IA - Intra-amniotic Infusion; IT - Intratracheal Infusion; Eso Lig. - Esophageal Ligation
p<0.05 vs. surgical controls
Figure 3.
Thymus. The CD4/CD25 positive cells (top panel) and gamma delta T lymphocytes (lower panel) in the fetal thymus are shown as percentages. Only after 7 days of contact of LPS with the fetal gut or the fetal lung the number of CD4 and CD25 positive cells was increased. The percentages of gamma delta T lymphocytes were increased in the same group of animals at both 2 days and 7 days after contact of LPS with the fetal lung and the fetal gut (* p<0.05 versus control). IA- intraamniotic, IT-intratracheal, Eso. Lig- esophageal ligation.
Posterior mediastinal lymph node
We evaluated the weight and lymphocyte types in the PMLN because lung lymph and gut drains through this node 21. Fetuses with lungs only exposed to LPS had an increased PMLN to body weight ratio at 2 and 7 d (Table 3). Amniotic and gut exposure also resulted in a larger PMLN at 7 d, but fetal LPS exposure that excluded lung and gut did not result in an increase in PMLN weight. The percentage of CD4 and CD8 positive lymphocytes increased in the PMLN after 2 and 7 d in all groups (Table 3). The double positive CD4/CD25 lymphocytes increased at 7 d with chorioamnion and gut exposure or lung only exposure to LPS, but not chorioamnion exposure alone (Fig. 4). A similar increase to Gamma Delta T lymphocytes occurred at 2d and 7 d. Therefore, the immune modulation of the PMLN required either the gut or lung exposure to LPS.
Figure 4.
Posterior mediastinal lymph node. The CD4/CD25 positive cells (top panel) and gamma delta T lymphocytes (lower panel) are shown as percentages. After 7 days of contact of LPS with the fetal gut or the fetal lung the number of CD4 and CD25 positive cells was increased in the posterior mediastinal lymph node. No increase was seen after 2 d of exposure The percentages of gamma delta T lymphocytes were increased at both 2 days and 7 days after contact of LPS with the fetal lung and the fetal gut but not after oesophageal occlusion (* p<0.05 versus control). IA- intraamniotic, IT-intratracheal, Eso. Lig- esophageal ligation.
TNF- alpha Responsiveness of lung monocytes in vitro
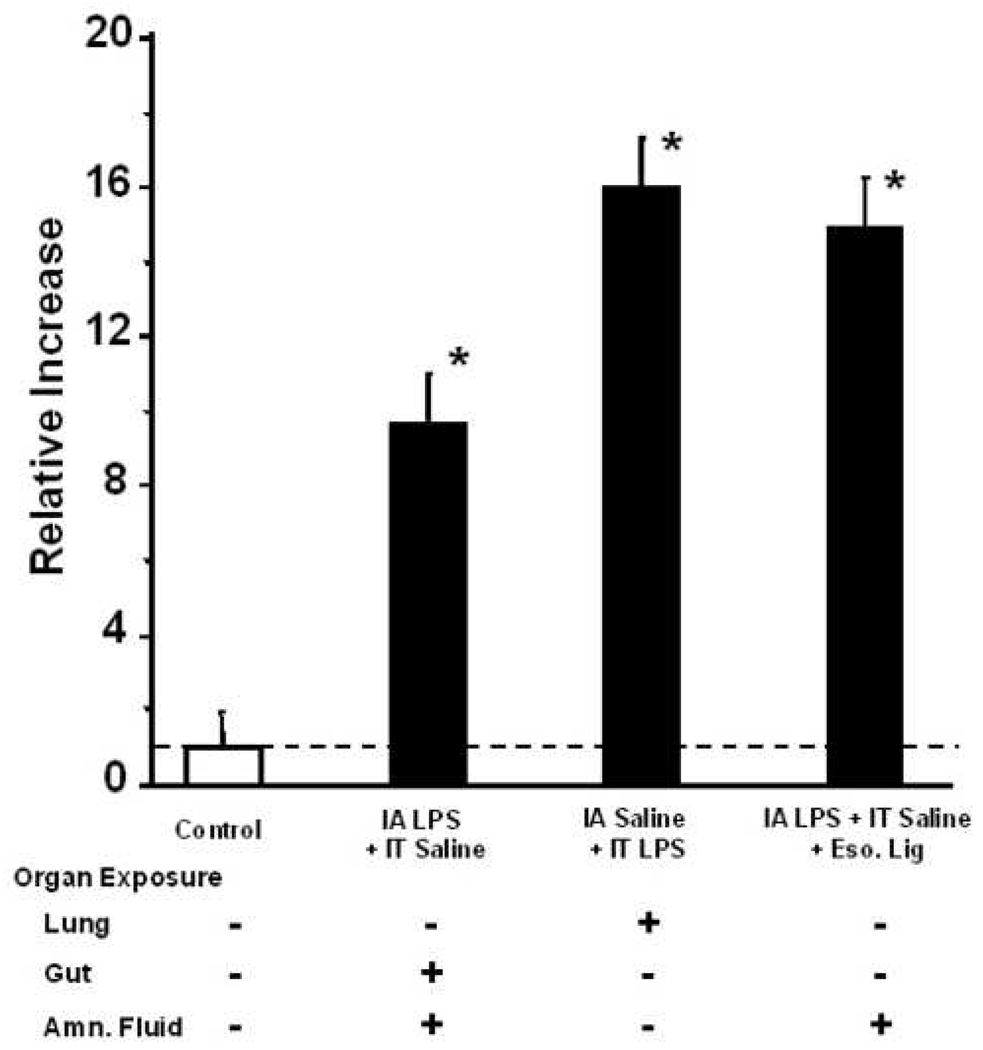
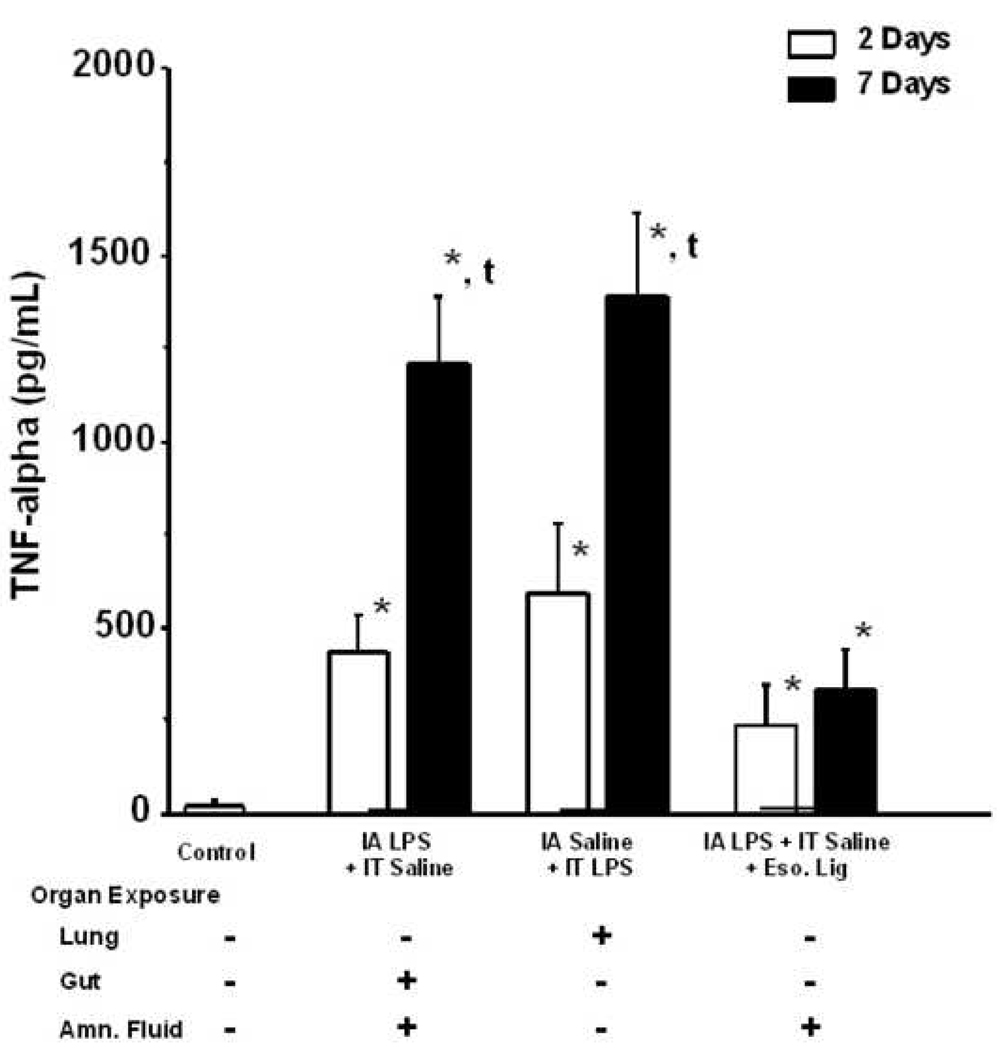
Monocytes recovered from lungs of control animals produced very little TNF-alpha on stimulation with LPS in culture. The concentration of TNF-alpha in cell culture media was increased after stimulation of lung macrophages with LPS in all groups and at all time points in comparison to control (Figure 5). The TNF-alpha concentration was increased after 7 d exposure of intra-amniotic LPS in comparison to 2d exposure for the lung and gut plus chorioamnionitis via the same routes. Monocytes from the esophagal ligation group with exclusion of lung and gut, had increased TNF-alpha levels relative to controls but less TNF-alpha at 7 d relative to the other groups. This result suggests that enhanced lung monocyte responsiveness is more dependent on lung and gut exposure to LPS than exposure of the chorioamnion alone.
Figure 5.
TNF-alpha concentration in cell culture media after stimulation of lung monocytes with 100 ng/mL LPS. The concentration of TNF-alpha in cell culture media was increased after stimulation of lung macrophages with LPS in all groups and at all time points in comparison to control. The TNF-alpha concentration was increased after 7 days exposure of intra-amniotic LPS in comparison to 2 days exposure via the same route. Similar results were obtained after intratracheal LPS exposure. The esophageal ligation resulted in a small increase in TNF-alpha production after 2 and 7 days relative to controls but less relative to the other LPS groups (p<0.05; * p<0.05 versus control; t < 0.05 versus respective 2 days group (t-test)). IA- intraamniotic, IT-intratracheal, Eso. Lig- esophageal ligation.
DISCUSSION
We surgically manipulated the fetal sheep to achieve targeted fetal exposures of LPS to the fetal lung, the amniotic compartment (fetal skin, chorioamnion, amniotic fluid) or the amniotic compartment and fetal gut. We explored the systemic versus local signaling route of intra-amniotic LPS-induced chorioamnionitis in the fetus in this series of experiments. The systemic indicators of fetal responses were the acute phase reactant SAA in the liver and neutrophils in the blood, and the three exposures caused similar increases in both indicators. Thus, the fetal immune system can detect LPS via lung or amniotic exposures. We previously reported that lung exposure by infusion of LPS into fetal lung fluid induced both lung inflammation and lung maturation 9. That observation is confirmed and extended to now include the signaling of a systemic response. In humans, chorioamnionitis and particularly funisitis is associated with a FIRS that is diagnosed in the experimental literature by increases in cord blood IL-6 3, 22, 23. There is very little known about the sources or ranges of fetal responses. In ventilated baboons for example, autoreactive T-lymphocytes may play a role in the development of BPD 24. The development of BPD was also accompanied by thymic cortical involution 25. In fetal sheep, the mRNA for the acute phase reactant SAA is increased in the liver at 2d and blood neutrophils and monocytes (gut exposure) increase at 7d.
The acute inflammatory response represents recognition of LPS by the innate immune system of the fetus. The preterm fetus has a poorly responsive innate immune system relative to the adult 19. TLR receptors are low in preterm fetal sheep 26, and lung and blood monocytes have low TNF–alpha, IL-6 and H2O2 responses to the TLR4 agonist LPS and other TLR agonists on in vitro challenge relative to alveolar macrophages from adult sheep 13, 18, 20. However, intra-amniotic LPS induces fetal lung monocytes to mature to alveolar macrophages that appear phenotypically mature and that respond to TLR agonists similarly to alveolar macrophages from adult sheep 20. Similarly, fetal blood monocytes that respond minimally to TLR agonists become responsive 7d after intra-amniotic LPS 20. We have referred to this induction of innate immune responsiveness as maturation 27. Fetal monocyte innate immune responses also can be paralyzed by a second exposure to intra-amniotic LPS 18, 20 - an immune regulatory phenomenon referred to as endotoxin tolerance 28. These effects of LPS induced chorioamnionitis in monocytes in fetal sheep blood and lungs no doubt represent just some of the fetal responses to inflammation. There are now multiple reports of how fetal exposures to presumptive infection/inflammation can alter immune responses in childhood. For example, elevated cytokines in cord blood predict an increase in asthma in children 29, as does chorioamnionitis in preterm infants 30.
To begin to explore other effects of LPS induced chorioamnionitis on the fetal immune system we measured CD4 and CD8 lymphocytes, CD4/CD25 lymphocytes, and gamma delta lymphocytes in the posterior mediastinal lymph node and the thymus. The fetal thymus contains mostly immature CD4− and CD8− thymocytes that mature within the thymus to CD4+/CD8+ thymocytes with subsequent selection to lymphocytes that express CD4 or CD8. We measured CD4+ and CD8+ cells, but did not measure the CD4+/CD8+ cells simultaneously. However, we showed striking increases in both CD4+ and CD8+ cells in the fetal thymus within 2d of intra-amniotic LPS exposure. These increases persisted to 7d and demonstrated an induced maturation of lymphocytes. The numbers of the activated CD4+/CD25+ regulatory T-cells also increased in the thymus by 7d. These cells are presumably appearing to damp down the inflammatory responses 31. Gamma delta T-cells are another class of mature lymphocytes that also increase with chorioamnionitis. Thus, independent of route of fetal exposure, the fetal thymus responds with large changes in lymphocyte populations suggesting both maturation and activation of lymphocytes. The short or long term effects of such acute changes in lymphocyte populations in the fetal thymus are unknown.
Similar changes in lymphocytes populations with increased CD4+, CD8+, CD4+/CD25+, and gamma delta T lymphocytes occurred in the posterior mediastinal lymph node, but reached statistical significance only for gut or lung exposures to LPS. The posterior mediastinal lymph node in sheep receives most of the lymph from the lung, as well as lymph from the gut. This lymph node is a site of dendritic cell signaling following inflammatory exposures 32. This lymph node also increased in size with the gut or lung exposures. This result suggests that the fetal immune system can distinguish between a local inflammatory stimulus and a systemic stimulus. The implications of the targeted stimulus may be that selected organ responses may result in specific adverse outcomes, which has to be tested in future experiments. For example, chorioamnionitis correlates with increased risks for BPD or necrotizing enterocolitis in some cohorts 33–35. These results suggest that alterations in fetal immune response may predate and program postnatal inflammatory responses in selected organs.
We also tested if the lung monocytes responded to LPS challenge in vitro with increased secretion of TNF-alpha. As expected, lung exposure to LPS induced a large increase in TNF-alpha at 7d. This is the maturation response that we reported previously for intra-amniotic LPS without tracheal diversion 17. The lung monocyte response suggests that direct contact of the lung with LPS matures the lung monocyte secretion of TNF-alpha in response to LPS. The group with tracheal diversion and esophageal ligation had a minimal increase in TNF-alpha secretion by lung monocytes at 7 d. A more surprising result is the comparable increased TNF-alpha response when the exposure was via the chorioamnion and gut and therefore presumably primarily was a gut effect. This exposure group had enlarged posterior mediastinal lymph nodes, but no lung inflammation. Nevertheless, the monocytes isolated from the lung had developed the ability to secrete TNF-alpha on LPS stimulation, presumably by either recruitment of monocytes from the blood or by retrograde signaling from the posterior mediastinal lymph node. The recruitment of monocytes may also explain the increase in monocytes in the cord blood. These results demonstrate the complexity of immune response in the naïve fetus when exposed to an inflammatory challenge.
There are limitations to this experiment. We did not isolate the gut and do not have a gut only exposure group. Such an experiment would further refine the assessments of local inflammatory responses. The intra-amniotic LPS exposure for the group with the tracheal bag and esophageal ligation resulted in higher LPS levels in amniotic fluid at 2d, presumably because fetal swallowing was prevented. Also, the amniotic fluid containing the LPS will contact the epithelium of the upper airway, oral cavity and pharynx, as well as the chorioamnion and fetal skin. We do not know which exposure from the amniotic LPS resulted in the systemic inflammatory and immune response. We did not perform more extensive immune assessments because of lack of sheep specific antibodies. This study supports the concept of chorioamnionitis as a “multi-organ disease” of the fetus. Chorioamnionitis was shown in epidemiological studies to be a risk factor for adverse outcomes with respect to the brain, lung, and gut with increased incidences of white matter disease, bronchopulmonary dysplasia and necrotizing enterocolitis 36–38. The common link between the different organs is the inflammatory response 2, 39, 40. Our model allows us to study effects of antenatal inflammation on the fetal immune system. We found that the exposure to a proinflammatory agent induced alveolar macrophages and modulated the function of monocytes and the newly induced alveolar macrophages 17, 18, 20. Surprisingly, our experimental data and clinical data suggest that the fetus can adapt to persisting inflammation or even viable bacteria in the clinical setting 4, 41. Therefore, understanding the mechanisms of fetal immunomodulation may be an approach to new therapies in perinatal medicine.
Acknowledgement
The excellent help of Helgi Jobe, Andrea Meyer and Sahofu Li are gratefully appreciated.
Supported by HL-KO8 70711, HL-65397, HD-12714, HD-57869 from the National Institute of Health, National Health and Medical Research Council of Australia, the Women and Infants' Research Foundation, Western Australia, the Dutch Scientific Research Organization and the Research School Oncology and Developmental Biology, University of Maastricht.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation: Targeted fetal exposure to LPS causes selective lung, liver, blood, thymic, and lymph node responses as part of a systemic inflammatory response.
References
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 2.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 3.Shimoya K, Taniguchi T, Matsuzaki N, et al. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod. 2000;15:2234–2240. doi: 10.1093/humrep/15.10.2234. [DOI] [PubMed] [Google Scholar]
- 4.Kramer BW, Jobe AH. The clever fetus: responding to inflammation to minimize lung injury. Biol Neonate. 2005;88:202–207. doi: 10.1159/000087583. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Newnham JP, Willet KE, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182:401–408. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- 7.Kramer BW, Moss TJ, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 8.Moss TJ, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189:1771–1776. doi: 10.1016/s0002-9378(03)00810-x. [DOI] [PubMed] [Google Scholar]
- 9.Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham JP, Jobe AH. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol. 2002;187:1059–1065. doi: 10.1067/mob.2002.126296. [DOI] [PubMed] [Google Scholar]
- 10.Sosenko IR, Kallapur SG, Nitsos I, et al. IL-1alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatr Res. 2006;60:294–298. doi: 10.1203/01.pdr.0000233115.51309.d3. [DOI] [PubMed] [Google Scholar]
- 11.Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186:1062–1068. doi: 10.1067/mob.2002.122293. [DOI] [PubMed] [Google Scholar]
- 12.Nijland MJ, Day L, Ross MG. Ovine fetal swallowing: expression of preterm neurobehavioral rhythms. J Matern Fetal Med. 2001;10:251–257. doi: 10.1080/714904334. [DOI] [PubMed] [Google Scholar]
- 13.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham J, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immunity. 2009 doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 14.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 15.Wilson TC, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Pulmonary and systemic induction of SAA3 after ventilation and endotoxin in preterm lambs. Pediatr Res. 2005;58:1204–1209. doi: 10.1203/01.pdr.0000185269.93228.29. [DOI] [PubMed] [Google Scholar]
- 16.Newnham JP, Kallapur SG, Kramer BW, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol. 2003;189:1458–1466. doi: 10.1067/s0002-9378(03)00758-0. [DOI] [PubMed] [Google Scholar]
- 17.Kramer BW, Joshi SN, Moss TJ, et al. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L345–L353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and Systemic Endotoxin Tolerance in Preterm Fetal Sheep Exposed to Chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- 19.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–57. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 20.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 21.Hedenstierna G, Lattuada M. Lymphatics and lymph in acute lung injury. Curr Opin Crit Care. 2008;14:31–36. doi: 10.1097/MCC.0b013e3282f2f4b5. [DOI] [PubMed] [Google Scholar]
- 22.Kramer BW, Kaemmerer U, Kapp M, et al. Decreased expression of angiogenic factors in placentas with chorioamnionitis after preterm birth. Pediatr Res. 2005;58:607–612. doi: 10.1203/01.PDR.0000175641.39056.7A. [DOI] [PubMed] [Google Scholar]
- 23.D'Alquen D, Kramer BW, Seidenspinner S, et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res. 2005;57:263–269. doi: 10.1203/01.PDR.0000148713.48218.86. [DOI] [PubMed] [Google Scholar]
- 24.Rosen D, Lee JH, Cuttitta F, Rafiqi F, Degan S, Sunday ME. Accelerated thymic maturation and autoreactive T cells in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;174:75–83. doi: 10.1164/rccm.200511-1784OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Felice C, Latini G, Del Vecchio A, Toti P, Bagnoli F, Petraglia F. Small thymus at birth: a predictive radiographic sign of bronchopulmonary dysplasia. Pediatrics. 2002;110:386–388. doi: 10.1542/peds.110.2.386. [DOI] [PubMed] [Google Scholar]
- 26.Hillman NH, Moss TJ, Nitsos I, et al. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63:388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 29.Macaubas C, de Klerk NH, Holt BJ, et al. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 2003;362:1192–1197. doi: 10.1016/s0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–884. doi: 10.1016/j.jaci.2008.01.030. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 34.Satar M, Turhan E, Yapicioglu H, Narli N, Ozgunen FT, Cetiner S. Cord blood cytokine levels in neonates born to mothers with prolonged premature rupture of membranes and its relationship with morbidity and mortality. Eur Cytokine Netw. 2008;19:37–41. doi: 10.1684/ecn.2008.0118. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Tallo E, Claure N, Bancalari E. Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol Neonate. 1997;71:292–298. doi: 10.1159/000244428. [DOI] [PubMed] [Google Scholar]
- 36.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis. 2003;16:349–355. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Dammann O, Leviton A. Biomarker epidemiology of cerebral palsy. Ann Neurol. 2004;55:158–161. doi: 10.1002/ana.20014. [DOI] [PubMed] [Google Scholar]
- 38.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 39.Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med. 2006;11:363–368. doi: 10.1016/j.siny.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]