Abstract
Peritoneal carcinomatosis is a frequent finding in gastric cancer associated with a poor prognosis. The features that enable gastric tumors to disseminate are poorly understood until now. Previously, we showed elevated mRNA levels of phosphoglycerate kinase 1 (PGK1), an ATP-generating enzyme in the glycolytic pathway, the chemokine receptor 4 (CXCR4), the corresponding chemokine ligand 12 (CXCL12) and β-catenin in specimens from gastric cancer patients with peritoneal carcinomatosis. In this study the influence of PGK1 on CXCR4 and β-catenin was assessed as well as the invasiveness of PGK1 overexpressing cancer cells. In this current study we found that PGK1 regulates the expression of CXCR4 and β-catenin at the mRNA and protein levels. On the other hand, CXCR4 regulates the expression of PGK1. Plasmid-mediated overexpression of PGK1 dramatically increased the invasiveness of gastric cancer cells. Interestingly, inhibition of CXCR4 in cells overexpressing PGK1 produced only a moderate reduction of invasiveness, suggesting that PGK1 itself has a critical role in tumor invasiveness. Immunohistochemistry in specimens from diffuse gastric cancer patients also revealed an overexpression of PGK1 in patients with development of peritoneal carcinomatosis. Therefore, PGK1 may be a crucial enzyme in peritoneal dissemination. Together these findings suggest that the enhanced expression of PGK1 and its signaling targets CXCR4 and β-catenin in gastric cancer cells promote peritoneal carcinomatosis. Thus, PGK1 may serve as prognostic marker and/or be a potential therapeutic target to prevent dissemination of gastric carcinoma cells into the peritoneum.
Keywords: PGK1, CXCR4, CXCL12, gastric cancer, peritoneal carcinomatosis, cancer dissemination
Introduction
Gastric cancer associated with peritoneal carcinomatosis is a frequent cause of cancer-related death worldwide 1,2. Presently, there is no effective therapy and the five-year survival rate is less than 2% 3. Previously we studied the features that enable gastric tumors to develop peritoneal dissemination. Comparative microarray analyses were performed using human specimens from consecutive gastric cancer patients with peritoneal carcinomatosis versus gastric cancer patients without peritoneal carcinomatosis. Tissues recovered from individuals with peritoneal dissemination demonstrated significantly higher mRNA levels of phosphoglycerate kinase 1 (PGK1), the chemokine receptor 4 (CXCR4), its ligand chemokine ligand 12 (CXCL12) and β-catenin 4. PGK1 is an ATP-generating enzyme of the glycolytic pathway that catalyzes the conversion of 1,3-diphosphoglycerate to 3-phosphoglycerate. In the nucleus, PGK1 affects DNA replication and repair 5. It is assumed that the glycolytic pathway and glycolysis-related genes play an important role in tumorigenesis.
Solid tumor cells employ glycolytic enzymes, such as PGK1, to generate ATP when their supply of oxygen is limited 6. Further, PGK1 is involved in the onset of malignancies, such as prostate, breast, pancreatic, multi-drug resistant ovarian cancer and as recently reported, also in gastric cancer 4,5,7–9. The human chemokine system contains about 50 known ligands and 20 G protein–coupled receptors. Chemokines and their receptors play important roles in migration and activation of leukocytes, angiogenesis and tumor growth 10. CXCR4, a seven-transmembrane G-protein-coupled chemokine receptor and its ligand CXCL12 also known as stromal-derived-factor-1 (SDF1) promote the progression and spreading of a number of malignant diseases including prostate, non–small-cell lung, pancreatic, breast and gastric cancer 2,4,11–14. CXCR4 is expressed on hematopoietic and epithelial cancer cells, where it may aid in establishing metastasis along a chemotactic gradient to organs expressing CXCL12 15,16. Moreover, CXCL12-expressing cancer cells may protect themselves from cytotoxic immune responses via induction of apoptosis in cytotoxic T cells and inducing a local Th1→Th2 shift in the tumor area 16–18. β-catenin, a 92-kDa protein, plays a central role in cadherin-based cell adhesion processes, as a downstream effector in the Wnt-signaling pathway, and also as a potential downstream target of PGK1 5,19. In gastric cancer, increased expression of β-catenin is associated with enhanced proliferation, invasiveness, metastasis, angiogenesis and drug resistance 4,20,21. There is a close relationship between the regulation of the CXCR4/CXCL12 axis, β-catenin and PGK1 in prostate cancer 5. Interestingly, as already mentioned above, we detected a strong overexpression of these genes in human primary gastric cancer associated with development of peritoneal carcinomatosis. Therefore we studied the relationship between peritoneal dissemination through PGK1 and the regulation within its signaling targets CXCR4, CXCL12 and β-catenin. Here, we report that PGK1 appears to be a crucial enzyme for peritoneal dissemination of gastric cancer in both CXCR4/CXCL12-dependent and by CXCR4/CXCL12-independent mechanisms.
Materials and Methods
Cell culture
The human gastric adenocarcinoma cell line MKN-45 was purchased (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum gold (Lonza, Basel, Switzerland). The cell line was selected due to the distinct expression of PGK1 and the known moderate expression of CXCR4, as described by Yasumoto et al 2.
Small interfering RNA knockdown of PGK1 and CXCR4
MKN-45 cells were seeded at 6×104 cells per well in 24-well plates 24h before transfection. For transient transfection, cells were incubated with a complex of Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA) and siRNA in Optimem® (Invitrogen) achieving final concentrations of 100nM siRNA. The used small interfering RNA (siRNA) was siGENOME® ON-TARGETplus™ SMARTpool PGK1 (L-006767-00-0005) and siGENOME® ON-TARGETplus SMARTpool™ CXCR4 (L-005139-00-0005) (Dharmacon, Chicago, IL). Each pool consisted of four siRNAs targeting the gene of interest. The siRNA ON-TARGETplus™ non-targeting Pool was used as a negative control. Ten hours after transfection medium was replaced by fresh culture medium. The cells were harvested at distinct intervals after transfection.
Transfection and overexpression of PGK1
Plasmid transfection was carried out using the plasmid vector pEF-IRES containing PGK1. Briefly, 6×105 MKN-45 cells were seeded in 24-well plates one day before transfection. Cells were transfected employing Tfx™-50 (Promega, Madison, WI) and 1 μg plasmid DNA was administered per well. The empty vector pEF-IRES was transfected, serving as a negative control. Transfected cells were selected with 5 μg/ml puromycin (AppliChem, Darmstadt, Germany) 24 h after transfection 22.
Quantitative Real-Time-PCR (qRT-PCR)
RNA was extracted using the RNeasy® Mini Kit (Qiagen, Hilden, Germany) assessing quality and quantity using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Real-time PCR and complementary DNA synthesis was performed as previously described 4. Relative amounts of each gene proportional to an internal β-actin control and hence fold changes were calculated using the equation 2-ΔΔCT, ΔCT=CT(gene)-CT(β-actin) and ΔΔCT=ΔCT(transfected)-ΔCT(control-transfected) 23. Sequence specific primers were used as follows: PGK1: 5′-CATACCTGCTGGCTGGATGG-3′ (forward)/5′-CCCACAGGACCATTCCACAC-3′ (reverse), CXCR4:5′-CAGTTTCAGCACATCATGGTTGG-3′ (forward)/5′-GTGACAGCTTGGAGATGATAATGC-3′ (reverse), β-catenin: 5′-GTCTTACCTGGACTCTGGAATCC-3′ (forward)/5′-GGTATCCACATCCTCTTCCTCAG-3′ (reverse).
Flow cytometry
Adherent MKN-45 cells were dissociated using Accutase (PAA, Pasching, Austria), washed twice in phosphate buffered saline at 4°C and resuspended using AccuMax (PAA). For every assay 5×105 cells were used. For extracellular staining a CXCR4 IgG2b monoclonal antibody (mAb) (R&D, Minneapolis, MN) was employed followed by labelling with a phycoerythrin (R-PE) conjugated secondary antibody (Ab) (BD, Franklin Lakes, NJ). Intracellular staining was conducted with the IntraPrep™ permeabilization reagent (Beckman Coulter, Fullerton, CA) according to the manufacturer’s instructions. A β-catenin Alexa Fluor 488 conjugated mAb (R&D) was used. Incubation of all Ab was conducted for 20min in the dark, followed by washing twice in flow cytometry buffer (PBS, 0.1% BSA, 0.1% sodium azide). Cytometric analysis was performed using an Epics XL-MCL Flow Cytometer (Beckman Coulter). Evaluation was performed by observing positive cells at the indicated excitation wavelengths, taking into account the zero compensation of the non specific fluorescence signal using MKN-45 cells previously incubated with matching isotype control Ab (R&D). Analysis was performed considering 2×105 events in the gate previously verified to contain intact MKN-45 cells. The specific fluorescence index (SFI; ratio of mean fluorescence intensity with antibody compared to mean fluorescence intensity with isotype antibody) was determined which underlined the specificity of all findings.
Western blot analysis
Cells were harvested, washed and lysed in RIPA Buffer (Pierce, Rockford, IL). For 5×105 cells 200μl buffer was used. PGK1 detection was performed with a polyclonal Ab (Abnova, Taipei, Taiwan) at 1:500 dilution and anti-species-specific horseradish peroxidase-conjugated secondary Ab (Jackson Immuno Research, Suffolk, UK).
Invasion assay
Blind well chemotaxis chambers and polycarbonate filters with 8-μm pore size (Neuro Probe, Gaithersburg, MD) were used for the assay. Previously polycarbonate filters were coated with growth factor reduced Matrigel™ (16,5μg/filter, BD Biosciences, Franklin Lakes, NJ). In the lower chamber recombinant human CXCL12 (10mM, R&D, Minneapolis, MN) was added as a chemoattractant to NIH 3T3 (supernatant obtained from a mouse embryonic fibroblast cell line) conditioned medium and 1×106 cells were added to the top chamber. The chambers were incubated at 37°C for 24h. The top side of the filters was cleaned and the invading cells were fixed with ethanol and stained by crystal violet. Invaded cells were counted using a microscope (Axiovert 200, Axiovision software, 200x Zeiss, Goettingen, Germany). A neutralizing anti-CXCR4 Ab (25μg/ml, R&D) added to the cells before seeding them in the top chamber was used to inhibit cell invasion. Each assay was performed using at least four replicates.
Cell-viability assay
Transfection was performed using 2×106 cells and a final concentration of 100nM siRNA for siPGK1 and non-targeting control siRNA, as described above. Cells in the supernatant and adherent cells were collected and stained with 0.4% trypan blue dye. Live and dead cells were determined by cell counting in a Neubauer cytometer. Each point in time was assessed in triplicate.
Patients and tissue specimens
Primary tumor samples from 10 patients who underwent laparotomy for gastrectomy were investigated. Peritoneal carcinomatosis in diffuse gastric cancer specimens was histologically confirmed in 6 of the 10 patients (5 women, 1 men; median age 69 years, range 29–80; TNM-categories according to the UICC: pT2-category (n=1), pT3-category (n=5), pN0-category (n=2), pN2-category (n=4), M1-category (n=6)). No peritoneal carcinomatosis was observed in 4 of the 10 patients (3 women, 1 man; median age 72 years, range 62–77; TNM-categories according to the UICC: pT2-category (n=1), pT3-category (n=3), pN1-categories (n=3), pN2-category (n=1), M0-category (n=4)). All of the tumor specimens were obtained at the Department of General, Visceral and Transplant Surgery at the University of Tübingen, Germany. The specimens were snap_frozen in liquid nitrogen and stored at −80 °C until use. Each tumor sample was subjected to frozen sectioning, stained with haematoxylin and eosin, and re-evaluated by an experienced surgical pathologist. All of patients provided informed consent to participate in the study, which was approved by the local ethics committee (168/2005).
Immunohistochemistry
For the immunohistochemical examination, 2 μm tissue slides from formalin fixed, paraffin embedded tumor tissue were used. In brief, sections were deparaffined in xylene, rehydrated, and epitop retrieval was performed in citrate buffer (pH6) using the Pascal pressure chamber (Dako, DakoCytomation GmbH, Hamburg, Germany) for 2 minutes at 125 °C. After blocking endogenous peroxidase with 3%H2O2, primary antibody (dilution 1:200; clone: sc-48342; Santa Cruz Biotechnology Inc, Santa Cruz, California, USA) was incubated for 60 minutes. Detection was carried out using Envision™ goat-anti-mouse secondary antibody (Dako, DakoCytomation GmbH, Hamburg, Germany) for 60 minutes followed by incubation with diaminobenzidine (DAB). Nuclear as well as cytoplasmic staining was considered specific, and a moderate to strong expression was considered as positive.
Statistical analyses
Mean and standard deviation (SD) were calculated for all variables. Inter-group statistical significance was determined using the Student’s t test, p < 0.05 was considered statistically significant.
Results
PGK1 regulates CXCR4 and β-catenin expression
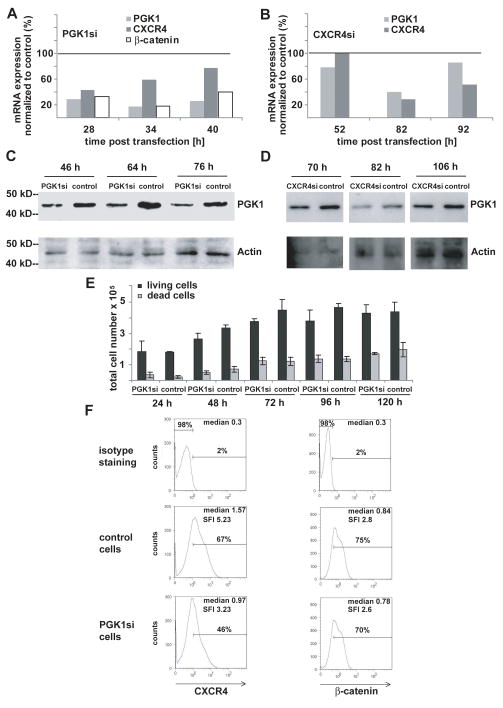
To detect that the CXCR4/CXCL12 axis and β-catenin are regulated by PGK1 expression, quantitative real time-PCR (qRT-PCR), flow cytometry and western blots were performed after siRNA targeting of PGK1 in MKN45 gastric adenocarcinoma cells. QRT-PCR revealed a considerable down-regulation of PGK1, CXCR4 and β-catenin expression (Fig. 1A). CXCL12 mRNA was not detectable in the MKN45 cell line. Western blot analysis was conducted for PGK1 and flow cytometry for CXCR4 and β-catenin with the objective to confirm the mRNA expression data. Western blot showed a considerable down regulation of PGK1 expression in siPGK1-treated cells compared to control cells using β-actin as a reference (Fig. 1C). β-catenin and CXCR4 protein expression were decreased in MKN45 siPGK1 cells (Fig. 1F). The distinct time intervals can be explained by the fact that the effects detected on mRNA levels are translated with a certain delay into effects on protein levels. Hence, the most pronounced effects on protein levels were observed at later points of time.
Figure 1. small interfering RNA-mediated PGK1 gene silencing reduces CXCR4 and β-catenin expression in MKN45 cells.
MKN45 cells treated with control siRNA or PGK1 siRNA pool (A) or CXCR4 siRNA pool (B) were assessed for PGK1, CXCR4 and β-catenin mRNA levels by qRT-PCR at 28, 34 and 40 h (A) or 52, 82 and 92 h (B) post transfection, mRNA levels are given as relative changes compared to corresponding MKN45 cells treated with non-targeting control siRNA resulting in 100%.
(C), Cell lysates of the MKN45 control cells or PGK1-siRNA cells were examined for PGK1 protein expression by immunoblot using β-actin as a reference. Lysates were prepared 46, 64 and 76 h post transfection. (D), Cell lysates of the MKN45 control cells or MKN45 CXCR4-siRNA cells were examined for PGK1 protein expression by Western blot using β-actin as a reference. Lysates were prepared 70, 82 and 106 h post transfection. (E), Cell proliferation and viability of PGK1siRNA and control siRNA treated cells were determined by the trypan blue dye exclusion method. Total cell numbers of living and dead cells are given at 24, 48, 72, 96 and 120 h and represent the mean numbers of three independent experiments. (F), CXCR4 and β-catenin protein levels in MKN45 siControls or MKN45 siPGK1 cells were determined by FACS analysis 76h post transfection. Representative FACS profiles are shown, and the respective medians of fluorescence and SFI values are shown within the histograms.
PGK1 and CXCR4 regulate their expression reciprocally
To determine whether the inhibition of CXCR4 regulates PGK1 expression, siRNA was used to decrease CXCR4 expression. Inhibition of CXCR4 reduced both, mRNA expression of PGK1 (Fig. 1B) and PGK1 protein levels (Fig. 1D), suggesting that reciprocal regulation exists between PGK1 and CXCR4.
Cell-viability assay
Overall proliferation and survival of the siPGK1-treated cells and the corresponding control siRNA treated cells were evaluated by the trypan blue assay over 4 days following transfection (Fig. 1E). The number of viable and cells unable to exclude the dye in both groups were comparable (the transfected with PGK1 and siControl). This suggests that the knockdown of PGK1 is not responsible for relevant differences in cell death.
Overexpression of PGK1 regulates CXCR4 and β-catenin expression
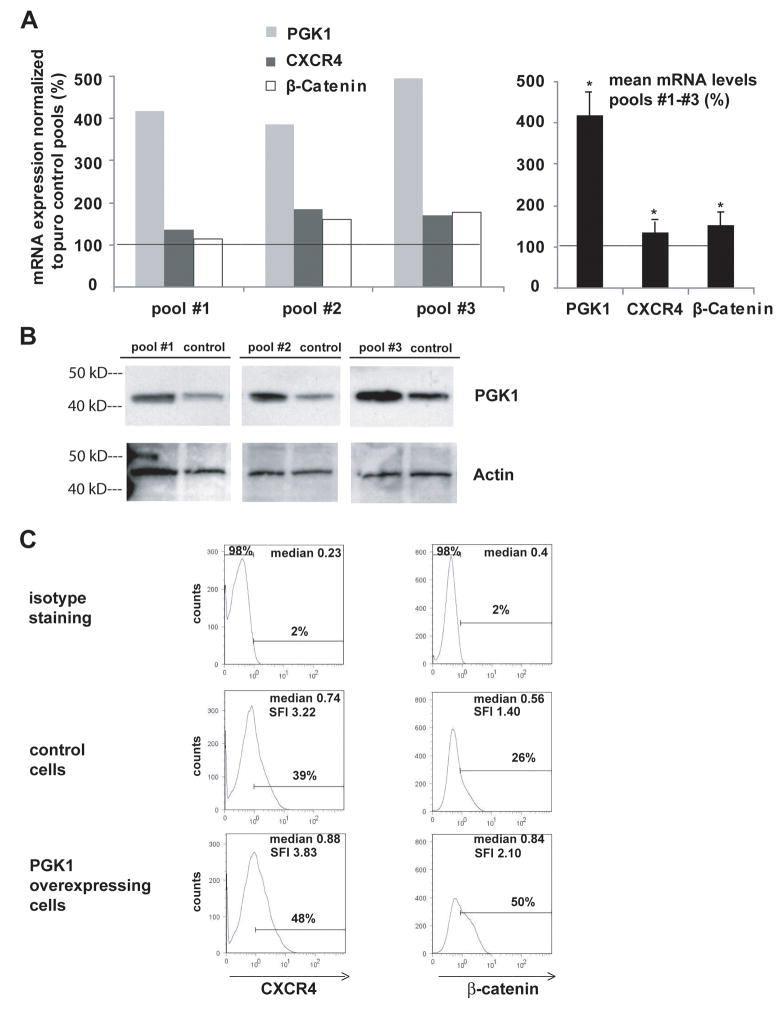
Since elevated PGK1 levels are a prominent feature of peritoneal dissemination in gastric cancer, overexpression of PGK1 was induced in MKN45 cells stably transfected with PGK1 pEF-IRES plasmid. Controls included cells transfected with an empty pEF-IRES vector and qRT-PCR, flow cytometry and western blots were conducted. QRT-PCR demonstrated significant increases in PGK1, CXCR4 and β-catenin expression (Fig. 2A, right panel). Western blot analysis revealed that overexpression of PGK1 in pEF-IRES cells was achieved using β-actin as a reference (Fig. 2B). In these cells, significantly higher protein levels of CXCR4 and β-catenin were detected compared to the corresponding controls (Fig. 2C). These data suggest that PGK1 is a regulatory molecule of CXCR4 and β-catenin.
Figure 2. PGK1 overexpression increases CXCR4 and β-catenin levels in MKN45 cells.
(A), PGK1 mRNA overexpression in MKN45 cells stably transfected with PGK1 pEF-IRES plasmid normalized to MKN45 pEF-IRES control pools (equals to 100%) as well as CXCR4 mRNA levels were assessed by qRT-PCR. Mean expression levels of PGK1, CXCR4 and β-catenin mRNA levels in pools #1 – #3 are presented in the right panel (*p<0.05). The left panel depicts the single pools. (B), Cell lysates of the MKN45 control pools or the MKN45 PGK1 pools were examined for PGK1 protein expression by immunoblot using β-actin as a reference. (C), MKN45/PGK1pools #1 – #3 or MKN45/control pools were assessed for CXCR4 and β-catenin protein by flow cytometry. Representative flow cytometry profiles of the MKN45/PGK1pools and MKN45/control pools are shown and the respective medians of fluorescence and SFI values are displayed within the histograms.
Overexpression of PGK1 increases the invasiveness of gastric cancer in vitro
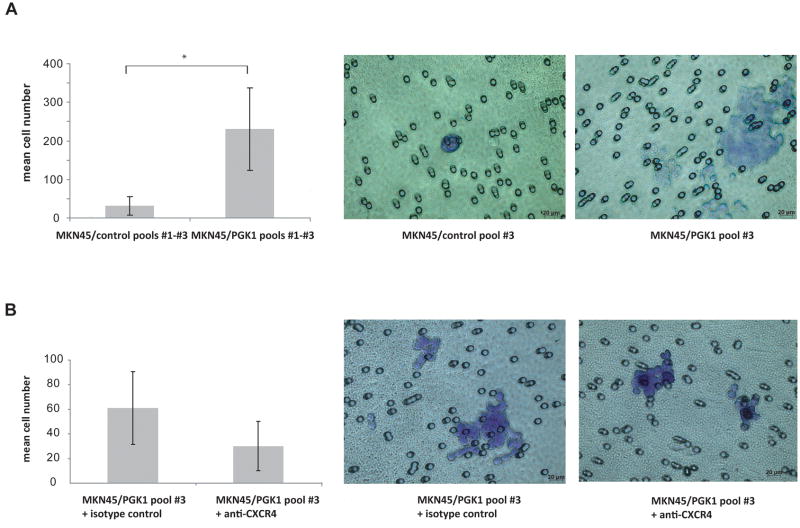
To test the hypothesis that PGK1 overexpression promotes invasion, invasion assays were performed in which a basement membrane matrix (e.g. Matrigel™) was used as a barrier and NIH-3T3 conditioned medium with recombinant human CXCL12 was used as the chemoattractant. PGK1 MKN45/pEF-IRES cells invaded dramatically stronger than MKN45/control cells (Fig 3A). To elucidate if the invasiveness of these cells was mainly caused by PGK1 or CXCR4, the assays were performed in the presence or absence of anti-CXCR4 specific blocking antibodies. Under these conditions, invasion was only moderately inhibited (Fig. 3B).
Figure 3. PGK1 overexpression dramatically increases the invasiveness of gastric cancer cells: modulation by inhibition of CXCR4.
(A), Invasion for 24h was assessed in three independent MKN45 puromycin control pools and in MKN45/PGK1pools #1–#3. Data are expressed as means of the control pools #1–#3 and MKN45/PGK1pools #1–#3 and SD (*p<0.05) (B), MKN45/PGK1pool#3 cells were treated with isotype control or anti-CXCR4 antibody. (A), (B), Representative photomicrographs of the results of the invasion assays are depicted.
Overexpression of PGK1 by immunohistochemistry in human diffuse gastric cancer
To further explore PGK1 expression in gastric cancer, immunohistochemical staining of diffuse primary gastric cancer specimens from 10 patients was performed. Peritoneal carcinomatosis was histologically confirmed in 6 of the 10 patients at the time of surgery. Five of these patients with peritoneal carcinomatosis showed a medium to strong nuclear staining for PGK1, whereas cytoplasmic staining was less intense or absent. Only in one sample where peritoneal carcinomatosis was histologically confirmed, a weak staining in a very few cells was detected. In patients where no peritoneal carcinomatosis was histologically confirmed, all of the samples showed only a weak or no staining for PGK1 (Fig. 4).
Figure 4. PGK1 overexpression in human diffuse gastric cancer with peritoneal carcinomatosis.
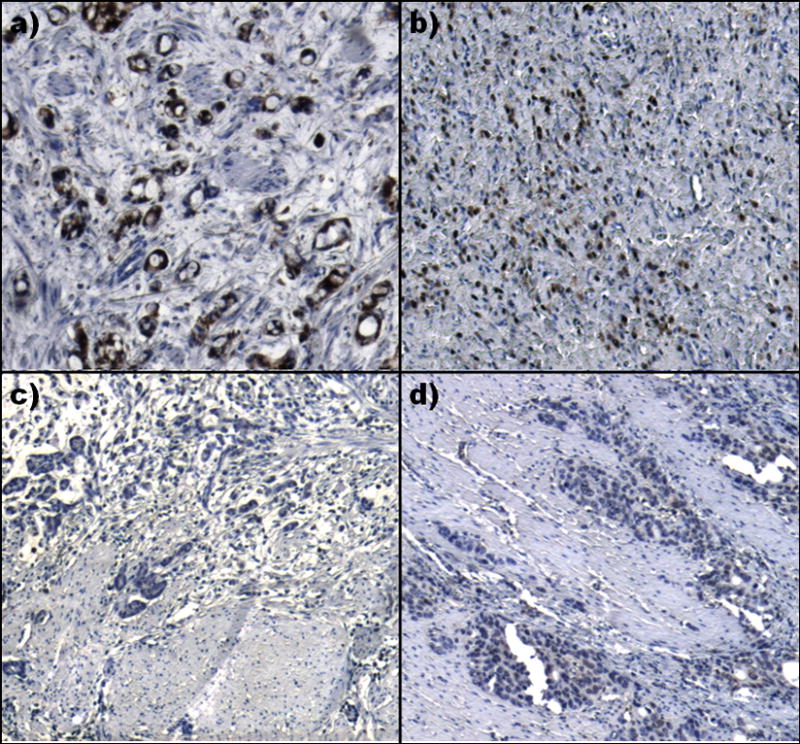
Immunohistochemical staining for PGK1 in diffuse gastric cancer patients with (a and b) and without (c and d) peritoneal carcinomatosis. a) and b) show a strong staining in most of the tumor cells whereas c) and d) reveal only a weak or no staining for PGK1. All images are 50-times magnified.
Discussion
In this investigation the relationship between tumorigenesis, glycolytic metabolism, and chemokine signaling was examined. Using siRNA targeting PGK1 in a gastric cancer cell line, we discovered that the expression of the chemokine receptor CXCR4 and the Wnt- pathway member β-catenin were linked to PGK1 expression. Flow cytometry confirmed the regulation of CXCR4 and β-catenin on protein level. Similarly, when CXCR4 was targeted by siRNA, PGK1 expression was diminished. Hence, PGK1 and CXCR4 are able to regulate each other in a co-stimulating way. Likewise, assuming that elevated levels of PGK1 are crucial for peritoneal dissemination in gastric cancer, the effects of overexpression of PGK1 were further evaluated. PGK1 overexpression in gastric cancer cells resulted in significantly elevated mRNA expression and protein levels of CXCR4 and β-catenin. In vitro invasion assays underlined these findings showing that cells invaded more dramatically when PGK1 was overexpressed. Blocking CXCR4 in the invasion assay revealed only a moderate inhibition of invading cells. Performing immunohistochemical staining of diffuse human primary gastric cancer specimens, we further detected that patients with peritoneal dissemination showed a moderate to stronger staining compared to the primary tumors without peritoneal dissemination. These results lead to two conclusions:
First, there is a direct connection between PGK1 signaling and CXCR4 and β-catenin expression. Second, overexpression of PGK1 leads to signaling through CXCR4 and perhaps through other unknown pathways resulting in changes in the metastatic rate, invasiveness and tumor growth in gastric cancer.
Furthermore it is known that results obtained using this kind of assay show a strong correlation between the ability of tumor cells to invade in vitro and their invasive behavior in vivo, which validates this assay as an index for invasive potential. Together, these data suggest that overexpression of PGK1 enhances gastric cancer cells to invade and metastasize in a CXCR4/CXCL12-dependent and furthermore particularly independent way. Advanced gastric cancer is often associated with metastasis and peritoneal carcinomatosis. Although the reasons for dissemination and invasiveness are not clear, several mechanisms may account for the complex phenomenon. One recently reported mechanism associated with peritoneal carcinomatosis was that elevated levels of CXCR4 were detected in gastric tumors combined with high concentrations of CXCL12 in malignant ascites fluids of these patients 2. It is assumed that CXCR4 positive cells are attracted along a chemotactic gradient to organs expressing its ligand CXCL12 16. Further CXCL12-expressing cancer cells may protect themselves from a cytotoxic immune response via induction of apoptosis in cytotoxic T cells and inducing a local Th1→Th2 shift in the tumor area 15,17,18. A second mechanism of cell proliferation, angiogenesis, tumor invasion and metastasis in gastric cancer is mediated through β-catenin 4,20,21. A third and novel mechanism is supported by the PGK1 data. Our previous work showed that patients with gastric cancer developing peritoneal carcinomatosis, compared to patients without further dissemination, displayed much higher mRNA levels of CXCR4/CXCL12, β-catenin and the glycolytic enzyme PGK1 4. Our new data concerning PGK1 and its regulatory effects, even though based on protein levels rather than activity per se, provide comprehensive evidence of the “Warburg effect”. In the 1950s Warburg quantified the impaired mitochondrial function and increased dependence on glycolysis as a source of energy, using ascitic cancer cells 24. Meanwhile it is largely accepted and shown that solid tumor cells employ glycolytic enzymes, such as PGK1, to produce ATP when their supply of oxygen is limited 6. PGK1 is known to be involved in the onset of several malignancies, such as breast, pancreatic and ovarian cancer 7–9. A significant association between PGK1 expression and the CXCR4/CXCL12 axis was recently reported in prostate cancer cell lines, where overexpression of PGK1 increased the metastatic rate 5. One of our most striking findings was that PGK1 seems to be a central molecule concerning tumor invasion and metastasis in gastric cancer (Fig. 5). Overexpression of PGK1 resulted in significantly elevated expression and protein levels of CXCR4 and β-catenin and enhanced invasiveness in Matrigel™ assays. High levels of CXCL12, via CXCR4 overexpression, might protect cancer cells (as already described) from immune responses and elevated CXCR4 levels multiplying the metastatic process 15. On the other hand, there is evidence, that low levels of extracellular CXCL12 may augment metastatic potential towards environments with higher levels of CXCL12 16. Since PGK1 was shown to regulate β-catenin in prostate cancer, it is possible that an enhanced expression of PGK1 is able to decrease cancer cell-cell-adhesion linked to Wnt signaling and thus increase cell migration 5. Those results previously obtained are underlined by our recent findings in human gastric cancer patients 4. In summary, the results presented here show that high levels of PGK1 are essential for tumor growth and metastasis. These data further show a direct relationship between PGK1 signaling CXCR4 and β-catenin expression and represent a promising pathway concerning peritoneal carcinomatosis and metastasis in gastric cancer. These findings support the comprehension between glucose metabolism and chemokine function and might serve as potential therapeutic targets to prevent metastasis of gastric carcinoma cells.
Figure 5. Pathway-model: PGK1 a promoter for peritoneal cancer dissemination.
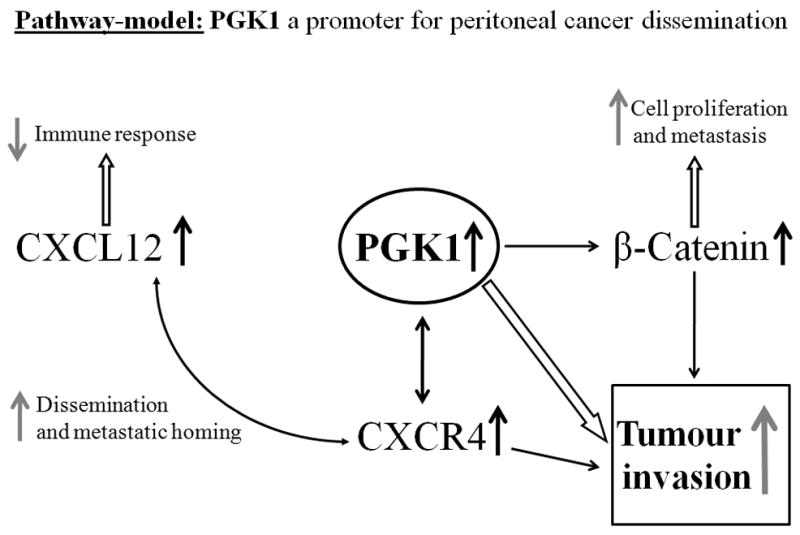
The figure represents a potential model of peritoneal dissemination in gastric cancer, via PGK1 signaling. PGK 1 overexpression leads to increased CXCR4/CXCL12 signaling which supports metastatic homing and tumor dissemination. Enhanced PGK1 expression influences tumor invasion by elevated CXCR4 and β-catenin levels. CXCR4 is reciprocally regulated by PGK1 and overexpression of CXCR4 allows a link to CXCL12 signaling and thus immune evasion mechanisms apart from direct influences. β-catenin furthermore exhibits its function in cell proliferation and metastasis. Therefore PGK1 might be a crucial enzyme in peritoneal dissemination and metastasis.
Acknowledgments
The authors gratefully acknowledge the input and contributions to the present study made by the participating technologists and staff, including Melanie Hauth, Matthias Wingert and Asghar Abbasi. This work was supported by the National Cancer Institute PO1 award CA93900 (R.S. Taichman), Department of Defense Awards PC060857 and PC073952 (R.S. Taichman).
Abbreviations in the text
- ATP
Adenosine triphosphate
- CXCR4
chemokine receptor 4
- CXCL12
chemokine ligand 12
- MKN45
gastric adenocarcinoma cell line
- mAb
monoclonal Antibody
- NIH 3T3
mouse embryonic fibroblast cell line
- PGK1
phosphoglycerate kinase 1
- qRT-PCR
quantitative real time PCR
- SDF1
stromal-derived-factor-1
- SFI
specific fluorescence index
- siRNA
small interfering RNA
Footnotes
No prior or Subsequent Publication: This manuscript contains original material that has neither been published or submitted to any other journal nor any similar paper in whole or in part has been or will be submitted or published in any other scientific journal while under your consideration.
Conflict of Interests: The authors declare no competing interests in any means or any conflicting financial interests.
Statement: PGK1 was proven to have regulating impacts on CXCR4, CXCL12 and β-catenin, furthermore PGK1 overexpression leads to more malignant phenotypes in gastric cancer. These mechanisms could be of crucial importance for peritoneal dissemination in gastric cancer.
Disclosure/Conflict of Interest
The authors declare no conflict of interest or conflicting financial interests.
Reference List
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi Y, Yoshie O, Saiki I. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181–7. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- 3.Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62. doi: 10.1016/s0002-9610(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 4.Zieker D, Konigsrainer I, Traub F, Nieselt K, Knapp B, Schillinger C, Stirnkorb C, Fend F, Northoff H, Kupka S, Brucher BL, Konigsrainer A. PGK1 a potential marker for peritoneal dissemination in gastric cancer. Cell Physiol Biochem. 2008;21:429–36. doi: 10.1159/000129635. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Wang J, Dai J, Jung Y, Wei CL, Wang Y, Havens AM, Hogg PJ, Keller ET, Pienta KJ, Nor JE, Wang CY, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67:149–59. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 6.Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta. 2004;1691:17–22. doi: 10.1016/j.bbamcr.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Duan Z, Lamendola DE, Yusuf RZ, Penson RT, Preffer FI, Seiden MV. Overexpression of human phosphoglycerate kinase 1 (PGK1) induces a multidrug resistance phenotype. Anticancer Res. 2002;22:1933–41. [PubMed] [Google Scholar]
- 8.Hwang TL, Liang Y, Chien KY, Yu JS. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259–72. doi: 10.1002/pmic.200500345. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics. 2005;4:1686–96. doi: 10.1074/mcp.M400221-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–15. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 11.Mosadegh B, Saadi W, Wang SJ, Jeon NL. Epidermal growth factor promotes breast cancer cell chemotaxis in CXCL12 gradients. Biotechnol Bioeng. 2008;100:1205–13. doi: 10.1002/bit.21851. [DOI] [PubMed] [Google Scholar]
- 12.Oonakahara K, Matsuyama W, Higashimoto I, Kawabata M, Arimura K, Osame M. Stromal-derived factor-1alpha/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol. 2004;30:671–7. doi: 10.1165/rcmb.2003-0340OC. [DOI] [PubMed] [Google Scholar]
- 13.Saur D, Seidler B, Schneider G, Algul H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–50. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 15.Schimanski CC, Galle PR, Moehler M. Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol. 2008;14:4721–4. doi: 10.3748/wjg.14.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–71. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 17.Colamussi ML, Secchiero P, Gonelli A, Marchisio M, Zauli G, Capitani S. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol. 2001;69:263–70. [PubMed] [Google Scholar]
- 18.Dunussi-Joannopoulos K, Zuberek K, Runyon K, Hawley RG, Wong A, Erickson J, Herrmann S, Leonard JP. Efficacious immunomodulatory activity of the chemokine stromal cell-derived factor 1 (SDF-1): local secretion of SDF-1 at the tumor site serves as T-cell chemoattractant and mediates T-cell-dependent antitumor responses. Blood. 2002;100:1551–8. [PubMed] [Google Scholar]
- 19.Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002;277:11336–44. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- 20.Lowy AM, Clements WM, Bishop J, Kong L, Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C, Groden J. beta-Catenin/Wnt signaling regulates expression of the membrane type 3 matrix metalloproteinase in gastric cancer. Cancer Res. 2006;66:4734–41. doi: 10.1158/0008-5472.CAN-05-4268. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761–6. [PubMed] [Google Scholar]
- 22.Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun. 1998;252:368–72. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.WARBURG O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]