Abstract
P53 wild-type and p53-null or mutant cells undergo a G2-phase cell cycle arrest in response to ionizing radiation (IR). In this study we examined the effect of heat shock protein 90 (HSP90) inhibitor geldanamycin (GA) on IR-induced G2 arrest in human colon adenocarcionoma cells with different p53 status. We show that GA treatment abrogates IR-induced G2 phase arrest in cells null or mutant for p53. Specifically, GA treatment pushed irradiated p53 signaling defective cells into a premature mitosis characterized by aberrant mitotic figures, increased γH2AX expression, and formation of micronucleated cells. Cells expressing wild-type p53 were resistant to GA-induced G2 checkpoint abrogation. Notably, GA treatment decreased levels of G2 regulatory proteins Wee1 and Chk1, and inhibitory phosphorylation of Cdc2, independent of p53 status. Further investigation identified p21 as the potential downstream effector of p53 that mediates resistance to G2 checkpoint abrogation. Clonogenic survival studies demonstrated higher sensitivity to GA alone or combination IR plus GA treatment in p53 and p21 null cells. Collectively, these data demonstrate potential mechanisms through which HSP90 inhibition can enhance the effects of ionizing radiation in p53 compromised cancer cells. Combination IR plus HSP90 inhibitor therapies may be particularly useful in treating cancers that lack wild-type p53.
Introduction
The tumor suppressor p53 is a transcription factor that plays a key role during the cellular response to DNA damage. P53 is mutated in over 50% of human cancers while its regulation and downstream effects are impaired in many other malignancies (Giono and Manfredi, 2006). Accordingly, therapeutic intervention that targets cells with compromised p53 function is considered an ideal strategy to combat many cancers. P53 levels increase after DNA damage resulting in transcriptional upregulation of genes involved in growth arrest, senescence or apoptosis such as P21waf1, PUMA, and Bax. P21 is a cyclin dependent kinase inhibitor that functions in growth arrest at both G1 and G2 phases of the cell cycle (Giono and Manfredi, 2006; Sherr, 1994). Cyclin dependent kinases in complex with their regulatory cyclins orchestrate the sequential transition through the phases of the cell cycle. P21 can bind directly to these complexes and inhibit their activity (Sherr, 1994).
Exposure to ionizing radiation (IR), a common cancer therapy, induces growth arrest in G1 and G2 phase (Iliakis et al., 2003). The widely held view is that these arrests constitute checkpoints that allow repair of sublethal DNA damage prior to continuing cell division. IR induces DNA double strand breaks (DSBs) which activate the protein kinase Ataxia Telangiectasia mutated (ATM) and to a lesser extent ATM and Rad3-related (ATR) protein (Iliakis et al., 2003). ATM phosphorylates many proteins including histone H2AX, p53, and checkpoint kinase 2 (Chk2) while ATR phosphorylates Chk1 among other substrates. Phosphorylation by ATM and activated Chk2 stabilizes p53, which can then promote expression of its downstream targets (Iliakis et al., 2003). Cell cycle arrest in G1 following IR depends largely on p53 and p21. In contrast, G2 arrest is initiated in p53 and p21-deficient cells though of shorter duration compared to normal cells, suggesting p53 and p21 are required for maintenance but perhaps not initiation of this G2 arrest (Bunz et al., 1998; Waldman et al., 1996). Due to defective p53 signaling many cancer cells lack G1 arrest and depend to a greater extent on G2 arrest as their primary response to DNA damage (Kawabe, 2004). Abrogation of G2 arrest leading to premature mitotic entry and mitotic death has emerged as a potential therapeutic strategy (Dixon and Norbury, 2002; Kawabe, 2004). Tumor cells treated with G2 abrogators such as caffeine, pentoxifylline and the Chk1 inhibitor UCN-01 have been shown to be sensitized to IR and other DNA damaging agents (Jackson et al., 2000; Russell et al., 1996; Sarkaria et al., 1999).
Heat shock protein 90 (HSP90) is a molecular chaperone critical for the correct folding and stability of many proteins involved in signal transduction, survival, oncogenic signaling, and cell cycle regulation (Whitesell and Lindquist, 2005). Geldanamycin (GA) and its analogs 17-AAG and 17-DMAG are ansamycin antibiotics that inhibit HSP90 by binding to the NH2-terminal ATP binding domain, leading to degradation of HSP90 clients (Whitesell and Lindquist, 2005). Previous reports demonstrated HSP90 inhibitors can sensitize cells to the cytotoxic effects of DNA damaging agents, including IR, primarily through downregulation of cell survival and cytoprotective factors (Arlander et al., 2003; Bisht et al., 2003; Bull et al., 2004; Dote et al., 2006; Rahmani et al., 2003; Robles et al., 2006). However GA has also been shown to abrogate G2 arrest in doxorubicin treated lymphoma and irradiated carcinoma cells (Bull et al., 2004; Dote et al., 2006; Robles et al., 2006; Sugimoto et al., 2007). Mechanistic studies on the enhancement of IR cytotoxicity by HSP90 inhibition, particularly those focusing on G2-checkpoint abrogation, are limited. In the current study we find that GA treatment abrogates IR-induced G2 cell cycle arrest in cells lacking a functional p53-p21 signaling pathway. Importantly, GA decreased the expression of G2 regulatory proteins Wee1 and Chk1, as well as inhibitory phosphorylation of Cdc2. Premature mitotic entry by GA treatment potentiated the development of micronucleated cells specifically in cells deficient for p53 or p21. Finally, decreased clonogenic survival was demonstrated in p53 and p21 deficient cells treated with GA alone or combination IR plus GA.
Results
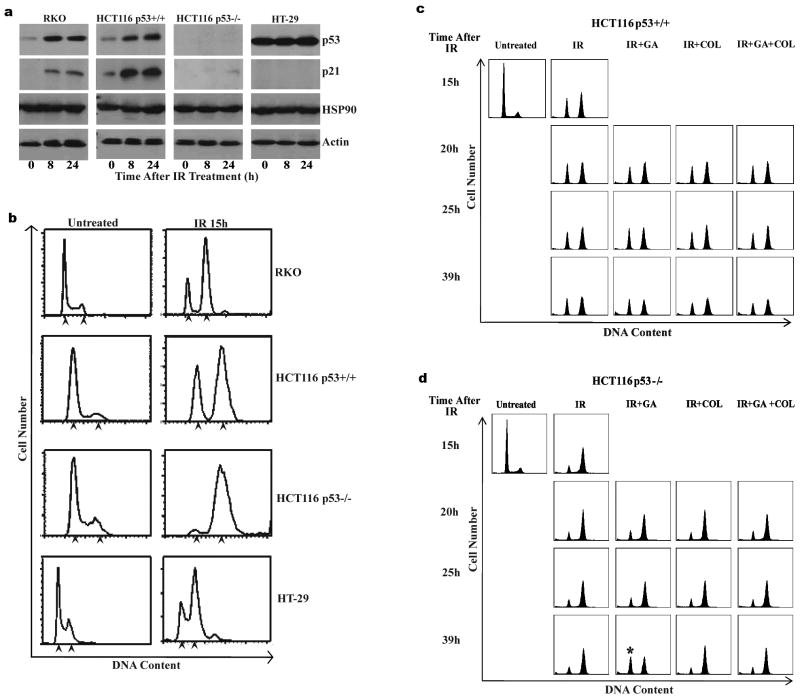
IR induces both G1 and G2-phase cell cycle arrests in cells expressing wild type p53 (p53wt). Previous reports have established a diminished G1 arrest in p53 deficient cells (Deng et al., 1995; Kastan et al., 1992). We monitored cell cycle profiles and p53/p21 expression in irradiated human colon carcinoma cell lines with different p53 status. An IR dose of (10Gy) was used for these studies because this dose caused a robust G2 arrest in all the cell lines we tested. P53, p21 and HSP90 expression was determined by immunoblotting cell lysates before and after IR treatment in RKO (p53wt), HCT116 p53+/+ (p53wt), HT-29 (p53 mutant; R273H) and HCT116 p53−/− (p53 null) cells. P53 and p21 expression increased in IR-treated RKO and HCT116 P53+/+ cells, but not in IR-treated HT-29 cells (Figure 1a). As expected, p53 was not detected in HCT116 P53−/− cells, though modest induction of p21 was observed following IR treatment, as previously described (Bunz et al., 1998). HSP90 expression was similar in all cell types and unaffected by IR treatment. DNA content analysis by flow cytometry demonstrated IR-induced G1 and G2/M arrest in p53 wild-type cells (HCT116 P53+/+ and RKO cells) but only a G2 arrest in cells null or mutant for p53 (HT-29 and HCT116 P53−/−) (Figure 1b).
Figure 1.
IR induces cell cycle arrest in human colon adenocarcinoma cells from different p53 backgrounds and GA abrogates IR induced G2 arrest in HCT116 p53−/− cells. RKO (p53 wildtype), HCT116 p53+/+ (p53 wildtype), HCT116 p53−/− (p53 null) and HT-29 (p53 mutant) cells were irradiated (10Gy). (a) Representative immunoblots from cell lysates collected 8h and 24h after irradiation probed using antibodies specific against p53, p21 and HSP90. Actin expression was detected as a loading control. Immunoblots are representative of three independent experiments. (b) Representative DNA content profiles of untreated and IR treated cells (15h). Cells were fixed, stained with propidium iodide and analyzed by flow cytometry for DNA content. DNA profiles are representative of three independent experiments. Arrowheads indicate 2N and 4N DNA content respectively on each DNA profile. (c) (d) Representative DNA content profiles from (c) HCT116 P53+/+ and (d) HCT116 P53−/− cells which were irradiated (10Gy) and subsequently treated with GA or GA/COL 15h later. Cells were fixed at times indicated after IR, stained with propidium iodide and analyzed by flow cytometry for DNA content. DNA profiles are representative of three independent experiments. Asterisk indicates increased G1 population observed in IR/GA treated HCT116 p53−/− cells.
We wished to examine the effect of HSP90 inhibition on G2 arrest in IR treated cells, and the potential involvement of p53 in this effect. To this end we treated HCT116 P53+/+ and HCT116 P53−/− with IR to establish G2 cell cycle arrest (15h). Cells were then treated with the HSP90 inhibitor geldanamycin (GA; 1μM) in the presence or absence of colcemid (COL), and cell cycle profile analyzed at time points after GA and/or COL addition. COL blocks progression through mitosis at metaphase and hence traps cells that have escaped G2 arrest and entered mitosis (Rieder and Palazzo, 1992). Treatment with GA, COL or a combination of both had no effect on the cell cycle profile of IR treated HCT116 P53+/+ cells compared to those treated with IR alone (Figure 1c). G1 cell cycle arrest was maintained in these cells as evidenced by the absence of detectable S phase cells. Furthermore, the percentage IR treated HCT116 p53+/+ cells accumulated with G2/M DNA content was unaffected by GA or COL treatment. In contrast, GA treatment decreased the percentage of IR treated HCT116 P53−/− cells with G2/M DNA content, coincident with an apparent increase in the G1 population (asterisk at 39h timepoint, Figure 1d). Importantly, this effect was blocked by COL treatment, demonstrating that this effect requires passage through mitosis. The addition of COL alone to irradiated HCT116 P53−/− cells had no effect on the DNA content profile.
Results of Figure 1 suggested GA could abrogate the G2/M arrest in HCT116 p53−/− cells, but not in HCT116 p53+/+ cells. To investigate this further, we examined the effect of GA on IR treated HCT116, RKO and HT29 cells using a similar mitotic-trap assay. First, cells were treated with IR followed by COL addition 15 hours later. At time-points after COL addition, cells were stained with propidium iodide for DNA content and an antibody specific for the mitotic marker phospho-Histone H3 (ser10) to mark cells in mitosis. Cells were then analyzed by flow cytometry. Treatment of asynchronous (no IR) cells with COL for 10 hours resulted in an accumulation of between 20–25% of cells in mitosis for the four cell types examined (data not shown). IR treatment of RKO, HCT116 p53+/+ and HCT116 p53−/− cells 15 hours prior to COL addition significantly reduced entry of cells into mitosis, though HCT116 p53−/− cells did begin to accumulate in mitosis 39h after IR treatment (13+/−4.9% of cells trapped in mitosis, Figure 2a). HT-29 cells displayed a more transient G2 arrest, beginning to accumulate in mitosis 20h after IR+COL treatment. To investigate the potential of GA to abrogate the IR induced G2 arrest, cells were treated with IR for 15h to establish G2 arrest followed by treatment with GA and COL. Analysis of the mitotic cell populations after treatment with IR and GA+COL demonstrated no significant increase in mitotic cells in irradiated HCT116 p53+/+ or RKO cells (Figure 2a and 2b). In contrast, GA+COL enhanced the mitotic population in IR treated HCT116 p53−/− cells, with 49.6+/−8.6% of cells in mitosis at the 39 h timepoint (24h after GA+COL addition, Figure 2a). Similarly, GA+COL enhanced the mitotic cell population in HT-29 cells (10h after GA; 45.6+/−3.5% for IR+GA+COL vs 25.8+/−5.3% for IR+COL alone). This ability of GA to abrogate G2 arrest and push HCT116 p53−/− cells into mitosis was dose-dependent, with maximal push into mitosis observed at 1μM GA (Supplementary Figure 1). Another Hsp90 inhibitor, 17-AAG, also abrogated IR induced G2 arrest in HCT116 p53−/− cells (Supplementary Figure 1). Collectively this data shows the ability of Hsp90 inhibitors to overcome an IR induced G2 arrest in cells with defective p53 signaling.
Figure 2.
GA pushes IR arrested p53 signaling defective human colon adenocarcinoma cells into mitosis. RKO (p53 wildtype), HCT116 p53+/+ (p53 wildtype), HCT116 p53−/− (p53 null) and HT-29 (p53 mutant) cells were irradiated (10Gy) and subsequently treated with COL alone or GA/COL 15h later. Cells were fixed at time points indicated and were stained with an antibody specific against the mitotic marker phospho-Histone H3 (Ser10) and propidium iodide. (a) Mitotic index was determined by flow cytometry as percentage of phospho-Histone H3 positive cells in population. Data represents results from three independent experiments. Error bars represent SEM of three independent experiments. (b) Representative flow cytometric dot plots of phospho-Histone H3 vs DNA content for treated HCT116 cells 39h after IR treatment. Mitotic population is indicated.
Passage of irradiated cells through the G2 restriction point is often associated with aberrant mitosis, extensive micronucleation, and mitotic catastrophe as a form of cell death (Jonathan et al., 1999). We observed HSP90 inhibition can prematurely push irradiated p53 signaling defective cells into mitosis. We were interested to determine the effects of this treatment on subsequent mitotic events in these cells. Initially we seeded HCT116 p53+/+ and p53−/− cells on coverslips and treated them with IR. GA was added 15h after IR treatment and the cells fixed 24h after GA addition. Cells were then stained with an antibody against phospho-Histone H3 (Ser10) to label mitotic cells and DAPI to visualize DNA. Mitotic figures in untreated HCT116 p53+/+ (data not shown) and HCT116 p53−/− cells appeared normal (Figure 3a). While IR treatment prevented passage of most HCT116 p53−/− cells into mitosis (Figure 2), those cells that did enter mitosis displayed aberrant mitotic figures including lagging, misaligned and fragmented chromosomes in metaphase cells (IR 39 h, Figure 3a). Irradiated HCT116 p53−/−cells that were subsequently treated with GA displayed similar mitotic defects in a high percentage of cells, in addition to mitotic cells displaying grossly fragmented chromosomes (IR+GA 39h, Figure 3a). DNA double strand breaks (DSBs) are the predominant form of IR induced DNA damage (Iliakis et al., 2003). We hypothesized that irradiated cells entering mitosis after GA treatment would do so in the presence of persistent DNA damage due to insufficient time for DNA repair. Phosphorylated Histone H2AX (γH2AX) is a marker for DSBs that localizes to sites of DNA damage in subnuclear foci (Burma et al., 2001). HCT116 p53+/+ and HCT116 p53−/− cells were seeded on coverslips and treated with IR (39h), GA(24h), or IR(15h) followed by GA (24h) and then fixed. Cells were co-stained with DAPI and anti γH2AX antibody. γH2AX foci were not detected in untreated HCT116 p53+/+ and HCT116 p53−/− cells, though treatment with GA alone for 24h appeared to increase γH2AX foci detection in both cell types to some extent (Figure 3b). As expected, a pronounced increase in γH2AX foci was seen in both cell types after IR alone or IR followed by GA treatment. Importantly, abundant γH2AX staining was detected in mitosis in HCT116 p53−/− cells treated with IR followed by GA, consistent with these cells entering mitosis in the presence of unrepaired DSBs (Figure 3b).
Figure 3.
Abberant mitotic figures and increased micronucleation observed in IR/GA treated HCT116 p53−/− cells. HCT116 p53+/+ (p53 wildtype) and HCT116 p53−/− (p53 null) cells were irradiated (10Gy) and subsequently treated with GA 15h later. (a) Representative fluorescent images of treated HCT116 p53−/− cells 39h after IR treatment displaying aberrant mitotic metaphase spreads in IR and IR/GA treated cells. Cells were stained with an antibody specific against phospho-Histone H3 (Ser10) and labeled with rhodamine conjugated goat anti mouse IgG to identify mitotic cells. DAPI was used to stain DNA. Images are representative of three independent experiments. (b) Representative fluorescent images of treated HCT116 p53−/− and HCT116 p53+/+ cells 39h after IR treatment (treatment with GA alone was for 24h) displaying γH2AX foci. Cells were stained with an antibody specific against γH2AX and labeled with rhodamine conjugated goat anti mouse IgG to identify DNA DSBs. DAPI was used to stain DNA. Arrowheads indicate mitotic cells. Images are representative of three independent experiments. (c) Representative fluorescent images of HCT116 p53−/− treated with IR/GA (fixed 39h after IR) displaying micronuclei. Cells were stained with DAPI to label DNA. Arrowheads indicate micronuclei. Images are representative of three independent experiments. (d) Quantification of micronucleated cells after IR treatment in the presence or absence of GA and/or COL. HCT116 p53+/+ and HCT116 p53−/− cells were irradiated (10Gy) or left untreated for 15h and then subsequently treated with GA and/or COL for 24h. Cells were fixed and DNA stained with DAPI. Percentage of micronucleated cells was determined by direct counting (500 cells counted per slide). Data is representative of three independent experiments. Error bars represent SEM of three independent experiments.
We speculated that premature mitotic entry with damaged DNA would result in increased death of HCT116 p53−/− cells. Aberrant mitosis leading to mitotic catastrophe is often characterized by extensive micronucleation and death by apoptotic and non-apoptotic mechanisms (Castedo et al., 2004). We quantified the percent micronucleated cells after IR and GA treatment. GA alone did not increase micronucleation in either HCT116 p53+/+ or HCT116 p53−/− cells. In contrast, IR alone (15–39h) increased micronucleation in both cell types, though this effect was most apparent in HCT116 p53−/− cells (Figure 3c and 3d). Subsequent GA treatment did not increase micronucleation in IR-treated HCT116 p53+/+ cells, however it did significantly increase micronucleation in IR-treated HCT116 p53−/− cells compared to IR treatment alone. As expected, the addition of COL blocked the development of micronuclei in both cell types confirming that micronucleation requires attempted passage through mitosis.
Passage from G2 phase into mitosis requires the activity of Cyclin B/Cdc2 complexes. Cdc2 is inactivated through phosphorylation at Tyr15 by Wee1 kinase (Norbury et al., 1991). In contrast, the phosphatase cdc25 activates Cdc2 by dephosphorylating Tyr15. Chk1 and Chk2 promote G2 arrest by inhibiting cdc25 (Iliakis et al., 2003; Kawabe, 2004). IR can promote a G2 arrest in cells with and without wild-type p53 by increasing Wee1, Chk1, and Chk2 activity. We wished to examine the mechanism by which GA abrogates the G2/M checkpoint in irradiated p53−/− cells. To this end, we monitored the levels of these G2/M checkpoint proteins in p53+/+ and p53−/− cells that were first irradiated followed by GA treatment. HCT116 p53+/+ and HCT116 p53−/− cells were treated with IR for 15h or left untreated. The cells were then treated with GA and lysates collected at time points after treatment. Immunoblotting revealed p-Cdc2(Tyr15) and Cyclin B1 levels increased in both cell types treated with IR alone, consistent with cells accumulating at the G2/M boundary. In contrast, both cell types treated with GA alone, or IR followed by GA, showed downregulation of p-Cdc2(Tyr15), Cdc2, Cyclin B1, Wee1 and Chk1 protein levels (Figure 4). We also examined the levels of Chk2, p53, and p21 in these cells. Chk2 expression was not affected by IR, GA, or IR+GA treatment in either cell type (Figure 4). P53 and p21 levels increased to comparable levels in HCT116 p53+/+ cells following treatment with IR alone or IR+GA, indicating p53 and p21 levels are unaffected by GA treatment. P53 was not detected and p21 only mildly increased in HCT116 p53−/− cells following IR or IR+GA treatment. In summary, the data show similar downregulation in the levels of various G2 cell cycle regulators in both cell types after IR and GA, while IR-induced p53 and p21 expression is unaffected by GA.
Figure 4.
GA treatment alters expression of cell cycle regulatory proteins in HCT116 p53+/+ and HCT116 p53−/− human colon adenocarcinoma cells. (a) Treatment schedule timeline used for immunoblot analysis (b) HCT116 p53+/+ (p53 wildtype) and HCT116 p53−/− (p53 null) cells were irradiated (10Gy) or left untreated and subsequently treated with GA 15h later. Immunoblotting was performed on cell lysates collected after treatment with antibodies specific against indicated proteins. Actin expression was detected as a loading control.
GA treatment 15 hrs after IR had no effect on p53 and p21 protein expression in HCT116 p53+/+ cells. Given that p21 maintains G2 arrest in IR-treated cells (Bunz et al., 1998; Waldman et al., 1996) we hypothesized that p53 dependent p21 expression may account for the differences observed in G2-checkpoint abrogation between p53 wild-type and p53 deficient cells. To investigate this, we used HCT116 p21−/− cells. To confirm the p21 status of these cells we treated HCT116 p21−/− cells with IR and examined p53 and p21 expression by immunoblotting. P53 levels increased following IR in these cells and p21 was not detected (Figure 5a). Similar to HCT116 p53−/− cells, IR treatment clearly induced an accumulation of HCT116 p21−/− cells with G2/M DNA content in the absence of apparent G1 arrest 15 hours after IR treatment (Figure 5b). We next performed a mitotic-trap assay to investigate the effect of IR and GA on G2 arrest in these cells. IR-treatment caused a relatively weak G2 arrest in HCT116 p21−/− cells, evidenced by the fact that 26.6% of IR-treated cells were found in mitosis 10 hr after COL addition (Figure 5c). Nonetheless, GA treatment after IR pushed HCT116 p21−/− cells into mitosis which was maximally observed 10 hours after GA treatment (Figure 5c). Mitotic figures in IR and IR+GA treated HCT116 p21−/− cells displayed aberrant chromosomal structures similar to HCT116 p53−/− cells (data not shown). Analysis of micronuceated cells demonstrated increased micronucleation in irradiated HCT116 p21−/− cells when treated with IR followed by GA compared to IR alone (Figure 5d). The effect of GA on micronucleation in these cells was not as significant as that seen in HCT116 p53−/− cells, most likely due to the weaker G2 arrest seen in p21−/− cells (Figure 5c). Overall this data shows that the absence of p21 expression also sensitizes HCT116 cells to GA-mediated abrogation of IR induced G2 arrest.
Figure 5.
GA abrogates IR induced G2 arrest in HCT116 p21−/− human colon adenocarcinoma cells. HCT116 p21−/− cells were treated with IR (10Gy). (a) Representative immunoblots from HCT116 p21−/− cell lysates collected 8h and 24h after IR probed using antibodies specific against p53, p21 and HSP90. Actin expression was detected as a loading control. Immunoblots are representative of three independent experiments. (b) Representative DNA content profiles of untreated and IR treated HCT116 p21−/− cells (15h). Cells were fixed, stained with propidium iodide and analyzed by flow cytometry for DNA content. DNA profiles are representative of three independent experiments. Arrowheads indicate 2N and 4N DNA content respectively on each DNA profile. (c) HCT116 p21−/− cells were irradiated (10Gy) and subsequently treated with COL alone or GA/COL 15h later. Cells were fixed at time points indicated and were stained with an antibody specific against the mitotic marker phospho-Histone H3 (Ser10) and propidium iodide. Mitotic index was determined by flow cytometry as percentage of phospho-Histone H3 positive cells in the population. Data represents results from three independent experiments. Error bars represent SEM of three independent experiments. (d) Quantification of micronucleated cells after IR treatment in the presence or absence of GA and/or COL. HCT116 p21−/− cells were irradiated (10Gy) or left untreated for 15h and then subsequently treated with GA and/or COL for 24h. Cells were fixed and DNA was stained with DAPI. Percentage of micronucleated cells was determined by direct counting (500 cells counted per slide). Data is representative of three independent experiments.
Finally, we examined the effect of IR and GA treatment on clonogenic survival of HCT116 p53+/+, HCT116 p53−/−, and HCT116 p21−/− cells. Cells were treated with IR at increasing doses and subsequently treated with GA 15 hours later. The cells were then serially diluted and replated in fresh media 24 hours after GA treatment. Decreased clonogenic survival correlated with increasing doses of IR in all cell types (Figure 6a and 6b). There were no significant differences in clonogenic survival between HCT116 p53+/+ cells and either HCT116 p53−/− or HCT116 p21−/− cells treated with IR alone. Interestingly, GA alone caused a greater decrease in clonogenic survival in HCT116 p53−/− and HCT116 p21−/− cells compared to HCT116 p53+/+ cells, and combination IR+GA treatment also had a greater killing effect in HCT116 p53−/− and HCT116 p21−/− cells compared to HCT116 p53+/+ cells. This was true at multiple GA doses tested (Supplementary Figure 2). Determination of synergy using isobologram analysis at multiple doses of GA and IR did not show apparent synergy between these two agents (data not shown). These data show a greater reduction in cell survival in p53 signaling defective cells when treated with GA alone or IR+GA compared to wild-type cells.
Figure 6.
GA decreases clonogenicity in HCT116 p53−/− and HCT116 p21−/− cells after IR treatment. (a)(b) HCT116 cells were irradiated (0–10Gy) and 15h later were treated with GA for 24h. Cells were replated and colony forming efficiency was determined by direct counting of colonies present after two weeks. Survival was expressed as percentage of untreated cells. Each data point represents counts from three independent experiments each performed in triplicate. Error bars represent SEM from three independent experiments.
Discussion
Many cancer cells have a defective G1 checkpoint and depend to a greater extent on G2 arrest as a DNA damage response (Kawabe, 2004). Abrogation of G2 arrest in cancer cells treated with DNA damaging agents specifically sensitizes these cells to mitotic cell death. G2 abrogators such as the Chk1 inhibitor UCN-01 sensitize cancer cells to chemotherapeutic drugs and radiotherapy (Arlander et al., 2003; Jackson et al., 2000; Kawabe, 2004). The vast majority of G2 abrogators investigated thus far target specific G2 regulators such as Chk1 or ATM (Jackson et al., 2000; Kawabe, 2004; Sarkaria et al., 1999). HSP90 inhibition offers a multitarget approach to G2 abrogation in addition to simultaneously targeting multiple oncogenic signaling pathways. The effects of HSP90 inhibitors appear to be specific to tumor cells which can at least partly be explained by an increased affinity of HSP90 for its inhibitors in cancer cells (Kamal et al., 2003). HSP90 is overexpressed in tumor cells and stabilizes multiple proteins that are critical to cancer cell survival and cancer progression (Whitesell and Lindquist, 2005). In this study we show that HSP90 inhibition abrogates IR induced G2 arrest in p53 signaling defective colon carcinoma cells leading to aberrant mitosis and subsequent development of micronucleated cells. We have also shown decreased Wee1 and Chk1 expression as the potential mechanism for G2 checkpoint abrogation by GA, and show p21 as a p53 dependent effector of resistance to GA induced mitotic entry in p53 wild-type cells.
Mitotic catastrophe is a general term for cell death that arises from aberrant mitosis, and is characterized by extensive micronucleation and death by apoptotic and non-apoptotic mechanisms (Castedo et al., 2004). Extensive DNA damage results in mis-segregated, fragmented, and lagging chromosomes which become encapsulated in nuclear envelopes and decondense to form micronuclei. It has been reported that mitotic catastrophe is the predominant form of cell death induced by IR (Jonathan et al., 1999). In this study we did not detect apoptosis by Anexin-V staining (data not shown) as an early event after IR, GA or IR/GA treatment however we have shown that HSP90 inhibition can potentiate the development of micronuclei after irradiation in p53 and p21 deficient cells. This is associated with the abrogation of IR induced G2 arrest and increased appearance of grossly aberrant mitotic figures in these cells. Typically, mitotic catastrophe occurs when cells exit mitosis after failed chromosomal segregation and enter into a micronucleated tetraploid interphase (Roninson et al., 2001). Indeed COL blocked micronucleation of irradiated cells in this study. However DNA content analysis (Fig 1D) suggested that HSP90 inhibition decreased the G2/M population in p53 deficient cells after IR treatment coincident with increases in the G1 population. This implies that a fraction of irradiated cells entering mitosis after GA treatment successfully undergo chromosomal segregation and cytokinesis. In this case it might be assumed that these cells have done so in the presence of DNA damage due to premature mitotic entry, though it is unclear whether they too have become micronucleated and/or continue to passage through further cell cycles. IR induces delayed genomic instability including chromosomal aberrations and micronuclei in the progeny of exposed cells multiple generations after the initial insult (Morgan, 2003a; Morgan, 2003b). A link between IR induced delayed chromosomal instability and delayed reproductive cell death has also been established (Limoli et al., 1997; Marder and Morgan, 1993). As such cells passing through mitosis may become non-viable during successive cell cycles.
GA treatment of irradiated p53-null and p21-null cells pushed these cells into premature mitosis, resulting in increased micronucleation. This implies that GA potentiates the killing effect of IR in p53- or p21-deficient cells, at least at early time points after treatment. However, while micronucleated cells can be used as an indicator of mitotic castastrophe following irradiation, their presence alone cannot accurately predict radiosensitivity. Loss of clonogenicity is a more accurate marker for the long-term outcome of radiation treatment (Pawlik and Keyomarsi, 2004). P53 and p21-deficient HCT116 cells were more sensitive to killing by GA alone or combination IR+GA treatment compared to the wild-type HCT116 cells in clonogenic survival studies. However, we saw no evidence for synergistic killing when combining IR+GA compared to IR or GA alone. These results suggest that other effects, beyond the initial push into mitosis and formation of micronucleated cells, also contribute to decreased survival in these cells. Previous reports have suggested GA and its analogs may enhance radiosensitivity through effects on survival/radioprotective factors such as ErbB2 and AKT (Bisht et al., 2003; Bull et al., 2004; Dote et al., 2006; Shintani et al., 2006). Thus, while premature mitotic entry and micronucleation may contribute to an initial killing response in IR+GA treated cells, long-term survival may ultimately reflect the effects of HSP90 inhibition on survival factors such as ErbB2 and AKT. Nonetheless, it is important that p53 and p21-deficient cells were more sensitive to HSP90 inhibition alone or in combination with IR in this study.
γH2AX is a commonly used indicator of DNA double strand breaks (DSBs). We observed abundant γH2AX expression in IR-treated p53−/− cells that entered mitosis after GA treatment. This indicates the cells entered mitosis prematurely in the face of damaged and broken chromosomes. Interestingly we also observed limited γH2AX expression in cells treated with GA alone. Previous reports describe decreased clearance of DSBs in irradiated cells treated with HSP90 inhibitors associated with decreased phosphorylation of DNA repair protein DNA-PKcs (DNA PK catalytic subunit) (Dote et al.,2006; Noguchi et al., 2006). It is presently unclear if similar mechanisms account for this increase in DSB detection in the current study.
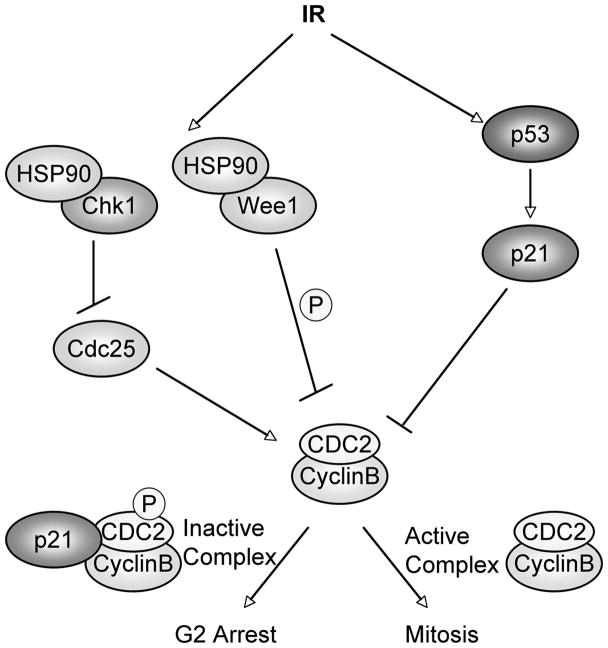
What is the mechanism by which HSP90 inhibition (GA) abrogates the G2/M checkpoint? To address this question we focused on HSP90 client proteins that establish and/or maintain the G2 arrest in IR treated cells. Cyclin B1 and Cdc2 form an active complex which regulates mitotic entry (Figure 7). IR arrests cells in G2 phase through multiple mechanisms which inhibit the activity of Cyclin B-Cdc2 complexes. IR activates Wee1, a kinase that can inhibit G2/M phase progression by phosphorylating Cdc2 at Tyr15 (inhibitory phosphorylation) (Norbury et al., 1991). IR also activates Chk1, which can inhibit G2/M phase progression by phosphorylating and inactivating the Cdc25 phosphatase (Iliakis et al., 2003; Kawabe, 2004). Cdc25 normally dephosphorylates Cdc2 at the Tyr15 residue thereby restoring Cdc2 activity. Wee1 and Chk1 have been identified as HSP90 client proteins in yeast and/or mammalian cells (Arlander et al., 2003; Goes and Martin, 2001). Indeed, we show in the current study that these proteins are decreased during GA treatment independently of p53 status. Depletion of Chk1 has been previously linked to abrogation of G2 arrest (Arlander et al., 2003), and inhibition of Wee1 by a small molecule inhibitor was also reported to abrogate G2 arrest in mammalian cells (Wang et al., 2004; Wang et al., 2001). It is therefore likely that decreased Wee1 and Chk1 contribute to the abrogation of G2 arrest and premature mitotic entry we observe in GA treated cells. However, while Wee1 and Chk1 levels were decreased in both p53+/+ and p53−/− cells treated with GA alone or IR+GA, only the p53−/− cells entered a premature mitosis. These findings indicate passage into mitosis is suppressed by a p53 dependent pathway in wild-type cells. IR treatment activates p53 in p53+/+ cells leading to increased levels of the cdk inhibitor p21 (Iliakis et al., 2003). P21 maintains Cyclin B/Cdc2 complexes in an inactive state and this prevents GA-treated cells from entering mitosis. In summary, our data suggest that GA treatment abrogates the G2 arrest in p53−/− cells and promotes premature mitotic entry through downregulation of Wee1 and Chk1. In p53+/+ cells the G2 arrest is maintained through upregulation of p21 and continued inhibition of Cyclin B/Cdc2 complexes.
Figure 7. Proposed Model for GA abrogation of IR induced G2 growth arrest.
IR can promote a G2 arrest in cells with and without wild-type p53 at least in part by increasing Wee1 and Chk1 activity. Passage from G2 phase into mitosis requires the activity of Cyclin B/Cdc2 complexes. Cdc2 is inactivated through phosphorylation at Tyr15 by Wee1 kinase. The phosphatase cdc25 activates Cdc2 by dephosphorylating Tyr15. Chk1 phosphorylates and thereby inhibits cdc25. IR activates Chk1 and Wee1 to promote p53-independent G2 arrest. IR also activates the p53 signaling pathway in cells expressing wild-type p53, leading to increased levels of p21. P21 can also inhibit Cdc2/Cyclin B complexes preventing entry into mitosis. GA binds and inhibits HSP90 leading to degradation of Wee1 and Chk1 which decreases inhibitory phosphorylation on Cdc2 allowing cells to enter mitosis. However, in p53 wild-type cells p21 maintains G2 arrest by retaining Cyclin B1/Cdc2 complexes in an inactive state.
G2 arrest is initiated and maintained by both p53 and p53 independent pathways after IR treatment (Bunz et al., 1998; Giono and Manfredi, 2006; Iliakis et al., 2003). This study suggests the p53 independent pathway that includes Wee1 and Chk1activation is critically dependent on HSP90 activity (Figure 7). In contrast the p53 dependent pathway that involves p53 activation of p21 appears fully functional when HSP90 is inhibited, since p53 and p21 remained at high levels in GA treated cells. This may seem surprising since p53 and p21 are recognized as HSP90 client proteins (Jascur et al., 2005; Sasaki et al., 2007). The degradation of p53 in HSP90 inhibitor treated cells is believed to be MDM2-dependent. IR stabilizes p53 through post translational modifications that block its interaction with MDM2 (Giono and Manfredi, 2006). In this study we first treated cells with IR to stabilize p53 and activate the G2 block followed by GA treatment 15h later. Thus stabilized p53 can remain active and resistant to degradation induced by GA treatment due to the stabilization mechanisms that block its interaction with MDM2. Our results suggest that this treatment schedule allows protection of p53+/+ colon carcinoma cells through maintenance of a G2 arrest while selectively killing p53−/− cells. Given that HSP90 inhibitors are currently undergoing clinical trials (Banerji et al., 2005; Grem et al., 2005) it is clear that further mechanistic studies will help to define the most effective strategies to combine IR and HSP90 inhibitors as clinically relevant treatment options.
Materials and Methods
Cells
HT-29 and RKO cells were from ATCC. HCT116 p53+/+, p53−/−, p21−/− were from Bert Vogelstein (John Hopkins). HT-29 and HCT116 were grown in McCoy’s 5a medium (100 units/mL penicillin, 100μg/mL streptomycin,10% fetal bovine serum (FBS)). RKO cells were grown in Eagle’s minimum essential medium (100 units/mL penicillin, 100μg/mL streptomycin, 10% FBS). Geldanamycin (GA) was from AG Scientific and used at final concentration of 1μM. Colcemid (COL) was from Calbiochem and used at 0.1μg/mL..
Immunoblots
Cell were lysed in 50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, phenylmethylsulfonyl fluoride, leupeptin. Lysates resolved by SDS-PAGE were transferred to nitrocellulose, blocked with 5% milk, incubated in primary antibody 1.5hr and secondary antibody 1hr, and visualized by chemiluminescence. Primary antibodies were: p53 (ab-2) (Calbiochem) HSP90(H-114) and Actin (Santa Cruz,); Wee1, p-Cdc2 Tyr15(10A11), Cdc2(POH1), Cyclin B1(V152), Chk1(2G1D5) and Chk2 (Cell Signaling Technology). Densitometriy was performed using NIH image J and values corrected for actin loading control. Significance was determined using student T test (p<0.05).
Immunofluorescence
Immunofluorescence was as described (Inoue et al., 2001). Briefly, cells were fixed on coverglass with 4% paraformaldehyde, blocked in 1% bovine serum albumin (BSA), 0.1% Triton X-100, incubated with primary antibodies 1.5h and rhodamine-conjugated second anibody 1 h. Primary antibodies were: phospho-H2AX (s139) (Upstate), phospho-Histone H3 Ser10 (6G3) (Cell signaling). Samples were visualized using a fluorescence microscope. Micronucleated cells were identified by DAPI staining and quantified (500 cells/coverslip).
Cell cycle
Cells were trypsinized and resuspended in PBS (0.1% BSA). 1.5 ×106 cells/mL were fixed in ethanol (4°C), resuspended in RNase A (0.7 mg/mL)/Propidium iodide (PI; 50 μg/mL) and incubated at 37°C (RT). Analysis was done using FACScanto flow cytometer (BD Biosciences) and data analyzed using Flowjo software.
Mitotic trap assay
Cells incubated in COL were trypsinized, resuspended in PBS (0.1% BSA), and fixed in 2% paraformaldehyde 10 min followed by incubation in cold methanol. Cells were incubated with anti-phospho-Histone H3 Ser10 (6G3) antibody 1.5h, washed and incubated with Alexa-488 conjugated anti-mouse IgG (Invitrogen). Cells resuspended in PBS (PI (50μg/mL)), RNase A (0.7mg/mL) were analyzed on BD FACScanto flow cytometer.
Clonogenicity assay
HCT116 cells were irradiated (0–10Gy) in triplicate followed by GA treatment 15h later. 24h after GA adddition cells were serially diluted and grown for 2 weeks. Colonies were stained with crystal violet. Colonies per cells plated were counted and statistical significance was determined by two-way ANOVA.
Supplementary Material
References
- Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. Journal of Biological Chemistry. 2003;278:52572–7. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Research. 2003;63:8984–95. [PubMed] [Google Scholar]
- Bull EE, Dote H, Brady KJ, Burgan WE, Carter DJ, Cerra MA, et al. Enhanced tumor cell radiosensitivity and abrogation of G2 and S phase arrest by the Hsp90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin. Clinical Cancer Research. 2004;10:8077–84. doi: 10.1158/1078-0432.CCR-04-1212. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–6. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–37. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dixon H, Norbury CJ. Therapeutic exploitation of checkpoint defects in cancer cells lacking p53 function. Cell Cycle. 2002;1:362–8. doi: 10.4161/cc.1.6.257. [DOI] [PubMed] [Google Scholar]
- Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Research. 2006;66:9211–20. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- Giono LE, Manfredi JJ. The p53 tumor suppressor participates in multiple cell cycle checkpoints. Journal of Cellular Physiology. 2006;209:13–20. doi: 10.1002/jcp.20689. [DOI] [PubMed] [Google Scholar]
- Goes FS, Martin J. Hsp90 chaperone complexes are required for the activity and stability of yeast protein kinases Mik1, Wee1 and Swe1. Eur J Biochem. 2001;268:2281–9. doi: 10.1046/j.1432-1327.2001.02105.x. [DOI] [PubMed] [Google Scholar]
- Grem JL, Morrison G, Guo XD, Agnew E, Takimoto CH, Thomas R, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–47. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- Inoue T, Geyer RK, Howard D, Yu ZK, Maki CG. MDM2 can promote the ubiquitination, nuclear export, and degradation of p53 in the absence of direct binding. J Biol Chem. 2001;276:45255–60. doi: 10.1074/jbc.M107477200. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–72. [PubMed] [Google Scholar]
- Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, et al. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol Cell. 2005;17:237–49. doi: 10.1016/j.molcel.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Current Opinion in Chemical Biology. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–10. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kawabe T. G2 checkpoint abrogators as anticancer drugs. Molecular Cancer Therapeutics. 2004;3:513–9. [PubMed] [Google Scholar]
- Limoli CL, Kaplan MI, Corcoran J, Meyers M, Boothman DA, Morgan WF. Chromosomal instability and its relationship to other end points of genomic instability. Cancer Res. 1997;57:5557–63. [PubMed] [Google Scholar]
- Marder BA, Morgan WF. Delayed chromosomal instability induced by DNA damage. Mol Cell Biol. 1993;13:6667–77. doi: 10.1128/mcb.13.11.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003a;159:567–80. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003b;159:581–96. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–63. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10:3321–9. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. International Journal of Radiation Oncology, Biology, Physics. 2004;59:928–42. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, et al. Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Research. 2003;63:8420–7. [PubMed] [Google Scholar]
- Rieder CL, Palazzo RE. Colcemid and the mitotic cycle. J Cell Sci. 1992;102(Pt 3):387–92. doi: 10.1242/jcs.102.3.387. [DOI] [PubMed] [Google Scholar]
- Robles AI, Wright MH, Gandhi B, Feis SS, Hanigan CL, Wiestner A, et al. Schedule-dependent synergy between the heat shock protein 90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin and doxorubicin restores apoptosis to p53-mutant lymphoma cell lines. Clinical Cancer Research. 2006;12:6547–56. doi: 10.1158/1078-0432.CCR-06-1178. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resistance Updates. 2001;4:303–13. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Russell KJ, Wiens LW, Demers GW, Galloway DA, Le T, Rice GC, et al. Preferential radiosensitization of G1 checkpoint-deficient cells by methylxanthines. Int J Radiat Oncol Biol Phys. 1996;36:1099–106. doi: 10.1016/s0360-3016(96)00432-4. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–82. [PubMed] [Google Scholar]
- Sasaki M, Nie L, Maki CG. MDM2 binding induces a conformational change in p53 that is opposed by heat-shock protein 90 and precedes p53 proteasomal degradation. J Biol Chem. 2007;282:14626–34. doi: 10.1074/jbc.M610514200. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–5. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Shintani S, Zhang T, Aslam A, Sebastian K, Yoshimura T, Hamakawa H. P53-dependent radiosensitizing effects of Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin on human oral squamous cell carcinoma cell lines. International Journal of Oncology. 2006;29:1111–7. doi: 10.3892/ijo.29.5.1111. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Sasaki M, Isobe Y, Tsutsui M, Suto H, Ando J, et al. Hsp90-inhibitor geldanamycin abrogates G(2) arrest in p53-negative leukemia cell lines through the depletion of Chk1. Oncogene. 2007 doi: 10.1038/sj.onc.1210978. [DOI] [PubMed] [Google Scholar]
- Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–6. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Decker SJ, Sebolt-Leopold J. Knockdown of Chk1, Wee1 and Myt1 by RNA interference abrogates G2 checkpoint and induces apoptosis. Cancer Biol Ther. 2004;3:305–13. doi: 10.4161/cbt.3.3.697. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Booher RN, Kraker A, Lawrence T, Leopold WR, et al. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001;61:8211–7. [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nature Reviews Cancer. 2005;5:761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.