Abstract
Photodynamic therapy (PDT) was discovered over one hundred years ago by observing the killing of microorganisms when harmless dyes and visible light were combined in vitro. Since then it has primarily been developed as a treatment for cancer, ophthalmologic disorders and in dermatology. However in recent years interest in the antimicrobial effects of PDT has revived and it has been proposed as a therapy for a large variety of localized infections. This revival of interest has largely been driven by the inexorable increase in drug resistance amongst many classes of pathogen. Advantages of PDT include equal killing effectiveness regardless of antibiotic resistance, and a lack of induction of PDT resistance. Disadvantages include the cessation of the antimicrobial effect when the light is turned off, and less than perfect selectivity for microbial cells over host tissue. This review will cover the use of PDT to kill or inactivate pathogens in ex vivo tissues and in biological materials such as blood. PDT has been successfully used to kill pathogens and even to save life in several animal models of localized infections such as surface wounds, burns, oral sites, abscesses and the middle ear. A large number of clinical studies of PDT for viral papillomatosis lesions and for acne refer to its anti-microbial effect, but it is unclear how important this microbial killing is to the overall therapeutic outcome. PDT for periodontitis is a rapidly growing clinical application and other dental applications are under investigation. PDT is being clinically studied for other dermatological infections such as leishmaniasis and mycobacteria. Antimicrobial PDT will become more important in the future as antibiotic resistance is only expected to continue to increase.
Keywords: Photodynamic therapy, photosensitizer, localized infection, bacteria, virus, fungus, skin, wound, burn
1. Introduction
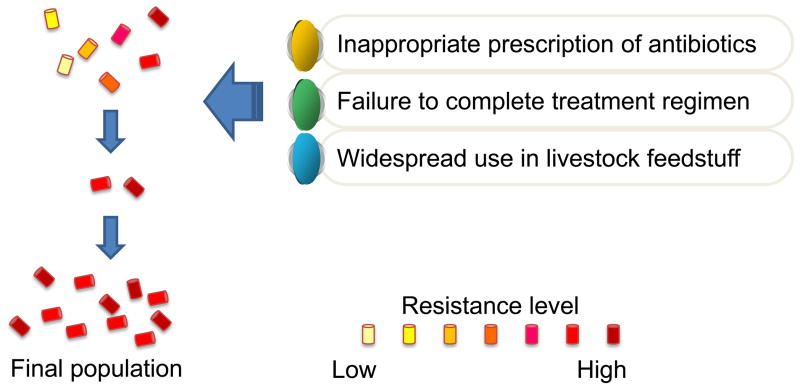
The rapidly increasing emergence of antibiotic resistance amongst many species of pathogenic bacteria may be bringing to an end a period extending over the past 50 years, termed “the antibiotic era” (1, 2). Bacteria replicate very rapidly and a mutation that helps a microbe survive in the presence of an antibiotic drug will quickly become predominant throughout the microbial population. Furthermore transferable genetic elements such as plasmids encoding resistance enzymes and efflux pumps can be transferred between species. The inappropriate prescription of antibiotics especially for viral diseases, the failure of some patients to complete their treatment regimen and the widespread use of antibiotics in livestock feedstuff only work together to exacerbate the problem by repeatedly selecting for the most resistant strains as illustrated in Figure 1 (3). The worldwide inexorable growth of multi-drug resistant bacteria has led to a major research effort to find alternative antibacterial therapeutics to which, it is hypothesized, bacteria will not be easily able to develop resistance.
Figure 1.
Problem of antibiotic resistance.
Photodynamic therapy (PDT) involves the use of non-toxic dyes or photosensitizers (PS) in combination with harmless visible light of the correct wavelength to excite the PS (4). In the presence of the oxygen, the excited state PS transfers energy or electrons to ground state molecular oxygen producing reactive oxygen species (ROS) such as singlet oxygen and hydroxyl radical that are able to kill cells (5). When the cells to be killed are pathogenic microorganisms the procedure is termed photodynamic inactivation (PDI) (6), lethal photosensitization (7) or in the dental field, photo-activated disinfection (PAD) (8).
Antimicrobial PDI may be a new approach to killing or eliminating pathogens that are infecting tissue (9). All studies that have examined the killing of antibiotic resistant bacteria by PDI have found them to be equally as susceptible as their naïve counterparts (10) (or even more susceptible (11). Moreover it has not as yet been possible to artificially induce resistance to PDI in any microbes where it has been tested (12).
Because the delivery of visible light to living tissue is almost by definition a localized process, PDT for infections is likely to be applied exclusively to localized disease, as opposed to systemic infections such as bacteremia. In contrast to PDT for cancer, where the PS is usually injected into the bloodstream and accumulates in the tumor, we believe that PDT for localized infections will be carried out by local delivery of the PS into the infected area by methods such as topical application, instillation, and interstitial injection or aerosol delivery. The key issues to be addressed, therefore, will be the effectiveness of the treatment in destroying sufficient numbers of the disease-causing pathogens, whether effective selectivity of the PS for the microbes over mammalian cells can be achieved thus avoiding an unacceptable degree of PDT damage to host tissue in the area of infection, and the avoidance of regrowth of the pathogens from a few survivors following the treatment.
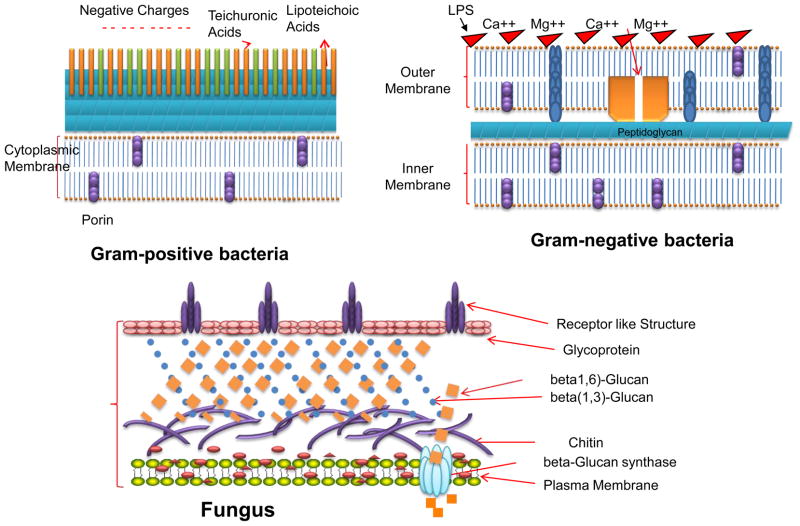
It has been known since the first days of PDT, early in the last century, that certain microorganisms can be killed by the combination of dyes and light in vitro (13). Throughout the years since those times there have been additional reports of bacteria being killed or inactivated by various combinations of PS and light (9). In the 1990s, it was observed that there was a fundamental difference in susceptibility to PDT between Gram-positive and Gram-negative bacteria (14). It was found that, in general, neutral, anionic or cationic PS molecules could efficiently kill Gram-positive bacteria, whereas only cationic PS or strategies that permeabilize the Gram-negative permeability barrier in combination with non-cationic PS are able to kill multiple logs of Gram-negative species. This difference in susceptibility between species in the two bacterial classifications was explained by their physiology, as the Gram-positive species have a cytoplasmic membrane surrounded by a relatively porous cell wall composed of peptidoglycan and lipoteichoic acid that allows PS to cross. The cell envelope of Gram-negative bacteria however consists of an inner cytoplasmic membrane and an outer membrane that are separated by the peptidoglycan-containing periplasm. The outer membrane forms an effective permeability barrier between the cell and its environment and tends to restrict the binding and penetration of many PS structures (15). Fungal cell walls have a relatively thick layer of beta-glucan and chitin that leads to a permeability barrier intermediate between Gram-positive and Gram-negative bacteria (see Figure 2 for a schematic depiction of the cell walls of these three classes of microbial cells.
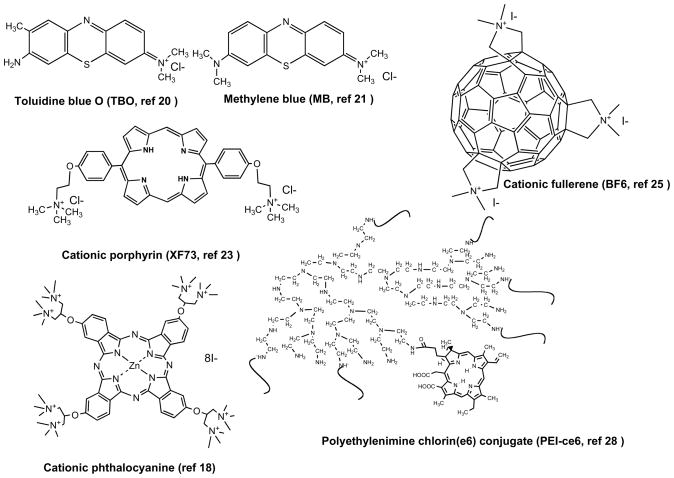
Figure 2.
Molecular structures of representative antimicrobial PS
Several approaches have been tested that allow PDI of Gram-negative bacterial species. The Israeli group of Nitzan and co-workers used the polycationic peptide polymyxin B nonapeptide (PMBN), which increased the permeability of the Gram-negative outer membrane and allowed PS that are normally excluded from the cell to penetrate to a location where the reactive oxygen species generated upon illumination can cause fatal damage (14). Another approach was taken by Bertoloni et al. (16) who found that the use of ethylenediaminetetraacetic acid (EDTA) to release LPS or the induction of competence with calcium chloride sensitized E. coli and Klebsiella pneumoniae to PDI by hematoporphyrin or zinc phthalocyanine. An approach adopted by several groups is to use a PS molecule with one or more intrinsic positive charges usually provided by quaternary nitrogen atoms (17–19). Phenothiazinium dyes such as toluidine blue O (20), methylene blue (21) and azure dyes (22) have been widely employed to carry out PDI of a large range of both Gram-positive, Gram-negative bacteria and also fungal cells. Cationic tetrapyrrole PS containing quaternary groups have been synthesized based on frameworks such as porphyrins (17, 23), phthalocyanines (18, 24), and even C60 fullerenes (25). The last approach that will be discussed involves covalently attaching a non-cationic PS molecules such as chlorin(e6) to a polymer molecule containing basic amino groups such as polylysine (26, 27) or polyethylenimine (28). Figure 3 illustrates the structural formulae of six representative molecules that possess cationic or basic groups and are highly active as antimicrobial PS. The mechanisms of action of all the cationic PS or basic polymer-PS conjugates is thought to be that of “self-promoted uptake pathway” (29). In this process cationic molecules first displace the divalent cations, Ca2+ and Mg2+ from their position on the outer membrane where they act as an anchor for the negatively charged LPS molecules. The weakened outer membrane becomes slightly more permeable and allows even more of the cationic PS to gain access thus steadily increasing the disorganization of the permeability barrier and increasing PS uptake with each additional binding (15).
Figure 3.
Structures of the cell walls of three different classes of microbial pathogens.
Another important observation that has been made about these cationic antimicrobial PS concerns their selectivity for microbial cells compared to host mammalian cells (30). It is thought that cationic molecules are only slowly taken up by host cells by the process of endocytosis, while their uptake into bacteria is relatively rapid. If illumination is performed at short intervals after PS application (minutes) then PDT damage to host tissue will be minimized.
2. Antimicrobial PDT ex vivo or in biological material
In order for antimicrobial PDT to be able to play any role as a therapeutic modality it has to be demonstrated that PS and light are able to effectively kill microbial cells in the type of environment where they are found in actual infections, i.e. surrounded by proteins, cells, blood, or tissue. This requirement has been addressed by carrying out PDI of microbial cells in vitro but using biological materials or ex vivo tissue to mimic the clinical environment. Figure 4 illustrates some of the biological tissues and materials that have been used in these types of experiments.
Figure 4.
Schematic depiction of the use of ex vivo biological tissues and materials to mimic the use of PDT to kill microbial cells in infections.
2.1 Proteins and cells
It was found that antibacterial PDI was considerable more efficient if the bacteria were incubated with the PS in saline or medium with a low protein concentration (31). Protein-rich media or addition of bovine serum albumin reduced the effectiveness of PDI. Wilson and Pratten (32) found that MRSA was killed tenfold less by aluminum disulfonated phthalocyanine and light in the presence of horse serum. Lambrechts et al (33) reported similar results, in which human plasma and serum both reduced the killing of S. aureus, P. aeruginosa and C. albicans using the cationic 5-phenyl-10,15,20-tris(N-methyl-4-pyridyl)porphyrin chloride and white light (30 mW cm-2). Street et al (34) asked whether PDI mediated by methylene blue (MB) could kill MRSA growing on an artificial skin construct composed of human-derived epidermal keratinocytes and dermal fibroblasts cultured at an air/media interface to form a stratified (epidermis and dermis), intact model of full thickness epithelialized human skin. Application of MB alone resulted in small reductions in MRSA viability from non-treated control while PDT treatment produced a significant (5.1 log10 reduction from control) immediately post-treatment and at 24 hours after treatment were almost sterile.
2.2 Blood sterilization
This has been an important use of PDT since 1992 when it was first approved by the Red Cross in Germany. Previously transmission of hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) had occurred However, there are still risks of infection associated with transfusion of cellular blood components, i.e. red blood cell (RBC) concentrates and platelet concentrates. This is due to the inability of serological tests to detect viral infection during the “window” soon after infection. Mohr et al reported (35) the inactivation of HBV, HCV, HIV, parvovirus B19 in plasma products with MB concentrations in the μM range. West Nile virus could also be inactivated (36). The dye thionine combined with yellow light illumination was proposed to be combined with UVB to sterilize platelet concentrates (37).
Ben-Hur and colleagues have studied the use of phthalocyanines and red light to inactivate pathogens in red blood cell concentrates (RBCC) (38). Under conditions leading to virus sterilization the blood borne parasites Trypanosoma cruzi (Chagas disease) and Plasmodium falciparum (malaria) could be eliminated to undetectable levels (> 4 log10 kill). RBC damage during treatment could be avoided by increasing the light fluence rate to 80 mW/cm2, and by including the free radical scavenger glutathione and the vitamin E derivative Trolox during light exposure procedures (39).
2.3 Skin
Maisch et al (40) examined penetration and antibacterial efficacy of XF73 (a cationic porphyrin PS) against MRSA using an ex vivo porcine skin model. They used both preincubation of bacteria in solution with XF73 and subsequent application on the ex vivo porcine skin, and also application of bacteria on the skin followed by an incubation with XF73 in a water-ethanol formulation for up to 60 min under occlusion. The localization of XF73 was restricted to the stratum corneum. Photoinactivation of pre-incubated S. aureus demonstrated >3 log10 reduction, while illumination after XF73 was delivered to the bacteria on the skin resulted in a approximately 1 log10 growth reduction independently of the antibiotic resistance pattern of used S. aureus strains.
Smijs et al (41) carried our similar studies with the dermatophyte, Trichophyton rubrum the commonest cause of superficial fungal infections. They employed an ex vivo model using human stratum corneum and inoculated T. rubrum microconidia. The PS used were 5,10,15-tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride (Sylsens B) and deuteroporphyrin monomethylester (DP mme) and two different incubation media, Dulbecco’s modified Eagle medium and distilled water. The PDT susceptibility depended on the time of PDT application after spore inoculation. A decrease in susceptibility was observed with increasing time of PDT application for both photosensitizers in DMEM. Changing the incubation medium to distilled water resulted in an increased fungicidal effect for Sylsens B and in a decreased effect for DP mme.
2.4 Teeth
The use of PDT to treat endodontic infections that are caused by bacterial biofilms has been studied in ex vivo-extracted teeth. Enterococcus faecalis is the pathogen most commonly associated with recurrent endodontic infections (42). Fonseca et al contaminated root canals in uniradicular teeth with E faecalis and incubated for 48 h at 35 degrees C followed by addition of a solution of 0.0125% toluidine blue for 5 min and irradiation using a 50-mW 660-nm. The mean decrease in CFU was 99.9% in the treated group, whereas in the controls an increase of 2.6% was observed. Soukos et al performed similar experiments with E. faecalis using methylene blue (25 μg/mL) for 5 minutes followed by exposure to 30 J/cm2 of 665 nm light using an optical fiber with multiple cylindrical diffusers that uniformly distributed light at 360 degrees. They obtained 53% killing and this increased to 97% after 222 J/cm2. Garcez et al used PDT to kill Gram-negative (Pseudomonas aeruginosa and Proteus mirabilis) bacteria growing as biofilms in root canals of extracted teeth. While Gram-negative species are not as commonly found as Gram-positives they are harder to kill, and moreover bioluminescence monitoring could be used to quantify infection. They used a conjugate between polyethylenimine and chlorin(e6) and 660-nm diode laser light delivered into the root canal via a 200-micron fiber, and this was compared and combined with standard endodontic treatment using mechanical debridement and antiseptic irrigation. Endodontic therapy alone reduced bacterial bioluminescence by 90% while PDT alone reduced bioluminescence by 95%. The combination reduced bioluminescence by >98%, and importantly the bacterial regrowth observed 24 hours after treatment was much less for the combination (P<0.0005) than for either single treatment.
2.5 Stomach
Helicobacter pylori organisms are spiral, microaerophilic, Gram-negative bacteria that colonize the gastric mucosa of the human stomach and secrete urease and other virulence factors that increase their pathogenicity. Ferrets are sometimes considered as an animal model of H. pylori infection as they are frequently naturally infected with a similar gastric helicobacter called H. mustelae. Millson et al (43) investigated the effect of topical PDT on explanted ferret gastric mucosa using one of five sensitizers (methylene blue (MB), toluidine blue O (TBO), phthalocyanine, hematoporphyrin derivative and 5-aminolavulinic acid), followed by irradiation with an appropriately tuned copper vapor pumped dye laser. A 90% reduction in counts of bacteria sensitized with 0.75 mg/kg TBO were seen after irradiation with 200 J/cm2. Concentrations of MB of 0.75 mg/kg and 7.5 mg/kg were not toxic to H. mustelae, but the further addition of 20 J/cm2 laser light reduced colony counts by more than 99%.
3. PDT in animal models of infection
Over the years, numerous studies have been designed to investigate the effects of PDT on prevention and treatment of infectious diseases utilizing animal models of infection, including mouse, rat, dog, pig, etc. The intent for the use of animals as models of disease is to establish an infection that mimics the real disease seen in the species of concern, usually humans. By duplicating as closely as possible the clinical infection, the reasons for the establishment of the infection can be researched and new treatments developed. It is clear that bacteria and fungi that are obtaining their essential nutrients from mammalian cells and tissue are very different from microorganisms growing exponentially in laboratory media. Many genes are transcribed differently in the so called “planktonic” phase cells that are growing logarithmically compared to the “stationary” phase or biofilm growing cells typical of an actual infection (44–46).
3.1. Monitoring PDT of infection by bioluminescence imaging
In vivo studies of PDT on infection models suffer from difficulties in monitoring the development of an infection in animal models and its response to treatment. Standard microbiological techniques used to follow infections in animal models frequently involve sacrifice of the animals, removal of the infected tissue, homogenization, serial dilution, plating and colony counting. These assays use a large number of animals, are time consuming, and often are not statistically reliable.
In order to facilitate the non-invasive monitoring of animal models of infection, we have developed a procedure that uses bioluminescent genetically engineered bacteria and a light sensitive imaging system to allow real-time visualization of infections. When these bacteria are treated with PDT in vitro, the loss of luminescence parallels the loss of colony-forming ability. We have developed several mouse models of localized infections that can be followed by bioluminescence imaging (BLI) (47).
BLI can be used either to track the course of an infection or monitor the efficacy of antimicrobial therapies. Bacterial pathogenesis appeared to be unaffected by the presence of the luciferase genes, and bioluminescence can be detected throughout the study period in animals. Further, the intensity of the bioluminescence measured from the living animal correlated well with the bacterial burden subsequently determined by standard protocols (48–50). Transposon-mediated integration of the luciferase operon into the bacterial chromosome means that reduction of luminescence from sites of infection in animals can be attributed to reduction of bacterial numbers rather than loss of plasmids.
3.2 Wound infections
Surgical wound infections account for 25% of nocosomial infections and frequently display some degree of antibiotic resistance. Species involved include S. aureus, enterococci, Gram (−) enteric bacilli. Patients who have intestinal surgery, who are neutropenic due to cancer chemotherapy or other medication or who have diabetes or other vascular disease are at increased risk of post surgical wound infection. It may be possible when these infected wounds need surgical intervention to apply topical PDT especially for drug resistant strains.
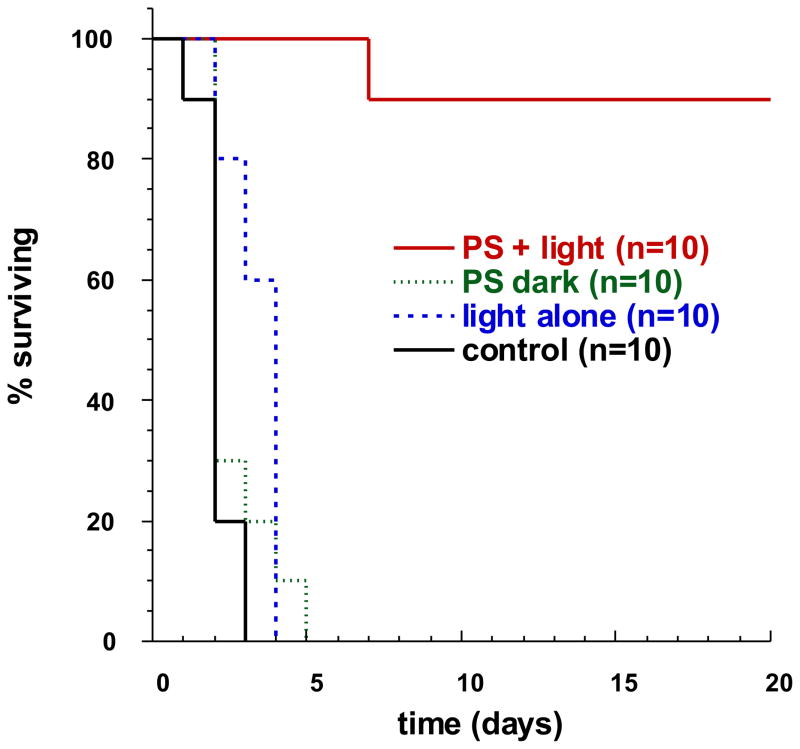
Hamblin et al. (51, 52) reported the first time the use of mouse wound infection models to investigate the effect of PDT on treating excisional wounds infected with Escherichia coli and Pseudomonas aeruginosa. Single wounds measuring 100 mm2 (8 mm×12.5 mm) were made on the backs of mice. Bioluminescent bacteria transduced with a plasmid containing a bacterial lux gene operon were used, allowing the infection to be monitored in real time by use of a sensitive charge-coupled camera. Polycationic photosensitizer conjugate was applied topically followed by red-light illumination at 665 nm for up to 240 J/cm2. A rapid light dose-dependent loss of luminescence was observed as measured by image analysis. For the P. aeruginosa infection, all 3 groups of non-treated control mice died within 5 days; in contrast, 90% of the PDT-treated mice survived as shown in the Kaplan-Meier plot in Figure 5.
Figure 5.
Kaplan-Meier plot of survival of mice with excisional wounds infected with Pseudomonas aeruginosa, and given either no treatment, light alone, PS (polylysine-ce6 conjugate) alone, or PDT with conjugate plus light.
Using similar mouse models, Wong at al. (53) and Zolifagehari et al (54) studied the effect of methylene blue and toluidine blue O mediated PDT on Vibrio vulnificus and methicillin-resistant S. aureus wound infections, respectively. Over 1 log of reduction in bacterial numbers was observed in both studies after 150 to 360 J/cm2 illumination of red light. For V. vulnificus infection, it was demonstrated that PDT could cure mice that would develop otherwise fatal sepsis.
3.3 Burn infections
The effect of burns in destroying the cutaneous barrier, rendering the affected tissue non-perfused, and depressing immune defenses, means that they very commonly become infected. In past years the majority of patients with serious burns died from infections. The introduction of topical antimicrobial treatments and early excision and skin grafting has reduced the death rate significantly. The ubiquitous pathogen P. aeruginosa, together with S. aureus, Candida and filamentous fungi are frequently responsible.
Using a guinea pig model, Orenstein et al (55) studied the effect of porphyrins on the eradication of S. aureus in burns. Guinea pigs, weighing about 400 g each, were shaved on the backs. A copper plate of 10 mm × 10 mm × 30 mm equipped with a 200 mm handle, heated to 150 °C, was placed on the shaved back of each mouse for 10 s, resulting a third degree burn. Wounds were infected with 108 CFU of S. aureus 15 min after the burning. It was noted that a reduction of 99% of the viable bacteria was achieved after the porphyrins was dropped on the eschar or injected into eschar but the therapy was not particularly dependent on light delivery.
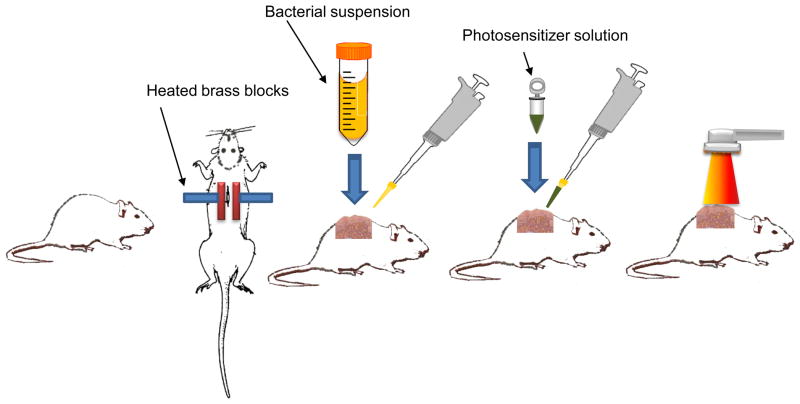
Mouse models (BALB/c mouse, 6–8weeks age) were employed by Lambrechts et al (56) and Dai et al (57) to evaluate the PDT for S. aureus and A. baumannii burn infections respectively and the procedure is illustrated in Figure 6. Burns were created on the backs of mice by applying two pre-heated (95 °C) brass blocks to the opposing sides of an elevated skin-fold on the backs of mice for 10 seconds. The brass block area was 20 mm X 10 mm giving a burned area of 200 mm2. Bioluminescent S. aureus or A. baumannii was applied to the burns 10 minutes after the creation of burn. When PDT was performed at 30 minutes after infection, over 3-log10 inactivation of A. baumannii was achieved, as quantified by luminescent imaging analysis (Figure 7). During the same period of time less than 0.9 log10 reduction of bacterial luminescence was observed in the dark control and the bacterial luminescence of the light alone control increased by a factor of 2 during this same period (Fig. 2). When PDT was performed 24 hours after infection, over 1 log10 inactivation of both S. aureus and A. baumannii was achieved. PDT did not delay wound healing in A. baumannii infected burns, but in S. aureus infected burns, delay of wound healing was observed in the PDT treated burns.
Figure 6.
Schematic depiction of the steps involved in performing antimicrobial PDT on a burn infection in mice.
Figure 7.
Dose response of bacterial luminescence from a representative mouse burn infected with A. baumannii and treated with PEI-ce6 and light (PDT) at 30 minutes after infection; a representative mouse burn infected with A. baumannii and treated with PEI-ce6 only at 30 minutes after infection (dark control); a representative mouse burn infected with A. baumannii and treated with light only at 24 hours after infection (light control).
3.4 Soft tissue infections
Although soft tissue infections are relatively rare they can have devastating consequences to patients. The spread can be rapid, the mortality rate is high (up to 50%), and frequently mutilating surgery is the only means of arresting the unrelenting course of the disease. The group includes such manifestations such as necrotizing fasciitis (S. aureus, Streptococci, or polymicrobial species); gas gangrene (Clostridium species), necrotizing cellulitis and Fournier’s gangrene (synergistic mixtures of aerobes and anaerobes). In these infections repeated excisions of affected tissue are frequently necessary and topical PDT could have a role to play in rapidly reducing the bacterial burden, and hence reducing the extent of surgical debridement.
A commonly used animal model of a localized soft-tissue infection is the intramuscular injection of a bacterial suspension into the mouse thigh muscle. This has been carried out with many bacterial species including E. coli, P. aeruginosa and S. aureus. Berthiaume et al. (58) evaluated the efficacy of antibody-targeted photolysis to kill bacteria in vivo using immunconjugates against P. aeruginosa. Initially, they mixed the bacteria with the tin(IV) chlorin e6–monoclonal antibody conjugate in vitro and injected the mixture into the subcutaneous dorsal area in mice. After infection, both specific and nonspecific conjugates were injected at the infection site. After a 15 min incubation period, the site was exposed to 630 nm light with a power density of 100 mW cm2 for 1600 s (total light 160 J/cm2). Illumination resulted in a greater than 75% decrease in the number of viable bacteria at sites treated with a specific conjugate, whereas normal bacterial growth was observed in animals that were untreated or treated with a non-specific conjugate. Gad et al (59) used luminescent bacteria and studied the PDT for S. aureus infection in soft tissue. One million (106) mid-log phase bioluminescent S. aureus cells suspended in 50 μL phosphate buffered saline (PBS) were injected 2 mm beneath the surface of the thigh muscle in neutropenic mice. PDT mediated with poly-lysine chlorine e6 conjugate was performed 24 hours after infection. There was a light dose dependent loss of luminescence not seen in the non-treated infections or those treated with light alone. PDT treated legs healed better than the infected legs without treatment.
3.5 Oral and dental infections
As more and more bacterial strains become resistant to antibiotics, the dental clinician often is faced with choosing alternatives to combat anaerobic bacteria that grow in the periodontal pocket and lead to periodontal diseases and tooth loss. A primary related concern for the implantologist is peri-implantitis, a condition in the region of the dental implant involving soft-tissue inflammation (peri-implant mucositis), bleeding, and suppuration, which can progress to fairly rapid bone loss.
In a pilot study, Shibli et al (60) reported the results on lethal photosensitization on ligature-induced peri-implantitis in male mongrel dogs (2 years age, average weight of 18 kg) with different implant surfaces. A total of 36 dental implants with 4 different surface coatings (9 commercially pure titanium surface; 9 titanium plasma-sprayed; 9 hydroxyapatite; and 9 acid-etched) were inserted in 6 male mongrel dogs 3 months after extraction of mandibular premolars. After a 14 months, dogs underwent surgical debridement of the dental implant sites and lethal photosensitization by combination of toluidine blue O (100 μg/mL) and illumination from a 685-nm diode laser at the energy of 200 J/cm2. Five months later, biopsies of the implant sites were dissected and prepared for ground sectioning and analysis. The percentage of bone fill was 26.70 to 48.28, and the percentage of reosseointegration was 15.83 to 25.25, depending on the different surface coating used.
Periodontal disease is the result of the collapse of teeth-supporting structures by the local action of periodontopathogenic microorganisms. These microorganisms release substances that strictly injure periodontal tissues, besides inducing tissue destruction by inflammatory and immunologic responses of the host. Komerik et al (61) investigated the use of toluidine blue-mediated photosensitization for killing organisms in the oral cavities of male Sprague-Dawley rats (weighing 200 g). The maxillary molar of each rat was inoculated with 2.5×108 CFU of P. gingivalis and immediately exposed to up to 48 J of 630-nm laser illumination in the presence of toluidine blue. Significant reduction in the number of viable P. gingivalis was observed after PDT. Radiographic analysis showed the bone loss in the PDT treated animals was found to be significantly less than that in the control groups.
Fernandes et al (62) and de Almeida et al (63, 64) studied the effect of PDT on treating periodontal diseases using normal rat and immunosuppressed rat models (treated with dexamethasone). Male Wistar rats (250–330 g) were used in the studies. Ligatures were placed on the first mandibular molar in rats to induce periodontitis. PDT mediated with methylene blue or toluidine blue-O was performed on the normal rats and immuno-suppressed rats at day 2 and day 7 post-infection respectively. Results indicated that less bone loss was found in the PDT group than the control groups. In addition, PDT showed better therapeutic efficacy in dexamethasone-inhibited rats than scaling and root planning (62).
Sigusch et al (65) performed a study on PDT for periodontal infections in beagle dogs (weight 15–20 kg). The animals were infected with P. gingivalis and Fusobacterium nucleatum in all subgingival areas. Two photosensitizers, chlorine e6 and BLC1010, were tested. The PDT procedure carried out with either of the photosensitizers in combination with 662 nm laser illumination caused a significant reduction in the clinical inflammation signs of redness and bleeding on probing, compared to the controls (light only and no treatment). Furthermore, PDT with chlorin(e6) caused a significant reduction in P. gingivalis infected sites, whereas there was a lack in suppression after PDT with BLC1010. F. nucleatum could hardly be reduced with chlorine e6, and only to a certain extent with BLC 1010 and laser only. In the control groups, the P. gingivalis infected test sites did not change.
Mucocutaneous oropharyngeal candidiasis is one of the most common manifestations of human immunodeficiency virus (HIV) infection, occurring in up to 84% of HIV-infected patients, and is considered to be an independent predictor of immunodeficiency in patients with acquired immune deficiency syndrome (AIDS). Candida albicans is the most often isolated organism from patients. In a normal healthy adult population an estimated 40% are oral carriers, and most cases of mucocutaneous oropharyngeal candidiasis are endogenously acquired. Teichert et al (66) evaluated the efficacy of using methylene blue (MB)–mediated PDT to treat oral candidiasis in an immunosuppressed mouse model, mimicking what is found in human patients. Seventy-five experimental beige nude mice with severe combined immunodeficiency disease (SCID) were inoculated 3 times a week by swabbing the oral cavity with a C albicans–coated Calgis type 4 swab for a period of 4 weeks. On treatment day, mice were cultured for baseline fungal growth and received a topical oral cavity administration of 0.05 mL MB solution at various concentrations from 250 to 500 μg/mL. After 10 minutes, the mice were recultured and underwent light activation with 664 nm of diode laser light with a cylindrical diffuser. The results indicated a MB dependent effect of PDT, and completed eradication of from oral cavity was achieved when 450–500 μg/mL MB was used.
Lin et al (67) examined the effect of toluidine blue (TB)-mediated PDT on oral wound infections in rats. Male Wistar rats weighing 150–180 g were used in this study. Excisional wounds (1×2 mm2) were made on the palate of the first maxillary molar with the gingiva and connective tissue beneath cut off. After the blood had been blocked, 20 μL Streptococcus spp. or Actinomyces viscosus suspension containing 2×108 CFU was injected into each wound. At 24 hours post-infection, 10 μL TB solution was topically applied to the wound, which was subsequently irradiated with a 635-nm diode laser for up to 48 J/cm2. It was observed that, when 48 J/cm2 light had been delivered, approximately 97% killing of bacteria was achieved. Accelerated wound healing was found in the PDT treated group than the untreated control group.
3.6 Leishmaniasis
Leishmaniasis causes substantial mortality and morbidity in the developing world, with endemic levels in 88 countries. Depending on the genetic background of the host and species of the Leishmania parasite, the resulting infection may be restricted to cutaneous sites, involve the multiplication of microorganisms in the mucous membranes (as in muco-cutaneous leishmaniasis), or spread throughout the reticuloendothelial system, as in visceral leishmaniasis. Recently, cutaneous leishmaniasis has raised considerable concerns because about 1% of the US service members deployed to the Middle East have contracted this disease.
Akilov et al (68–70) reported the use of a mouse model to study the effcicay of PDT for cutaneous leishmaniasis. Approximately 1×106 metacyclic parasites in 20 μL of PBS were inoculated intradermally into each ear of 6 to 8-week-old BALB c female mice. PDT was performed 3 weeks after infection. Photosensitizers used included (3,7-Bis(N,N-dibutylamino) phenothiazinium bromide (PPA904) and δ-aminolevulinic acid-derived protoporphyrin IX (ALA). Infected sites were illuminated using a 665-nm non-coherent light source or a 635-nm diode laser. Mice were sacrificed 5 days after PDT and the load of parasites was quantified. It was indicated that PDT with PPA904 exhibit a high parasiticidal effect in-vivo against cutaneous leishmaniasis [35, 37]. In-vivo PDT with ALA resulted in significant reduction of the parasite loads but also vigorous tissue destruction.
3.7 Mycobacterial infection
Mycobacterium tuberculosis is a major public health problem and manifests as latent infection or progressive contagious disease. The predominance of these infected cases is in the latent form; the remainder is active and often contagious. Although only 10% of infected people develop active tuberculosis, the death rate of about 2 million a year is among the highest for infectious diseases worldwide.
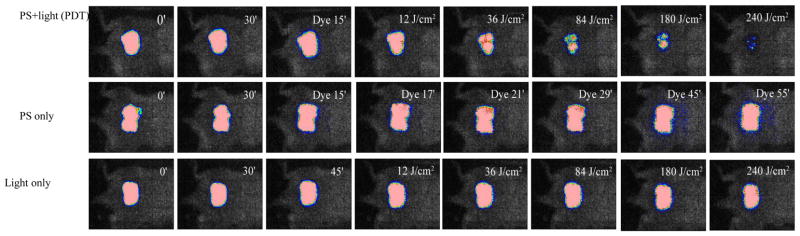
O’Riordan et al (6, 71) developed a mouse model of localized mycobacterial infection and used PDT to treat this infection in vivo. Male BALB/c mice (6–8 weeks old) were used. One full thickness incisional skin wound was made in a line along the dorsal surface of each mouse and a subcutaneous pocket made with a fine-tipped sterile forceps. The collagen implants were placed at either side of the dorsal midline and the incisions then closed with nylon sutures. Three PS were tested in the studies: Verteporfin (lipid-formulated benzoporphyrin derivative monoacid ring A), benzo[a]phenothiazinium chloride, and benzo[a]phenoselenazinium chloride. Real-time fluorescence monitoring technique was used to track the delivery of the PS to the infected sites as shown in Figure 8. When 105 Mycobacterium bovis BCG were present in the in vivo-induced granulomas, a significant reduction in viable mycobacterial cells was demonstrated in PDT-treated granulomas compared to those of non-treated controls.
Figure 8.
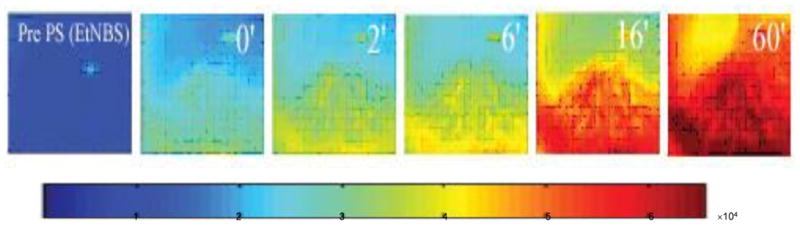
Real time monitoring accumulation of PS (EtNBS) in subcutaneous granuloma site in BALB/c mice by fluorescence imaging. Fluorescence intensity increased linearly immediately after PS injection up to 60 min, suggesting steady, time dependent delivery of EtNBS to the collagen implants.
3.8 Otitis media
Otitis media with effusion (OME) is the most common disease of childhood with the exception of viral upper respiratory infections. Despite the popular use of antibiotics, complications of otitis media with significant morbidity still occur. It is obvious that otitis media is a major health problem, especially among children. One of the most important factors that contribute to the development of OME is bacterial infection.
By using a gerbil model (Meriones unguiculatus, weighing 50 g each), Jung et al (72) evaluate the antibacterial effects of PDT in vivo on Haemophilus influenzae and Streptococcus pneumoniae, the common bacterial species causing OME. Bacteria solution of 20 μL (107 CFU/mL) was injected through the bullae under sterile conditions. PDT was performed two days after the infection by injecting 20 μL of Photogem (1 mg/mL, a hematoporphyin derivative) solution into the bullae followed by 632-nm laser illumination from a sterilized fiber tip for 90 J. PDT was effective in killing S. pneumoniae in 87% of the infected bullae with OME, whereas it was effective in eradicating H. influenzae in 50% of the infected bullae with OME.
3.9 Osteomyelitis
Bisland et al (73) studied PDT as a possible treatment for osteomyelitis using a bioluminescent strain of biofilm-producing S. aureus grown onto kirschner wires (K-wire). S. aureus-coated K-wires were exposed to methylene blue (MB) or 5-aminolevulinic acid (ALA)-mediated PDT either in vitro or following implant into the tibial medullary cavity of Sprague-Dawley rats. The progression of S. aureus biofilm was monitored non-invasively using bioluminescence and expressed as a percentage of the signal for each sample immediately prior to treatment. S. aureus infections were subject to PDT 10 days post inoculation. Treatment comprised administration of ALA (300 mg/kg IP followed 4 h later by light (635 +/− 10 nm; 75 J cm2 delivered transcutaneously via an optical fiber placed onto the tibia and resulted in significant delay in bacterial growth and inhibited biofilm formation on implants in bone.
3.10 Virus infections
Smetana et al (74) infected Hartley Guinea pigs (200–300 g), Inoculation of HSV on the backs of guinea pigs resulted in a local infection starting after 24 hours, manifested as reddening and swelling for up to 3 days. At 3–6 days vesicles were formed, followed by the appearance of crusts during the second week. Complete healing occurred at 3–4 weeks after infection. Exposure to light or ALA only at various times after infection had no obvious effect on the clinical manifestations. When treated with ALA-PDT immediately or up to 6 hours after infection there was a dramatic effect. Duration of vesicles’ appearance was very short and healing started on the third day. Crusting time, however, was longer and the diameter of the crusts was 2 cm instead of 0.3–0.5 cm in the controls. The clinical observations were confirmed by titrating HSV isolated after infection. In control or ALA alone animals the HSV titer reached a peak of 5 log10 PFU 4 days after infection. However, when ALA administration was followed by light exposure no HSV could be isolated. ALA-PDT 2 days after infection had no effect on the HSV titer.
4. PDT for clinical infectious disease
The ability of light-drug combinations to kill microorganisms has been known for over 100 years. Although reports of the photodynamic inactivation of viruses appeared in 1928, long before chemotherapeutic antiviral drugs, the first clinical trial in humans-the topical treatment of herpes genitalis-did not take place until the early 1970s (75).
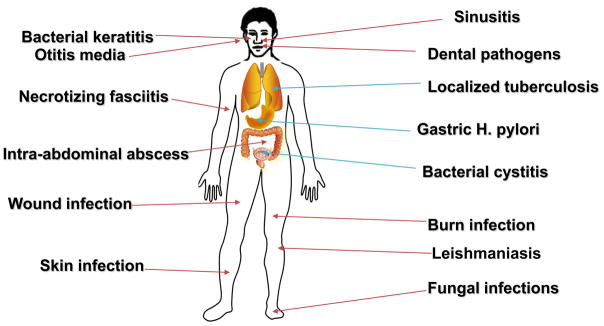
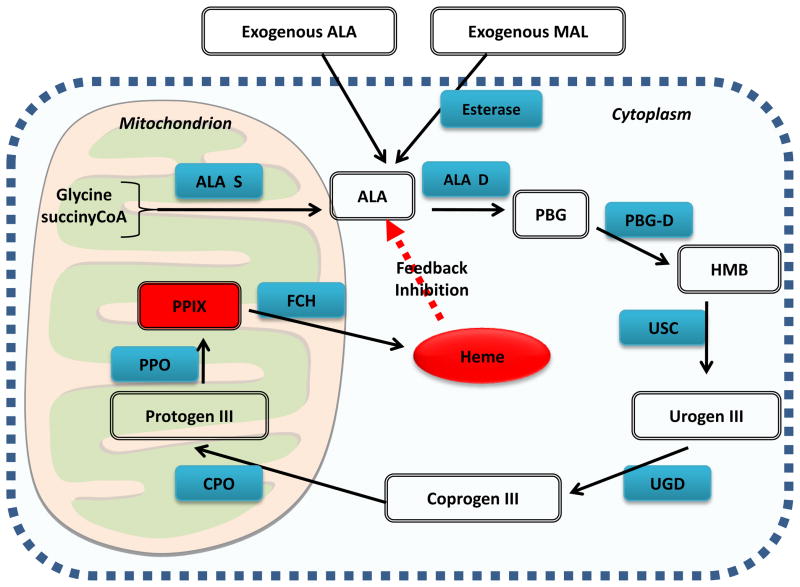
Figure 9 shows the wide range of infectious diseases that PDT may be especially suited to deal with. Many clinical applications of antimicrobial PDT (especially in the skin) involve topical application of the precursor amino-acid, 5-aminolevulinic acid (or more recently the ALA-methyl ester known as methyl aminolevulinate, MAL) in a process that leads to accumulation of the photosensitizer protoporphyrin IX in cells that are equipped with heme biosynthesis enzymes as illustrated in Figure 10. It is at present uncertain to what extent ALA-PDT of dermatological infections achieves its clinical success by killing the actual microorganisms responsible for the infection, and to what extent ALA-PDT kills the host cells or tissue that harbor the infectious microbes.
Figure 9.
Candidate infectious diseases for PDT. A wide variety of localized infections could be clinically treated by antimicrobial PDT.
Figure 10.
ALA or MAL-induced PPIX. Schematic illustrating the interaction of the heme biosynthesis pathway with exogenous ALA or MAL to give intracellular PPIX.
Abbreviations are ALA-D = ALA dehydratase; ALA-S = ALA synthetase; Coprogen III = coproporphyrinogen III; CPO = coproporphyrinogen oxidase; FCH = ferrochelatase; HMB = hydroxymethylbilane, PBG-D = porphobilinogren deaminase; protogen III = protoporphyrinogen; PPO = protoporphyrinogen oxidase; Urogen III = uroporphyrinogen III; UCS = uroporphyrinogen cosynthase, UGD = uroporphyrinogen decarboxylase.
4.1 Localized bacterial infection
There is one report of PDT being clinically used to treat localized bacterial infections by topical administration of PS and light in patients by Lombard et al (76). They intraoperatively treated 5 patients with brain abscesses after craniotomy and surgical drainage by instilling hematoporphyrin into the abscess bed and illuminating 5 minutes afterwards to give a positive clinical response.
A group in Russia has reported using PDT to treat “purulent wounds” (77) and suppurative soft-tissue infections” (78), but the papers are in Russian with no abstracts so obtaining details is difficult.
Photopharmica (www.photopharmica.com), a company based in UK, has carried out clinical trials of topical PDT mediated by a phenothiazinium derivative (PP904) in non-healing leg ulcers infected with bacteria. Improved wound healing and microbial reductions were found but these data have so far only been presented at conferences (79).
4.2 Acne
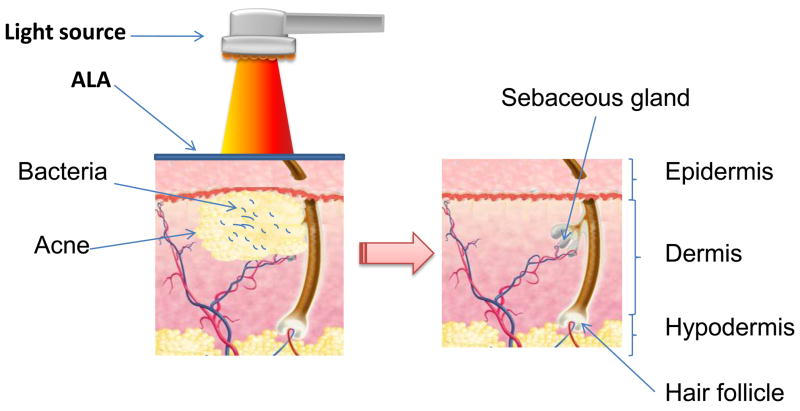
Acne vulgaris is a multifactorial disease. Propionibacterium acnes has been found in the sebaceous glands of patients and is considered a major (but not the only cause) of the disease. Common therapies for acne include both topical and systemic antibacterial therapies (80). Phototherapy without added PS (using lasers or blue light alone) began to be used in 1990s to clear lesions and to improve recent and old scarring (81). Laser therapy is beneficial for people with nodular and cystic acne. The bacterium responsible for acne (P. acnes) has long been known to naturally accumulate red- uorescent porphyrins (82). This property has been used to follow the response of patients to therapy by uorescence photography of the face (83). Ashkenazi et al. (84) confirmed that a particular strain of P. acnes was capable of producing endogenous porphyrins with no need for addition of any aminolevulinic acid (ALA) precursors, and Ramsted et al (85) showed that even more porphyrins were accumulated in the presence of ALA or MAL especially if the temperature was raised.
PDT after ALA, and more recently, methyl aminolevulinate (MAL) has been applied to the skin has been shown to be a safe and effective modality for the treatment of acne vulgaris (80, 86) as illustrated in Figure 11. The first clinical trial using ALA-PDT with 550–570-nm broadband light source in the treatment of acne vulgaris was reported by Hongcharu et al (87) in 2000. MAL-PDT also proved to be an efficient treatment for inflammatory acne (88). Wiegell et al. (89) found that there was no significant differences in the response rate between ALA-PDT and MAL-PDT. The efficacy of topical application of indocyanine green (ICG) dye in combination with the NIR diode laser (803 or 809 nm) phototherapy for treatment of acne vulgaris was demonstrated by Tuchin et al (90). PDT with an intralesional injection of ALA showed a de nite statistical superiority in raising the specificity of the treatment and shortening the incubation time compared with conventional ALA-PDT (91). Photoactivation with blue light, red light (630+/−63 nm) (92), yellow light, broadband or halogen light, or pulsed dye laser devices (93) can all yield significant long-term improvement (94). Taylor reported that topical short-contact (90 min or less) application of ALA or MAL and illumination with a noncoherent light source at 2–4 week intervals for a total of two to four treatments produced the greatest clinical effect (95).
Figure 11.
Schematic depiction of ALA-PDT for acne. The infalammation and the bacteria in the sebaceous gland are destroyed.
4.3 Other dermatologic infections (96–105)
Unlike acne, rosacea is a dermatological condition in which the etiology is less firmly attributed to actual infection but is frequently treated with antibiotics (97). Rosacea has been proposed to be associated with the presence of the Demodex follicularum skin mite in a condition know as demodicosis (106, 107). Gallo et al attributed the pathogenesis of rosacea to the excessive production of antimicrobial peptides such as cathelicidin (108). Bryld and Jemec (109) treated patients with rosacea with MAL-PDT and red light given one to four times and achieved good results in 10 out of 17 patients, and fair results in another 4 patients. However these same authors were unable to find any changes in bacterial flora of the skin after MAL-PDT (96). Katz and Patel reported (110) a case of a 45-year-old woman who presented with facial erythema, papules, pustules, and flushing consistent with severe rosacea, who had failed standard pharmacologic treatments. She received 6 sessions of ALA-PDT given at 2-week intervals. Improvement was evident after the second treatment and was considered “excellent” after the sixth treatment. Improvement continued and no flares were observed 1 month after the final treatment.
Darras-Vercambre et al. (98) reported the first cases of photodynamic treatment of erythrasma, a superficial cutaneous infection. Illumination (80 J/cm2) by red light (broad band, peak at 635 nm) without exogenous photosensitizing molecules achieved a complete recovery for some patients. Calzavara-Pinton et al (103) applied 20% ALA preparation in Eucerin cream under an occlusive dressing to skin lesions of interdigital mycosis of the feet caused by Candida or Trichophyton species followed by irradiation of 75 J/cm2 of broad-band red light. Interdigital lesions of the other foot served as control (treated with only light or only ALA). Clinical and microbiological recovery was seen in six out of nine patients after one (four cases) or four (two cases) treatments. However, after 4 weeks, recurrences were seen in four patients.
4.4 PDT for viral infections
Papillomatosis, caused by human papillomatosis virus (HPV), has been treated by systemic and topical PDT in several anatomic locations. Recurrent respiratory papillomatosis (RRP), which is caused by HPV types 6 and 11, is the most common benign neoplasm of the larynx among children (111). Conventional therapies cannot prevent multiple recurrences. Systemic PDT with dihematoporphyrin ether (4.25 mg/kg) was tested in 48 patients who received 50 J of 630 nm laser light 48 h after application of the drug (112). There was notable improvement with a signi cant decrease in papilloma growth rate compared to control patients. Similar results were reported by Abramson et al. (113) and by Bujia et al.(114). Abramson et al. treated thirty-three patients with moderate to severe recurrent laryngeal papillomatosis with 2.5 mg/kg of dihematoporphyrin ether intravenously either 48 or 72 hours prior to photoactivation with an argon pump dye laser system. Statistical analysis showed 50% patients got a significant decrease (113).
HPV infection is also the course of genital warts also known as condyloma accuminata. They occur on the male or female external genitalia, around the anus, and in women HPV infects the uterine cervix where (if the virus is type 16 or 17) it can lead to development of cervical intraepithelial neoplasia (CIN) and cervical cancer. Ichimura et al. showed PDT (with a 630-nm YAG-OPO laser after 60 h of polyhematoporphyrin ether/ester 2 mg/kg IV) was effective not only in improving the cytological and histological measures when treating CIN but also for eradicating cervical HPV (115).
Abdel-Hady et al. (116) used topical ALA-PDT to treat high-grade vulval intraepithelial neoplasia (VIN 2–3) lesions but observed a short-term response in only one third of cases. Unifocal lesions were found more responsive than multifocal and pigmented lesions. They measured HPV infection; HLA expression; and immune infiltrating cells in VIN biopsies from responders and non-responders. There was a greater likelihood of HPV positivity associated with a lack of response of VIN to PDT, and VIN non-responders were more likely to show HLA class I loss compared with responders. There was a significant increase of CD8 infiltration (cytotoxic T-cells) in post-treatment VIN responders compared with non-responders. High-risk HPV infection and lack of cell-mediated immunity may play a role in the observed poor response of lower genital lesions to topical PDT.
Topical ALA or MAL-PDT has been used to treat condyloma in the vulva, vagina, and penis. Selective accumulation of PPIX fluorescence was demonstrated in the condylomata (117, 118). Chen et al (119) reported a randomized clinical trial comparing ALA-PDT vs. CO2 laser vaporization in treatment of condylomata acuminata: Sixty-five patients were allocated to receive 20% ALA solution under occlusive dressing for 3 h followed by irradiation with the helium-neon laser at a dose of 100 J/cm2 while patients were treated with the CO2 laser. After one treatment, the complete removal rate was 95% in the ALA-PDT group and 100% in the control group. After two treatments with ALA-PDT, the complete removal rate in the treatment group was 100%. The recurrence rate for ALA-PDT group was 6.3% which was significantly lower than that in control group (19.1%, P < 0.05). Moreover, the proportion of patients with adverse effects in the ALA-PDT group (13.9%) was also significantly lower than that in CO2 laser group (100%, P < 0.05). The side-effects in patients treated with ALA-PDT mainly included mild burning and/or stinging restricted to the illuminated area. However Szeimies et al (120) reported no difference in recurrence rates when ALA-PDT was combined with CO2 laser. Herzinger et al (121) performed a small open study using topical 5-ALA and red light (630 nm) in nine men with genital condylomata and a history of at least one previous unsuccessful conventional treatment. Complete cure was achieved in three patients, one of whom experienced a relapse after 3 weeks while 3 patients showed partial responses, and 3 showed no response. Wang et al (122) treated 164 patients with intraurethral condylomata with topical ALA followed by intraurethral light delivery through a cylindrical fiber. The complete response rate was 95% and the recurrence rate was 5% after 6–24 months of follow-up.
Cutaneous warts known as verrucae vulgaris or verrucae plana (also caused by HPV) have been treated with ALA-PDT. In particular when they occur on soles of feet (plantar warts) they cause problems that necessitate treatment. Schroeter et al (123) treated 31 patients with 48 plantar warts with ALA (mean incubation time of 6.8 hours), and the mean treatment time was 18.7 minutes per wart. Each wart was treated an average of 2.3 times, with a median fluence of 100 cm2. Forty-two of 48 (88%) warts showed a complete response. A trend was found between total clearance and size of the warts, age of the patient, and the mean treatment time. No significant side effects were seen postoperatively. Stender et al (124, 125) found ALA-PDT with white light repeated 1 (W1) or 3 (W3) times to be better than ALA-PDT with red (R3) or blue (B3) light repeated 3 times and also better than standard cryotherapy (CRYO) in 30 patients with 250 recalcitrant warts: 73% of the warts treated with W3 were completely healed, 71% after W1, 42% after R3, 23% after B3 and 20% after CRYO. No scars were observed in the ALA-PDT treated areas and patients treated for foot warts were all able to walk after the treatment. No recurrences in completely responding ALA-PDT treated warts were observed after 12 months of follow-up. However ALA-PDT for warts is painful and in 17% of patients it was reported as severe or unbearable requiring pharmacological pain relief (126).
Molluscum contagiosum (MC) is a viral infection of the skin or occasionally of the mucous membranes. MC has no animal reservoir, infecting only humans. The infecting human MC virus is a DNA poxvirus called the molluscum contagiosum virus (MCV). There are four types of MCV, MCV-1 to −4; MCV-1 is the most prevalent and MCV-2 is seen usually in adults and often sexually transmitted. MC has been successfully treated with ALA-PDT in HIV-positive individuals (127, 128).
In the 1970’s there was a burst of popularity in treating Herpes simplex lesions by topical PDT ((129, 130) and reviewed in (131)). Several dyes (of which the most popular choice was neutral red) were topically applied to oral or genital herpes lesions followed by illumination generally with white light. However this practice diminished after Myers et al (132) carried out a controlled clinical trial showing no therapeutic effects in 96 patients and a possible adverse effect on orolabial lesions. In addition to this, concern was raised about the possible carcinogenic effect of the treatment (133).
4.5 Leishmaniasis
Leishmaniasis is a disease caused by protozoan parasites that belong to the genus Leishmania and is transmitted by the bite of certain species of sand fly (subfamily Phlebotominae). Cutaneous leishmaniasis is the most common form of leishmaniasis. There are as yet no standard treatment guidelines for leishmaniasis. Compared with topical paromomycin, significant results were found in the patients who received weekly PDT therapy (10% ALA preparation, red light with a wavelength of 633 nm, light dose of 100J/cm2) (134). PDT of 75 J/cm2 red light performed 12 weeks also showed good results (135). Sohl, Kauer et al. reported that excellent results were achieved with PDT on a patient with facial cutaneous Leishmania tropica infection which proved to be resistant to various therapeutic regimes (136). PDT was showed to be more effective than topical paromomycin and methylbenzethonium chloride in the therapy of cutaneous leishmaniasis (137).
4.6 Dental infections - periodontitis and endodontics
Dental infections are the largest growth area of clinical antimicrobial PDT. This is because three companies are actively involved in clinical trials and are marketing what is still a relatively unknown therapy. Ondine Biopharma (www.ondinebiopharma.com) in North America is using methylene blue (MB) and 660-nm light for treating periodontitis (and nasal MRSA decontamination) while HELBO Photodynamic Systems (www.helbo.at) in Austria is using tolduidine blue O (TBO) and 635-nm light to treat periodontitis and endodontic infection and Denfotex (www.denfotex.com) in UK also uses TBO and 635-nm light to treat endodontics, periodontitis and caries..
Periodontitis is a disease caused by bacterial infection in the dental pocket accompanied with the inflammation of connected tissues and resorption of alveolar bone. Cytokine profiles are of considerable value when studying disease course during treatment. PDT was found to have similar effects on crevicular TNF-alpha and RANKL levels, compared to scaling and root planning treatment alone in patients with aggressive periodontitis (138). The additional application of a single treatment of PDT to scaling and root planing resulted in significantly higher reduction of bleeding scores than following scaling and root planing alone (139). In patients with chronic periodontitis, clinical outcomes of conventional subgingival debridement can be improved by adjunctive PDT, which was performed with a diode laser (660 nm, 100mW/cm2, in combination with phenothiazine chloride (140). Christodoulides et al. also showed patients with chronic periodontitis resulted in a significantly higher reduction in bleeding scores compared to scaling and root planing alone (141). Recently, Qin et al reported that TBO-mediated PDT with 1 mg/mL of TBO plus 12 J/cm2 red laser irradiation could effectively treat periodontitis in vivo and has high potential in clinical application (142).
Another dental application of antimicrobial PDT is in the sterilization of the endodontic root canal in patients who are being treated for necrotic pulp and periapical lesions. In this case PDT can be combined with standard endodontic therapy of mechanical debridement and chemical antimicrobials such as hypochlorite and hydrogen peroxide. Garcez et al. (143) analyzed the antimicrobial effect of PDT using a polyethylenimine chlorin(e6) conjugate and 660-nm light in association with standard endodontic treatment in 20 patients. At the end of the first session, the root canal was filled with Ca(OH)(2), and after 1 week, a second session of the therapies was performed. Endodontic therapy gave a mean reduction of 1.08 log. The combination with PDT significantly enhanced the reduction (1.83 log, p = 0.00002). The second endodontic session gave a similar diminution to the first (1.14 log), and the second PDT was significantly more effective than the first. The second total reduction was significantly higher than the second endodontic therapy. The total first + second reduction (3.19 log) was significantly different from the first combination. Pinheiro et al (144) used TBO mixed with a urea peroxide preparation and red light added to mechanical instrumentation to sterilize root canals in children with deciduous teeth with necrotic pulps. The instrumentation resulted in a reduction of 82.59% of viable bacteria, and, after PDT, the microbial reduction observed was 98.37% (P=0.0126).
4.7 Gastric Helicobacter pylori infection
Helicobacter pylori colonizes the mucus layer of the human stomach and may cause peptic ulcer and adenocarcinoma. Increasing reports mention the emergence of antibiotic resistance to conventional triple drug therapy (145) prompting the search for alternative treatments (146). A preliminary clinical trial was carried out in 13 patients using oral 5-ALA (20 mg/kg) and, 45 minutes later, a zone of gastric antrum was illuminated through an endoscope with a blue laser (410 nm, 50 J/cm2) (147). They demonstrated the greater eradication of HP in biopsies from illuminated areas compared to control zones.
Hamblin et al (148) demonstrated that H. pylori naturally accumulates the photoactive porphyrins, coproporphyrin and protoporphyrin and this means the bacterial cells are exceptionally sensitive to photoinactivation without any added PS, especially when blue light is employed. Ganz et al (149) went on to show that blue light (405 nm, 40 J/cm2) could be delivered to a 1-cm diameter spot in the gastric antrum via optical fiber passed through an upper GI endoscope in patients with proven H. pylori infection. They took weighed biopsies from treated and control spots and colonies quantitatively were cultured. On average of 90% of the CFU were destroyed. This group then went on to demonstrate that whole stomach illumination with 405-nm light was feasible and safe (150). A novel light source consisting of laser diodes and diffusing fibers delivered 408-nm illumination at a total optical power of 12W and provided escalating total fluences to the whole stomach. Eighteen adults (10 female) with H. pylori infection were treated at three U.S. academic endoscopy centers. Quantitative bacterial counts were obtained from biopsies taken from the antrum, body, and fundus, and serial urea breath tests. The largest reduction in bacterial load was in the antrum (>97%), followed by body (>95%) and fundus (>86%). There was a correlation between log reduction and initial bacterial load in the antrum. There was no dose-response seen with increasing illumination times. The urea breath test results indicated that the bacteria repopulated in days following illumination.
5 Conclusion and future outlook
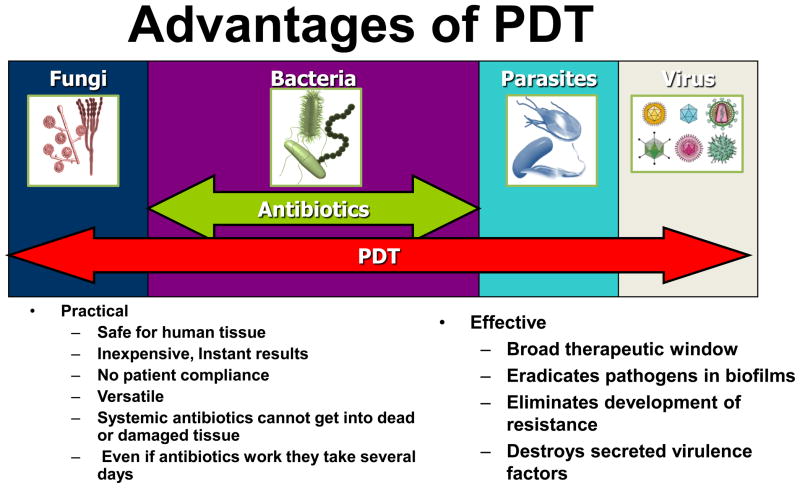
The never-ending world-wide rise in drug-resistance amongst many classes of pathogenic microbes leading to lessening effectiveness of standard antibiotic, antiviral and anti-parasitic therapies will only continue to give rise to international concern. The day may arrive when infections return as the chief cause of premature death, as indeed they have been throughout most of human history. This worrying phenomenon has led to an astonishing research effort both in academic laboratories and in small companies on new, alternative antimicrobial technologies that do not rely on the selective pharmacology and cell biology approaches that have given us 60 years of very successful antibiotics. In our opinion one of the most important examples of these new technologies is antimicrobial PDT and its many advantages are summarized in Figure 12. To the extent that the PS can be targeted to the microbial cell and the light can be targeted to the infected tissue area, PDT has double selectivity. Nevertheless it is important to realize that many infectious diseases will continue to need systemic therapy.
Figure 12.
Schematic depiction of the advantages of PDT for localized infections compared to antibiotic drugs.
One important topic in PDT, which has not so far been much investigated in PDT for infectious disease, is its role in stimulating the host immune system. It is reasonably well established that when PDT is used to treat cancer, it possesses a particular ability to increase the host immune response against the cancer (151). This property is proposed to be due to the PDT-induced killing of tumor cells creating or releasing a mixture of tumor antigens and cellular danger signals at the same time as the acute inflammatory response caused by PDT attracts, activates and matures dendritic cells and other cellular components of both the innate and adaptive immune systems. In principle this same process should operate when infections are treated by PDT, but we are aware of only one paper by Abdel-Hady (previously discussed (116)) that even comes close to approaching this topic. This avenue could be a fruitful field for further study.
Another beneficial property of antimicrobial PDT that is not commonly seen in other antimicrobial therapies is its ability to photo-destroy secreted virulence factors. Most of the molecules that act as secreted virulence factors are proteins or enzymes and it is well known that proteins in solution are highly vulnerable to oxidation of sensitive aminoacid residues such as cysteine, methionine, tryptophan, tyrosine and histidine. This ability of PDT to destroy secreted virulence factors has been shown for lipolysaccharide and Pseudomonas proteases (152). Hamblin et al proposed (52) the PDI of protease and other secreted virulence factors could explain the better wound healing observed when P. aeruginosa infected wounds were sterilized by PDT compared to being sterilized by silver nitrate.
One bottleneck in the wider application of PDT for clinical infections is the lack of highly effective antimicrobial PS with clinical approval. The phenothiazinium dyes (MB and TBO) and ALA or MAL-induced PPIX are the only PS that have been widely used in patients for infectious disease. While these do have some efficacy depending on the type of microbe and the anatomical location of the infection, those who study antimicrobial PDT know that optimized PS molecules are available that have hundreds or even thousands of time the potency, but have never been subjected to the costly toxicological and safety studies necessary for approval for human use. The rapid increase in recent years in the clinical use of PDT for periodontitis gives just a foretaste of the number of infections that could be clinically treated in the future.
Acknowledgments
Research in the Hamblin laboratory is supported by the NIH (grant RO1AI050875) and the US Air Force MFEL program (contract FA9550-04-1-0079). T. Dai was supported by the Bullock-Wellman Postdoctoral Fellowship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell SG. Antibiotic resistance: is the end of an era near? Neonatal Netw. 2003;22:47–54. doi: 10.1891/0730-0832.22.6.47. [DOI] [PubMed] [Google Scholar]
- 2.Poole MD. Are we facing the end of the antibiotic era? Ear Nose Throat J. 1993;72:433. [PubMed] [Google Scholar]
- 3.Harrison JW, Svec TA. The beginning of the end of the antibiotic era? Part II. Proposed solutions to antibiotic abuse. Quintessence Int. 1998;29:223–229. [PubMed] [Google Scholar]
- 4.Mroz P, Hamblin MR. Advances in photodynamic therapy: basic, translational and clinical. Norwood, MA: Artech House; 2008. [Google Scholar]
- 5.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 6.O’Riordan K, Sharlin DS, Gross J, Chang S, Errabelli D, Akilov OE, Kosaka S, Nau GJ, Hasan T. Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrob Agents Chemother. 2006;50:1828–1834. doi: 10.1128/AAC.50.5.1828-1834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004;3:412–418. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 8.Bergmans L, Moisiadis P, Huybrechts B, Van Meerbeek B, Quirynen M, Lambrechts P. Effect of photo-activated disinfection on endodontic pathogens ex vivo. Int Endod J. 2008;41:227–239. doi: 10.1111/j.1365-2591.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem. 2009;9:947–983. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 11.Tang HM, Hamblin MR, Yow CM. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J Infect Chemother. 2007;13:87–91. doi: 10.1007/s10156-006-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem Photobiol Sci. 2002;1:468–470. doi: 10.1039/b200977c. [DOI] [PubMed] [Google Scholar]
- 13.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 14.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 15.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertoloni G, Rossi F, Valduga G, Jori G, van Lier J. Photosensitizing activity of water-and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol Lett. 1990;59:149–155. doi: 10.1111/j.1574-6968.1990.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 17.Lazzeri D, Rovera M, Pascual L, Durantini EN. Photodynamic studies and photoinactivation of Escherichia coli using meso-substituted cationic porphyrin derivatives with asymmetric charge distribution. Photochem Photobiol. 2004;80:286–293. doi: 10.1562/2004-03-08-RA-105. [DOI] [PubMed] [Google Scholar]
- 18.Segalla A, Borsarelli CD, Braslavsky SE, Spikes JD, Roncucci G, Dei D, Chiti G, Jori G, Reddi E. Photophysical, photochemical and antibacterial photosensitizing properties of a novel octacationic Zn(II)-phthalocyanine. Photochem Photobiol Sci. 2002;1:641–648. doi: 10.1039/b202031a. [DOI] [PubMed] [Google Scholar]
- 19.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown ST. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 20.Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg Med. 2001;29:165–173. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 21.Wainwright M, Crossley KB. Methylene Blue--a therapeutic dye for all seasons? J Chemother. 2002;14:431–443. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- 22.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 23.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kussovski V, Mantareva V, Angelov I, Orozova P, Wohrle D, Schnurpfeil G, Borisova E, Avramov L. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett. 2009;294:133–140. doi: 10.1111/j.1574-6968.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- 25.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamblin MR, O’Donnell DA, Murthy N, Rajagopalan K, Michaud N, Sherwood ME, Hasan T. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941–951. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 27.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–1410. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George S, Hamblin MR, Kishen A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem Photobiol Sci. 2009;8:788–795. doi: 10.1039/b809624d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitzan Y, Balzam-Sudakevitz A, Ashkenazi H. Eradication of Acinetobacter baumannii by photosensitized agents in vitro. J Photochem Photobiol B. 1998;42:211–218. doi: 10.1016/s1011-1344(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 32.Wilson M, Pratten J. Lethal photosensitisation of Staphylococcus aureus in vitro: effect of growth phase, serum, and pre-irradiation time. Lasers Surg Med. 1995;16:272–276. doi: 10.1002/lsm.1900160309. [DOI] [PubMed] [Google Scholar]
- 33.Lambrechts SA, Aalders MC, Verbraak FD, Lagerberg JW, Dankert JB, Schuitmaker JJ. Effect of albumin on the photodynamic inactivation of microorganisms by a cationic porphyrin. J Photochem Photobiol B. 2005;79:51–57. doi: 10.1016/j.jphotobiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Street CN, Pedigo L, Gibbs A, Loebel NG. Antimicrobial photodynamic therapy for the decolonization of methicillin-resistant Staphylococcus aureus from the anterior nares. Proc SPIE. 2009;7380 doi: 10.1117/1112.828279. [DOI] [Google Scholar]
- 35.Mohr H, Bachmann B, Klein-Struckmeier A, Lambrecht B. Virus inactivation of blood products by phenothiazine dyes and light. Photochem Photobiol. 1997;65:441–445. doi: 10.1111/j.1751-1097.1997.tb08586.x. [DOI] [PubMed] [Google Scholar]
- 36.Mohr H, Knuver-Hopf J, Gravemann U, Redecker-Klein A, Muller TH. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion. 2004;44:886–890. doi: 10.1111/j.1537-2995.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- 37.Mohr H, Redecker-Klein A. Inactivation of pathogens in platelet concentrates by using a two-step procedure. Vox Sang. 2003;84:96–104. doi: 10.1046/j.1423-0410.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Hur E, Geacintov NE, Studamire B, Kenney ME, Horowitz B. The effect of irradiance on virus sterilization and photodynamic damage in red blood cells sensitized by phthalocyanines. Photochem Photobiol. 1995;61:190–195. doi: 10.1111/j.1751-1097.1995.tb03959.x. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Hur E, Barshtein G, Chen S, Yedgar S. Photodynamic treatment of red blood cell concentrates for virus inactivation enhances red blood cell aggregation: protection with antioxidants. Photochem Photobiol. 1997;66:509–512. doi: 10.1111/j.1751-1097.1997.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 40.Maisch T, Bosl C, Szeimies RM, Love B, Abels C. Determination of the antibacterial efficacy of a new porphyrin-based photosensitizer against MRSA ex vivo. Photochem Photobiol Sci. 2007;6:545–551. doi: 10.1039/b614770d. [DOI] [PubMed] [Google Scholar]
- 41.Smijs TG, Bouwstra JA, Schuitmaker HJ, Talebi M, Pavel S. A novel ex vivo skin model to study the susceptibility of the dermatophyte Trichophyton rubrum to photodynamic treatment in different growth phases. J Antimicrob Chemother. 2007;59:433–440. doi: 10.1093/jac/dkl490. [DOI] [PubMed] [Google Scholar]
- 42.Rocas IN, Siqueira JF, Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30:315–320. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Millson CE, Wilson M, MacRobert AJ, Bown SG. Ex-vivo treatment of gastric Helicobacter infection by photodynamic therapy. J Photochem Photobiol B. 1996;32:59–65. doi: 10.1016/1011-1344(95)07190-3. [DOI] [PubMed] [Google Scholar]
- 44.Wood TK. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ Microbiol. 2009;11:1–15. doi: 10.1111/j.1462-2920.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- 46.Jain A, Gupta Y, Agrawal R, Khare P, Jain SK. Biofilms--a microbial life perspective: a critical review. Crit Rev Ther Drug Carrier Syst. 2007;24:393–443. doi: 10.1615/critrevtherdrugcarriersyst.v24.i5.10. [DOI] [PubMed] [Google Scholar]
- 47.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. J Photochem Photobiol B. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocchetta HL, Boylan CJ, Foley JW, Iversen PW, LeTourneau DL, McMillian CL, Contag PR, Jenkins DE, Parr TR., Jr Validation of a noninvasive, real-time imaging technology using bioluminescent escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001;45:129–137. doi: 10.1128/AAC.45.1.129-137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis KP, Joh D, Bellinger-Kawahara C, Hawkinson MJ, Purchio TF, Contag PR. Monitoring bioluminescent Staphylococcus aureus infections in living mice using a novel luxABCDE construct. Infect Immun. 2000;68:3594–3600. doi: 10.1128/iai.68.6.3594-3600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, Purchio TF, Caparon MG, Lipsitch M, Contag PR. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun. 2001;69:3350–3358. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol. 2002;75:51–57. doi: 10.1562/0031-8655(2002)075<0051:rcowib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–1725. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong TW, Wang YY, Sheu HM, Chuang YC. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49:895–902. doi: 10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, Nitzan Y. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol. 1997;19:307–314. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 56.Lambrechts SA, Demidova TN, Aalders MC, Hasan T, Hamblin MR. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem Photobiol Sci. 2005;4:503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai T, Tegos GP, Lu Z, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (N Y) 1994;12:703–706. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 59.Gad F, Zahra T, Francis KP, Hasan T, Hamblin MR. Targeted photodynamic therapy of established soft-tissue infections in mice. Photochem Photobiol Sci. 2004;3:451–458. doi: 10.1039/b311901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shibli JA, Martins MC, Nociti FH, Jr, Garcia VG, Marcantonio E., Jr Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary histologic study in dogs. J Periodontol. 2003;74:338–345. doi: 10.1902/jop.2003.74.3.338. [DOI] [PubMed] [Google Scholar]
- 61.Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandes LA, de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Martins TM, Okamoto T, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats. J Clin Periodontol. 2009;36:219–228. doi: 10.1111/j.1600-051X.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 63.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol. 2008;79:2156–2165. doi: 10.1902/jop.2008.080103. [DOI] [PubMed] [Google Scholar]
- 64.de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol. 2007;78:566–575. doi: 10.1902/jop.2007.060214. [DOI] [PubMed] [Google Scholar]
- 65.Sigusch BW, Pfitzner A, Albrecht V, Glockmann E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76:1100–1105. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 66.Teichert MC, Jones JW, Usacheva MN, Biel MA. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:155–160. doi: 10.1067/moe.2002.120051. [DOI] [PubMed] [Google Scholar]
- 67.Lin J, Bi LJ, Zhang ZG, Fu YM, Dong TT. Toluidine blue-mediated photodynamic therapy of oral wound infections in rats. Lasers Med Sci. 2009 doi: 10.1007/s10103-009-0700-5. [DOI] [PubMed] [Google Scholar]
- 68.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem Photobiol Sci. 2007;6:1067–1075. doi: 10.1039/b703521g. [DOI] [PubMed] [Google Scholar]
- 69.Akilov OE, Kosaka S, O’Riordan K, Hasan T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp Dermatol. 2007;16:651–660. doi: 10.1111/j.1600-0625.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 70.Akilov OE, Yousaf W, Lukjan SX, Verma S, Hasan T. Optimization of topical photodynamic therapy with 3,7-bis(di-n-butylamino)phenothiazin-5-ium bromide for cutaneous leishmaniasis. Lasers Surg Med. 2009;41:358–365. doi: 10.1002/lsm.20775. [DOI] [PubMed] [Google Scholar]
- 71.O’Riordan K, Akilov OE, Chang SK, Foley JW, Hasan T. Real-time fluorescence monitoring of phenothiazinium photosensitizers and their anti-mycobacterial photodynamic activity against Mycobacterium bovis BCG in in vitro and in vivo models of localized infection. Photochem Photobiol Sci. 2007;6:1117–1123. doi: 10.1039/b707962a. [DOI] [PubMed] [Google Scholar]
- 72.Jung JY, Seung Kwon P, Chul Ahn J, GeR, Suh MW, Rhee CK. In vitro and in vivo photodynamic therapy of otitis media in gerbils. Laryngoscope. 2009 doi: 10.1002/lary.20568. [DOI] [PubMed] [Google Scholar]
- 73.Bisland SK, Chien C, Wilson BC, Burch S. Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem Photobiol Sci. 2006;5:31–38. doi: 10.1039/b507082a. [DOI] [PubMed] [Google Scholar]
- 74.Smetana Z, Malik Z, Orenstein A, Mendelson E, Ben-Hur E. Treatment of viral infections with 5-aminolevulinic acid and light. Lasers Surg Med. 1997;21:351–358. doi: 10.1002/(sici)1096-9101(1997)21:4<351::aid-lsm6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 75.Moore C, Wallis C, Melnick JL, Kuns MD. Photodynamic treatment of herpes keratitis. Infect Immun. 1972;5:169–171. doi: 10.1128/iai.5.2.169-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lombard GF, Tealdi S, Lanotte MM. The treatment of neurosurgical infections by lasers and porphyrins. In: Jori G, Perria CA, editors. Photodynamic Therapy of Tumors and other Diseases. Padova, Italy: Edizione Libreria Progetto; 1985. pp. 363–366. [Google Scholar]
- 77.Tolstykh PI, Stranadko EF, Koraboev UM, Urinov A, Tolstykh MP, Terekhova RP, Volkova NN, Duvanskii VA. Experimental study of photodynamic effect on bacterial wound microflora. Zh Mikrobiol Epidemiol Immunobiol. 2001:85–87. [PubMed] [Google Scholar]
- 78.Stranadko EF, Koraboev UM, Tolstykh MP. Photodynamic therapy in suppurative diseases of soft tissue. Khirurgiia (Mosk) 2000:67–70. [PubMed] [Google Scholar]
- 79.Brown SB. Clinical developments in antimicrobial PDT. 12th International Photodynamic Association World Congress; Seattle. 2009. [Google Scholar]
- 80.Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007;22:67–72. doi: 10.1007/s10103-006-0420-z. [DOI] [PubMed] [Google Scholar]
- 81.Houk LD, Humphreys T. Masers to magic bullets: an updated history of lasers in dermatology. Clin Dermatol. 2007;25:434–442. doi: 10.1016/j.clindermatol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Johnsson A, Kjeldstad B, Melo TB. Fluorescence from pilosebaceous follicles. Arch Dermatol Res. 1987;279:190–193. doi: 10.1007/BF00413256. [DOI] [PubMed] [Google Scholar]
- 83.Meffert H, Gaunitz K, Gutewort T, Amlong UJ. Therapy of acne with visible light. Decreased irradiation time by using a blue-light high-energy lamp. Dermatol Monatsschr. 1990;176:597–603. [PubMed] [Google Scholar]
- 84.Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003;35:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 85.Ramstad S, Le Anh-Vu N, Johnsson A. The temperature dependence of porphyrin production in Propionibacterium acnes after incubation with 5-aminolevulinic acid (ALA) and its methyl ester (m-ALA) Photochem Photobiol Sci. 2006;5:66–72. doi: 10.1039/b512837d. [DOI] [PubMed] [Google Scholar]
- 86.Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC. Phototherapy in the treatment of acne vulgaris: what is its role? Am J Clin Dermatol. 2004;5:211–216. doi: 10.2165/00128071-200405040-00001. [DOI] [PubMed] [Google Scholar]
- 87.Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000;115:183–192. doi: 10.1046/j.1523-1747.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 88.Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using methyl aminolaevulinate: a blinded, randomized, controlled trial. Br J Dermatol. 2006;154:969–976. doi: 10.1111/j.1365-2133.2005.07107.x. [DOI] [PubMed] [Google Scholar]
- 89.Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate. J Am Acad Dermatol. 2006;54:647–651. doi: 10.1016/j.jaad.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 90.Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB. A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment. Lasers Surg Med. 2003;33:296–310. doi: 10.1002/lsm.10211. [DOI] [PubMed] [Google Scholar]
- 91.Ryou JH, Lee SJ, Park YM, Kim HO, Kim HS. Acne-photodynamic therapy with intra-lesional injection of 5-aminolevulinic acid. Photodermatol Photoimmunol Photomed. 2009;25:57–58. doi: 10.1111/j.1600-0781.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- 92.Hong SB, Lee MH. Topical aminolevulinic acid-photodynamic therapy for the treatment of acne vulgaris. Photodermatol Photoimmunol Photomed. 2005;21:322–325. doi: 10.1111/j.1600-0781.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 93.Gold MH. Photodynamic Therapy with Lasers and Intense Pulsed Light. Facial Plast Surg Clin North Am. 2007;15:145–160. doi: 10.1016/j.fsc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Nestor MS. The use of photodynamic therapy for treatment of acne vulgaris. Dermatol Clin. 2007;25:47–57. doi: 10.1016/j.det.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 95.Taylor MN, Gonzalez ML. The practicalities of photodynamic therapy in acne vulgaris. Br J Dermatol. 2009;160:1140–1148. doi: 10.1111/j.1365-2133.2009.09054.x. [DOI] [PubMed] [Google Scholar]
- 96.Bryld LE, Jemec GB. The bacterial flora of the skin surface following routine MAL-PDT. J Dermatolog Treat. 2006;17:222–223. doi: 10.1080/09546630600830570. [DOI] [PubMed] [Google Scholar]
- 97.Ceilley RI. Advances in the topical treatment of acne and rosacea. J Drugs Dermatol. 2004;3:S12–22. [PubMed] [Google Scholar]
- 98.Darras-Vercambre S, Carpentier O, Vincent P, Bonnevalle A, Thomas P. Photodynamic action of red light for treatment of erythrasma: preliminary results. Photodermatol Photoimmunol Photomed. 2006;22:153–156. doi: 10.1111/j.1600-0781.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 99.Yung A, Stables GI, Fernandez C, Williams J, Bojar RA, Goulden V. Microbiological effect of photodynamic therapy (PDT) in healthy volunteers: a comparative study using methyl aminolaevulinate and hexyl aminolaevulinate cream. Clin Exp Dermatol. 2007;32:716–721. doi: 10.1111/j.1365-2230.2007.02472.x. [DOI] [PubMed] [Google Scholar]
- 100.Calzavara-Pinton PG, Venturini M, Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J Photochem Photobiol B. 2005;78:1–6. doi: 10.1016/j.jphotobiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 101.Donnelly RF, McCarron PA, Tunney MM. Antifungal photodynamic therapy. Microbiol Res. 2008;163:1–12. doi: 10.1016/j.micres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Donnelly RF, McCarron PA, Tunney MM, David Woolfson A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B. 2006 doi: 10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 103.Calzavara-Pinton PG, Venturini M, Capezzera R, Sala R, Zane C. Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid. Photodermatol Photoimmunol Photomed. 2004;20:144–147. doi: 10.1111/j.1600-0781.2004.00095.x. [DOI] [PubMed] [Google Scholar]
- 104.Kim YJ, Kim YC. Successful treatment of pityriasis versicolor with 5-aminolevulinic acid photodynamic therapy. Arch Dermatol. 2007;143:1218–1220. doi: 10.1001/archderm.143.9.1218. [DOI] [PubMed] [Google Scholar]
- 105.Sotiriou E, Koussidou T, Patsatsi A, Apalla Z, Ioannides D. 5-Aminolevulinic acid-photodynamic treatment for dermatophytic tinea pedis of interdigital type: a small clinical study. J Eur Acad Dermatol Venereol. 2008 doi: 10.1111/j.1468-3083.2008.02783.x. [DOI] [PubMed] [Google Scholar]
- 106.Moravvej H, Dehghan-Mangabadi M, Abbasian MR, Meshkat-Razavi G. Association of rosacea with demodicosis. Arch Iran Med. 2007;10:199–203. [PubMed] [Google Scholar]
- 107.Skrlin J, Richter B, Basta-Juzbasic A, Matica B, Ivacic B, Cvrlje M, Kucisec N, Baucic A. Demodicosis and rosacea. Lancet. 1991;337:734. doi: 10.1016/0140-6736(91)90318-j. [DOI] [PubMed] [Google Scholar]
- 108.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 109.Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21:1199–1202. doi: 10.1111/j.1468-3083.2007.02219.x. [DOI] [PubMed] [Google Scholar]
- 110.Katz B, Patel V. Photodynamic therapy for the treatment of erythema, papules, pustules, and severe flushing consistent with rosacea. J Drugs Dermatol. 2006;5:6–8. [PubMed] [Google Scholar]
- 111.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–1247. doi: 10.1097/MLG.0b013e31816a7135. [DOI] [PubMed] [Google Scholar]
- 112.Shikowitz MJ, Abramson AL, Freeman K, Steinberg BM, Nouri M. Efficacy of DHE photodynamic therapy for respiratory papillomatosis: immediate and long-term results. Laryngoscope. 1998;108:962–967. doi: 10.1097/00005537-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 113.Abramson AL, Shikowitz MJ, Mullooly VM, Steinberg BM, Amella CA, Rothstein HR. Clinical effects of photodynamic therapy on recurrent laryngeal papillomas. Arch Otolaryngol Head Neck Surg. 1992;118:25–29. doi: 10.1001/archotol.1992.01880010029011. [DOI] [PubMed] [Google Scholar]
- 114.Bujia J, Feyh J, Kastenbauer E. Photodynamic therapy with derivatives from hemotoporphyrines for recurrent laryngeal papillomatosis of the children. Early results. An Otorrinolaringol Ibero Am. 1993;20:251–259. [PubMed] [Google Scholar]
- 115.Ichimura H, Yamaguchi S, Kojima A, Tanaka T, Niiya K, Takemori M, Hasegawa K, Nishimura R. Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial neoplasia. Int J Clin Oncol. 2003;8:322–325. doi: 10.1007/s10147-003-0354-4. [DOI] [PubMed] [Google Scholar]
- 116.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G, Kitchener HC, Hampson IN. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–196. [PubMed] [Google Scholar]
- 117.Ross EV, Romero R, Kollias N, Crum C, Anderson RR. Selectivity of protoporphyrin IX fluorescence for condylomata after topical application of 5-aminolaevulinic acid: implications for photodynamic treatment. Br J Dermatol. 1997;137:736–742. [PubMed] [Google Scholar]
- 118.Fehr MK, Chapman CF, Krasieva T, Tromberg BJ, McCullough JL, Berns MW, Tadir Y. Selective photosensitizer distribution in vulvar condyloma acuminatum after topical application of 5-aminolevulinic acid. Am J Obstet Gynecol. 1996;174:951–957. doi: 10.1016/s0002-9378(96)70332-0. [DOI] [PubMed] [Google Scholar]
- 119.Chen K, Chang BZ, Ju M, Zhang XH, Gu H. Comparative study of photodynamic therapy vs CO2 laser vaporization in treatment of condylomata acuminata: a randomized clinical trial. Br J Dermatol. 2007;156:516–520. doi: 10.1111/j.1365-2133.2006.07648.x. [DOI] [PubMed] [Google Scholar]
- 120.Szeimies RM, Schleyer V, Moll I, Stocker M, Landthaler M, Karrer S. Adjuvant photodynamic therapy does not prevent recurrence of condylomata acuminata after carbon dioxide laser ablation-A phase III, prospective, randomized, bicentric, double-blind study. Dermatol Surg. 2009;35:757–764. doi: 10.1111/j.1524-4725.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 121.Herzinger T, Wienecke R, Weisenseel P, Borelli C, Berking C, Degitz K. Photodynamic therapy of genital condylomata in men. Clin Exp Dermatol. 2006;31:51–53. doi: 10.1111/j.1365-2230.2005.01935.x. [DOI] [PubMed] [Google Scholar]
- 122.Wang XL, Wang HW, Wang HS, Xu SZ, Liao KH, Hillemanns P. Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata. Br J Dermatol. 2004;151:880–885. doi: 10.1111/j.1365-2133.2004.06189.x. [DOI] [PubMed] [Google Scholar]
- 123.Schroeter CA, Pleunis J, van Nispen tot Pannerden C, Reineke T, Neumann HA. Photodynamic therapy: new treatment for therapy-resistant plantar warts. Dermatol Surg. 2005;31:71–75. doi: 10.1111/j.1524-4725.2005.31011. [DOI] [PubMed] [Google Scholar]
- 124.Stender IM, Lock-Andersen J, Wulf HC. Recalcitrant hand and foot warts successfully treated with photodynamic therapy with topical 5-aminolaevulinic acid: a pilot study. Clin Exp Dermatol. 1999;24:154–159. doi: 10.1046/j.1365-2230.1999.00441.x. [DOI] [PubMed] [Google Scholar]
- 125.Stender IM, Na R, Fogh H, Gluud C, Wulf HC. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet. 2000;355:963–966. doi: 10.1016/S0140-6736(00)90013-8. [DOI] [PubMed] [Google Scholar]
- 126.Stender IM, Borgbjerg FM, Villumsen J, Lock-Andersen J, Wulf HC. Pain induced by photodynamic therapy of warts. Photodermatol Photoimmunol Photomed. 2006;22:304–309. doi: 10.1111/j.1600-0781.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 127.Gold MH, Boring MM, Bridges TM, Bradshaw VL. The successful use of ALA-PDT in the treatment of recalcitrant molluscum contagiosum. J Drugs Dermatol. 2004;3:187–190. [PubMed] [Google Scholar]
- 128.Scheinfeld N. Treatment of molluscum contagiosum: a brief review and discussion of a case successfully treated with adapelene. Dermatol Online J. 2007;13:15. [PubMed] [Google Scholar]
- 129.Roome AP, Tinkler AE, Hilton AL, Montefiore DG, Waller D. Neutral red with photoinactivation in the treatment of herpes genitalis. Br J Vener Dis. 1975;51:130–133. doi: 10.1136/sti.51.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaufman RH, Gardner HL, Brown D, Wallis C, Rawls WE, Melnick JL. Herpes genitalis treated by photodynamic inactivation of virus. Am J Obstet Gynecol. 1973;117:1144–1146. doi: 10.1016/0002-9378(73)90768-0. [DOI] [PubMed] [Google Scholar]
- 131.Bockstahler LE, Lytle CD, Hellman KB. A review of photodynamic therapy for herpes simplex: benefits and potential risks. N Y J Dent. 1975;45:148–157. [PubMed] [Google Scholar]
- 132.Myers MG, Oxman MN, Clark JE, Arndt KA. Failure of neutral-red photodynamic inactivation in recurrent herpes simplex virus infections. N Engl J Med. 1975;293:945–949. doi: 10.1056/NEJM197511062931901. [DOI] [PubMed] [Google Scholar]
- 133.Friedrich EG, Jr, Kaufman RH, Lynch PJ, Woodruff D. Vulvar histology after neutral red photoinactivation of herpes simplex virus. Obstet Gynecol. 1976;48:564–570. [PubMed] [Google Scholar]
- 134.Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial. Clin Exp Dermatol. 2006 doi: 10.1111/j.1365-2230.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- 135.Gardlo K, Horska Z, Enk CD, Rauch L, Megahed M, Ruzicka T, Fritsch C. Treatment of cutaneous leishmaniasis by photodynamic therapy. J Am Acad Dermatol. 2003;48:893–896. doi: 10.1067/mjd.2003.218. [DOI] [PubMed] [Google Scholar]
- 136.Sohl S, Kauer F, Paasch U, Simon JC. Photodynamic treatment of cutaneous leishmaniasis. J Dtsch Dermatol Ges. 2007;5:128–130. doi: 10.1111/j.1610-0387.2007.06177.x. [DOI] [PubMed] [Google Scholar]
- 137.Gonzalez U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008:CD005067. doi: 10.1002/14651858.CD005067.pub3. [DOI] [PubMed] [Google Scholar]
- 138.de Oliveira RR, Schwartz-Filho HO, Novaes AB, Garlet GP, de Souza RF, Taba M, Scombatti de Souza SL, Ribeiro FJ. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: cytokine profile in gingival crevicular fluid, preliminary results. J Periodontol. 2009;80:98–105. doi: 10.1902/jop.2009.070465. [DOI] [PubMed] [Google Scholar]
- 139.Chondros P, Nikolidakis D, Christodoulides N, Rossler R, Gutknecht N, Sculean A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci. 2008 doi: 10.1007/s10103-008-0565-z. [DOI] [PubMed] [Google Scholar]
- 140.Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol. 2008;35:877–884. doi: 10.1111/j.1600-051X.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 141.Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rossler R, Sculean A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol. 2008;79:1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 142.Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontal Res. 2008;43:162–167. doi: 10.1111/j.1600-0765.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 143.Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod. 2008;34:138–142. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pinheiro SL, Schenka AA, Neto AA, de Souza CP, Rodriguez HM, Ribeiro MC. Photodynamic therapy in endodontic treatment of deciduous teeth. Lasers Med Sci. 2008 doi: 10.1007/s10103-008-0562-2. [DOI] [PubMed] [Google Scholar]
- 145.Savarino V, Zentilin P, Pivari M, Bisso G, Raffaella Mele M, Bilardi C, Borro P, Dulbecco P, Tessieri L, Mansi C, Borgonovo G, De Salvo L, Vigneri S. The impact of antibiotic resistance on the efficacy of three 7-day regimens against Helicobacter pylori. Aliment Pharmacol Ther. 2000;14:893–900. doi: 10.1046/j.1365-2036.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- 146.Jodlowski TZ, Lam S, Ashby CR., Jr Emerging therapies for the treatment of Helicobacter pylori infections. Ann Pharmacother. 2008;42:1621–1639. doi: 10.1345/aph.1L234. [DOI] [PubMed] [Google Scholar]
- 147.Wilder-Smith CH, Wilder-Smith P, Grosjean P, van den Bergh H, Woodtli A, Monnier P, Dorta G, Meister F, Wagnieres G. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg Med. 2002;31:18–22. doi: 10.1002/lsm.10066. [DOI] [PubMed] [Google Scholar]
- 148.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC, 3rd, Lindmark WR, McGrath JR, Hamblin MR. Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: A pilot clinical trial. Lasers Surg Med. 2009;41:337–344. doi: 10.1002/lsm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Komerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]