Abstract
LSVT® LOUD (Lee Silverman Voice Treatment) is efficacious in the treatment of speech disorders in idiopathic Parkinson's disease (IPD), particularly hypophonia. Functional imaging in patients with IPD has shown abnormalities in several speech regions and changes in these areas immediately following treatment. This study serves to extend the analysis by correlating changes of regional neural activity with the main behavioral change following treatment, namely, increased vocal intensity. Ten IPD participants with hypophonia were studied before and after LSVT LOUD. Cerebral blood flow during rest and reading conditions were measured by H2 15O‐positron emission tomography. Z‐score images were generated by contrasting reading with rest conditions for pre‐ and post‐LSVT LOUD sessions. Neuronal activity during reading in the pre‐ versus post‐LSVT LOUD contrast was correlated with corresponding change in vocal intensity to generate correlation images. Behaviorally, vocal intensity for speech tasks increased significantly after LSVT LOUD. The contrast and correlation analyses indicate a treatment‐dependent shift to the right hemisphere with modification in the speech motor regions as well as in prefrontal and temporal areas. We interpret the modification of activity in these regions to be a top–down effect of LSVT LOUD. The absence of an effect of LSVT LOUD on the basal ganglion supports this argument. Our findings indicate that the therapeutic effect of LSVT LOUD in IPD hypophonia results from a shift in cortical activity to the right hemisphere. These findings demonstrate that the short‐term changes in the speech motor and multimodal integration areas can occur in a top–down manner. Hum Brain Mapp, 2010. © 2009 Wiley‐Liss, Inc.
Keywords: Parkinson's disease, hypophonia, cerebral blood flow, speech motor system, performance correlation
INTRODUCTION
Speech and voice disorders affect 90% of patients with Idiopathic Parkinson's disease (IPD) (Duffy, 2005). The dysarthria reported in IPD includes reduced loudness (hypophonia), monopitch, hoarseness, a breathy voice quality, and imprecise articulation (Duffy, 2005), likely related to the hypokinetic state (Ramig et al., 2001a, b, 2004; Sapir et al., 2002, 2007). Abnormalities in various measures of speech such as decreased maximum forces of the articulatory muscles, decreased force rise time, shorter duration of sustained phonation, longer pause duration, and decreased vocal sound pressure level (SPL), and an overall reduction in speech intelligibility have been reported (Gentil et al., 2003; Ramig et al., 2004; Sapir et al., 2007). In addition, abnormalities in the auditory system that also adversely affect speech production in IPD are reported. These include reduced ability to follow rapid intensity fluctuations (Guehl et al., 2008) and abnormal auditory‐motor integration (Kofler et al., 2001; Sabate et al., 2008).
Although the neural correlates of speech have been studied extensively by functional imaging in healthy controls, only two neuroimaging studies have compared the speech motor areas in patients with IPD with healthy participants (Pinto et al., 2004a; Liotti et al., 2003). When compared to healthy participants, those with IPD demonstrated an increased regional cerebral blood flow (rCBF) in the premotor areas during speech tasks (Pinto et al., 2004a; Liotti et al., 2003). The rCBF response in the primary motor cortex and cerebellum appears variable with both decreases (Pinto et al., 2004a) and increases (Liotti et al., 2003) reported. The authors of both these studies interpret these findings as pretreatment abnormalities and hypothesize that an altered recruitment of the motor regions result in an increased involvement of the premotor and prefrontal cortices during speech (Liotti et al., 2003; Pinto et al., 2004a).
Dysarthia in IPD can be treated pharmacologically, surgically, and by behavioral speech therapy. A behavioral therapy that has been shown to be effective in treating hypophonia in IPD is LSVT®LOUD (Lee Silverman Voice Treatment). LSVT LOUD is organized around a simple but powerful therapeutic principle: increasing vocal loudness (targeting amplitude of respiratory‐laryngeal movement) in individuals with IPD will retrain the sensory motor processes involved in disordered speech communication (Fox et al., 2002). The training mode of LSVT LOUD requires high effort (self‐perceived effort) and intensive training (16 individual 60‐min treatment sessions in 1 month) consistent with principles of motor learning, skill acquisition, and neural plasticity (Kleim and Jones, 2008; Schmidt and Lee, 1999; Verdolini et al., 1999). Treatment sessions consist of two parts: daily tasks and a speech hierarchy. Daily tasks increase vocal loudness through multiple repetitions of sustained vowels (“ah”), high/low‐pitch range exercises, and functional phrases. The speech hierarchy systematically improves functional communication by training patients to maintain improved vocal loudness (achieved in daily tasks) for longer periods of speaking and in more complex speaking situations (e.g., progressing from words to conversational speech).
LSVT LOUD has been shown to be effective in the short term and long term (up to 2 years) in improving hypokinetic dysarthria, especially hypophonia (Ramig et al., 2001a, b, 2004; Suchowersky et al., 2006). A system‐wide improvement across the speech production network, as well as improved self‐monitoring, clinically referred to as recalibration of the auditory system have been documented following LSVT LOUD and are thought to contribute to the overall improvement in functional communication (Fox et al., 2006).
There exists yet another disparity between behavioral and imaging‐based examination of the effect of treatment on speech disorders in IPD. Although many studies have examined the speech outcome following pharmacological, surgical, and behavioral treatments by perceptual and acoustic measurements [reviewed by Pinto et al. (2004b)], few have examined the changes in the speech motor areas by functional imaging. A significant improvement of variability in pitch and loudness, measures of respiration, and comprehensibility have been reported following pharmacological treatment (De Letter et al., 2007a, b; Pinto et al., 2004b). Although surgical interventions such as stimulation of the subthalamic nucleus (STN) have improved motor performances and vocal tremor, this procedure has also been shown to have either no effect or at times a worsening effect on perceptual and acoustic parameters related to prosody, articulation, vocal SPL, intensity, and intelligibility of speech (Pinto et al., 2004b; D'Alatri et al., 2008; Narayana et al., 2009).
Few functional imaging studies have reported on the changes that occur in the speech motor system in IPD immediately following various treatments (Rektorova et al., 2007; Pinto et al., 2004a; Liotti et al., 2003). A functional magnetic resonance imaging (fMRI) study of speech in females with IPD on levodopa, demonstrated significantly higher blood oxygen level dependent signal in the right primary sensorimotor cortex (SM1) compared to healthy controls (Rektorova et al., 2007). This right‐sided shift was attributed to the pharmacological treatment effect as well as compensatory mechanisms. By measuring rCBF with positron emission tomography (PET), the speech motor network in IPD was shown to become more similar to that of normal controls following STN stimulation (Pinto et al., 2004a), and a LSVT LOUD‐dependent right‐sided functional reorganization of brain activation pattern was also reported (Liotti et al., 2003). Our objective was to further examine the immediate mechanisms of action underlying the successful behavioral treatment such as LSVT LOUD. We were especially interested in identifying a possible neural mechanism for the auditory recalibration observed following LSVT LOUD (Fox et al., 2006).
Although within‐subject conditional contrast analyses, used in previous studies, are powerful and widely used, they are not always an adequate image analysis strategy (Fox et al., 2000). Conditional contrast analysis is based on the assumption that only the brain regions that are activated differently during one task are isolated. Isolating desired behaviors during imaging presents a significant challenge. An alternative technique, called performance‐correlation analysis using the principle that the intensity of brain activations is highly correlated with the frequency with which the neural elements are used during the imaging epoch was introduced for imaging (Silbersweig et al., 1995). It was further developed for speech motor studies (Braun et al., 1997; Fox et al., 2000; Ingham et al., 2004; Raboyeau et al., 2004; Jodzio et al., 2005; Rektorova et al., 2007), but thus far has not been applied to examine treatment outcomes. We therefore sought to apply performance correlation analyses to further investigate the brain correlates and the mechanisms of action characterizing successful LSVT LOUD treatment.
On this basis, this study had the following objectives: we sought to (1) replicate earlier findings of abnormalities in speech motor areas in individuals with IPD, (2) identify the neural correlates of a successful LSVT LOUD treatment by specifying the components of the speech system that directly correlate with the outcome of LSVT LOUD, and (3) propose a mechanism of action of LSVT LOUD based on the imaging findings reported below. Because the primary goal of LSVT LOUD is to increase vocal loudness, and loudness is a part of intonation and prosody that mainly activate speech motor areas in the right hemisphere (Ross and Monnot, 2008), we hypothesized that greater activation would be seen in the right hemisphere speech motor areas following LSVT LOUD. Loudness monitoring, another major component of LSVT LOUD, is predominantly regulated by the auditory cortices, especially in the right hemisphere (Ross and Monnot, 2008; Brancucci and San Martini, 2003; Brancucci et al., 2005). Therefore, we hypothesized that a voice treatment like LSVT LOUD would also target the auditory system, and such recruitment could indicate a possible neural mechanism for the auditory recalibration observed clinically following LSVT LOUD.
METHODS
Participants
Ten right‐handed participants (8 men, 2 women; age 60 ± 11 years) with a diagnosis of IPD and speech and voice disorder were recruited for this study (see Table I for motor and speech characteristics). Symptom severity was mild to moderate with an average Unified Parkinson's Disease Rating Scale (UPDRS) score of 51 ± 12, range, 36–78. The reported speech symptoms (item 5 of UPDRS) were an average of 2.2 (range, 2–3). The average score of a speech motor examination (item 18 of UPDRS) was 1.9 (range, 1–3) indicative of speech that is monotone, slurred but understandable. The more severe participants had poor intelligibility. Mean disease onset was 4.6 years (range, 1.5–7 years). The participants had no history of any hearing impairment or other neurologic or psychiatric diseases. Individuals with IPD were considered for LSVT LOUD if they had significant hypophonia but could increase vocal loudness on command (5–10 dB SPL at 30 cm). All participants underwent a laryngeal examination to rule out abnormalities that might affect the participation and treatment outcomes (e.g., gastric reflux and vocal fold paralysis). All participants were on levodopa and the medication status was kept consistent throughout the period of the study. Written informed consent was obtained from all participants, and all procedures were approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio.
Table I.
Demographics and clinical characteristics of participants with IPD
| Subject | Age (years) | Sex | Duration of PD (years) | UPDRS total | UPDRS (item 5) | UPDRS (item 18) |
|---|---|---|---|---|---|---|
| 1 | 64 | F | 6 | 49 | 2 | 2 |
| 2 | 52 | F | 3 | 48 | 2 | 1 |
| 3 | 54 | M | 7 | 78 | 3 | 3 |
| 4 | 62 | M | 4 | 52 | 2 | 1 |
| 5 | 58 | M | 3 | 61 | 2 | 2 |
| 6 | 70 | M | 4 | 38 | 3 | 3 |
| 7 | 65 | M | 3 | 36 | 2 | 2 |
| 8 | 56 | M | 4 | 46 | 2 | 1 |
| 9 | 82 | M | 5 | 47 | 2 | 2 |
| 10 | 40 | M | 1.5 | 58 | 2 | 2 |
UPDRS, Unified Parkinson's Disease Rating Scale; UPDRS (Item 5), speech symptoms reported by the patients; UPDRS (item 18), speech motor signs recorded by the clinician.
Voice Therapy
LSVT LOUD was administered according to the prescribed program (Ramig et al., 1995, 1996; Smith et al., 1995; Ramig et al., 2004) by a speech‐language pathologist trained and certified in this method.
Behavioral Data Acquisition
Vocal SPL, the acoustic correlate of vocal loudness was measured for sustained vowel phonation, reading, and spontaneous conversation before, during and at the end of LSVT LOUD using a sound level meter (RadioShack®) placed at a distance of 30 cm from the participants' mouth. Speech during PET sessions was recorded using a digital audiotape (DAT) recording system with microphone placed 20 cm from the participants' mouth. Speech data were digitized at 44 KHz with a low‐pass filter set at 22 KHz and filtered using a noise reduction process from Adobe Audition (v. 1.5) to reduce background noise in the signal and further analyzed using TF32 speech analysis tool (http://userpages.chorus.net/cspeech/) for loudness measurements.
Imaging Method
PET acquisition
PET data were acquired with a CTI EXACT HR+ scanner (Knoxville, TN). Sixty‐three contiguous slices (2.5‐mm thick) in a transaxial field of view of 15.5 cm were acquired. Images were corrected by measured attenuation using 68Ge/68Ga transmission scans and reconstructed at an in‐plane resolution of 7‐mm full width at half maximum (FWHM) and an axial resolution of 6.5‐mm FWHM. Water labeled with oxygen‐15 (H2 15O, half‐life 122 s) was administered intravenously (555 MBq H2 15O dose/scan) and cerebral blood flow (CBF) was measured using a bolus technique (Fox et al., 2000, 2006). Participants' heads were immobilized in the PET scanner using individually fitted, thermally molded, plastic face masks (Fox and Raichle, 1984). Each subject was studied in two sessions: before LSVT LOUD and immediately after completion of LSVT LOUD. During both sessions, the participants underwent four measurements of CBF during paragraph reading at habitual voice level and during eyes open rest (two repetitions each). The participants read standard passages used in speech and voice assessments: “The Rainbow” (Fairbanks, 1960) and “The Grandfather” (Darley et al., 1975) passages. The passages were displayed on a computer monitor screen placed in front of patients' eyes. In the eyes open rest condition, patients were asked to lie still while looking at a crosshair on the monitor and maintain a relaxed state.
MRI acquisition
An anatomical MRI scan (Elscint 1.9T; Haifa, Israel) was also acquired for each subject for the purposes of spatial transformation of the PET data and parametric image display. A 3D‐gradient recalled acquisition in a steady‐state [GRASS] sequence was acquired with scan repetition time (TR) of 33 ms, an echo time (TE) of 12 ms, flip angle 60° as a 256 × 256 × 127 volume with a spatial resolution of 1 mm3.
Image data preprocessing
Image preprocessing was performed using previously validated methods and in‐house software. PET images were corrected for head motion using the MCFLIRT tool in FSL 4.0 (http://www.fmrib.ox.ac.uk/fsl/) and PET and MRI images were spatially transformed relative to the stereotaxic atlas of Talairach and Tournoux (1988) (Lancaster et al., 1995, 1997). Regional tissue uptake of 15O‐water was globally normalized to whole rCBF brain mean value with images scaled to a mean of 1,000 counts. These value and spatially normalized images were tri‐linearly interpolated, re‐sampled (60 slices, 8 mm3 voxels), and Gaussian filtered to a final resolution of 9.9 mm (FWHM). Further data analyses were performed using MIPS software (Medical Image Processing Station, Research Imaging Center, UT Health Science Center at San Antonio, TX) and MANGO (Multi Analysis GUI, Research Imaging Center, UT Health Science Center at San Antonio, TX).
PET Analyses
Conditional contrast analysis
For each subject and session, voxel‐by‐voxel pairwise contrasts were generated to identify regional changes present during overt paragraph reading relative to rest. Task‐specific, within‐subject regional changes were then averaged across individuals. A maxima and minima search (Fox et al., 1988; Fox and Mintun, 1989; Mintun et al., 1989) was then used to identify local extrema within a search volume measuring 1000 mm3. A gamma 1 statistic measuring skewness and gamma 2 statistic measuring kurtosis of the distribution of the extrema established before post hoc analysis were used as an omnibus test to assess overall significance. We confirmed that for the reading versus rest contrasts, the gamma 2 statistic for all the masked voxels and for the extrema set were significant. The group‐mean subtraction images from both sessions were then converted to statistical parametric images of z scores (SPI{z}). The Bonferroni correction was applied to correct for the number of positive extrema locations that were reported to have a P value <0.05. Laterality indices were calculated for pre‐ and post‐LSVT LOUD sessions as the ratio of volumes of all reported significant activations in each hemisphere (after applying the Bonferroni correction) using the formula:
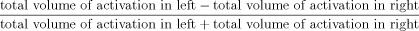 .
.
Volume of interest analysis
To further confirm the hemodynamic differences between pre‐ and post‐LSVT LOUD conditions, the brain regions that appeared to change following LSVT LOUD were probed in the value normalized PET data. Cubic VOIs (side of 9 mm) were placed at the center‐of‐mass of bilateral primary motor cortices (M1), supplementary motor area (SMA), pre‐SMA, dorsal premotor cortices (PMd), auditory cortices, globus pallidus (GP), right dorsolateral prefrontal cortex (DLPFC), and precueneus. The mean value normalized PET counts (VNC) were derived for the above locations during rest and reading conditions, both pre‐ and post‐LSVT LOUD time points from each subject. A paired Student's t‐test was performed to identify significant changes in CBF in these regions during reading condition contrasted with rest before and following LSVT LOUD.
Performance correlation analysis
A statistical parametric image of r values (SPI{r}) was computed as a voxel‐wise correlation of CBF with the loudness measure during reading using previously described method (Fox et al., 2000). Pre‐LSVT LOUD‐reading was contrasted with post‐LSVT LOUD reading and the CBF difference image was correlated with respective change in vocal SPL. SPI{r} was analyzed for speech performance effects first by an omnibus (whole‐brain) test and, if omnibus significance was proven, then a post hoc (regional) test was done and local extrema were identified. The SPI{r} was converted to SPI{z}, and P values were assigned from the Z distribution and corrected for the number of positive extrema. The volumes of significant correlations in various brain regions in both the hemispheres meeting the above criteria were calculated and graphed. A laterality index was calculated as the ratio of volumes of all significant positive correlation of CBF with vocal SPL during reading in each hemisphere using the formula
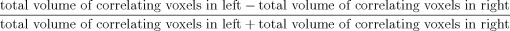 .
.
RESULTS
Behavioral Effects of LSVT LOUD
Voice and speech measures acquired immediately before and after LSVT LOUD (SPL at 30 cm) showed highly significant improvement (see Fig. 1). An ANOVA revealed significantly higher post‐LSVT LOUD SPL for all the three speech conditions: sustained phonation post‐ (M = 84.9 ± 1.7 dB) versus pre‐LSVT LOUD (M = 74.6 ± 4.9 dB), P ≤ 0.0005, F = 51.75, paragraph reading post‐ (M = 76 ± 1.7 dB) versus pre‐LSVT LOUD (M = 68.3 ± 2.5 dB), P ≤ 0.0002, F = 64.72, and spontaneous conversation post‐ (M = 73.7 ± 1.3 dB) versus pre‐LSVT LOUD (M = 66.7 ± 2.7 dB), P ≤ 0.00001, F = 74.37. No significant change was observed in the duration of sustained phonation between post‐LSVT LOUD (M = 20.1 ± 8.3 s) versus pre‐LSVT LOUD (M = 16.3 ± 11.2 s), P = 0.37, F = 0.88. The treatment related increase in vocal SPL confirmed perceptual judgments of the LSVT clinician regarding improved hypophonia and intelligibility. ANOVA of SPL measurements during paragraph reading in PET sessions showed significant increase following LSVT LOUD similar to out of scanner changes (pre‐LSVT LOUD M = 65.6 ± 3.2 dB vs. post‐LSVT LOUD M = 73.6 ± 2.5 dB) P ≤ 0.0007, F = 16.55.
Figure 1.
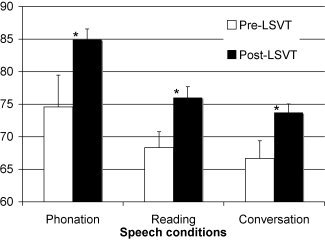
Behavioral data: changes in measure of loudness as sound pressure level (SPL) in decibels (dB) during sustained phonation, reading, and conversation pre and post‐LSVT LOUD. *Post‐LSVT LOUD loudness was significantly different (P < 0.0005).
PET Results
Conditional contrast
Table II and Figures 2, 3, 4 identify the brain regions showing a significant change during paragraph reading compared with rest both before and after LSVT LOUD. After correcting for multiple positive extrema, only maxima with z score ≥3.6, cluster volume ≥150 mm3 and P ≤ 0.00014, one‐tailed were identified as significant and are reported here.
Table II.
Comparison of regions activated during paragraph reading contrasted with rest, before and following LSVT LOUD in patients with IPD
| Lobe | Region | Pre‐voice treatment | Post‐voice treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | Cluster size (mm3) | Z score | P value | X | Y | Z | Cluster size (mm3) | Z score | P value | ||
| Frontal | |||||||||||||
| Left | M1‐mouth (BA 4) | −44 | −16 | 40 | 896 | 5.85 | P < 0.00003 | ||||||
| Dorsal premotor cortex (BA 6) | −42 | −8 | 44 | 984 | 6.87 | P < 0.00003 | −40 | −6 | 46 | 848 | 4.32 | P < 0.00003 | |
| Supplementary motor cortex (BA 6) | −4 | −6 | 62 | 848 | 6.19 | P < 0.00003 | −4 | 4 | 58 | 992 | 6.61 | P < 0.00003 | |
| Pre‐SMA/rostral SMA (BA 6/8) | −3 | 12 | 48 | 600 | 4.41 | P < 0.00003 | |||||||
| Cingulate cortex (BA 24) | −8 | 13 | 30 | 328 | 3.77 | 0.000080 | |||||||
| Inferior frontal gyrus (BA 47) | −50 | 18 | 0 | 520 | 4.32 | P < 0.00003 | |||||||
| Right | M1‐mouth (BA 4) | 48 | −6 | 34 | 864 | 4.03 | P < 0.00003 | 48 | −14 | 34 | 784 | 4.68 | P < 0.00003 |
| 54 | −12 | 30 | 864 | 4.87 | P < 0.00003 | ||||||||
| Dorsal premotor cortex (BA 6) | 30 | −11 | 42 | 584 | 4.32 | P < 0.00003 | |||||||
| Supplementary motor cortex (BA 6) | 1 | 2 | 56 | 808 | 5.02 | P < 0.00003 | 4 | −4 | 58 | 896 | 6.06 | P < 0.00003 | |
| Pre‐SMA/rostral SMA (BA 6/8) | 14 | 14 | 48 | 424 | 4.54 | P < 0.00003 | |||||||
| Middle frontal gyrus (BA 9/46) | 28 | 38 | 22 | 584 | 4.03 | P < 0.00003 | |||||||
| Temporal | |||||||||||||
| Left | Superior temporal gyrus (BA 22) | −50 | 8 | −6 | 728 | 3.92 | 0.000040 | −46 | 10 | −4 | 680 | 3.85 | 0.000060 |
| Middle temporal gyrus (BA 21) | −46 | −34 | −6 | 512 | 3.43 | 0.000300 | −46 | −28 | −10 | 264 | 3.34 | 0.000420 | |
| Right | Superior temporal gyrus (BA 22) | 51 | −10 | 0 | 592 | 3.71 | 0.000100 | ||||||
| Superior temporal gyrus (BA 22) | 50 | −20 | 4 | 608 | 3.71 | 0.000100 | |||||||
| Parietal | |||||||||||||
| Right | Precuneus (BA 7) | 24 | −70 | 36 | 360 | 2.75 | 0.003010 | 28 | −68 | 36 | 608 | 3.74 | 0.000090 |
| Occipital | |||||||||||||
| Left | Lingual gyrus (BA 17) | −20 | −94 | −12 | 864 | 9.56 | P < 0.00003 | −8 | −94 | −8 | 992 | 7.48 | P < 0.00003 |
| Mid/inferior occipital gyrus (BA 18) | −18 | −90 | 16 | 768 | 3.69 | 0.000110 | −28 | −88 | −4 | 992 | 6.06 | P < 0.00003 | |
| Fusiform gyrus (BA 19) | −36 | −74 | −14 | 976 | 5.47 | P < 0.00003 | −40 | −78 | −12 | 880 | 4.32 | P < 0.00003 | |
| Right | Lingual gyrus (BA 17) | 14 | −94 | 0 | 880 | 6.61 | P < 0.00003 | 16 | −90 | 0 | 952 | 6.21 | P < 0.00003 |
| Mid/inferior occipital gyrus (BA 18) | 22 | −94 | −4 | 960 | 6.76 | P < 0.00003 | 8 | −86 | −6 | 1000 | 7.22 | P < 0.00003 | |
| Cerebellum | |||||||||||||
| Left | Lobule V1 (declive) | −16 | −72 | −20 | 872 | 4.87 | P < 0.00003 | −10 | −64 | −16 | 920 | 5.48 | P < 0.00003 |
| Lobule IV/V (culmen) | −30 | −50 | −24 | 768 | 4.49 | P < 0.00003 | −18 | −43 | −24 | 312 | 3.56 | 0.000190 | |
| Right | Lobule V1 (declive) | 12 | −68 | −18 | 1000 | 6.57 | P < 0.00003 | 16 | −62 | −18 | 968 | 5.59 | P < 0.00003 |
| Lobule IV/V (culmen) | 34 | −60 | −26 | 840 | 5.02 | P < 0.00003 | 6 | −52 | −20 | 776 | 4.87 | P < 0.00003 | |
M1, primary motor cortex; BA, Brodmann area; x, y, z, talairach co‐ordinate system; SMA, supplementary motor area.
Figure 2.
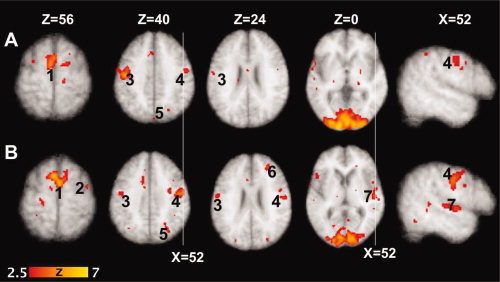
Activation pattern during paragraph reading in individuals with IPD hypophonia. Top panel A: Pre LSVT LOUD and bottom panel B: Post‐LSVT LOUD. (1) Bilateral SMA, (2) right PMd, (3) left primary motor cortex (M1‐mouth), (4) right primary motor cortex (M1‐mouth), (5) right parietal cortex (BA 7), (6) right dorsolateral prefrontal cortex (BA 9), and (7) right superior temporal cortex. The figures in the last column are coronal sections (at x = 52) show increased right M1 activation, as well as appearance of right superior temporal gyrus activation post‐LSVT LOUD during a speech task.
Figure 3.
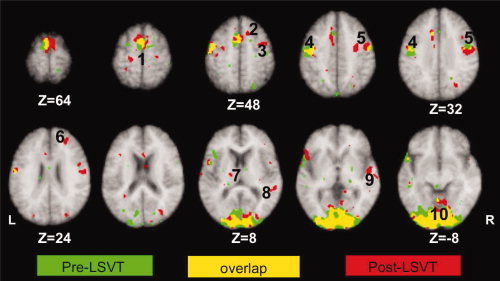
Comparison of activation patterns during paragraph reading in individuals with IPD hypophonia pre and post‐LSVT LOUD. Green: activations during paragraph reading pre LSVT LOUD; red: activations during paragraph reading post‐LSVT LOUD; yellow: overlap of activations between two imaging sessions. L, left hemisphere; R, right hemisphere. (1) SMA, (2) rostral or pre‐SMA, (3) dorsal premotor cortex, (4) left primary motor cortex (M1‐mouth), (5) right primary motor cortex (M1‐mouth), (6) right dorsolateral prefrontal cortex (BA 9), (7) left thalamus, (8) right superior temporal cortex, (9) right superior temporal sulcus, and (10) bilateral visual cortices. Notice no change in SMA, left M1, and visual areas following LSVT LOUD.
Figure 4.
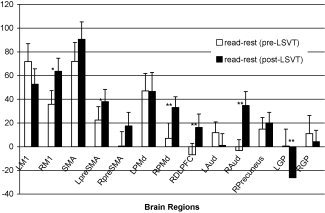
Volume of interest analysis: value normalized counts from speech conditions contrasted with rest in select brain regions that showed significant changes following LSVT LOUD. Post‐LSVT LOUD changes were significant in RPMd, RDLPFC, R Aud, and LGP areas. L and RM1, left and right primary mouth motor cortex; SMA, bilateral supplementary motor area; L and R preSMA, left and right rostral SMA; L and RPMd, left and right dorsal premotor areas; RDLPFC, right dorsolateral prefrontal cortex; L and R Aud, left and right auditory cortices; RPrecuneus, right precuneus; L and RGP, left and right globus pallidus. **Means P < 0.05 and * indicates P < 0.01.
Pre LSVT LOUD conditional contrast (reading‐rest)
Bilateral activations were observed in the primary mouth motor cortex (M1‐mouth, Brodmann area (BA 4), supplementary motor cortex (SMA, BA 6), dorsal premotor cortex (PMd, BA 6), visual areas [lingual (BA 17), middle and inferior occipital (BA 18), and fusiform (BA 19) gyri], and cerebellum (lobules 1V, V, and V1). In the left hemisphere, activations in anterior cingulate cortex (ACC, BA 24), rostral SMA (BA 6/8), superior temporal (BA 22), and middle temporal gyri (BA 21) were observed. In addition, activation in the right precuneus (BA 7) was also observed. The laterality index measured as the ratio of volume of activation in each hemisphere was 0.23, indicating a slightly greater volume of activation in the left hemisphere.
Post‐LSVT LOUD conditional contrast (reading‐rest)
Following LSVT LOUD, bilateral activations were still present in SMA, PMd, visual areas, and cerebellum. In addition, bilateral activations were observed in superior and middle temporal gyri. Left‐sided activations were observed in inferior frontal gyrus (BA 47). Left‐sided activations that were present pre‐LSVT LOUD in the M1‐mouth, rostral SMA, and ACC did not reach significance post‐treatment. However, several right‐sided activations were present following LSVT LOUD in areas such as middle frontal gyrus (BA 9 and 46), rostral SMA and notably in superior and middle temporal gyri. The activated volumes in various brain regions in the two hemispheres showed a rightward shift, which is reflected in the laterality index of −0.13.
Volume of interest analysis
VOI analysis comparing the pre‐ versus post‐LSVT LOUD contrasts of reading with rest showed significantly (P < 0.05) increased hemodynamic response in right DLPFC, PMd, and the auditory cortex following the voice treatment. Right M1 and left rostral SMA showed trends (P ≤ 0.01) of increased CBF (see Fig. 4). Following LSVT LOUD, there was also a significant decrease in CBF in left globus pallidus.
Performance correlation
Brain regions with an r‐value ≥ 0.5, z‐score ≥ 2.8, and P ≤ 0.0025 (after correcting for multiple positive extrema) are reported (Figs. 5 and 6 and Table III). Brain regions were predominantly on the right side with M1‐mouth, M1‐hand, PMd, middle frontal gyrus (BA 9), inferior frontal gyrus (BA 44/45), middle and inferior temporal gyri (BA 22, 21), primary sensory cortex (S1‐BA 3), precuneus (BA 7), inferior parietal lobule (BA 40), and ventral anterior nucleus (VAN) of thalamus showing increases in CBF corresponding with increasing vocal SPL. In the left hemisphere, pre‐SMA, precuneus (BA 7), paracentral lobule (BA 5), posterior cingulate (BA 31), and VAN of thalamus indicated a positive rCBF correlation with vocal SPL. The volumes of activity that significantly correlated with loudness in various brain regions in the two hemispheres are shown in Figure 6. More brain regions and greater volumes in the right hemisphere were seen corresponding to a laterality index of −0.50 that once again indicates a shift to the right hemisphere.
Figure 5.
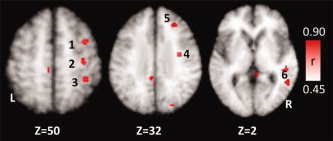
Positive correlation of cerebral activity with treatment outcome (SPL) following LSVT LOUD in individuals with IPD. L, left hemisphere; R, right hemisphere. (1) Right dorsal premotor cortex, (2) right M1‐hand, (3) LEFT precuneus (BA 7), (4) right M1‐mouth, (5) right DLPFC, and (6) right superior temporal gyrus.
Figure 6.
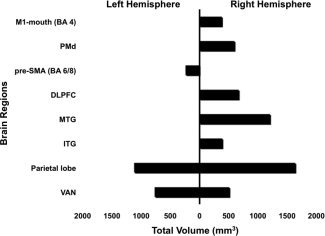
Volumes in mm3 of significant correlations (r‐value ≥ 0.5, z‐score ≥ 2.8, and P ≤ 0.0025) in various brain regions that correlated with loudness following LSVT LOUD in left and right hemispheres. M1‐mouth, primary motor cortex, mouth; PMd, dorsal premotor areas; SMA, supplementary motor area; DLPFC, dorsolateral prefrontal cortex; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; VAN, ventral anterior nucleus.
Table III.
Brain regions showing positive correlations with treatment
| Lobe | Region | X | Y | Z | Cluster size (mm3) | Z score | r value | P value |
|---|---|---|---|---|---|---|---|---|
| Frontal | ||||||||
| Left | Pre‐SMA/rostral SMA (BA 6/8) | −14 | 12 | 54 | 240 | 2.93 | 0.53 | 0.00171 |
| Right | M1‐mouth (BA 4) | 34 | −10 | 32 | 384 | 3.71 | 0.67 | 0.00011 |
| Dorsal premotor cortex (BA 6) | 34 | 0 | 52 | 600 | 3.26 | 0.59 | 0.00056 | |
| M1‐hand (BA 4) | 34 | −22 | 50 | 504 | 3.01 | 0.54 | 0.00130 | |
| Middle frontal gyrus (BA 9) | 26 | 30 | 32 | 672 | 2.90 | 0.52 | 0.00190 | |
| Inferior frontal gyrus (BA 44/45) | 48 | 14 | 20 | 472 | 3.13 | 0.56 | 0.00087 | |
| Temporal | ||||||||
| Right | Inferior Temporal Gyrus (BA 21) | 46 | −2 | −11 | 392 | 2.88 | 0.52 | 0.00201 |
| Middle temporal gyrus (BA 22) | 50 | −34 | 2 | 552 | 3.37 | 0.61 | 0.00038 | |
| Middle temporal gyrus (BA 21) | 49 | −54 | 6 | 656 | 3.64 | 0.65 | 0.00013 | |
| Parietal | ||||||||
| Left | Precuneus (BA 7) | −17 | −50 | 58 | 208 | 2.88 | 0.52 | 0.00197 |
| posterior cingulate (BA 31) | −7 | −46 | 36 | 576 | 3.15 | 0.57 | 0.00081 | |
| Paracentral Lobule (BA 5) | −4 | −32 | 48 | 336 | 2.87 | 0.52 | 0.00208 | |
| Right | Primary sensory cortex (BA 3) | 22 | −34 | 57 | 256 | 2.91 | 0.52 | 0.00184 |
| Precuneus (BA 7) | 22 | −48 | 55 | 336 | 3.38 | 0.61 | 0.00036 | |
| Inferior Parietal Lobule (BA 40) | 36 | −40 | 50 | 640 | 3.25 | 0.58 | 0.00058 | |
| Precuneus (BA 31) | 0 | −72 | 26 | 408 | 3.55 | 0.64 | 0.00019 | |
| Sub cortical | Thalamus | |||||||
| Left | Ventral posterior lateral nucleus (VPLN) | −2 | −2 | 10 | 768 | 3.50 | 0.63 | 0.00023 |
| Right | Ventral anterior nucleus (VAN) | 0 | −12 | 18 | 512 | 2.96 | 0.53 | 0.00153 |
SMA, supplementary motor area; BA, Brodmann area; x, y, z, talairach co‐ordinate system; M1, primary motor cortex.
DISCUSSION
Data presented above replicate earlier reports of abnormal activations in the speech motor areas in individuals with IPD. Our data demonstrate, for the first the time, the use of performance correlation as a powerful analytical tool to identify the components of the speech motor system directly involved in a successful behavioral treatment. Successful treatment with LSVT LOUD resulted in a rightward shift in the cortical speech motor and premotor systems as well as in the association areas mediating multimodal integration. We interpret the modification of activity in these regions during speech to be a top–down effect of LSVT LOUD. This deduction is further supported by the absence of a direct effect of LSVT LOUD on the basal ganglion (BG). These effects of LSVT LOUD are discussed under two sections: (1) neural correlates of successful voice treatment and (2) brain regions that remained unchanged following treatment.
Neural Correlates of Successful Voice Therapy
Previous neuroimaging studies of speech in IPD that examined the speech motor regions (Pinto et al., 2004a; Liotti et al., 2003; Rektorova et al., 2007) have reported abnormal increases or decreases in neural activity in several areas of the speech system and interpreted these findings as pretreatment abnormalities. Consistent with previous studies, our data show that in the pretreatment session, the conditional contrasts revealed similar significant activations in the motor, premotor, and subcortical areas as well as the sensory areas such as the superior temporal gyrus (STG), parietal cortex, and visual cortices.
The data presented here indicate that the primary effect of LSVT LOUD broadly consists of two major components: (1) recruitment of right‐sided regions during speech and (2) a top–down mechanism of action of voice therapy. The right‐sided shift is evident from the laterality indices calculated on the conditional contrast as well the performance correlation data. Pre‐LSVT LOUD, the activations were predominantly in the left hemisphere as indicated by the laterality index of 0.23 and post‐LSVT LOUD, the laterality index during speech was −0.13, indicating greater volumes of activated cortical and subcortical areas in the right hemisphere. The CBF in regions correlating with vocal SPL also showed a strong rightward shift with a laterality index of −0.5.
The right‐sided regions that were mainly modified by treatment were M1‐mouth, DLPFC, PMd, and auditory cortices (Figs. 4 and 6). Consistent with our hypothesis, we attribute this right shift to be a direct result of motor and sensory loudness training during LSVT LOUD. Such an effect is thought to result in an increased modulation of prosody and the gain processing of speech sounds. Loudness along with tone and pitch are nonsegmental parts of speech and are elements of speech prosody (Seddoh and Robin, 2001). Both lesion and imaging studies have shown right hemispheric dominance in overall comprehension and production of speech as well as in comprehension and expression of global aspects of prosodic speech such as tone, pitch, and loudness (Belin et al., 1998a; Riecker et al., 2002; Borod et al., 2002; Mitchell and Crow, 2005; Hesling et al., 2005; Nakhutina et al., 2006; Ross and Monnot, 2008). Frontal lobes (M1, PMd, insula) and parietal lobes, as well as inferior, middle, and superior temporal sulci have been shown to be activated by prosody. Similarly, the right hemispheric frontal‐parietal‐temporal cortical network has been shown to be involved in selective attention to auditory stimuli (Paus et al., 1997; Belin et al., 1998a, b). Indeed, consistent with our hypothesis, these were the regions that showed significant right shift in this study.
A finding of specific interest in this study is the increased activation seen in the auditory cortices, especially in the right hemisphere after LSVT LOUD. It is well documented that patients with IPD have difficulty integrating sensory information into the motor commands and show a mismatch between internal sense of effort (calibration of sensory signals internally) and motor output (Solomon et al., 2000; Solomon and Robin, 2005). This has been shown to be true in the somatosensory (Solomon and Robin, 2005) as well the auditory (Kühn et al., 2004; Sabate et al., 2008) systems in IPD. Existence of such abnormal sensory‐motor integration can easily explain the altered self‐assessment of loudness that is frequently observed in individuals with IPD (Fox et al., 2006). Through loudness training, LSVT LOUD is thought to recruit the auditory cortex, especially on the right side and result in sensory calibration particularly of the auditory system. Such targeted increase in activity in the auditory cortex and improved communication of this region with other speech motor areas is thought to augment sensory motor integration and improve feedback. Such a phenomenon supports the hypothesis that the auditory calibration that is observed following treatment is a result of increased recruitment of right auditory cortex.
This study provides evidence that the main effect of LSVT LOUD is to directly modify the cortical motor, auditory, and prefrontal areas during speech. These areas, in turn, are thought to modulate the activity of subcortical regions during speaking tasks. In this aspect, LSVT LOUD can be considered to be a speech motor treatment paradigm resulting in a top–down treatment effect. Differential effects of pharmacotherapy (from the limbic system to the cortex, i.e., bottom–up) and psychotherapy (from the cortical areas to the limbic system, i.e., top–down) on the cortico‐limbic system have been demonstrated by neuroimaging in depression (Mayberg, 2003; Mayberg et al., 1999; Petersen, 2006). In contrast to the cortical or top–down modulation of speech motor system by behavioral treatment such as LSVT LOUD, the surgical and pharmacological treatments in IPD can be thought to be effective via a direct effect on the subcortical speech regions, that is, a bottom–up effect. In this case, the subcortical regions in turn modify activity of cortical regions such as M1, SMA, and PMd during speech. The top–down modulation of the speech motor system by LSVT LOUD can also explain the distributed effects of improved articulation (Fox et al., 2006; Sapir et al., 2007), facial expression (Spielman et al., 2003), and swallowing (El Sharkawi et al., 2002) that are reported following LSVT LOUD. Similarly, positive effects of LSVT LOUD that are documented in disorders other than IPD, such as ataxic dysarthria (Sapir et al., 2003), stroke, and cerebral palsy (Fox et al., 2006) can be explained on the basis of top–down therapeutic effect of LSVT LOUD.
The following discussion will examine the individual speech motor, auditory, and multimodal association areas directly modified by LSVT LOUD and will also examine the role of the thalamus in increasing loudness.
Major Cortical Areas Modified by LSVT LOUD
Primary motor areas
In our study, the right M1‐mouth showed significantly greater activation during speech post‐LSVT LOUD (Figs. 3 and 4) and a strong correlation with treatment outcome (Figs. 5 and 6). Such a change in M1‐mouth region is likely a direct effect of LSVT LOUD. Similar findings have been found in IPD following STN stimulation where improvements in clinical, acoustic, and biomechanical parameters of dysarthria were demonstrated following restored activation of bilateral M1‐mouth area during speech (Pinto et al., 2004a). Such compensatory changes in the non‐dominant primary motor areas have been demonstrated in other neurological disorders as well, such as stuttering (Fox et al., 2000; Ingham et al., 2004), and stroke (Riecker et al., 2002). We conclude that the vocal exercises of LSVT LOUD directly modulate the activity of primary mouth motor cortices, especially on the right side.
Primary and secondary auditory areas
Another cortical area that was significantly altered following LSVT LOUD was the right auditory region (BA 21, 22). Although the role of left auditory areas (BA 22) appears to be related mainly to discriminating linguistic components of speech, the right auditory regions have been shown to have more diverse functions. Price et al. (1992, 1996) showed a linear increase in activity in the right anterior STG with increased word rate. Right auditory cortex has been shown to have a role in pitch judgement, processing complex harmonic structures, and timbre discrimination (Zatorre and Binder, 2000; Perry et al., 1996, 1999). Right STG has been shown to be active in conjunction with right prefrontal and premotor areas in working memory tasks of pitch (Zatorre et al., 1994) and auditory tones (Perry et al., 1993). Increased bilateral activation of primary auditory cortices has been shown in IPD individuals under both DBS‐off and DBS‐on conditions and is thought to be associated with the auditory feedback perception (Pinto et al., 2004a). In stuttering, right temporal lobe (BA 21, 22) activity has been shown to correlate negatively with stuttering rate (Ingham et al., 2004; Brown et al., 2005) and is thought to be a result of an increased inhibition from the primary motor cortices, an abnormal efference copy (Brown et al., 2005). Such data indicate the existence of connections between primary motor and auditory cortices. This supports our conclusion that compensatory or plastic connections exist between the motor cortical areas and auditory areas and that these connections can be activated during speech motor training programs such as LSVT LOUD.
Dorsolateral prefrontal cortex
The right DLPFC also showed significant increase in activity during speech and correlated with treatment outcome (Figs. 4, 5, 6). A previous DBS study in IPD has shown abnormal hyperactivity in bilateral DLPFC in participants with IPD during speech tasks that was reversed following stimulation of STN (Pinto et al., 2004a). The authors conclude that the combined hyperactivity of DLPFC and the rostral SMA to be a compensatory phenomenon due to the disease process, which becomes normalized following STN‐DBS. In the previous LSVT LOUD imaging study (Liotti et al., 2003), the DLPFC activation was seen in speech tasks only post treatment. Here, the authors concluded that the DLPFC activation post‐LSVT LOUD undergoes normalization similar to the limb motor system in IPD, due to reestablishment of BG‐thalamic inputs to the prefrontal cortex.
In both these studies, the primary effect of treatment was thought to be at the level of the BG. However, DLPFC is not usually active in healthy participants during speech motor tasks. Furthermore, behavioral correlation in our study indicates that as speech became more normal, CBF in the right DLPFC increased. Thus, our data indicate that the recruitment of an alternative fronto‐striatal loop able to affect pallidal output (Alexander et al., 1986; Liotti et al., 2003) is a more reasonable explanation. This effect of LSVT LOUD can be viewed as a component of the top–down modulation of the speech motor network. Thus, LSVT LOUD modifies the activity in DLPFC, which, in turn, activates motor and subcortical connections (basal ganglia‐thalamic inputs) for an effective speech outcome. The absence of correlation between the activity in the basal ganglia and the treatment (see below) supports this conclusion. Through its connection to the parietal and temporal areas (Passingham, 1997), DLPFC can augment somatosensory and auditory feedback and improve communication of these regions with motor areas. Such a top–down effect on sensory systems can explain not only the auditory recalibration seen following LSVT, but also the distributed effects of LSVT LOUD (see above).
Thalamus
In this study, activity in ventroposterior lateral nucleus on the left side and VAN on the right hemisphere during speech correlated positively with treatment. Thalamus is a major component of the extrapyramidal motor system connecting the cortical motor areas with the BG, the cerebellum, and the thalamocortical pathway and is involved in movement initiation. The effects of LSVT LOUD on thalamic nuclei can be considered to be secondary downstream modulation by cortical areas such as the DLPFC and premotor cortices.
Brain Regions That Remained Unchanged Following Treatment
Regions of the speech motor network such as left primary motor, premotor, auditory cortices, and right GP continue to show activation during speech after LSVT LOUD. Bilateral parietal association areas (BA 7 and 31), visual cortices, and cerebellum were also active during speech after treatment. These findings indicate that the left motor cortex continues to be specialized for the control of movements of face and support higher order aspects of speech production. Following stimulation of STN, the abnormal activation in left PMd during speech are reported to be normalized (Pinto et al., 2004a). However, our data suggest that even after successful outcome following LSVT LOUD, individuals with IPD continue to activate left PMd during speech. Caudal SMA (Talirach coordinate y ≤ 0), thought to be a premovement sensory integration and articulatory planning area (Loucks et al., 2007; Passingham, 1997; Passingham et al., 1989), was active bilaterally during speech tasks after LSVT LOUD. However, there was no correlation between CBF in caudal SMA and the behavioral outcome. Parietal association areas (BA 5, 7, and 40) that play a crucial role in the processing of somatosensory and visual information required for motor movement (Deiber et al., 1991; Jenkins et al., 2000; Playford et al., 1992) continued to be active during speech in IPD after voice treatment. The above findings support our conclusion that bilateral parietal association areas (both superior and inferior) continue to be important in speech production in patients with IPD following the voice treatment.
Another main finding in this study is the absence of correlation of activity in BG with treatment outcome. Although activation of BG has been shown to be important in motor preparation, execution (Chesselet and Delfs, 1996), and rescaling movement dimensions in limb‐motor tasks, including velocity, strength, and force (Turner and Anderson, 1997; Turner et al., 1998), there is evidence that it does not primarily mediate motor learning (Boecker et al., 1998; Grafton et al., 1992; Jenkins et al., 1994). Basal ganglia activity has been shown not to correlate with increasing movement frequency (Blinkenberg et al., 1996; Jenkins et al., 1997) or force (Dettmers et al., 1995). In earlier studies in IPD, BG activation was absent during speech tasks both with DBS‐on and DBS‐off conditions (Pinto et al., 2004a). This was thought to be due to presence of activity in BG during all conditions, therefore suppressed during intergroup contrasts. As pointed out earlier, this is a major drawback of contrast analysis. In the previous LSVT LOUD study, a treatment‐specific effect was found in right putamen and caudate nucleus in the phonation task, and it was speculated that LSVT LOUD might restore function in the BG (Liotti et al., 2003). Our finding is consistent with both IPD speech studies. Basal ganglia activation reported during speech post‐LSVT LOUD indicates its direct role during speech tasks such as phonation and paragraph reading. But its activity is modified posttreatment, not directly as speculated by Liotti et al. (2003), but rather indirectly via alternate fronto‐striatal loop that modify pallidal output.
In conclusion, speech appears to be a complex motor task in individuals with IPD requiring several motor and prefrontal areas. Even after a successful behavioral treatment (e.g., LSVT LOUD), speech continues to be a complex and challenging task requiring these areas, especially in the right hemisphere. The changes reported here are immediately following the completion of treatment. It is important to follow up with imaging to see if the right‐sided shift is also a marker of long‐lasting therapeutic effect of LSVT LOUD.
Acknowledgements
The authors thank Betty Heyl (PET technologist), Paul Jerabek, Ph.D., Morgan Stratton, Amanda Sullivan (Radiotracer preparation), Dianne Johnson (subject screening), and Richard Holt, M.D.(Laryngeal examination).
REFERENCES
- Alexander GE, DeLong MR, Strick PL ( 1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Belin P, McAdams S, Smith B, Savel S, Thivard L, Samson S, Samson Y ( 1998a): The functional anatomy of sound intensity discrimination. J Neurosci 18: 6388–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, Samson Y ( 1998b): Lateralization of speech and auditory temporal processing. J Cogn Neurosci 10: 536–540. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law L ( 1996): Rate dependence of regional cerebral activation during performance of a repetitive motor task: A PET study. J Cereb Blood Flow Metab 16: 794–803. [DOI] [PubMed] [Google Scholar]
- Boecker H, Dagher A, Ceballos‐Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ ( 1998): Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: Investigations with H2 15O PET. J Neurophysiol 79: 1070–1080. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard PW, Theodore WH ( 2000): Activation of language cortex with automatic speech tasks. Neurology 55: 1151–1157. [DOI] [PubMed] [Google Scholar]
- Borod JC, Bloom RL, Brickman AM, Nakhutina L, Curko EA ( 2002): Emotional processing deficits in individuals with unilateral brain damage. Appl Neuropsychol 9: 23–36. [DOI] [PubMed] [Google Scholar]
- Brancucci A, San Martini P ( 2003): Hemispheric asymmetries in the perception of rapid (timbral) and slow (nontimbral) amplitude fluctuations of complex tones. Neuropsychology 17: 451–457. [DOI] [PubMed] [Google Scholar]
- Brancucci A, Babiloni C, Rossini PM, Romani GL ( 2005): Right hemisphere specialization for intensity discrimination of musical and speech sounds. Neuropsychologia 43: 1916–1923. [DOI] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, Carson RE, Ludlow CL ( 1997): Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain 120 ( Pt 5): 761–784. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT ( 2005): Stuttered and fluent speech production: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Delfs JM ( 1996): Basal ganglia and movement disorders: An update. Trends Neurosci 19: 417–422. [DOI] [PubMed] [Google Scholar]
- D'Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR ( 2008): Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. J Voice 22: 365–372. [DOI] [PubMed] [Google Scholar]
- Darley F, Aronson A, Brown J ( 1975): Motor Speech Disorders. Philadelphia: W.B. Saunders Inc. [Google Scholar]
- De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, Boon P ( 2007a): The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson's disease. Clin Neurol Neurosurg 109: 495–500. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, Estercam I, Van Maele G, De Bodt M, Boon P, Van Borsel J ( 2007b): Levodopa‐induced modifications of prosody and comprehensibility in advanced Parkinson's disease as perceived by professional listeners. Clin Linguist Phon 21: 783–791. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS ( 1991): Cortical areas and the selection of movement: A study with positron emission tomography. Exp Brain Res 84: 393–402. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS ( 1995): Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–815. [DOI] [PubMed] [Google Scholar]
- Didic M, Ceccaldi M, Poncet M ( 1998): Progressive loss of speech: A neuropsychological profile of premotor dysfunction. Eur Neurol 39: 90–96. [DOI] [PubMed] [Google Scholar]
- Duffy JR ( 2005): Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 2nd ed St. Louis, MO: Mosby. [Google Scholar]
- El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, Pawlas A, Baum S, Werner C ( 2002): Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT LOUD): A pilot study. J Neurol Neurosurg Psychiatry 72: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G ( 1960): The rainbow passage. In: Voice and Articulation Drillbook, Vol. 2 New York: Harper and Row. [Google Scholar]
- Fox CM, Morrison CE, Ramig LO, Sapir S ( 2002): Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson's Disease. Am J Speech‐Lang Pathol 11: 111–123. [Google Scholar]
- Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG ( 2006): The science and practice of LSVT LOUD/LOUD: Neural plasticity‐principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin Speech Lang 27: 283–299. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME ( 1984): Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol 51: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA ( 1989): Noninvasive functional brain mapping by change‐distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med 30: 141–149. [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Reiman EM, Raichle ME ( 1988): Enhanced detection of focal brain responses using intersubject averaging and change‐distribution analysis of subtracted PET images. J Cereb Blood Flow Metab 8: 642–653. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL ( 2000): Brain correlates of stuttering and syllable production. A PET performance‐correlation analysis. Brain 123: 1985–2004. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Fox SP, Sandoval H, Kochunov P, Capaday C, Lancaster JL ( 2006): Intensity modulation of TMS‐induced cortical excitation: Primary motor cortex. Hum Brain Mapp 27: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentil M, Pinto S, Pollak P, Benabid AL ( 2003): Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian dysarthria. Brain Lang 85: 190–196. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME ( 1992): Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci 12: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Burbaud P, Lorenzi C, Ramos C, Bioulac B, Semal C, Demany L ( 2008): Auditory temporal processing in Parkinson's disease. Neuropsychologia 46: 2326–2335. [DOI] [PubMed] [Google Scholar]
- Hesling I, Clément S, Bordessoules M, Allard M ( 2005): Cerebral mechanisms of prosodic integration: Evidence from connected speech. Neuroimage 24: 937–947. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Xiong J, Zamarripa F, Hardies LJ, Lancaster JL ( 2004): Brain correlates of stuttering and syllable production: Gender comparison and replication. J Speech Lang Hear Res 47: 321–341. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE ( 1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14: 3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Passingham RE, Brooks DJ ( 1997): The effect of movement frequency on cerebral activation: A positron emission tomography study. J Neurol Sci 151: 195–205. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ ( 2000): Self‐initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123 ( Pt 6): 1216–1228. [DOI] [PubMed] [Google Scholar]
- Jodzio K, Drumm DA, Nyka WM, Lass P, Gasecki D ( 2005): The contribution of the left and right hemispheres to early recovery from aphasia: A SPECT prospective study. Neuropsychol Rehabil 15: 588–604. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA ( 2008): Principles of experience‐dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res 51: S225–S239. [DOI] [PubMed] [Google Scholar]
- Kofler M, Müller J, Wenning GK, Reggiani L, Hollosi P, Bösch S, Ransmayr G, Valls‐Solé J, Poewe W ( 2001): The auditory startle reaction in parkinsonian disorders. Mov Disord 16: 62–71. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Sharott A, Trottenberg T, Kupsch A, Brown P ( 2004): Motor cortex inhibition induced by acoustic stimulation. Exp Brain Res 158: 120–124. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg HS, and Fox PT ( 1995): A modality‐independent approach to spatial normalization of tomographic images of the human brain. Human Brain Mapp 3: 209–223. [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC ( 1997): Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward‐transform method. Human Brain Mapp 5: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Ramig LO, Vogel D, New P, Cook CI, Ingham RJ, Ingham JC, Fox PT ( 2003): Hypophonia in Parkinson's disease: Neural correlates of voice treatment revealed by PET. Neurology 60: 432–434. [DOI] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL ( 2007): Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. Neuroimage 36: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS ( 2003): Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimized treatment. Br Med Bull 65: 193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Fox PT, Raichle ME ( 1989): A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab 9: 96–103. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Crow TJ ( 2005): Right hemisphere language functions and schizophrenia: The forgotten hemisphere? Brain 128( Pt 5): 963–978. [DOI] [PubMed] [Google Scholar]
- Nakhutina L, Borod JC, Zgaljardic DJ ( 2006). Posed prosodic emotional expression in unilateral stroke patients: Recovery, lesion location, and emotional perception. Arch Clin Neuropsychol 21: 1–13. [DOI] [PubMed] [Google Scholar]
- Narayana S, Jacks A, Robin DA, Poizner H, Zhang W, Franklin C, Liotti M, Vogel D, Fox PT ( 2009): A non‐invasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson's disease. Am J Speech Lang Pathol 18: 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R ( 1997): The frontal lobes and voluntary action Oxford Psychology Series 21 New York: Oxford University Press. [Google Scholar]
- Passingham RE, Thaler DE, Chen Y ( 1989): Supplementary motor cortex and self initiated movement In: Ito M, editor. Neural Programming. Karger: Basel; pp 13–24. [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC ( 1997): Time related changes in neural systems underlying attention and arousal during performance of an auditory vigilance task. J Cogn Neurosci 9: 392–408. [DOI] [PubMed] [Google Scholar]
- Perry DW, Petrides M, Alivisatos B, Zatorre RJ, Evans AC, Meyer E ( 1993): Functional activation of human frontal cortex during tonal working memory tasks. Soc Neurosci Abstr 18: 843. [Google Scholar]
- Perry DW, Zatorre RJ, Evans AC ( 1996): Co‐variation of CBF during singling with vocal fundamental frequency. Neuroimage 3: S315. [Google Scholar]
- Perry DW, Zatorre RJ, Petrides M, Alivisatos B, Meyer E, Evans AC ( 1999): Localization of cerebral activity during simple singing. Neuroreport 10: 3979–3984. [DOI] [PubMed] [Google Scholar]
- Petersen TJ ( 2006): Enhancing the efficacy of antidepressants with psychotherapy. J Psychopharmacol 20( 3 Suppl): 19–28. [DOI] [PubMed] [Google Scholar]
- Pinto S, Thobois S, Costes N, Le Bars D, Benabid AL, Broussolle E, Pollak P, Gentil M ( 2004a): Subthalamic nucleus stimulation and dysarthria in Parkinson's disease: A PET study. Brain 127: 602–615. [DOI] [PubMed] [Google Scholar]
- Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin‐Dowsey P, Auzou P ( 2004b): Treatments for dysarthria in Parkinson's disease. Lancet Neurol 3: 547–556. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ ( 1992): Impaired mesial frontal and putamen activation in Parkinson's disease: A positron emission tomography study. Ann Neurol 32: 151–161. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise R, Ramsay S, Friston K, Howard D, Patterson K, Frackowiak R ( 1992): Regional response differences within the human auditory cortex when listening to words. Neurosci Lett 146: 179–182. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ ( 1996): Hearing and saying. The functional neuro‐anatomy of auditory word processing. Brain 119 ( Pt 3): 919–931. [DOI] [PubMed] [Google Scholar]
- Raboyeau G, Marie N, Balduyck S, Gros H, Demonet JF, Cardebat D ( 2004): Lexical learning of the English language: A PET study in healthy French participants. Neuroimage 22: 1808–1818. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, Thompson LL, Horii Y ( 1995): Comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res 38: 1232–1251. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, O'Brien C, Hoehn M, Thompson L ( 1996): Intensive speech treatment for patients with Parkinson's disease: Short‐and long‐term comparison of two techniques. Neurology 47: 1496–1504. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Fox C, Countryman S ( 2001a): Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson's disease: A comparison with untreated patients and normal age‐matched controls. Mov Disord 16: 79–83. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O'Brien C, Hoehn M, Thompson LL ( 2001b): Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. J Neurol Neurosurg Psychiatry 71: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Fox C, Sapir S ( 2004): Parkinson's disease: Speech and voice disorders and their treatment with the Lee Silverman Voice Treatment. Semin Speech Lang 25: 169–180. [DOI] [PubMed] [Google Scholar]
- Rektorova I, Barrett J, Mikl M, Rektor I, Paus T ( 2007): Functional abnormalities in the primary orofacial sensorimotor cortex during speech in Parkinson's disease. Mov Disord 22: 2043–2051. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Grodd W, Ackermann H ( 2002): Reorganization of speech production at the motor cortex and cerebellum following capsular infarction: A follow‐up functional magnetic resonance imaging study. Neurocase 8: 417–423. [DOI] [PubMed] [Google Scholar]
- Ross ED, Monnot M ( 2008): Neurology of affective prosody and its functional‐anatomic organization in right hemisphere. Brain Lang 104: 51–74. [DOI] [PubMed] [Google Scholar]
- Sabate M, Llanos C, Rodriguez M ( 2008): Integration of auditory and kinesthetic information in motion: Alterations in Parkinson's disease. Neuropsychology 22: 462–468. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Hoyt P, Countryman S, O'Brien C, Hoehn M ( 2002): Speech loudness and quality 12 months after intensive voice treatment (LSVT LOUD) for Parkinson's disease: A comparison with an alternative speech treatment. Folia Phoniatr Logop 54: 296–303. [DOI] [PubMed] [Google Scholar]
- Sapir S, Spielman J, Ramig LO, Hinds SL, Countryman S, Fox C, Story B ( 2003): Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on ataxic dysarthria: A case study. Am J Speech Lang Pathol 12: 387–399. [DOI] [PubMed] [Google Scholar]
- Sapir S, Spielman JL, Ramig LO, Story BH, Fox C ( 2007): Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with idiopathic Parkinson disease: Acoustic and perceptual findings. J Speech Lang Hear Res 50: 899–912. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD ( 1999): Motor control and learning: A behavioral emphasis. Chapter 13, 4th Edition Illinois: Human Kinetics. [Google Scholar]
- Seddoh SA, Robin DA ( 2001): Neurogenic disorders of prosody. Treating disordered speech motor control, 2nd ed In: Vogel D, Cannito MP, editors. Texas: Pro‐ed Publishers; pp 277–320. [Google Scholar]
- Silbersweig DA, Stern E, Frith CD, Cahill C, Holmes A, Grootebank S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ ( 1995): A functional neuroanatomy of hallucinations in schizophrenia. Nature 378: 176–179. [DOI] [PubMed] [Google Scholar]
- Smith ME, Ramig LO, Dromey C, Perez KS, Samandari R ( 1995): Intensive voice treatment in Parkinson disease: Laryngostroboscopic findings. J Voice 9: 453–459. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Robin DA ( 2005): Perceptions of effort during handgrip and tongue elevation in Parkinson's disease. Parkinsonism Relat Disord 11: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NP, Robin DA, Luschei ES ( 2000): Strength, endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res 43: 256–267. [DOI] [PubMed] [Google Scholar]
- Spielman JL, Borod JC, Ramig LO ( 2003): The effects of intensive voice treatment on facial expressiveness in Parkinson disease: Preliminary data. Cogn Behav Neurol 16: 177–188. [DOI] [PubMed] [Google Scholar]
- Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ ( 2006): Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Neuroprotective strategies and alternative therapies for Parkinson disease (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 66: 976–982. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers. [Google Scholar]
- Turner RS, Anderson ME ( 1997): Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol 77: 1051–1074. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM ( 1998): Motor subcircuits mediating the control of movement velocity: A PET study. J Neurophysiol 80: 2162–2176. [DOI] [PubMed] [Google Scholar]
- Verdolini K, Hess MM, Titze IR, Bierhals W, Gross M ( 1999): Investigation of vocal fold impact stress in human subjects. J Voice 13: 184–202. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Binder JR ( 2000): Functional and structural imaging of the human auditory system In: Toga AW, Mazziotta JC, editors. Brain Mapping the Systems. San Diego, CA: Academic Press; pp 365–402. [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E ( 1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14: 1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]


