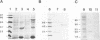Abstract
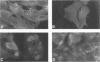
In enteropathogenic Escherichia coli, the eaeA gene produces a 94-kDa outer membrane protein called intimin which has been shown to be necessary but not sufficient to produce the attaching-and-effacing lesion. The purpose of this study was to characterize the intimin specified by the eaeA allele of the enterohemorrhagic E. coli (EHEC) serotype O157:H7 strain CL8 and to determine its role in adherence. The carboxyl-terminal 266 amino acids of the CL8 intimin were expressed as a protein fusion with glutathione S-transferase, which was used to raise antiserum in rabbits. The antiserum reacted in Western immunoblots with a 97-kDa outer membrane protein of EHEC strains of serogroups O5, O26, O111, and O157 and enteropathogenic E. coli strains of serogroups O55 and O127. Surface labelling of CL8 with 125I showed that intimin was surface exposed. An eaeA insertional inactivation mutant of CL8 was produced and was designated CL8-KO1. Total adherence of CL8-KO1 to HEp-2 cells was not significantly different from that of CL8, but CL8-KO1 gave a negative result in the fluorescent actin staining test. The eaeA gene expressed alone in E. coli HB101 also gave a negative fluorescent actin staining test result. The eaeA gene of CL8 was able to complement the eaeA deletion mutation in CVD206. We conclude that the product of the EHEC eaeA gene is a 97-kDa surface-exposed protein and propose that it be designated intiminO157. Sherman et al. described a 94-kDa outer membrane protein which played an important role in adherence of E. coli O157:H7 (Infect. Immun. 59:890-899, 1991). Western immunoblotting and indirect fluorescent antibody studies showed that the protein described by Sherman et al. is not intimin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebakhee G., Louie M., De Azavedo J., Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992 Feb 1;70(1):63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991 Dec;59(12):4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dytoc M., Soni R., Cockerill F., 3rd, De Azavedo J., Louie M., Brunton J., Sherman P. Multiple determinants of verotoxin-producing Escherichia coli O157:H7 attachment-effacement. Infect Immun. 1993 Aug;61(8):3382–3391. doi: 10.1128/iai.61.8.3382-3391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Collins J. E., Duimstra J. R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986 Mar;51(3):953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Kaper J. B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991 Dec;59(12):4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Mapping and topographic localization of epitopes of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1991 Oct;59(10):3424–3433. doi: 10.1128/iai.59.10.3424-3433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Cockerill F., 3rd, Soni R., Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991 Mar;59(3):890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Karmali M. Attaching and effacing adherence of Vero cytotoxin-producing Escherichia coli to rabbit intestinal epithelium in vivo. Infect Immun. 1988 Apr;56(4):756–761. doi: 10.1128/iai.56.4.756-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth I. K., Chapman C., Birden R., Brittingham J., Jackson C., Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986 Oct;154(4):712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- Yu J., Kaper J. B. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992 Feb;6(3):411–417. doi: 10.1111/j.1365-2958.1992.tb01484.x. [DOI] [PubMed] [Google Scholar]