Abstract
Bordetella pertussis, the causative agent of whooping cough, has been shown recently to enter and survive in epithelial cells and macrophages in vitro. In the present study, we show that B. pertussis is cytotoxic for J774A.1 cells, a monocyte-macrophage cell line, and for murine alveolar macrophages. We demonstrate that cell cytotoxicity mediated by B. pertussis occurred through apoptosis, as shown by changes in nuclear morphology and by host cell DNA fragmentation. Parental strains and a mutant deficient in pertussis toxin expression are able to induce apoptosis, whereas avirulent mutant or adenylate cyclase-hemolysin-deficient mutants are not cytotoxic. Both adenylate cyclase and hemolytic activities are required for programmed cell death. These results show that induction of apoptosis is dependent on the expression of adenylate cyclase-hemolysin. The infection of murine alveolar macrophages in primary culture with B. pertussis leads to apoptosis, suggesting that this process might be relevant in vivo. The ability of B. pertussis to promote cell death may be important for the initiation of infection, bacterial survival, and escape of the host immune response.
Full text
PDF






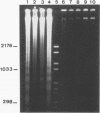
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine R., Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990 Jun;58(6):1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Aricò B., Scarlato V., Monack D. M., Falkow S., Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991 Oct;5(10):2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Bromberg K., Tannis G., Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991 Dec;59(12):4715–4719. doi: 10.1128/iai.59.12.4715-4719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. P., Bramhall J., Graves S., Bonavida B., Wisnieski B. J. Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem. 1989 Sep 15;264(26):15261–15267. [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Coote J. G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992 Feb;8(2):137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- Dowd D. R., Miesfeld R. L. Evidence that glucocorticoid- and cyclic AMP-induced apoptotic pathways in lymphocytes share distal events. Mol Cell Biol. 1992 Aug;12(8):3600–3608. doi: 10.1128/mcb.12.8.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Endoh M., Nagai M., Ueda T., Yoshida Y., Nakase Y. Cytopathic effect of heat-labile toxin of Bordetella parapertussis on aortic smooth muscle cells from pigs or guinea pigs. Microbiol Immunol. 1988;32(4):423–428. doi: 10.1111/j.1348-0421.1988.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn T. M., Shahin R., Mekalanos J. J. Characterization of vir-activated TnphoA gene fusions in Bordetella pertussis. Infect Immun. 1991 Sep;59(9):3273–3279. doi: 10.1128/iai.59.9.3273-3279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Fiederlein R. L., Glasser L., Galgiani J. N. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect Immun. 1987 Jan;55(1):135–140. doi: 10.1128/iai.55.1.135-140.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Nordensson K., Wilson L., Akporiaye E. T., Yocum D. E. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992 Nov;60(11):4578–4585. doi: 10.1128/iai.60.11.4578-4585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The steady state in cellular immunity. II. Immunological complaisance in murine pertussis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):417–426. doi: 10.1038/icb.1967.40. [DOI] [PubMed] [Google Scholar]
- Hanski E., Farfel Z. Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. J Biol Chem. 1985 May 10;260(9):5526–5532. [PubMed] [Google Scholar]
- Hanski E. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci. 1989 Nov;14(11):459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Mechanisms for inhibition of the catalytic activity of adenylate cyclase by the guanine nucleotide-binding proteins serving as the substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1986 Apr 15;261(11):5215–5221. [PubMed] [Google Scholar]
- Khelef N., Danve B., Quentin-Millet M. J., Guiso N. Bordetella pertussis and Bordetella parapertussis: two immunologically distinct species. Infect Immun. 1993 Feb;61(2):486–490. doi: 10.1128/iai.61.2.486-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelef N., Sakamoto H., Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog. 1992 Mar;12(3):227–235. doi: 10.1016/0882-4010(92)90057-u. [DOI] [PubMed] [Google Scholar]
- Lanotte M., Riviere J. B., Hermouet S., Houge G., Vintermyr O. K., Gjertsen B. T., Døskeland S. O. Programmed cell death (apoptosis) is induced rapidly and with positive cooperativity by activation of cyclic adenosine monophosphate-kinase I in a myeloid leukemia cell line. J Cell Physiol. 1991 Jan;146(1):73–80. doi: 10.1002/jcp.1041460110. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Roberts A. L., Finn T. M., Knapp S., Mekalanos J. J. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990 Aug;58(8):2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Ewanowich C. A., Bhargava A., Peppler M. S., Kenimer J. G., Brennan M. J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992 Jun;60(6):2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessnick S. L., Lyczak J. B., Bruce C., Lewis D. G., Kim P. S., Stolowitz M. L., Hood L., Wisnieski B. J. Localization of diphtheria toxin nuclease activity to fragment A. J Bacteriol. 1992 Mar;174(6):2032–2038. doi: 10.1128/jb.174.6.2032-2038.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Taichman N. S., Lally E. T., Wahl S. M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991 Sep;59(9):3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Masure H. R. Modulation of adenylate cyclase toxin production as Bordetella pertussis enters human macrophages. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6521–6525. doi: 10.1073/pnas.89.14.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade B. D., Kind P. D., Ewell J. B., McGrath P. P., Manclark C. R. In vitro inhibition of murine macrophage migration by Bordetella pertussis lymphocytosis-promoting factor. Infect Immun. 1984 Sep;45(3):718–725. doi: 10.1128/iai.45.3.718-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Mouallem M., Farfel Z., Hanski E. Bordetella pertussis adenylate cyclase toxin: intoxication of host cells by bacterial invasion. Infect Immun. 1990 Nov;58(11):3759–3764. doi: 10.1128/iai.58.11.3759-3764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Symes P., Conboy M., Weiss A. A., Hewlett E. L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987 Oct 15;139(8):2749–2754. [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roberts M., Fairweather N. F., Leininger E., Pickard D., Hewlett E. L., Robinson A., Hayward C., Dougan G., Charles I. G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991 Jun;5(6):1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Rogel A., Hanski E. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. Translocation of the toxin across the membrane. J Biol Chem. 1992 Nov 5;267(31):22599–22605. [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Steed L. L., Setareh M., Friedman R. L. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukoc Biol. 1991 Oct;50(4):321–330. doi: 10.1002/jlb.50.4.321. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P. DNA fragmentation induced in macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J Biol Chem. 1990 Aug 25;265(24):14476–14480. [PubMed] [Google Scholar]
- Weiss A. A., Goodwin M. S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989 Dec;57(12):3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wels W., Baldrich M., Chakraborty T., Gross R., Goebel W. Expression of bacterial cytotoxin genes in mammalian target cells. Mol Microbiol. 1992 Sep;6(18):2651–2659. doi: 10.1111/j.1365-2958.1992.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Witvliet M. H., Burns D. L., Brennan M. J., Poolman J. T., Manclark C. R. Binding of pertussis toxin to eucaryotic cells and glycoproteins. Infect Immun. 1989 Nov;57(11):3324–3330. doi: 10.1128/iai.57.11.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A. Programmed cell death in infectious diseases. Trends Microbiol. 1993 Jun;1(3):114–117. doi: 10.1016/0966-842x(93)90118-b. [DOI] [PubMed] [Google Scholar]
- van't Wout J., Burnette W. N., Mar V. L., Rozdzinski E., Wright S. D., Tuomanen E. I. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect Immun. 1992 Aug;60(8):3303–3308. doi: 10.1128/iai.60.8.3303-3308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]