Abstract
Treatment of vancomycin-resistant Enterococcus (VRE) infections is limited by the paucity of effective antibiotics. Administration of broad-spectrum antibiotics promotes VRE colonization by down-regulating homeostatic innate immune defenses. Intestinal epithelial cells and Paneth cells express antimicrobial factors upon direct or indirect stimulation of the Toll-like receptor (TLR)-MyD88-mediated pathway by microbe-derived molecules. Here, we demonstrate that the TLR5 agonist flagellin restores antibiotic-impaired innate immune defenses and restricts colonization with VRE. Flagellin stimulates the expression of RegIIIγ, a secreted C-type lectin that kills Gram-positive bacteria, including VRE. Systemic administration of flagellin induces RegIIIγ expression in intestinal epithelial cells and Paneth cells along the entire length of the small intestine. Induction of RegIIIγ requires TLR5 expression in hematopoietic cells and is dependent on IL-22 expression. Systemic administration of flagellin to antibiotic-treated mice dramatically reduces VRE colonization. By enhancing mucosal resistance to multi-drug resistant organisms, flagellin administration may provide a clinically useful approach to prevent infections in patients treated with broad-spectrum antibiotics.
Introduction
Vancomycin-resistant Enterococcus (VRE) infections have increased at an alarming rate in the last two decades [1, 2]. Infection with VRE is associated with increased mortality, healthcare costs, and hospital stays and, thus, places significant burdens on the healthcare system [3]. Exposure to certain antibiotics leads to increased risk for VRE colonization and infection [4-7]. Although the reason for this association has been cited as reduced competition for colonization due to the depletion of commensal bacteria during antibiotic treatment, additional mechanisms have also been implicated. Recent studies demonstrate that the intestinal microbial flora plays a critical role in regulating mucosal innate immunity [8-12]. Antimicrobial proteins (AMPs) produced by the intestinal epithelium are effector molecules that provide a first-line of defense against invading commensal and pathogenic organisms. Intestinal epithelial cells and Paneth cells directly respond to bacterial products via TLR-MyD88-mediated pathways by secreting AMPs to kill bacteria [9, 13-15]. In addition, cells of the adaptive and innate immune system produce cytokines, such as IL-22, that regulate the expression of AMPs by intestinal epithelial cells [16].
The antimicrobial protein RegIIIγ is a secreted C-type lectin with bactericidal activity against Gram-positive bacteria and is induced by intestinal commensal bacteria [13]. We have previously demonstrated that RegIIIγ kills VRE and Listeria monocytogenes in the lumen of the small intestine [17, 18]. Neutralization of RegIIIγ activity with polyclonal antiserum specific to RegIIIγ increases VRE survival in the murine intestine. Antibiotic-mediated depletion of commensal bacteria dramatically reduces expression of RegIIIγ leading to increased susceptibility to VRE; however, the delivery of recombinant RegIIIγ to the intestinal lumen re-establishes VRE killing [18]. Furthermore, the loss of commensal flora can be compensated by oral administration of lipopolysaccharide (LPS), which restores RegIIIγ expression and, thus, promotes VRE clearance [18]. These studies establish the important role of RegIIIγ in limiting early stages of VRE colonization.
A major concern with therapeutic use of TLR agonists, in particular LPS, is the potential to initiate inflammation and sepsis. Unlike LPS, bacterial flagellin, the ligand for TLR5, induces little TNF-α, IL-1α, and RANTES and does not lead to acute lung injury and sepsis upon administration of moderate doses [19, 20]. Moreover, systemically administered flagellin stimulates the intestinal innate immune system and protects against destruction of the intestinal epithelium by radiation, chemicals, and invasive pathogens [19, 21]. Unlike most other TLR ligands, flagellin is a protein and, consequently, would likely be degraded upon oral ingestion. Thus, previous studies have administered flagellin systemically. Furthermore, TLR5 expression is limited to a subset of dendritic cells in the lamina propria [22] and to the basolateral surface of epithelial cells and, therefore, is not accessible to flagellin in the intestinal lumen [23].
Herein, we demonstrate that TLR5 activation restores innate immune deficits that follow antibiotic-mediated depletion of commensal bacteria. Flagellin potently induces RegIIIγ expression in intestinal epithelial cells and Paneth cells along the length of the small intestine. Activation of TLR5-expressing hematopoietic cells and expression of IL-22 are required for flagellin-mediated RegIIIγ induction. We show that systemically administered flagellin protects antibiotic-treated mice against VRE colonization. These results suggest that flagellin administration may have a therapeutic role in the prevention of antibiotic-associated intestinal infections.
Methods
Mice and bacteria
C57BL/6 mice (6–8 weeks old) and IL-10Rβ-deficient breeding pairs were purchased from Jackson Laboratories. TLR5-deficient mice and IL-22-deficient mice were provided by R. Flavell (Yale University, New Haven, CT) [24]. Mice were maintained in a specific pathogen-free barrier facility at Memorial Sloan-Kettering Cancer Center Research Animal Resource Center. Experiments followed approved institutional guidelines. Age-matched, sex-matched controls were used for all experiments. An ATCC isolate of vancomycin-resistant Enterococcus faecium (stock #700221) was used for oral infections.
Flagellin
For most experiments, commercially available flagellin (InVivoGen), derived from Salmonella typhimurium, was used. For the dose-response experiment, flagellin was also purified from S. typhimurium ATCC strain 15277 using a previously described procedure [25]. Contaminating LPS was removed from the flagellin preparation by serial passage through Detoxi-Gel AffinityPak Columns (Thermo Scientific). The concentration of LPS was determined to be less than 2 pg per μg of flagellin.
Treatment with antibiotics and flagellin
Drinking water was supplemented with 1 g/L of vancomycin (Sigma), 0.5 g/L of neomycin sulfate (Sigma), and 1 g/L of metronidazole (Baxter) and provided to mice for 7 days. Unless noted otherwise, flagellin was administered by intraperitoneal injection of 15 μg for 3 days starting on day 5 of antibiotic treatment. Mice were sacrificed 24 hours after the last flagellin injection.
Sample collection
For the duodenum, a 2 cm segment was excised 1 cm distal to the pylorus. For the jejunum, a 2 cm segment was collected from the exact middle of the small intestine measured longitudinally. For the ileum, a 2 cm segment located 1 cm proximal to the ileocecal valve was excised. The luminal content was flushed with PBS prior to tissue processing.
Real-time PCR and Western blot analysis
Freshly isolated tissue was homogenized in Trizol reagent (Invitrogen). The manufacturer’s protocol for total RNA and protein extraction using Trizol was followed. DNase-treated RNA was reverse transcribed using Oligo(dT) primers and SuperScript II Reverse Transcriptase (Invitrogen). Real-time PCR was performed with gene-specific Quantitect primer assays (Qiagen) and the DyNAmo SYBR Green qPCR Kit (Finnzymes). Signals were normalized to GAPDH mRNA transcript levels, and gene expression relative to MNV-treated controls was quantitated with ΔΔCt analysis unless noted otherwise.
The extracted protein was reconstituted in a buffer of 8 M urea, 1% SDS, 0.15 M Tris-HCl at pH 7.5. Identical amounts of protein were loaded on a 4 – 12% SDS-Page gel (Nupage Bis-Tris Gel;Invitrogen) and transferred to a nitrocellulose membrane. RegIIIγ and the loading control protein were detected by rabbit polyclonal RegIIIγ-specific antiserum and mouse anti-β-tubulin antibodies (Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated anti-rabbit (GE Healthcare) and anti-mouse antibodies (Santa Cruz Biotechnology). Immunostaining was revealed by chemiluminescence (GE Healthcare).
Immunohistochemistry
Freshly isolated tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Sections of 5 μm thickness were deparaffinized and immunohistochemically stained for RegIIIγ using the EnVision + System-HRP Kit (DakoCytomation). Polyclonal RegIIIγ-specific antiserum (1:1000) was diluted in PBS with 1% BSA for staining. Sections were counterstained with hematoxylin.
Generation of BM chimeric mice
WT (CD45.1) or TLR5-deficient (CD45.2) recipient mice were lethally irradiated with 950 rads. Irradiated mice were injected via tail vein with 5 × 106 bone marrow (BM) cells derived from WT or TLR-5-deficient donor mice. Chimerism was assessed in spleen and intestinal lamina propria 7 weeks after engraftment by FACS analysis at which time the mice were used for experiments. Lamina propria leukocytes were harvested according to a previously described protocol [26]. Percent reconstitution was assessed by surface staining with APC-labeled CD45.1-specific antibody and PerCP-Cy5.5-labeled CD45.2-specific antibody with flow cytometric analysis by BD LSR II (BD Biosciences). Mice exhibited at least 90% reconstitution with donor-derived BM cells in the intestinal lamina propria (data not shown).
VRE infection
Mice were infected with 1010 colony-forming units of VRE via oral gavage on day 7 of antibiotic treatment. Twenty-four hours later mice were sacrificed. The distal half of the small intestine was flushed with 10 mL of PBS to collect the luminal content for plating. The intestinal wall was homogenized in PBS containing 0.1% Triton X-100 for plating. Samples were plated in serial dilutions on Enterococcosel Agar (Difco) plates supplemented with 8 μg/mL vancomycin. Colonies surrounded by a brownish-black zone were counted.
Statistical Analysis
Prism software was used to perform the statistical analysis. P-values ≤ 0.05 were considered significant.
Results
Flagellin induces RegIIIγ expression in vivo
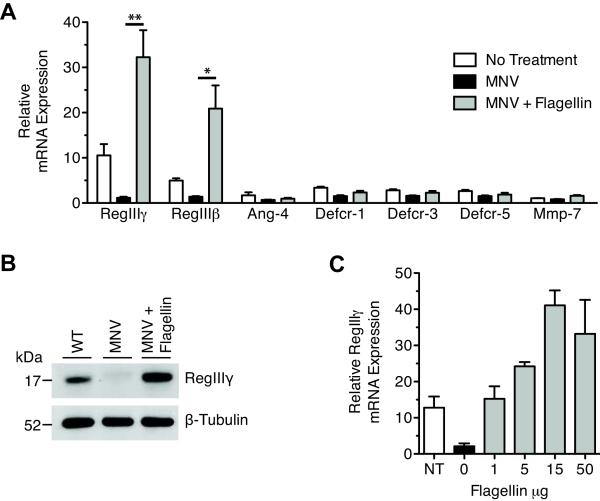
To determine whether flagellin can boost intestinal innate immune defense following broad-spectrum antibiotic administration, we measured mRNA levels of AMPs, previously shown to be regulated by commensal bacteria, in the distal small intestine of antibiotic-treated mice [13]. Mice were treated with a combination of metronidazole, neomycin, and vancomycin (MNV) in the drinking water. MNV-treated mice were given intraperitoneal injections of flagellin derived from Salmonella typhimurium, after which messenger RNA transcripts encoding AMPs were quantified in the small intestine. Quantitative PCR (qPCR) analysis showed a 30-fold induction of RegIIIγ mRNA transcripts in response to flagellin administration (Figure 1A). Western blot analysis also revealed substantial increases in RegIIIγ protein expression (Figure 1B). Similarly, mRNA levels of RegIIIβ, a RegIII family member that shares approximately 70% homology with RegIIIγ, was also strongly upregulated by flagellin (Figure 1A). Antibiotics or flagellin did not induce significant differences in the expression of other AMPs, including defensin related-cryptdins (Defcr), angiogenin-4 (Ang-4), and matrilysin (MMP-7) (Figure 1A). Because RegIIIγ directly mediates resistance to VRE infection, we focused on further characterization of the RegIIIγ response.
Figure 1.
Flagellin potently induces RegIIIγ and RegIIIβ in antibiotic-treated mice. A, Mice were treated with metronidazole, neomycin and vancomycin (MNV) for 7 days. Starting on day 5 of antibiotic treatment, mice received 15 μg of flagellin per day via intraperitoneal injection for 3 days. Mice were sacrificed 24 hours after the last flagellin injection. Transcriptional expression of RegIIIγ, RegIIIβ, Ang-4, Defcr-1, Defcr-3, Defcr-5, and MMP-7 was measured by quantitative real-time PCR. Expression levels were normalized to GAPDH and determined relative to MNV-treated mice. Values are representative of at least two experiments and are expressed as mean ± SEM (n=4–9, *p<0.05, **p<0.01, statistical analysis by one-way ANOVA with Bonferroni correction). B. Protein extracts from the distal ileum were analyzed by Western blotting with RegIIIγ-specific antiserum and anti-β-tubulin as a loading control. Shown are representative samples from two experiments (n = 6 for each group). C, On day 7 of MNV treatment, mice received 0, 1, 5, 15, or 50 μg of flagellin intraperitoneally. RegIIIγ mRNA message levels were measured 24 hours later by quantitative real-time PCR and were normalized to GAPDH (n=5 for each group).
To determine if flagellin-induced RegIIIγ expression is dose-dependent, we administered single doses of varying amounts of flagellin to MNV-treated mice. RegIIIγ mRNA levels were significantly increased at doses as low as 1 μg per mouse compared to MNV-treated mice receiving no flagellin. Induction of RegIIIγ by flagellin plateaued at a dose of 15 μg per mouse (Figure 1C).
Administration of flagellin alters the regional expression pattern of RegIIIγ in the small intestine
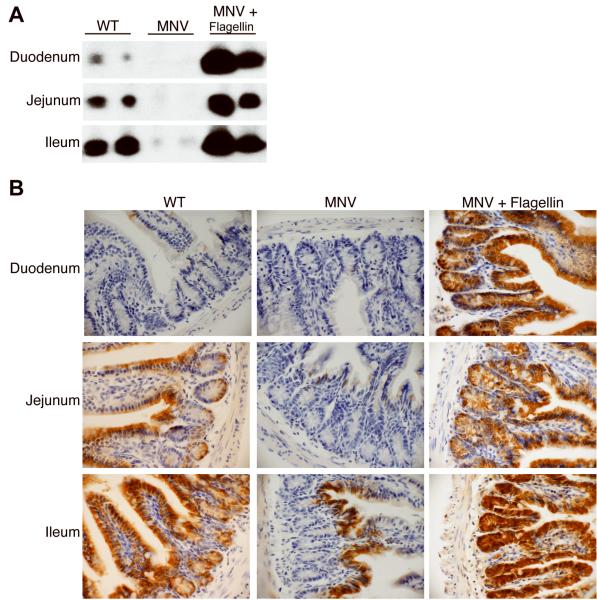
In the small intestine, the pattern of RegIIIγ expression, along with other AMPs, reflects the density of microbial colonization, which increases in the cephalocaudal direction [13]. Under normal circumstances, RegIIIγ expression increases along this axis with the highest expression in the distal ileum [13]. Because flagellin rapidly enters the blood stream when administered via intraperitoneal injection [27], we asked whether the systemic administration of flagellin to antibiotic-treated mice would alter the expression pattern of RegIIIγ in the small intestine. Western blot analysis of protein extracts from the duodenum, jejunum and ileum of MNV-treated mice showed that flagellin strongly upregulated RegIIIγ protein levels throughout the length of the small intestine (Figure 2A).
Figure 2.
Flagellin alters the regional expression of RegIIIγ in the small intestine. Mice were treated with metronidazole, neomycin and vancomycin (MNV) for 7 days, and on day 5, flagellin was given intraperitoneally at 15 μg per day for 3 days. Tissue from the duodenum, jejunum, and ileum were collected for Western blot analysis and immunohistochemistry. A, Protein extracts were analyzed by Western blotting using RegIIIγ-specific anti-serum. Each lane is a representative mouse from the indicated group. B, RegIIIγ was detected in paraffin-embedded tissue using immunohistochemistry with polyclonal RegIIIγ-specific antiserum. Positive cells were colorized brown (400-fold magnification). The data are representative of two independent experiments (n=6).
Immunohistochemical analysis of RegIIIγ protein expression revealed staining of epithelial cells and Paneth cells of the jejunum and ileum of wild-type mice; however, RegIIIγ was not detected in the duodenum (Figure 2B). In MNV-treated mice, RegIIIγ was absent from the Paneth cells throughout the length of the small intestine. Epithelial cells at the base of the villi in the ileum, but not within the intestinal crypts, continued to stain for RegIIIγ. Systemic administration of flagellin to MNV-treated mice induced RegIIIγ expression in epithelial cells and Paneth cells throughout the small intestine. Flagellin administration did not induce detectable inflammatory cell recruitment to the lamina propria of the small intestine, suggesting that RegIIIγ expression does not result from a general inflammatory response.
TLR5-expressing hematopoietic cells are required for induction of RegIIIγ by flagellin
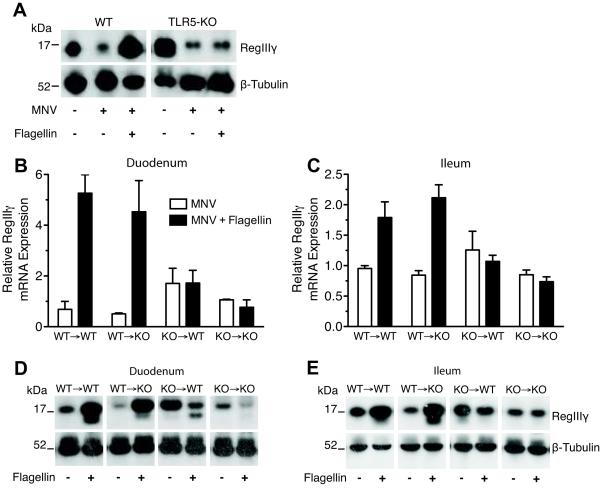
Because the flagellin used in these experiments was purified from Salmonella typhimurium, we needed to ensure that the response to flagellin is mediated by TLR5 and not by contaminating bacterial products. To address this possibility, we measured RegIIIγ protein levels in TLR5-deficient mice and wild-type (WT) control mice receiving MNV supplemented with flagellin injections. While WT controls treated with both MNV and flagellin showed an increase in RegIIIγ protein levels compared to MNV only-treated WT mice, MNV-treated TLR5-deficient mice did not increase RegIIIγ expression after flagellin administration (Figure 3A). RegIIIγ protein levels in TLR5-deficient mice were comparable to WT controls and were similarly downregulated upon antibiotic treatment.
Figure 3.
TLR5-mediated signaling is required in hematopoietic cells for flagellin-induced RegIIIγ expression. Mice were treated with metronidazole, neomycin, and vancomycin (MNV) for 7 days. After 5 days of MNV treatment, mice received 3 doses of 15 μg/day of flagellin via intraperitoneal injection. A, Western blot analysis of protein extracts from the distal ileum was used to assess differences between WT and KO mice using RegIIIγ-specific anti-serum. Each lane is a representative mouse from the indicated group. The data are representative of two independent experiments (n=5). B - E, bone marrow (BM) chimeric mice were treated with MNV and flagellin as described above approximately seven weeks after lethal irradiation and BM transfer. RegIIIγ mRNA levels were measured in the duodenum (B) and ileum (C) by quantitative real-time PCR, normalized to GAPDH, and expressed as mean ± SEM. RegIIIγ protein levels in the duodenum (D) and ileum (E) were assessed by Western blot analysis using RegIIIγ-specific anti-serum. Each lane of the Western blots is a representative mouse from the indicated group. Data are representative of two independent experiments (n=2–3).
In order to determine if intestinal epithelial cells are directly or indirectly activated by flagellin, we generated bone marrow (BM) chimeric mice by transferring BM from TLR5-deficient or WT mice into lethally irradiated WT or TLR5-deficient recipient mice. After mice were treated with MNV and flagellin as previously described, Western blot analysis and qPCR were performed on tissue harvested from the duodenum and ileum. Flagellin induced RegIIIγ expression in TLR5-deficient mice reconstituted with BM from WT mice (Figure 3B-E). These mice expressed TLR5 exclusively in hematopoietic cells, including lymphoid- and myeloid-derived cells. However, RegIIIγ was not induced by flagellin in WT mice reconstituted with TLR5-deficient BM in which TLR5 was expressed predominately in non-hematopoietic tissues, including epithelial and stromal cells. These results show that flagellin-mediated RegIIIγ upregulation requires TLR5 activation in cells of hematopoietic lineage.
Flagellin-mediated RegIIIγ induction requires IL-22
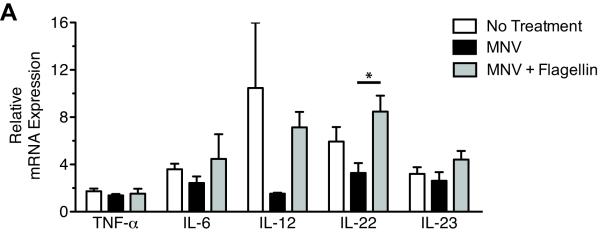
Because flagellin-mediated RegIIIγ induction does not involve direct stimulation of TLR5-expressing epithelial cells, we reasoned that a cytokine produced by flagellin-activated hematopoietic cells transmits signals to epithelial cells to induce RegIIIγ. Therefore, in the distal ileum, we measured TNF-α, IL-6, IL-12, and IL-23, which have been shown to be induced by flagellin in vivo and are known to have direct and indirect effects on epithelial cells [19-21, 28]. We also determined if flagellin induces IL-22, a cytokine that stimulates RegIIIγ and RegIIIβ upregulation during intestinal infection with murine pathogen Citrobacter rodentium [29]. Analysis of mRNA transcripts for these cytokines in the small intestine revealed that flagellin modestly induced expression of IL-22 (Figure 4). In addition, IL-12 transcript levels followed a similar trend, but the level of induction did not reach statistical significance.
Figure 4.
Flagellin induces a variety of cytokines. Mice were treated with metronidazole, neomycin, and vancomycin (MNV) for 7 days. Three doses of flagellin (15μg/day) were administered intraperitoneally to mice beginning on day 5 of MNV treatment. Messenger RNA was extracted from the distal ileum to assess cytokine induction after flagellin treatment using quantitative real-time PCR. IL-12 refers to IL-12p35, and IL-23 refers to IL23p19. Data were pooled from two independent experiments with a total of four mice per group. Levels were normalized to GAPDH and are expressed as mean ± SEM (*p < 0.05, statistical analysis by one-way ANOVA with Bonferroni correction)
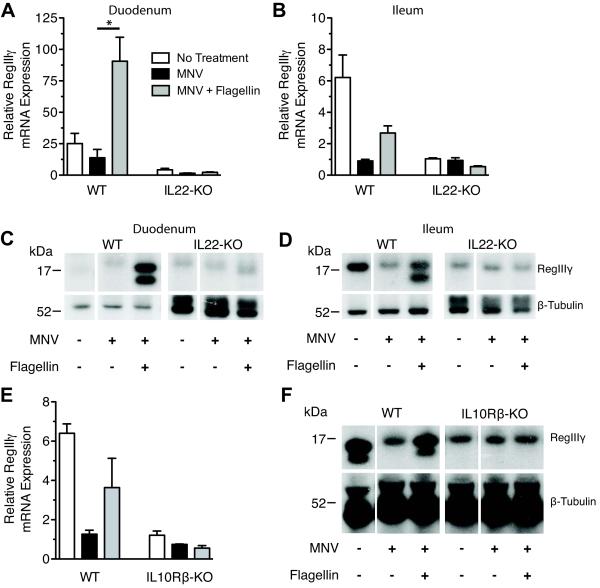
IL-22 is an IL-10-related cytokine produced by innate and adaptive immune cells that targets epithelial cells of the digestive, respiratory, urinary, and integumentary systems where the heterodimeric IL-22 receptor is highly expressed [30]. IL-22 has been shown to directly activate innate immune defenses and promote proliferation and survival of epithelial cells [24]. To determine if IL-22 plays a role in flagellin-mediated RegIIIγ induction, we compared RegIIIγ expression in MNV-treated WT and IL-22-deficient mice after flagellin administration. Untreated IL-22-deficient mice expressed low RegIIIγ protein and mRNA transcript levels in the distal ileum compared to WT mice, suggesting that IL-22 contributes to homeostatic expression of RegIIIγ (Figure 5B and D). Flagellin did not induce RegIIIγ expression in duodenum or ileum of IL-22-deficient mice, indicating that TLR5 stimulation of RegIIIγ expression is dependent on IL-22 (Figure 5A-D). The administration of recombinant IL-22 to MNV-treated mice also induced RegIIIγ expression in epithelial cells throughout the small intestine in the same pattern as flagellin administration (Supplementary Figure S1A-C).
Figure 5.
IL-22 is required for flagellin-mediated RegIIIγ expression. Wild-type (WT) and IL-22-deficient (IL-22-KO) or IL-10Rβ-deficient (IL-10Rβ-KO) mice were administered metronidazole, neomycin, and vancomycin (MNV) for 7 days. Mice received 15μg flagellin intraperitoneally on day 6 and 7 of MNV treatment. Tissue from the duodenum (A, C) and ileum (B, D - F) were collected for mRNA and protein extraction. A, B, and E, Quantitative PCR was used to evaluate RegIIIγ mRNA transcript expression. Levels were normalized to GAPDH and expressed as mean ± SEM (*p<0.05, statistical analysis by one-way ANOVA with Bonferroni correction). C, D, and B, Western blot analysis with RegIIIγ-specific anti-serum was used to assess RegIIIγ protein levels. Each lane is a representative sample from a mouse from the indicated group. A - D, Data were pooled from two independent experiments (n=8–10).
To confirm the requirement of IL-22 signaling for RegIIIγ induction, we measured RegIIIγ levels in mice lacking the IL-10Rβ chain of the heterodimeric IL-22R. These mice are deficient in both IL-10 and IL-22 signaling. RegIIIγ protein and mRNA transcript levels were low in the ileum of IL-10Rβ-deficient mice and not affected by antibiotic or flagellin administration compared to wild-type controls (Figure 5E and F).
The enhancement of innate immune defense by flagellin correlates with decreased VRE colonization
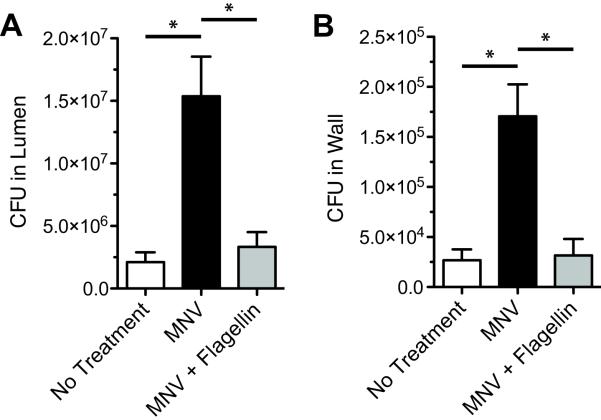
To determine if flagellin-induced RegIIIγ expression in the small intestine restores resistance to VRE colonization in mice treated with antibiotics, mice receiving MNV were given flagellin intraperitoneally and inoculated with VRE via oral gavage. Higher levels of VRE were recovered from the wall and lumen of the distal small intestine of MNV-treated mice compared to untreated mice (Figure 6A and B). However, flagellin administration to MNV-treated mice significantly reduced VRE survival in the intestinal lumen and wall compared to mice treated only with MNV. These results demonstrate that administering flagellin to antibiotic-treated mice prior to VRE infection can reduce VRE colonization to levels observed in mice not treated with antibiotics.
Figure 6.
Flagellin reduces susceptibility to VRE colonization in mice treated with broad-spectrum antibiotics. Mice were treated with metronidazole, neomycin and vancomycin (MNV) for 7 days. Starting on day 5 of antibiotic treatment, mice received 15 μg of flagellin per day via intraperitoneal injection for 3 days. On day 7 of antibiotic treatment, mice were inoculated with 1010 CFUs of VRE by oral gavage. The luminal content (A) and intestinal wall (B) of the distal half of the small intestine were collected for bacterial counts 24 hours post-inoculation. Data was compiled from three independent experiments and expressed as mean ± SEM (n=12–14, *p<0.001, statistical analysis by one-way ANOVA with Bonferroni correction).
Discussion
Recent studies have revealed that antibiotic treatment compromises the innate immune system of the intestinal mucosa by depleting commensal microbes that normally stimulate epithelial cells to produce homeostatic levels of AMPs [9, 18]. Here, we asked whether the TLR5 ligand flagellin could restore innate immune deficits caused by treatment with broad-spectrum antibiotics. We show that flagellin administration induces RegIIIγ in epithelial cells along the entire length of the small intestine of antibiotic-treated mice. The extensive upregulation of RegIIIγ was likely caused by systemic stimulation of TLR5 and secretion of IL-22. In contrast, RegIIIγ expression is limited to the ileum under steady-state conditions due to the higher density of bacteria in the distal small intestine compared to more proximal regions [9, 13]. Our results also show that TLR5-expressing hematopoietic cells mediate flagellin-induced RegIIIγ expression. In accordance, we found that IL-22 is also required for the response, suggesting that flagellin-stimulated hematopoietic cells produce IL-22, which signals intestinal epithelial cells to express RegIIIγ. Consistent with previous studies demonstrating that oral LPS induces RegIIIγ in the small intestine and enhances resistance to VRE infection, we demonstrate that systemically administered flagellin reduces susceptibility to VRE colonization in antibiotic-treated mice.
Although we have previously demonstrated that antibody-mediated blockade of RegIIIγ significantly diminishes in vivo killing of both VRE and Listeria monocytogenes [17, 18], RegIIIγ may not solely mediate flagellin’s protective effects. RegIIIβ is also upregulated in response to flagellin administration. Although RegIIIβ has no reported bactericidal activity, it binds peptidoglycan and may contribute to flagellin-mediated protection against VRE [9]. Flagellin induces several cytokines that may promote VRE clearance. In addition, MyD88-mediated signals in the gut promote repair and maintenance of the mucosal barrier [11, 19]. Together these responses may also contribute to flagellin-mediated resistance to VRE colonization.
Both hematopoietic and non-hematopoietic cells of the intestine respond to flagellin via the TLR5-MyD88-mediated pathway [23, 31]. In the case of hematopoietic cells, the high level of TLR5 expression in the small intestine has been attributed to a subset of CD11chiCD11bhi lamina propria dendritic cells important for IgA production and Th17 development [22, 32]. Given that RegIIIγ expression under normal conditions depends on direct detection of commensal bacteria by intestinal epithelial cells via the TLR-MyD88-mediated pathway [9, 17], we were surprised to find that TLR5 activation of hematopoietic cells is required for flagellin-mediated RegIIIγ expression. Our results using systemic flagellin administration support an alternative pathway of RegIIIγ induction. IL-22-mediated RegIIIγ expression induced by the presence of TLR ligands within subepithelial tissues, as opposed to stimulation from the luminal side of the epithelium, may play a critical role in alerting epithelial cells to the loss of mucosal integrity or presence of systemic infection. While RegIIIγ expression regulated by apical TLR stimulation is tightly controlled [18], IL-22 expression may be more indiscriminant with respect to stimulation by a variety of microbe-derived molecules once the epithelial barrier has been breached [29, 33]. Further work is needed to identify the hematopoietic cell subsets responsible for flagellin-induced RegIIIγ expression. The candidates for the source of IL-22 include Th17 cells, γδ-T cells, NK cells, and lymphoid tissue-inducer cells [34-38].
The protective effects of flagellin against numerous challenges, including lethal irradiation, chemical damage, and infectious agents, have been well described [19, 21]; however, the mechanism of TLR5-mediated protection has remained undefined. Prophylactic systemic administration of a TLR5 agonist results in dramatic survival after lethal irradiation [21]. High-dose ionizing radiation causes massive cell loss in the hematopoietic system and intestinal mucosa, which leads to invasion by commensal bacteria and fatal septicemia [39, 40]. NFκB activation protects against lethal irradiation by initiating anti-apoptotic pathways in radiosensitive tissue [41]. The radioprotective effects of flagellin are thought to be mediated by this mechanism [21]; however, based on our study, it is also possible that RegIIIγ expression induced by TLR5 activation bolsters the impaired mucosal barrier by directly killing invading bacteria.
The therapeutic use of TLR ligands has been approached cautiously due to the potential to stimulate undesired inflammatory responses. Most efforts to manipulate the immune system have targeted the adaptive arm, with a focus on enhancing long-term immunity. The innate immune system, however, can exert rapid and broad defense against invading organisms, and, if properly timed, might be exploited as an approach to ameliorate several clinical problems. For example, TLR ligands have been used as adjuvants in a variety of vaccines including two hepatitis B virus vaccines which use TLR4 agonists to induce a robust memory response [42]. One challenge, however, to the therapeutic use of flagellin is that repeated administration induces antibodies that eventually block TLR5 activation [43]. Therefore, targeting TLR5 might require the development agonists that do not stimulate neutralizing antibody responses.
Moderate stimulation of TLR5 has not been shown to induce severe sepsis; however, this does not exclude the possibility that flagellin administration may induce inflammation. In controlled trials, flagellin has been administered to humans with little side effects [44-46]. In spite of this, it is possible that certain subsets of patients will experience adverse reactions to flagellin treatment. Flagellin-mediated IL-22 induction may exacerbate skin plaques in psoriasis patients [47]. Also, stimulation of TLR5 is suspected to play a role in the pathogenesis of inflammatory bowel disease [48, 49]. Thus, the therapeutic use of flagellin will require extensive clinical study with a particular focus on potential complications resulting from accentuated inflammatory responses.
The commensal flora of the gut plays a critical role in the development and maintenance of a healthy intestinal mucosa. Treatment with broad-spectrum antibiotics greatly diminishes the intestinal microbial flora leading to increased susceptibility to a variety of bacterial infections, including VRE and Clostridium difficile [4, 7, 50]. The depletion of commensal bacteria results in diminished innate immune defenses, most notably RegIIIγ, due to reduced activation of Toll-like receptors [9, 17, 18]. Our experiments provide further evidence for the critical role of TLRs in RegIIIγ expression and for the ability of TLR activation to re-establish AMP expression which antibiotics have impaired. Our results suggest that flagellin may have therapeutic potential and may prevent intestinal invasion with resistant microbes in patients treated with broad-spectrum antibiotics. RegIIIγ induction along the small intestine may provide an approach to restrict potentially pathogenic bacteria in the intestinal lumen, thereby limiting colonization and dissemination, both within and between individuals.
Supplementary Material
Supplemental Figure S1 (Online Only)
Administration of recombinant IL-22 induces RegIIIγ in the small intestine Mice were treated with metronidazole, neomycin, and vancomycin (MNV) for 7 days. On day 7, mice were treated with 4 μg of recombinant IL-22 (Genscript) or PBS via intraperitoneal injection. Mice were sacrificed 12 hours post-injection, and intestinal segments from the duodenum (A) and ileum (B, C) were collected and processed for analysis. A and B, Recombinant IL-22 (rmIL-22) induced upregulation of RegIIIγ mRNA transcripts in the small intestine measured by qPCR. Levels were normalized to GAPDH and expressed as mean ± SEM. C, RegIIIγ-expressing cells were detected in paraffin-embedded tissue from the distal ileum by immunohistochemistry using polyclonal RegIIIγ-specific antiserum. Positive cells were colorized brown (400-fold magnification; n=2)
Acknowledgements
This work was supported by NIH grants AI39031 and AI42135 to EGP. MK and NS were supported by the National Institutes of Health Medical Scientist Training Program grant GM07739 to the Weill Cornell/RU/MSKCC Tri-Institutional MD-PhD Program. CU was supported by a post-doctoral fellowship from the Cancer Research Institute. LAZ was supported by a post-doctoral fellowship from the American Cancer Society. RAF is an investigator of the Howard Hughes Medical Institute.
Financial Support: National Institutes of Health (grants AI39031 and AI42135 to EGP, GM07739 to MK and NS); CU by the Cancer Research Institute; LAZ by the American Cancer Society
Footnotes
Potential conflicts of interests: none reported
Presented in part: 109th General Meeting of the American Society for Microbiology, Philadelphia, PA, May 17 – 21, 2009
References
- 1.Centers for Disease Control Nosocomial enterococci resistant to vancomycin--United States, 1989–1993. MMWR. 1993;42:597–9. [PubMed] [Google Scholar]
- 2.Hidron A, Edwards J, Patel J, et al. NHSN Annual Update: Antimicrobial□Resistant Pathogens Associated With Healthcare□Associated Infections: Annual Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82–9. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 4.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000;343:1925–32. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridkin SK, Edwards JR, Courval JM, et al. The effect of vancomycin and third-generation cephalosporins on prevalence of vancomycin-resistant enterococci in 126 U.S. adult intensive care units. Ann Intern Med. 2001;135:175–83. doi: 10.7326/0003-4819-135-3-200108070-00009. [DOI] [PubMed] [Google Scholar]
- 6.Husni R, Hachem R, Hanna H, Raad I. Risk factors for vancomycin-resistant Enterococcus (VRE) infection in colonized patients with cancer. Infect Control Hosp Epidemiol. 2002;23:102–3. doi: 10.1086/502016. [DOI] [PubMed] [Google Scholar]
- 7.Pultz NJ, Stiefel U, Subramanyan S, Helfand MS, Donskey CJ. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J Infect Dis. 2005;191:949–56. doi: 10.1086/428090. [DOI] [PubMed] [Google Scholar]
- 8.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–20. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 9.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Pamer EG. Immune responses to commensal and environmental microbes. Nat Immunol. 2007;8:1173–8. doi: 10.1038/ni1526. [DOI] [PubMed] [Google Scholar]
- 13.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 15.Vora P, Youdim A, Thomas L, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 16.Kolls JK, McCray PB, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandl K, Plitas G, Mihu C, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008;180:8280–5. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 20.Eaves-Pyles T, Murthy K, Liaudet L, et al. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 2001;166:1248–60. doi: 10.4049/jimmunol.166.2.1248. [DOI] [PubMed] [Google Scholar]
- 21.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–30. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 23.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 24.Zenewicz L, Yancopoulos G, Valenzuela D, Murphy A, Karow M, Flavell R. Interleukin-22 but Not Interleukin-17 Provides Protection to Hepatocytes during Acute Liver Inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim GF, Fleet GH, Lyons MJ, Walker RA. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–4. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov I, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Sanders CJ, Yu Y, Moore DA, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–8. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 28.Feuillet V, Medjane S, Mondor I, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–92. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Valdez P, Danilenko D, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 30.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Sanders CJ, Moore DA, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180:7184–92. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu S, Jang MH, Chevrier N, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 33.Aujla S, Chan Y, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanos SL, Bui VL, Mortha A, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satohtakayama N, Vosshenrich C, Lesjeanpottier S, et al. Microbial Flora Drives Interleukin 22 Production in Intestinal NKp46+ Cells that Provide Innate Mucosal Immune Defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 38.Godinez I, Raffatellu M, Chu H, et al. IL-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2008:42. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller CP, Hammond CW, Tompkins M. The incidence of bacteremia in mice subjected to total body x-radiation. Science. 1950;111:540–1. doi: 10.1126/science.111.2890.540. [DOI] [PubMed] [Google Scholar]
- 40.Vincent JG, Veomett RC, Riley RF. Relation of the indigenous flora of the small intestine of the rat to post-irradiation bacteremia. J Bacteriol. 1955;69:38–44. doi: 10.1128/jb.69.1.38-44.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Meng A, Lang H, et al. Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res. 2004;64:6240–6. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- 42.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 43.Nempont C, Cayet D, Rumbo M, Bompard C, Villeret V, Sirard JC. Deletion of flagellin’s hypervariable region abrogates antibody-mediated neutralization and systemic activation of TLR5-dependent immunity. J Immunol. 2008;181:2036–43. doi: 10.4049/jimmunol.181.3.2036. [DOI] [PubMed] [Google Scholar]
- 44.Whittingham S, Buckley JD, Mackay IR. Factors influencing the secondary antibody response to flagellin in man. Clin Exp Immunol. 1978;34:170–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Rowley MJ, Mackay IR. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969;5:407–18. [PMC free article] [PubMed] [Google Scholar]
- 46.Rowley MJ, Wistar R, Mackay IR. Measurement of antibody-producing capacity in man. V. Immunoglobulin classes of antibodies to flagellin. Immunology. 1972;22:475–84. [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng Y, Danilenko D, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 48.Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–63. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 49.Rhee SH, Im E, Riegler M, Kokkotou E, O’brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–5. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–92. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 (Online Only)
Administration of recombinant IL-22 induces RegIIIγ in the small intestine Mice were treated with metronidazole, neomycin, and vancomycin (MNV) for 7 days. On day 7, mice were treated with 4 μg of recombinant IL-22 (Genscript) or PBS via intraperitoneal injection. Mice were sacrificed 12 hours post-injection, and intestinal segments from the duodenum (A) and ileum (B, C) were collected and processed for analysis. A and B, Recombinant IL-22 (rmIL-22) induced upregulation of RegIIIγ mRNA transcripts in the small intestine measured by qPCR. Levels were normalized to GAPDH and expressed as mean ± SEM. C, RegIIIγ-expressing cells were detected in paraffin-embedded tissue from the distal ileum by immunohistochemistry using polyclonal RegIIIγ-specific antiserum. Positive cells were colorized brown (400-fold magnification; n=2)