Abstract
Cancer cells often acquire a constitutively active nuclear factor-κB (NF-κB) program to promote survival, proliferation and metastatic potential by mechanisms that remain largely unknown. Extending observations from an immunologic setting, we demonstrate that microRNA-146a and microRNA-146b (miR-146a/b) when expressed in the highly metastatic human breast cancer cell line MDA-MB-231 function to negatively regulate NF-κB activity. Lentiviral-mediated expression of miR-146a/b significantly downregulated interleukin (IL)-1 receptor-associated kinase and TNF receptor-associated factor 6, two key adaptor/scaffold proteins in the IL-1 and Toll-like receptor signaling pathway, known to positively regulate NF-κB activity. Impaired NF-κB activity was evident from reduced phosphorylation of the NF-κB inhibitor IκBα, reduced NF-κB DNA-binding activity and suppressed expression of the NF-κB target genes IL-8, IL-6 and matrix metalloproteinase-9. Functionally, miR-146a/b-expressing MDA-MB-231 cells showed markedly impaired invasion and migration capacity relative to control cells. These findings implicate miR-146a/b as a negative regulator of constitutive NF-κB activity in a breast cancer setting and suggest that modulating miR-146a/b levels has therapeutic potential to suppress breast cancer metastases.
Keywords: NF-κB, miRNA-146, metastatic breast cancer
All unstimulated normal cell types, with the exception of B cells, maintain members of the nuclear factor-κB (NF-κB) family of transcription factors sequestered within inactive cytoplasmic complexes by the IκB inhibitor proteins (Bonizzi et al., 2004; Hoffmann and Baltimore, 2006). NF-κB-activating signals, classically originating from members of the interleukin (IL)-1, Toll-like or tumor-necrosis factor receptor superfamily, promote the phosphorylation and proteasome-mediated degradation of IkB proteins, facilitating the release and nuclear translocation of NF-κB. This activation of NF-κB is typically transient and limited in magnitude by numerous negative feedback loops (Akira and Takeda, 2004; Bonizzi et al., 2004; Hayden and Ghosh, 2004; Hoffmann and Baltimore, 2006). However, it has been realized for some time that many cancers have acquired a constitutively active NF-κB program, functioning to promote such features as their proliferation, survival, angiogenesis and metastasis (Karin, 2006; Van Waes, 2007; Sethi et al., 2008).
The mechanisms underlying constitutive NF-κB activity in cancer cells remain poorly understood, although the signal transduction pathway producing classical NF-κB activation has been extensively studied (Bonizzi et al., 2004; Hoffmann and Baltimore, 2006; Van Waes, 2007; Sethi et al., 2008). In this regard, IRAK1 (IL-1 receptor-associated kinase) and TRAF6 (tumour-necrosis factor receptor-associated factor 6), two linearly linked adaptor/scaffold components coupling signals originating from the IL-1 and the Toll-like receptor superfamily to NF-κB activation (Cao et al., 1996a, b; Taganov et al., 2007), were recently proposed to be targeted by microRNA-146a and microRNA-146b (miR-146a/b) as part of an NF-κB-induced negative feedback loop in stimulated monocytes (Taganov et al., 2006, 2007; Sonkoly et al., 2008). In addition, a recent study has implicated the loss of miR-146a expression in the progression of hormone-refractory prostate cancer (Lin et al., 2008). Given the ability of microRNAs (miRs) to orchestrate cellular functions by modulating the level of their targeted proteins by either translational arrest or transcript degradation, a capacity often deregulated in cancers by aberrant miR expression (Bartel, 2004; Karres et al., 2007; Ma et al., 2007), we were prompted to evaluate the consequences of miR-146a/b expression in human breast cancer cells where constitutive NF-κB activity has been associated with aggressive breast cancer clinical behavior (Zhou et al., 2005).
The highly metastatic breast cancer cell line MDA-MB-231 with its constitutively active NF-κB program was infected with miR-146a, miR-146b and control viruses following lentivirus production as previously described (Coppe et al., 2006). Viral titers were adjusted to yield similar expression levels of the two miRs, as judged by northern analysis shown in Figure 1a. The miR-146b probe showed some cross-hybridization to miR-146a (Figure 1a, right panel) as expected from their sequences (Taganov et al., 2006). The lentiviruses expressed 10- to 30-fold higher levels of miR-146a or miR-146b relative to the low endogenous level of miR-146a/b in control infected cells.
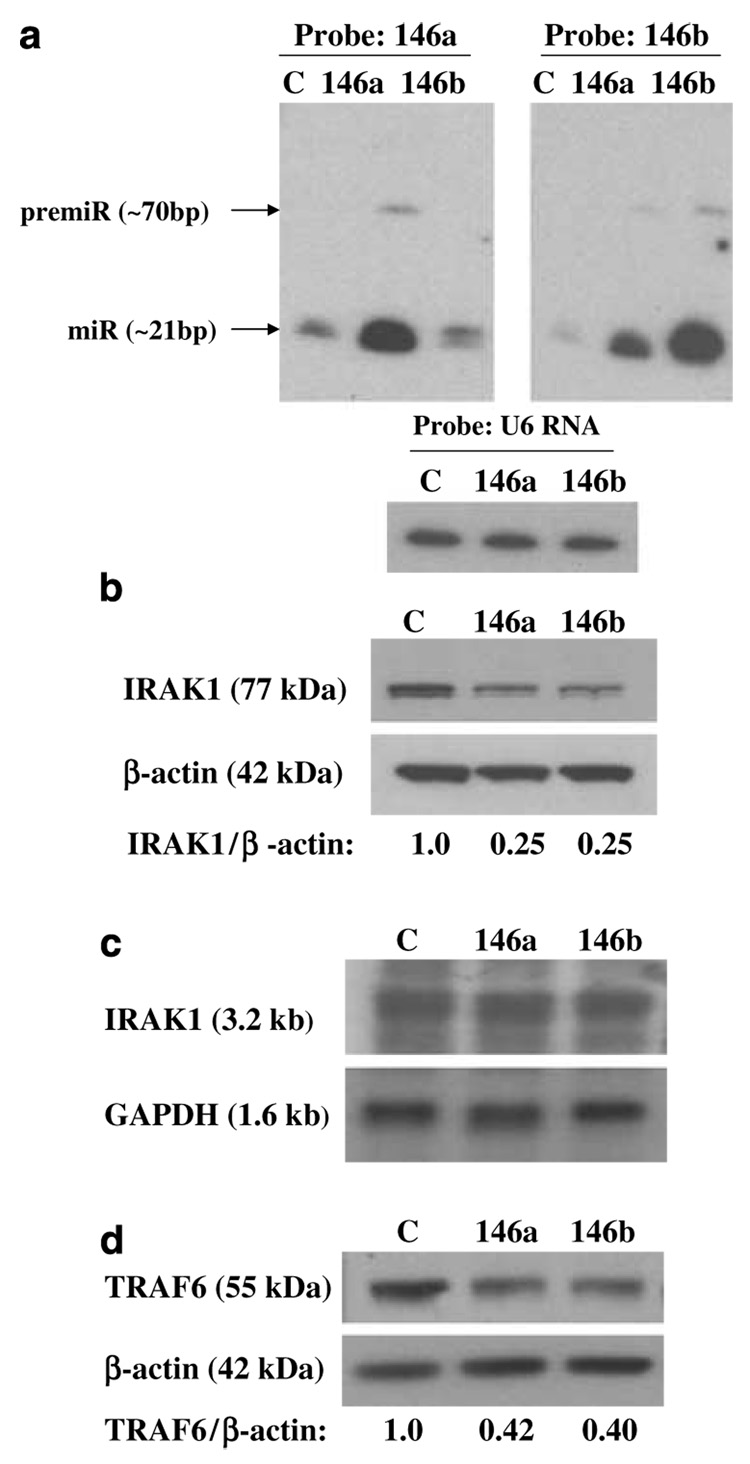
Figure 1. IRAK1 and TRAF6 protein levels are reduced in MDA-MB-231 cells overexpressing miRNA-146a and miR-146b.
(a) Northern blot of total RNA prepared from control MDA-MB-231 cells (C), miR-146a-expressing MDA-MB-231 cells (146a) and miR-146b-expressing MDA-MB-231 cells (146b) was probed with an antisense miR-146b DNA oligo (right panel), stripped and reprobed with an antisense miR-146a DNA oligo (left panel). Northern blot was performed as previously described (Scott et al., 2007). Mature and pre-miRNA species are noted. RNA loading was confirmed by probing for the small RNA species, U6 RNA (bottom panel). The lentiviral miR-146a/b expression vectors were constructed by cloning 146 bp of pri miR-146a sequence and 120 bp of pri miR-146b sequence that included the terminal loop, the upper stem and the lower stem with 10 bp of upstream/downstream flanking genomic sequence into a site under cytomegalovirus promoter control. (b) Western blot of total protein lysates prepared from MDA-MB-231 cell pools as described in Figure 1a was probed for IRAK1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The ratio of IRAK1 to β-actin band intensity for each lysate was normalized to the control ratio as shown below the western panel. (c) Northern blot using total RNA isolated from MDA-MB-231 cells as described in (a) was probed for IRAK1. Reprobing the blot for GAPDH established equal RNA loading. The IRAK1 3.2-kb transcript size was deduced from the ethidium-stained gel 18S (1.9 kb) and 28S (5.0 kb) bands. (d) Western blot of total protein lysates as described in (b) was probed for TRAF6 (sc-7221; Santa Cruz Biotechnology). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IRAK1, IL-1 receptor-associated kinase; miRNA, microRNA; TRAF6, tumour-necrosis factor receptor-associated factor 6.
Evaluating the impact of ectopically expressed miR-146a and miR-146b on IRAK1 protein levels, Figure 1b shows that miR-146a/b-expressing cells had IRAK1 levels reduced to approximately 25% of control levels. To determine whether this reduced IRAK1 protein level was accompanied by a corresponding suppression of IRAK1 mRNA, northern analysis was performed. As shown in Figure 1c, miR-146a/b-expressing and control MDA-MB-231 cells had essentially identical IRAK1 mRNA levels, suggesting that miR-146a/b suppressed IRAK1 mRNA translation but did not promote IRAK1 mRNA decay in these cells. The miR-146a/b expression also suppressed TRAF6 protein levels, although to a lesser extent (~40%) than IRAK1 protein suppression (Figure 1d).
As IRAK1 and TRAF6are essential components of the Toll-like and IL-1 receptor signaling networks that activate NF-κB, the consequences of their suppression by miR-146a/b in MDA-MB-231 cells were examined. Phosphorylation of IκBα is a key measure of NF-κB activation. As shown in Figure 2a, although total protein levels of both IκBα and the NF-κB p65/Rel A component were similar between miR-146a/b-expressing and control MDA-MB-231 cells, phosphorylation of IκBα on serine 32, which is essential for its degradation, was reduced to approximately 40% and 20% of control levels in miR-146a and miR-146b cells, respectively.
Figure 2. MDA-MB-231 cells overexpressing miR-146a/b have reduced phosphorylation of the NF-κB inhibitor IκBα and reduced NF-κB DNA-binding activity.
(a) Western blot of total protein lysates prepared from MDA-MB-231 cell pools as described in Figure 1a was probed for phosphorylated IκBα protein (Ser 32; Cell Signaling Technology, Danvers, MA, USA), stripped and reprobed for total IκBα protein (top panel). The ratio of phosphorylated band intensity to total band intensity was normalized as in Figure 1b and shown below the western panel. Western blot in the lower panel shows that levels of NF-κB (65 kDa) are equal in control cells and miR-146a/b-expressing cells relative to β-actin. (b) EMSA for binding to a consensus NF-κB DNA oligo probe using control (C), miR-146a (146a) and miR-146b (146b) nuclear extracts loaded in duplicate lanes (left panel). The position of the gel origin, NF-κB gel shifted band and free probe are indicated. Right panel shows specificity of NF-κB DNA binding as antibodies to the p65 and p50 subunits of NF-κB supershifted the NF-κB band (p65 Ab and p50 Ab lanes) whereas a control antibody (con Ab, an estrogen receptor antibody) had no effect. Bottom panel: western blot of nuclear extracts used for EMSA probed for HDAC2 (Santa Cruz Biotechnology) shows equal protein content of the extracts. HDAC2, histone deacetylase 2; miR, microRNA; NF-κB, nuclear factor-κB; EMSA, electrophoretic gel shift assays.
To assess NF-κB transcriptional activity, nuclear extracts prepared from miR-146a/b-expressing and control cells were measured for NF-κB binding to a consensus NF-κB response element by electrophoretic mobility shift assays. Control nuclear extracts possessed approximately three- to four-fold greater NF-κB DNA-binding activity compared to nuclear extracts from miR-146a/b-expressing cells (Figure 2b, left panel). The identity of the shifted band as the p65/p50 heterodimer, the predominate form of the dimeric NF-κB transcriptional complex, was confirmed by antibodies to these proteins that supershifted the NF-κB band relative to a control antibody that had no influence on the band mobility (Figure 2b, right panel). To confirm that the nuclear extracts contained equal amounts of protein, they were analysed by western blotting for histone deacetylase 2, an abundant and salt-extractable nuclear protein (Figure 2b, bottom panel). Taken together, these results indicate that miR-146a/b expression significantly reduced NF-κB activity in these human breast cancer cells.
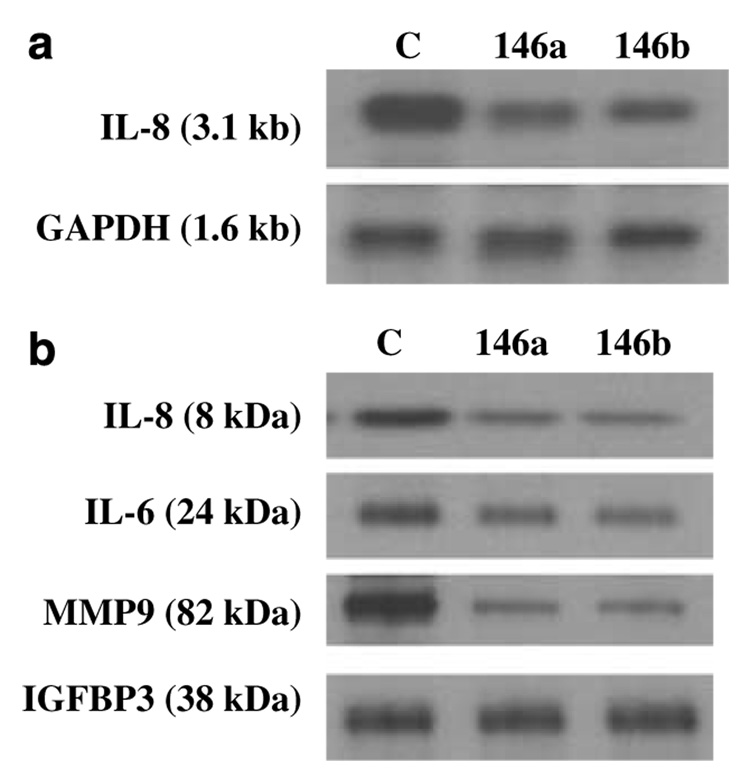
Consequences of the reduced level of NF-κB activity in miR-146a/b-expressing MDA-MB-231 cells were assessed by determining the expression level of NF-κB target genes contributing to the aggressive phenotype of these cells. As shown in Figure 3a, northern analysis of IL-8, a cytokine upregulated in many aggressive breast cancer cells (Benoy et al., 2004), demonstrated a significant mRNA reduction (~70%) in miR-146a/b-expressing MDA-MB-231 cells compared to control cells. Examination of conditioned medium by western blotting showed that miR-146a/b expression reduced secreted IL-8 levels by ~75% relative to control levels (Figure 3b). Likewise, western analysis of conditioned media for NF-κB responsive proteins IL-6 and the metalloproteinase MMP-9 demonstrated significantly reduced expression in miR-146a/b-expressing cells relative to control cells, as shown in Figure 3b. Equal loading was established by normalization to IGFBP3 levels, a secreted protein with no reported dependence on NF-κB activation.
Figure 3. IL-8 mRNA level together with IL-8, IL-6and MMP-9 protein levels are downregulated in cells overexpressing miR-146a/b.
(a) Northern blot prepared as described in Figure 1a was probed for IL-8. RNA loading was established by probing the blot with GAPDH. (b) Western blot using TCA-precipitated proteins from conditioned media containing 0.2% serum collected over 24 h was probed for IL-8 (R&D Systems, Minneapolis MN, USA), IL-6 (R&D Systems), MMP-9 (NeoMarkers, Fremont, CA, USA) and IGFBP3 (Santa Cruz Biotechnology). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; miR, microRNA; MMP-9, matrix metalloproteinase 9; TCA, trichloroacetic acid; IGFBP3, insulin like growth factor binding protein-3.
Given that NF-κB activity is known to enhance the survival and metastatic potential of cancer cells (Karin, 2006; Van Waes, 2007; Sethi et al., 2008), the functional consequences of NF-κB suppression in miR-146a/b-expressing MDA-MB-231 cells were explored using cell motility and Matrigel invasion assays under conditions of serum starvation. Typical fields from the Matrigel invasion assays, shown in Figure 4a, demonstrate that the ability to invade a basement membrane was significantly compromised in the miR-146a and miR-146b cells relative to control cells. Quantifying these results, it was shown that the miR-146a- and miR-146b-overexpressing cells respectively possessed only 25% and 20% invasion capacity relative to control cells (Figure 4b). In a migration assay experiment, miR-146a/b-expressing cells displayed only 45% and 38% of control migration capacity, respectively (Figure 4c). Interestingly, although compromised in their motility and invasiveness, the miR-146a/b-overexpressing cells exhibited no proliferation impairment or apoptosis sensitivity relative to control cells (data not shown), a result consistent with previous studies demonstrating that NF-κB activation does little to alter the proliferative or apoptotic index of MDA-MB-231 cells (Monks and Pardee, 2006; Zheng et al., 2006).
Figure 4. MDA-MB-231 cells overexpressing miR-146a/b have significantly reduced capacity to invade through Matrigel and migrate.
(a) Typical field of a Matrigel invasion insert showing the reduced number of invading miR-146a- and miR-146b-expressing cells compared to control cells (8 µm BioCoat Growth Factor Reduced Matrigel Invasion Chambers; BD Biosciences, San Jose, CA, USA). Cells were plated in triplicate at 1 × 105 cells per insert in DMEM media with 10 ng/ml recombinant EGF in DMEM media used as the chemoattractant in the lower chambers for both invasion and migration assays. Following 20 h of incubation, inserts were processed according to the manufacturer’s recommendations using 1% Toluidine Blue. Stained membranes were photographed using an Olympus I × 70 microscope at × 70. b Quantification of invasion achieved by the miR-146a and miR-146b cells as a percentage of that achieved by control cells. Cell counts were determined from the average of five random fields (each field capturing approximately 6% of total membrane area). (c) Quantification of migration through 8-µm pore inserts (BD Biosciences) by miR-146a- and miR-146b-expressing cells as a percentage of that achieved by control cells. All cell-invasion and migration assays were repeated in three independent experiments using the lentiviral-infected miR-146a, miR-146b and control cell pools. DMEM, Dulbecco’s modified Eagle’s medium; miR, microRNA; EGF, epidermal growth factor.
The human breast cancer cell line MDA-MB-231 has been extensively used to study essential components of the metastatic process (Sliva et al., 2002; Cicek et al., 2005; Monks and Pardee, 2006; Zheng et al., 2006). Results presented here demonstrate that ectopic expression of miR-146a/b in this cell line sharply curtailed NF-κB activity and significantly reduced its ability to invade a basement membrane-like extracellular matrix, a key event in cancer metastasis. Although this observed reduction in MDA-MB invasiveness likely derives in large part from miR-146a/b-targeted suppression of the upstream NF-κB signaling proteins IRAK1 and TRAF6, suppression of additional miR-146a/b targets, reflecting the well-known pleiotropic nature of miRs (Bartel, 2004), would be anticipated to contribute to this altered metastatic phenotype. Preliminary studies using IkB-α siRNA to suppress a proximal negative regulator of NF-κB activity demonstrated that while restoring the invasiveness of miR-146-treated cells from 30% to approximately 65% of control levels (data not shown), complete restoration of this metastatic property was not realized suggesting that other signaling pathways contributing to cell invasion are also impacted by miR-146. In this regard, it is noteworthy that epidermal growth factor receptor (EGFR), as a predicted target of miR-146 (miRGen database), coupled with the use of 10 ng/ml EGF as chemoattractant in the cell-invasion assays, is such a candidate pathway potentially impacted by miR-146 expression in MDA-MB-231 cells.
Given the multiplicity of signals the aberrant regulation of which could provide a cancer cell with constitutively active NF-κB, it was perhaps surprising that expression of miR-146a/b in the highly metastatic human breast cancer cell line MDA-MB-231 produced suppression of NF-κB activity, although many tumor cells express IL-1 and Toll-like receptors (Singer et al., 2003; Merrell et al., 2006) and are thus potentially vulnerable to IRAK1 and TRAF6 suppression. MDA-MB-231 cells, in particular, have been shown to express the IL-1 receptor as well as Toll-like receptors 4 and 9 (Singer et al., 2003; Merrell et al., 2006). Future studies evaluating the susceptibility of various other cancer cell lines and tumors with constitutively active NF-κB programs to modulation by miR-146a/b expression will likely provide deeper insights and therapeutic opportunities for treating metastatic disease, a problem that has remained largely intractable.
Acknowledgements
This work was supported in part by NIH grants R01-CA36773, P01-AG025901, P50-CA58207 and R37-AG09909 as well as Hazel P Munroe memorial funding to the Buck Institute.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996a;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6is a signal transducer for interleukin-1. Nature. 1996b;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Cicek M, Fukuyama R, Welch DR, Sizemore N, Casey G. Breast cancer metastasis suppressor 1 inhibits gene expression by targeting nuclear factor-kappaB activity. Cancer Res. 2005;65:3586–3595. doi: 10.1158/0008-5472.CAN-04-3139. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- Monks NR, Pardee AB. Targeting the NF-kappaB pathway in estrogen receptor negative MDA-MB-231 breast cancer cells using small inhibitory RNAs. J Cell Biochem. 2006;98:221–233. doi: 10.1002/jcb.20789. [DOI] [PubMed] [Google Scholar]
- Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem. 2007;282:1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- Sethi G, Sung B, Aggarwal BB. Nuclear factor-{kappa}B activation: from bench to bedside. Exp Biol Med. 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, et al. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res. 2003;9:4877–4883. [PubMed] [Google Scholar]
- Sliva D, Rizzo MT, English D. Phosphatidylinositol 3-kinase and NF-kappaB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J Biol Chem. 2002;277:3150–3157. doi: 10.1074/jbc.M109579200. [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Rodrik V, Toschi A, Shi M, Hui L, Shen Y, et al. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J Biol Chem. 2006;281:15862–15868. doi: 10.1074/jbc.M600660200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12 Suppl 1:S37–S46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]