Abstract
Objectives
To test whether secretory immunoglobulin A (sIgA) to human immunodeficiency virus (HIV) antigens in breast milk of HIV-positive women is associated with protection against HIV transmission among breast-fed infants.
Study design
Nested, case-control design in which HIV-specific sIgA was measured in breast milk collected from 90 HIV-positive women enrolled in a study in Lusaka, Zambia. Milk samples were selected to include 26 HIV-positive mothers with infected infants (transmitters) and 64 mothers with uninfected infants (nontransmitters).
Results
HIV-specific sIgA was detected more often in breast milk of transmitting mothers (76.9%) than in breast milk of nontransmitting mothers (46.9%, P = .009). There were no significant associations between HIV-specific sIgA in breast milk and other maternal factors, including HIV RNA quantities in breast milk, CD4 count, and plasma RNA quantities.
Conclusions
HIV-specific sIgA in breast milk does not appear to be a protective factor against HIV transmission among breast-fed infants.
Human immunodeficiency virus (HIV) can be transmitted from an HIV-infected mother to her child through breast-feeding. This poses a serious dilemma for health policy–makers. Although complete avoidance of breast-feeding would eliminate the risk of breast-feeding–associated transmission, breast milk substitutes are unaffordable, unavailable, unacceptable, and unsafe for many HIV-infected women in low resource settings. The quantity of viral RNA and cell-associated viral DNA in breast milk from HIV-positive women strongly predicts mother-to-child transmission among breast-fed infants.1–3 What is puzzling, however, is why the majority of breast-fed infants born to HIV-positive mothers remain uninfected despite prolonged exposure. Although breast milk is clearly a route of transmission, human milk is also a rich source of a multitude of innate and specific immune factors that may play a role in modulating the risk of infection.4 Better understanding of anti-infective properties of breast milk may assist with development of interventions to reduce risk of transmission while preserving other health benefits of breast-feeding.
Secretory immunoglobulin A (sIgA) is the predominant immunoglobulin in breast milk and is associated with passive immunity to other (non-HIV) pathogens among breast-fed infants.5 HIV-specific IgA antibodies are detected in breast milk of a high proportion of HIV-positive, lactating women.6–8 In an in vitro model, sIgA purified from colostrum was able to block one of the pathways involved in HIV penetration across mucosa, that is, transcytosis through epithelial cells,9 suggesting that sIgA may be related to decreased infectivity of breast milk. Although persistence of HIV-specific IgA and IgM in breast milk was associated with reduced transmission in one study in Rwanda,10 no protective association was observed in two other studies.8,11
HIV-specific sIgA has been detected frequently in cervicovaginal samples from exposed but persistently uninfected cohorts of high-risk women,12–14 suggesting a role for these mucosal responses in resistance to HIV. Purified sIgA from exposed-uninfected women has been shown to neutralize a variety of HIV subtypes and phenotypes15,16 and to block transcytosis.17 However, one study among uninfected sex workers from the Gambia found no evidence of specific mucosal antibody responses.18 Given these inconsistent findings, we investigated within our cohort of breast-feeding infants born to HIV-positive mothers in Zambia whether detection of HIV-specific sIgA was associated with lower risk of transmission.
METHODS
Breast milk samples were collected by manual expression from 100 HIV-positive women. All women were participants in the Zambia Exclusive Breast-feeding Study (ZEBS), which was a clinical trial undertaken in Lusaka, Zambia, to test the safety and efficacy of short, exclusive breast-feeding for reduction of HIV transmission and infant mortality rates.19 In brief, HIV-infected pregnant women who intended to breast-feed were recruited during pregnancy. They and their infants were given single-dose nevirapine for prevention of transmission. Women were counseled to breast-feed exclusively until the child was 4 months of age. Half of the women were randomly assigned to abruptly stop all breast-feeding at 4 months and the other half to continue with exclusive breast-feeding to 6 months, with gradual weaning and continued breast-feeding for a duration based on the mother’s informed choice. Infants were followed with regular study visits for up to 24 months after delivery to determine HIV transmission. Infant heel-stick blood samples were collected at birth, at monthly intervals to 6 months, and at 3-month intervals to 24 months. These samples were tested for HIV-1 DNA by using real-time polymerase chain reaction (PCR)20 to determine the child’s HIV status. CD4 counts (FACSCount system, BD Immunocytometry Systems, San Jose, Calif) and plasma viral load (Roche Amplicor® version 1.5, Roche, Branchburg, NJ) were measured in maternal samples collected at enrollment during pregnancy. All participants signed informed consent for participation in the study, which was approved by the institutional review boards of the institutions of the investigators.
Breast milk samples collected at the visit scheduled 1 week after delivery were tested if available, and, if not, the sample collected 5 weeks after delivery was tested. Milk was processed within 4 hours of collection and was kept cold until processing. The milk was centrifuged at 400g and the cell pellet removed. The supernatant and lipid portions of the milk were mixed together before aliquoting and were stored at −70°C until use. The fluid portions of breast milk samples were tested to quantify HIV-1 RNA by using an ultrasensitive assay with a lower limit of detection of 50 copies/mL (Roche Amplicor® version 1.5, Roche, Branchburg, NJ).20
Breast milk concentration of sIgA was measured after the thawed, stored samples had been centrifuged at 25,000 RCF for 30 minutes at 4°C to remove the lipid layer.21 All samples were processed in the same way without access to information about the other laboratory and clinical findings. The supernatants were diluted 1:5000 in sterile saline solution or PBS just before use. Concentration of HIV-specific sIgA was measured with the use of a modified Calypte Biomedical HIV-1 enzyme immunoassay (Calypte Biomedical Corporation, Berkeley, Calif), based on a recombinant HIV-1 envelope protein, as previously described.12,13,22 Samples with 25 μL of sample buffer are added to the well and incubated at 37°C for 1 hour. If antibodies to HIV envelope proteins are present in the sample, they bind to the antigen immobilized to the well. The sample buffer significantly reduces the non-specific binding of antibodies and other proteins to the wells. A wash step removes the unbound material. Antibodies are detected through the use of a modified enzyme immunoassay. In the first step, samples are incubated 1 hour in the Calypte Biomedical HIV-1–coated microtiter strips with their buffer. After absorption of antibodies, a washing step is performed. A specific horseradish peroxidase–conjugated anti-human IgA, (Jackson ImmunoResearch Lab, West Grove, Pa) is added to each well and incubated. After a second washing step, TMB, as chromogen and urea hydrogen peroxide, as substrate for peroxidase, are added. Stop solution containing 1 mol/L H2SO4 blocks the reaction. The absorbance values are determined at 450 nm. The distribution of sIgA among the HIV-negative controls was examined, and a value greater than 2 standard deviations above the mean absorbance value was considered a cut-off to define a positive HIV-specific IgA value (337 units).
Using a case-control design, breast milk samples were selected to include 36 HIV-positive women who transmitted HIV to their infants and a random sample of 64 women who did not transmit. A child with a confirmed positive HIV DNA PCR test at any time during follow-up was defined as HIV-infected. Ten of the infected infants with a positive PCR at birth were presumed to have intrauterine-acquired HIV infection and were excluded from this analysis. Of the remaining 26 infants, 22 were positive by their 2-month visit and 4 were positive at later visits. Children with a negative PCR test at 24 months (or at least 1 month after all breast-feeding had ended for those without 24-month samples) and who had no positive PCR tests were defined as uninfected. Breast milk was also collected and tested from 17 HIV-negative women from the same community as control.
The proportions of transmitters and nontransmitters with detectable HIV-specific IgA were compared through the use of χ2 tests. For other comparisons between groups, Wilcoxon tests were used for continuous variables and χ2 tests for categorical variables. Multivariable analysis was done with the use of logistic regression.
RESULTS
HIV-specific sIgA was detected more often in breast milk of transmitting mothers than in breast milk of nontransmitting mothers. Twenty of 26 (76.9%) HIV-positive mothers with infected children had detectable HIV-specific sIgA in their breast milk compared with 30 of 64 (46.9%) of mothers of uninfected children (P = .009) (Table I). Differences in the timing of sample collection between transmitting and nontransmitting mothers did not account for the association, and there continued to be a significantly greater likelihood of detecting HIV-specific sIgA in breast milk of transmitters (19/24, 79.2%) than of nontransmitters (23/47, 48.9%) when the analysis was confined to 1-week samples only (P = .01).
Table I.
Characteristics of the mother-child pairs and HIV-specific sIgA in breast milk of 90 HIV-positive mothers in Lusaka, Zambia
| Transmitting mothers n = 26 | Nontransmitting mothers n = 64 | P value | |
|---|---|---|---|
| Samples collected at: | |||
| 1-week visit | 24 | 47 | .05 |
| 5-week visit | 2 | 17 | |
| n (%) birth weight <2500 grams | 4/25 (16.0) | 7/64 (10.9) | .51 |
| n (%) firstborn child | (7.7) | 11 (17.2) | |
| 2nd–4th child | 18 (69.2) | 39 (60.9) | |
| 5th + child | 6 (23.1) | 14 (21.9) | .51 |
| Mean (standard deviation) maternal age in years | 26.5 (4.9) | 25.2 (5.1) | .25 |
| Mean (standard deviation) maternal CD4 count | 210 (101) | 410 (202) | <.0001 |
| n (%) CD4 count <200 | 12 (46.2) | 13 (20.3) | |
| 200–499 | 14 (53.8) | 29 (45.3) | |
| ≥500 | 0 | 22 (34.4) | .001 |
| Median viral load | 135,232 | 47,196 | .03 |
| n (%) Maternal plasma viral load | |||
| <50,000 copies/mL | 7 (26.9) | 34 (53.1) | |
| ≥50,000 copies/mL | 19 (73.1) | 30 (46.9) | .02 |
| n (%) Breast milk viral load | |||
| <50 copies/mL | 2 (7.7) | 39 (60.9) | |
| ≥50 copies/mL | 24 (92.3) | 25 (39.1) | <.0001 |
| Median copy number if detectable | 1687 | 240 | .04 |
| n (%) Any breast problems (mastitis, abscess, cracked nipples) | 2 (7.7) | 1 (1.6) | .14 |
| n (%) positive for HIV-specific sIgA in breast Milk | 20 (76.9) | 30 (46.9) | .009 |
HIV-specific sIgA was detected more often in breast milk of mothers of low-birth-weight (<2500 grams) infants (10/11, 90.9%) than in breast milk of mothers of normal-weight infants (>2500 grams) (39/78, 50.0%) (P = .01). The greater likelihood of detecting HIV-specific sIgA among the transmitters persisted after stratification for birth weight. Other clinical characteristics were not significantly associated with detection of HIV-specific sIgA (Table II).
Table II.
Factors associated with detection of HIV-specific sIgA in breast milk of 90 HIV-positive mothers in Lusaka, Zambia
| n | n (%) with detectable HIV-specific sIgA | P value | |
|---|---|---|---|
| Sample collected at 1-week visit | 71 | 42 (59.2) | |
| 5-week visit | 19 | 8 (42.1) | .18 |
| Birth weight <2500 grams | 11 | 10 (90.9) | |
| ≥2500 grams | 78 | 39 (50.0) | .01 |
| Firstborn child | 13 | 9 (69.2) | |
| 2nd–4th child | 57 | 32 (56.1) | |
| 5th + child | 20 | 9 (45.0) | .39 |
| Maternal age <20 years | 10 | 6 (60.0) | |
| 20–29 years | 59 | 35 (59.3) | |
| ≥30 years | 21 | 9 (42.9) | .41 |
| Maternal CD4 count <200 | 25 | 15 (60.0) | |
| 200–499 | 43 | 26 (60.5) | |
| ≥500 | 22 | 9 (40.9) | .28 |
| Maternal plasma viral load | |||
| <50,000 copies/mL | 41 | 24 (58.5) | |
| ≥50,000 copies/mL | 49 | 26 (53.1) | .60 |
| Breast milk viral load | |||
| <50 copies/mL | 41 | 23 (56.1) | |
| ≥50 copies/mL | 49 | 27 (55.1) | .92 |
Quantities of HIV RNA measured in breast milk and the proportions with detectable HIV RNA in milk were similar among those with and without HIV-specific sIgA in the study population overall. Within the subgroup of transmitting mothers, there was a nonsignificant trend toward lower HIV RNA quantities in breast milk (median, 427 copies/mL; n = 20) in the presence of detectable HIV-specific IgA than in its absence (median, 5754 copies/mL; n = 6), but within the subgroup of nontransmitting mothers, the median HIV RNA quantities were <50 copies/mL regardless of whether or not HIV-specific IgA was detected (Figure). Detection of HIV RNA in breast milk was strongly related to transmission, with 92.3% of transmitting mothers having HIV RNA quantities >50 copies/mL in breast milk compared with 39.1% of nontransmitting mothers (P < .001). Restricting to mothers with detectable HIV RNA in breast milk, median RNA quantities were significantly higher (1687 copies/mL, n = 24) among transmitters than among nontransmitters (240 copies/mL, n = 26, P = .04). Higher concentrations of HIV RNA in breast milk were significantly correlated with plasma viral load and lower maternal CD4 count.
Figure.
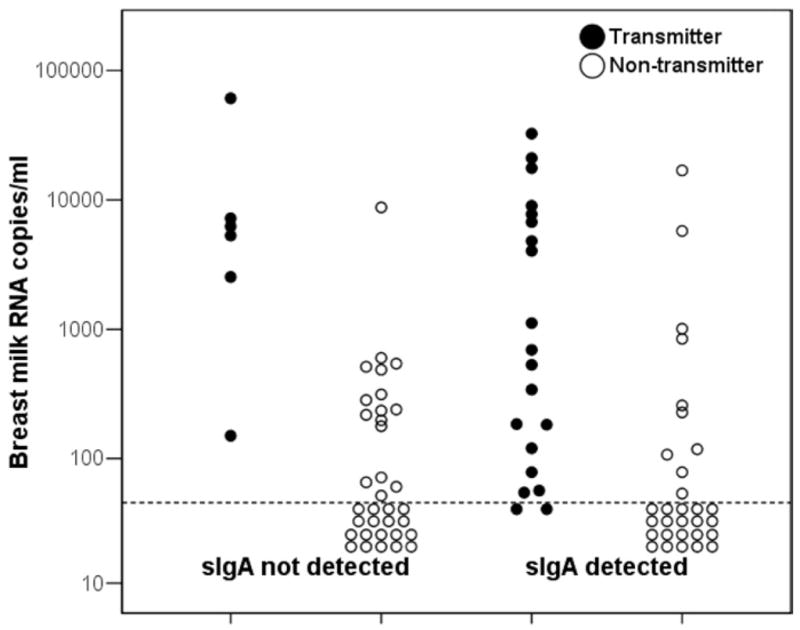
Breast milk HIV RNA concentrations among 26 HIV-positive transmitter mothers (solid dots) and 64 nontransmitter mothers (open circles) by whether or not HIV-specific sIgA could be detected in the milk.
Neither maternal CD4 count nor plasma viral load was associated with detection of HIV-specific IgA in breast milk. Breast milk from 15 of 25 (60.0%) women with CD4 counts less than 200 cells/mm3 had detectable sIgA compared with 26 of 43 (60.5%) with CD4 counts between 200 and 499 and 9 of 22 (40.9%) with CD4 counts of 500 cells/mm3 or greater. For women with plasma HIV RNA quantities less than 50,000 copies/mL, sIgA was detected among 24 of 41 (48.0%) compared with 26 of 49 (53.1%) women with higher plasma viral loads (Table II). There continued to be no association among transmitters and nontransmitters separately.
In a multivariable logistic regression model, HIV-specific IgA in breast milk remained significantly associated with transmission (odds ratio [OR], 6.93; 95% CI, 1.54 to 31.1) after adjusting for breast milk HIV RNA quantity (OR, 4.78 for each log10 increase; 95 CI, 2.03 to 11.21) and maternal CD4 count (OR, 0.48 for each 100 increase in CD4 count; 95% CI, 0.30 to 0.77). Maternal viral load was excluded because it was not associated with transmission, once breast milk RNA quantity was taken into account. However, if this variable was included, there was no change in the association between HIV-specific IgA and transmission.
DISCUSSION
We hypothesized a priori that HIV-specific sIgA in breast milk may be a protective factor in breast milk of HIV-positive mothers that may help to account for the relative inefficiency of HIV transmission through this route. Our data did not support this hypothesis, and HIV-specific sIgA was detected more frequently among transmitting compared with nontransmitting mothers. Our results are in contrast to studies among HIV-exposed but uninfected persons that have suggested a protective effect of IgA against HIV.12 We did not measure the neutralizing activity of the milk-derived IgA in these samples (because of small volume of samples). However, in previous studies measuring HIV-specific sIgA with this same method among exposed-uninfected adults, mucosal and systemic IgA are characterized by cross-clade HIV-1–specific neutralizing activity.15,16,23–25 The sIgA that we measured in milk may be qualitatively or quantitatively different from that among exposed-uninfected adults and may be inadequate to reduce the infectivity of milk or to offer sufficient passive immunity to the breast-feeding infant. In this regard, it is important to underline that IgA with capacity to neutralize primary HIV isolates detected among exposed-uninfected adults, in contrast to that among chronically infected adults, recognizes well-defined epitopes within gp41 restricted to aa 582–588 (QARILAV) and corresponds to the leucin-zip motif in the alpha-helic region.16,26,27 Limitations in the quantity of available material prevented us from mapping the epitope specificities of the nonprotective milk IgA.
We observed a significant association between detection of HIV-specific sIgA and low birth weight. This is consistent with findings that total sIgA concentration is greater in the milk of mothers of preterm infants.28 Hypothetically, this association could have masked a protective effect of HIV-specific sIgA in our study. However, even after stratification for birth weight, transmitters continued to be more likely than nontransmitters to have detectable HIV-specific sIgA. Since preterm and low-birth-weight infants are generally found to be at greater risk of acquisition, these findings are consistent with the notion that this compensatory mechanism in preterm infants does not appear to help to protect them from acquisition of HIV. We measured HIV-specific sIgA in milk at 1 and 5 weeks after birth and not at later ages. It is theoretically possible that persistence of HIV-specific sIgA in later milk may be more informative than measurements in early samples.
A possible reason for our findings may be that more active viral replication at mucosal sites or greater viral diversity is associated both with increased sIgA detection and with HIV transmissibility. In addition, under certain circumstances, IgA from HIV-infected individuals can enhance HIV infection in vitro.29
We excluded infected children who had detectable HIV DNA in their samples collected on the day of birth, generally assumed to indicate intrauterine transmission,30 which is unlikely to be influenced by postnatal factors. All infants were breast-fed, and most infected infants in our study had detectable infection by 2 months of age, making it difficult to distinguish postpartum- and intrapartum-acquired infections. Failure to include sufficiently large numbers of late postnatal infections may have limited our capacity to uncover associations unique to breast-feeding transmission. However, both postnatal and intrapartum transmission involve exposure to maternal mucosal fluids and are thought to occur across mucosal surfaces31 and thus may be influenced by immune factors in mucosal compartments.
We did not observe declines in the detection of HIV-specific sIgA among women with low CD4 counts or high plasma viral load. In contrast to observations among uninfected individuals, there usually is a predominance of IgG with defects in sIgA among individuals with HIV infection in mucosal compartments.32,33 We might have expected, therefore, that the deficits in sIgA production would increase with advanced disease and increasing systemic immunosuppression. We did not find this association. It is possible that local immune responses in breast milk are preserved despite systemic immunosuppression. Alternatively, true deficits may have been counterbalanced by increased likelihood of detection with longer and more extensive systemic viral infection.
Although the immunomodulatory properties of breast milk have been long appreciated with reference to pediatric infectious disease,34 only a limited number of milk factors have been investigated in relation to HIV transmission.4 We previously observed associations between higher concentrations of alpha-defensins in breast milk and reduced HIV transmission.35 Several experimental studies have described HIV inhibitory factors in breast milk,36–39 yet clinical studies have not observed consistent correlations between concentrations of innate immune factors in breast milk, such as secretory leukocyte inhibitor (SLPI), lactoferrin, lysozyme, interleukin-8 and chemokines, and reduced vertical HIV transmission.40–42 A specific oligosaccharide epitope in breast milk, Lewis X component, was recently discovered to be able to bind to dendritic cell–specific ICAM3-grabbing nonintegrin (DC-SIGN), thereby inhibiting HIV transfer to CD4 cells.43 Further study of other breast milk parameters, including of HIV-specific cellular immune responses44,45 and innate immune factors, remains important to further understanding of how to make breast-feeding safer.
Acknowledgments
Supported in part by NICHD grants 39611 and 40777 and by grants from Instituto Superiore di Sanita’ “Programma Nazionale di Ricerca sull’ AIDS”; by Centro di Eccellenza CISI; and by the EMPRO and AVIP EC WP6 Projects.
- HIV
Human immunodeficiency virus
- sIgA
Secretory immunoglobulin A
- PCR
Polymerase chain reaction
References
- 1.Lewis P, Nduati RW, Kreiss J, John GC, Richardson BA, Mbori-Ngacha D, et al. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–9. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillay K, Coutsoudis A, York D, Kuhn L, Coovadia HM. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immun Defic Syndr. 2000;24:330–6. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–7. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kourtis AP, Butera S, Ibegbu C, Beled L, Duerr A. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis. 2003;3:786–93. doi: 10.1016/s1473-3099(03)00832-6. [DOI] [PubMed] [Google Scholar]
- 5.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–8. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Van de Perre P, Hitimana DG, Lepage P. Human immunodeficiency virus antibodies of IgG, IgA, and IgM subclasses in milk of seropositive mothers. J Pediatr. 1988;113:1039–41. doi: 10.1016/s0022-3476(88)80578-x. [DOI] [PubMed] [Google Scholar]
- 7.Belec L, Bouquety JC, Georges AJ, Siopathis MR, Martin PM. Antibodies to human immunodeficiency virus in the breast milk of healthy, seropositive women. Pediatrics. 1990;85:1022–6. [PubMed] [Google Scholar]
- 8.Duprat C, Mohammed Z, Datta P, Stackiw W, Ndinya-Achola JO, Kreiss JK, et al. Human immunodeficiency virus type 1 IgA antibody in breast milk and serum. Pediatr Infect Dis J. 1994;13:603–8. doi: 10.1097/00006454-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, et al. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retroviruses. 1997;13:1179–85. doi: 10.1089/aid.1997.13.1179. [DOI] [PubMed] [Google Scholar]
- 10.Van de Perre P, Simonon A, Hitimana DG, Dabis F, Msellati P, Mukamabano B, et al. Infective and anti-infective properties of breastmilk from HIV-1 infected women. Lancet. 1993;341:914–8. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 11.Becquart P, Hocini H, Levy M, Sepou A, Kazatchkine MD, Belec L. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. J Infect Dis. 2000;181:532–9. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nature Med. 1997;3:1250–7. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 13.Kaul R, Trabattoni D, Bwayo J, Arienti D, Zagliani A, Mwangi FM, et al. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–9. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 14.Beyrer C, Artenstein AW, Rugpao S, Stephens H, VanCott TC, et al. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. J Infect Dis. 1999;179:59–67. doi: 10.1086/314556. [DOI] [PubMed] [Google Scholar]
- 15.Devito C, Hinkula J, Kaul R, Lopalco L, Bwayo J, Plummer F, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14:1917–20. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 16.Devito C, Hinkula J, Kaul R, Kimani J, Kiama P, Lopalco L, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immun Defic Syndr. 2002;30:413–20. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Belec L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, et al. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J Infect Dis. 2001;184:1412–22. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 18.Dorrell L, Hessell AJ, Wang M, Whittle H, Sabally S, Rowland-Jones S, et al. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. AIDS. 2000;14:1117–22. doi: 10.1097/00002030-200006160-00008. [DOI] [PubMed] [Google Scholar]
- 19.Thea DM, Vwalika C, Kasonde P, Kankasa C, Sinkala M, Semrau K, et al. Issues in the design of a clinical trial with a behavioral intervention: the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–65. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh MK, Kuhn L, West J, Semrau K, Decker D, Thea DM, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol. 2003;41:2465–70. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottcher MF, Jenmalm MC, Garofalo RP, Bjorksten B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:157–62. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 22.Lo Caputo S, Trabattoni D, Vichi F, Piconi S, Lopalco L, Villa ML, et al. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS. 2003;17:531–9. doi: 10.1097/00002030-200303070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Broliden K, Hinkula J, Devito C, Kiama P, Kimani J, Trabbatoni D, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 24.Kaul R, Plummer F, Clerici M, Bomsel M, Lopalco L, Broliden K. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15:431–2. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- 25.Mazzoli S, Lopalco L, Salvi A, Trabattoni D, Lo Caputo S, Semplici F, et al. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999;180:871–5. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- 26.Clerici M, Barassi C, Devito C, Pastori C, Piconi S, Trabattoni D, et al. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS. 2002;16:1731–41. doi: 10.1097/00002030-200209060-00004. [DOI] [PubMed] [Google Scholar]
- 27.Pastori C, Barassi C, Piconi S, Longhi R, Villa ML, Siccardi AG, et al. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J Biol Regul Homeostat Agents. 2000;14:15–21. [PubMed] [Google Scholar]
- 28.Gross SJ, Buckley RH, Wakil SS, McAllister DC, David RJ, Faix RG. Elevated IgA concentration in milk produced by mothers delivered of pre-term infants. J Pediatr. 1981;99:389–93. doi: 10.1016/s0022-3476(81)80323-x. [DOI] [PubMed] [Google Scholar]
- 29.Janoff EN, Wahl SM, Thomas K, Smith PD. Modulation of human immunodeficiency virus type 1 infection of human monocytes by IgA. J Infect Dis. 1995;172:855–8. doi: 10.1093/infdis/172.3.855. [DOI] [PubMed] [Google Scholar]
- 30.Bryson YJ, Luzuriaga K, Sullivan JL, Wara DW. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–7. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 31.Van de Perre P. Mother-to-child transmission of HIV-1: the ‘all mucosal’ hypothesis as a predominant mechanism of transmission. AIDS. 1999;13:1133–8. doi: 10.1097/00002030-199906180-00018. [DOI] [PubMed] [Google Scholar]
- 32.Belec L, Dupre T, Prazuck T, Tevi-Benissan C, Kanga JM, Pathey O, et al. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis. 1995;172:691–7. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 33.Artenstein AW, VanCott TC, Sitz KV, Robb ML, Wagner KF, Veit SC, et al. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: a comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–71. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 34.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12:664–71. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, et al. Alpha-defensins in the prevention of HIV transmission among breast-fed infants. J Acquir Immun Defic Syndr. 2005;39:138–42. [PMC free article] [PubMed] [Google Scholar]
- 36.Newburg DS, Viscidi RP, Ruff A, Yolken RH. A human milk factor inhibits binding of human immunodeficiency virus to the CD4 receptor. Pediatr Res. 1992;31:22–8. doi: 10.1203/00006450-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Orloff SL, Wallingford JC, McDougal JS. Inactivation of human immunodeficiency virus type I in human milk: effects of intrinsic factors in human milk and of pasteurization. J Hum Lactat. 1993;9:13–7. doi: 10.1177/089033449300900125. [DOI] [PubMed] [Google Scholar]
- 38.Newburg DS, Linhardt RJ, Ampofo SA, Yolken RH. Human milk glycosaminoglycans inhibit HIV glycoprotein gp120 binding to its host cell CD4 receptor. J Nutr. 1995;125:419–24. doi: 10.1093/jn/125.3.419. [DOI] [PubMed] [Google Scholar]
- 39.Moriuchi M, Moriuchi H. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J Immunol. 2001;166:4231–6. doi: 10.4049/jimmunol.166.6.4231. [DOI] [PubMed] [Google Scholar]
- 40.Semba RD, Kumwenda N, Taha TE, Hoover DR, Quinn TC, Lan Y, et al. Mastitis and immunological factors in breast milk of human immunodeficiency virus-infected women. J Hum Lactat. 1999;15:301–6. doi: 10.1177/089033449901500407. [DOI] [PubMed] [Google Scholar]
- 41.Semba RD, Kumwenda N, Taha TE, Hoover DR, Lan Y, Eisinger W, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–4. doi: 10.1128/cdli.6.5.671-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becquart P, Gresenguet G, Hocini H, Kazatchkine MD, Belec L. Secretory leukocyte protease inhibitor in colostrum and breast milk is not a major determinant of the protection of early postnatal transmission of HIV. AIDS. 1999;13:2599–602. doi: 10.1097/00002030-199912240-00018. [DOI] [PubMed] [Google Scholar]
- 43.Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TB, Pollakis G, et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115:3256–64. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbaj S, Edwards BH, Ghosh MK, Semrau K, Cheelo S, Thea DM, et al. Human immunodeficiency virus-specific CD8(+) T cells in human breast milk. J Virol. 2002;76:7365–73. doi: 10.1128/JVI.76.15.7365-7373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohman BL, Slyker J, Mbori-Ngacha D, Bosire R, Farquhar C, Obimbo E, et al. Prevalence and magnitude of human immunodeficiency virus (HIV) type 1-specific lymphocyte responses in breast milk from HIV-1-seropositive women. J Infect Dis. 2003;188:1666–74. doi: 10.1086/379374. [DOI] [PubMed] [Google Scholar]


