Abstract
For intraoperative consultation of mucinous adenocarcinoma involving the ovary, it would be useful to have approaching methods in addition to the traditional limited microscopic findings in order to determine the nature of the tumors. Mucinous adenocarcinomas involving the ovaries were evaluated in 91 cases of metastatic mucinous adenocarcinomas and 19 cases of primary mucinous adenocarcinomas using both an original algorithm (unilateral ≥10 cm tumors were considered primary and unilateral <10 cm tumors or bilateral tumors were considered metastatic) and a modified cut-off size algorithm. With 10 cm, 13 cm, and 15 cm size cut-offs, the algorithm correctly classified primary and metastatic tumors in 82.7%, 87.3%, and 89.1% of cases and in 80.6%, 84.9%, and 87.1% of signet ring cell carcinoma (SRC) excluded cases. In total cases and SRC excluded cases, 98.0% and 97.2% of bilateral tumors were metastatic and 100% and 100% of unilateral tumors <10 cm were metastatic, respectively. In total cases and SRC excluded cases, 68.4% and 68.4% of unilateral tumors ≥15 cm were primary, respectively. The diagnostic algorithm using size and laterality, in addition to clinical history, preoperative image findings, and operative findings, is a useful adjunct tool for differentiation of metastatic mucinous adenocarcinomas from primary mucinous adenocarcinomas of the ovary.
Keywords: Adenocarcinoma, Mucinous; Ovarian Neoplasms; Pimary; Metastatic
INTRODUCTION
The incidence of primary mucinous adenocarcinoma is low. Many previously diagnosed cases are now retrospectively regarded as metastatic mucinous adenocarcinomas or borderline mucinous tumors. Primary mucinous adenocarcinoma is characterized by a large unilateral ovarian mass with a smooth external surface. Young age, an expansile growth pattern, a complex papillary pattern, necrotic luminal debris, and histologic areas resembling benign and borderline mucinous tumors are characteristic of primary mucinous adenocarcinoma. Metastatic mucinous adenocarcinoma is more likely bilateral and shows a multinodular external surface. The cut surface of the metastatic lesion varies from completely solid to multicystic, mimicking the primary ovary mass. Findings of ovarian capsular implants, vascular invasion, a nodular growth pattern, and infiltrative growth of individual glands or single cells on microscopic examination favor metastasis (1-5). The most important and helpful finding to differentiate metastatic from primary cancers is knowledge of the clinical history of a primary malignancy.
Seidman et al. proposed an algorithm based on tumor size and laterality in which unilateral ≥10 cm were considered as primary mucinous and all others as metastatic. This algorithm correctly classified mucinous adenocarcinomas in 84% of all 194 cases, including 100% of primary origin tumors and 77% of all metastatic tumors (4). By adjusting the size criterion to 13 cm, the performance of the algorithm was improved with correct classification of 87% of tumors, including 98% of primary tumors and 82% of metastatic tumors (6).
In Korea, ovarian malignancy is the 9th in frequency with annual incidence of 1,300 cases (7). Primary mucinous adenocarcinoma of the ovary is diagnosed in less than 10% of ovarian malignancies (8). Intraoperative distinction between primary and metastatic mucinous adenocarcinomas on frozen sections is challenging and has potential for misdiagnosis (9). For intraoperative consultation of mucinous adenocarcinoma involving the ovary, it is useful to have additional methods other than the limited traditional microscopic findings to determine the nature of the ovarian mucinous tumors. Because ovarian mucinous tumors are rarely encountered and notorious for difficulty in distinction between primary and metastatic tumors. Herein, we have re-evaluated the usefulness of the simple algorithm using the size and laterality criterion with 10 cm, 13 cm, and 15 cm cut-off values, for differentiation of primary mucinous adenocarcinoma from metastatic mucinous adenocarcinoma.
MATERIALS AND METHODS
Cases which were diagnosed as either primary mucinous adenocarcinoma or metastatic adenocarcinoma with mucinous differentiation from 1996 January to 2005 December were retrieved from archives at Catholic University of Korea, Kangnam St. Mary's Hospital and St. Mary's Hospital. All slides were reviewed and reclassified according to the 2003 WHO classification. Metastatic adenocarcinoma with more than focal mucin production was classified as metastatic adenocarcinoma with mucinous differentiation. Metastatic mucinous adenocarcinoma with an unknown primary site, borderline mucinous tumors, and microinvasive mucinous adenocarinomas were excluded. Clinicopathologic data regarding age, tumor size, laterality, and follow up data were collected. Clinicopathologic data from most metastatic tumors were reported previously (10).
We used a simple algorithm using size and laterality criteria that classified all bilateral mucinous carcinomas as metastatic, unilateral mucinous carcinomas <10 cm as metastatic, and unilateral mucinous carcinoma ≥10 cm as primary. We also used a modified algorithm with size cut-offs of 13 cm and 15 cm. Results using the different algorithms were compared with the final diagnosis in each case. Overall survival (OS) was defined as the date from the time of the first diagnosis of ovarian malignancy to death from any cause, or to the date of last contact.
Analyses of overall survival were performed using Kaplan-Meier survival curves. All eligible patients were included in the analyses of OS and all causes of death were included in the calculation of survival.
RESULTS
Clinical characteristics
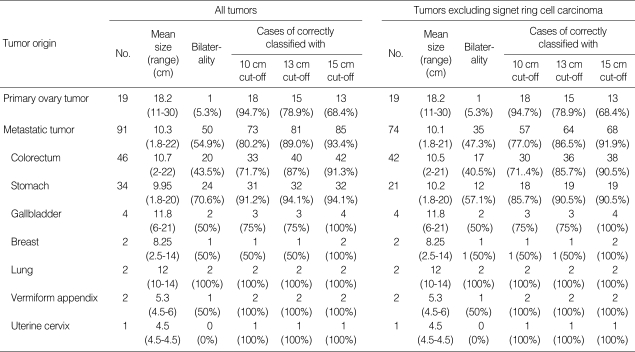
A total of 91 cases of metastatic mucinous adenocarcinoma and 19 cases of primary mucinous adenocarcinoma from 1996 to 2006 were included in this study to evaluate the algorithm using the size and laterality criteria. The mean age of patients with metastatic mucinous adenocarcinoma was 47 yr (median 45, range 19-76) and the mean age of patients with primary mucinous adenocarcinoma was 42 yr (median 40, range 16-71). All primary mucinous adenocarcinomas were surgically staged, except for one case. Stages included stage I in 12 cases, stage III in 5 cases, and stage IV in 1 case. Metastatic mucinous adenocarcinoma cases included those from the colorectum in 46 cases, stomach in 34 cases, gallbladder in 4 cases, breast in 2 cases, lung in 2 cases, vermiform appendix in 2 cases, and uterine cervix in 1 case. Among metastatic mucinous adenocarcinomas, 17 cases (18.7%) showed more than 50% of signet ring cell differentiation. These 17 cases of signet ring cell carcinoma (SRC) included 13 cases from the stomach and 4 cases from the colorectum.
Size and laterality
Primary mucinous adenocarcinomas were unilateral in 94.7% (18/19) of cases. Metastatic mucinous adenocarcinomas were bilateral in 54.9% (50/91) of cases. Primary mucinous adenocarcinomas had a mean size of 18.2 cm (range, 11-30) and metastatic mucinous adenocarcinomas had a mean size of 10.3 cm (range, 1.8-22).
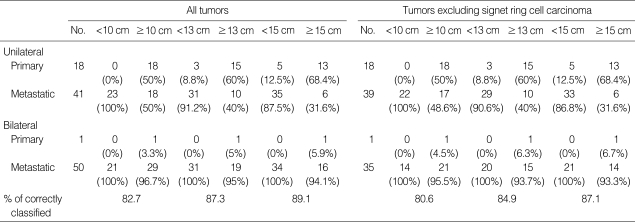
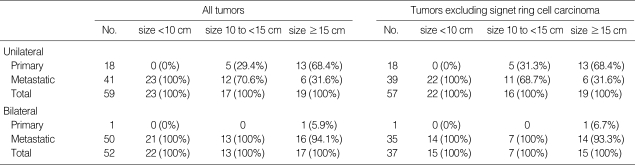
The distribution of primary and metastatic mucinous adenocarcinomas classified by tumor size (10 cm, 13 cm, and 15 cm of cut-off values) and laterality (unilateral and bilateral) is shown in Table 1. With the 10 cm size cut-off, the algorithm correctly classified primary vs. metastatic mucinous adenocarcinoma in 82.7% of cases (91/110), including 94.7% for primary and 80.2% for metastatic. With the 13 cm size cut-off, the algorithm correctly classified primary vs. metastatic in 87.3% of cases (96/110), including 78.9% for primary and 89.0% for metastatic. With the 15 cm size cutoff, the algorithm correctly classified primary vs. metastatic in 89.1% of cases (98/110), including 68.4% for primary and 93.4% for metastatic (Tables 1, 2). Of 74 mucinous adenocarcinomas with a size of <10 cm or with bilateral involvement, 73 cases (98.6%) were metastatic. Of 19 mucinous adenocarcinomas with a size of ≥15 cm and unilateral involvement, 13 cases (68.4%) were primary. Of 17 mucinous adenocarcinomas with a size between 10 cm and 15 cm and unilateral involvement, 5 cases (29.4%) were primary and 12 cases (70.6%) were metastatic (Table 3). We also evaluated these algorithms with ovarian mucinous adenocarcinoma excluding SRC, since SRC has characteristic histologic findings that can be easily diagnosed. With the 10 cm size cut-off, the algorithm correctly classified primary vs. metastatic mucinous adenocarcinoma in 80.6% of cases (75/93), including 94.7% for primary and 77.0% for metastatic. With the 13 cm size cut-off, the algorithm correctly classified primary vs. metastatic in 84.9% of cases (79/93), including 78.9% for primary and 86.5% for metastatic. With the 15 cm size cut-off, the algorithm correctly classified primary vs. metastatic in 87.1% of cases (81/93), including 68.4% for primary and 91.9% for metastatic (Tables 1, 2). Of 58 mucinous adenocarcinomas with a size of <10 cm or with bilateral involvement, 57 cases with a size of ≥15 cm and unilateral involvement, 13 cases (68.4%) were primary. Of 16 mucinous adenocarcinomas with a size between 10 cm and 15 cm and unilateral involvement, 5 cases (31.3%) were primary and 11 cases (68.7%) were metastatic (Table 3).
Table 1.
Distribution of primary/metastatic mucinous adenocarcinomas based on size with 10 cm, 13 cm, and 15 cm cut off, respectively, and laterality
Table 2.
Performance of the algorithm (size of 10 cm cut off and laterality) and modified algorithms (size of 13 cm and 15 cm cut off, respectively, and laterality)
Table 3.
Distribution of primary/metastatic mucinous adenocarcinomas classified by size <10 cm, 10 to <15 cm and ≥15 cm, and laterality
Survival
With a median follow-up period of 11 months (range, 1-122), 73 out of 110 (66.4%) patients died. Of 19 patients with primary mucinous adenocarcinoma, 17 patients were treated with surgery and adjuvant chemotherapy and two patients were treated with surgery only. The 5-yr overall survival rates were 87% for primary mucinous adenocarcinoma and 6% for metastatic mucinous adenocarcinoma (Fig. 1).
Fig. 1.
Overall survival curves of primary and metastatic mucinous adenocarcinomas.
DISCUSSION
Mucinous adenocarcinomas of the ovary are usually large, unilateral, smooth surfaced, multilocular or unilocular cystic masses containing watery or viscous mucoid materal. They are bilateral in 5% of cases (2, 11, 12). The proportion of primary mucinous adenocarcinoma in primary ovarian epithelial tumors has been recently reported as 2.4-6.7% (4, 13), which is lower than the previously reported proportion of primary mucinous adenocarcinoma (mean 12%, range 6-25%) (4). The low frequency of primary mucinous adenocarcinoma can be related to a wide variety in the appearance of metastatic mucinous adenocarcinomas of the ovaries. It has been recently recognized that many metastatic carcinomas have been misclassified as primary. Some mucinous tumors that would have been diagnosed as mucinous adenocarcinomas are diagnosed as intraepithelial and microinvasive mucinous carcinomas and are categorized as mucinous borderline tumors. Pseudomyxoma peritonei involving ovaries are known to be of gastrointestinal origin (not ovarian) (4).
The prognosis and treatment modalities of primary mucinous adenocarcinomas and metastatic mucinous adenocarcinomas are quite different and an accurate diagnosis is essential for an effective treatment. Because areas of malignancy may be limited, a generous sampling of all mucinous cystic tumors to include up to one histologic section per 1-2 cm of tumor diameter has been recommended to make a diagnosis (14). Sometimes, immunohistochemical staining for CK7, CK20, CDX2, and Dpc4 are helpful to differentiate metastatic from primary tumors (15-17). However, at the time of intraoperative consultation, it is not practical to examine multiple sections or to perform immunohistochemical staining. Therefore, a simple algorithm is useful that correctly classifies a high proportion of cases on the basis of easily assessible gross features (4).
A performance evaluation of the algorithm to distinguish primary from metastatic tumors using originally proposed criteria (bilateral tumors of any size, or unilateral tumor <10 cm=metastatic; unilateral tumor ≥10 cm=primary) demonstrated excellent diagnostic performance. Overall, 84-90% of all tumors were correctly classified (4, 6, 13). Using the same criteria, we found that 91 out of 110 cases (82.7%) were correctly classified. In the subgroup of unilateral tumors ≥10 cm, the proportion of primary mucinous adenocarcinomas varied from 62% to 82% (4, 13). This discrepancy reflects differences in metastatic ovarian tumor sizes between the study populations. Delay prior to visiting hospital for detection of ovarian metastasis leads to larger sized tumors (13).
In our subgroup of unilateral tumors ≥10 cm, the proportion of primary mucinous adenocarcinomas was only 50%. By adjusting the size criterion to 13 cm, and 15 cm, 87.3% (96 out of 110 cases) and 89.1% (98 out of 110 cases) of cases, respectively, were correctly classified. In the unilateral tumor subgroup with cut-off values of ≥13 cm and ≥15 cm, the proportion of primary mucinous adenocarcinoma increased to 60% and 68.4%, respectively. In Korea, gastric cancer metastasis to the ovary is common. Many cases show characteristic signet ring cell features of which diagnosis is easily made. Therefore we evaluated mucinous adenocarcinoma excluding SRC using the algorithms with cut-offs of 10 cm, 13 cm, and 15 cm. The results revealed similar trends with the results of total cases (Tables 1-3).
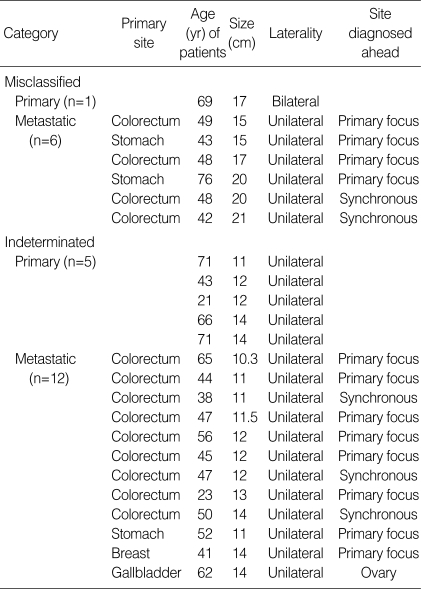
Using the algorithm with a size cut off of 15 cm, 89.1% and 87.1% of total cases and SRC excluded cases were correctly classified respectively. All unilateral tumors <10 cm were metastatic but unilateral tumor sizes of 10 cm to <15 cm were metastatic in 70.6% and 68.7% of total cases and SRC excluded cases, respectively. This result supports the need for the modification suggested by Khunamornpong et al. (13) (all bilateral tumors or unilateral tumors <10 cm are metastatic, unilateral tumors ≥15 cm are primary, and unilateral tumors with sizes between 10 cm and 15 cm are indeterminate). Application of this modified algorithm resulted in correct classification of 86 out of 93 cases (92.5%) and 70 out of 77 cases (90.9%), respectively, and 17 cases (15.5%) and 16 cases (17.2%) remained undetermined. Misclassified cases included 1 case of bilateral primary mucinous adenocarcinoma and 6 cases of ≥15 cm unilateral metastatic mucinous adenocarcinomas (4 from the colorectum and 2 from the stomach). Among 6 misclassified and 12 indeterminated metastatic tumors, primary malignancies were detected ahead of an ovarian mass in 5 cases and synchronously with an ovarian mass in 12 cases (Table 4). According to these data, a complete clinical history and a careful search for possible primary tumors in the operation field, especially in the gastrointestinal tract, is important. When surgeons submit intraoperative frozen samples for examination, they should provide pathologists a clinical history and intraoperative findings, such as the status of the opposite ovary.
Table 4.
Cases of misclassified and indeterminate by modified algorithms with size <10 cm, 10-15 cm, ≥15 cm and laterality
In conclusion, an algorithm using size and laterality is a useful adjunct tool for differentiation of metastatic mucinous adenocarcinoma from primary mucinous adenocarcinoma of the ovary. However, clinicopathologic evaluation is important, especially when the tumor is unilateral with a size between 10 cm and 15 cm.
References
- 1.Harrison ML, Jameson C, Gore ME. Mucinous ovarian cancer. Int J Gynecol Cancer. 2008;18:209–214. doi: 10.1111/j.1525-1438.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Riopel MA, Ronnett BM, Kurmann RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol. 1999;23:617–635. doi: 10.1097/00000478-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27:985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Young RH, Scully RE. Differential diagnosis of ovarian tumors based primarily on their patterns and cell types. Semin Diagn Pathol. 2001;18:161–235. [PubMed] [Google Scholar]
- 6.Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol. 2008;32:128–138. doi: 10.1097/PAS.0b013e3180690d2d. [DOI] [PubMed] [Google Scholar]
- 7.Baker P, Oliva E. A practical approach to intraoperative consultation in gynecological pathology. Int J Gynecol Pathol. 2008;27:353–365. doi: 10.1097/PGP.0b013e31815c24fe. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health and Welfare. Annual report of the central cancer registry in Korea. 2002. [Google Scholar]
- 9.Koonings PP, Campbell K, Mishell DR, Jr, Grimes DA. Relative frequency of primary ovarian neoplasm: a 10-year review. Obstet Gynecol. 1989;74:921–926. [PubMed] [Google Scholar]
- 10.Lee SJ, Bae JH, Lee AW, Tong SY, Park YG, Park JS. Clinical characteristics of metastatic tumors to the ovaries. J Korean Med Sci. 2009;24:114–119. doi: 10.3346/jkms.2009.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with 'pseudomyxoma peritonei'. Am J Surg Pathol. 2000;24:1447–1464. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Guerrieri C, Högberg T, Wingren S, Fristedt S, Simonsen E, Boeryd B. Mucinous borderline and malignant tumors of the ovary. A clinicopathologic and DNA ploidy study of 92 cases. Cancer. 1994;74:2329–2340. doi: 10.1002/1097-0142(19941015)74:8<2329::aid-cncr2820740818>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Khunamornpong S, Suprasert P, Pojchamarnwiputh S, Na Chiangmai W, Settakorn J, Siriaunkgul S. Primary and metastatic mucinous adenocarcinomas of the ovary: Evaluation of the diagnostic approach using tumor size and laterality. Gynecol Oncol. 2006;101:152–157. doi: 10.1016/j.ygyno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Lee KR, Tavassoli FA, Prat J, Dietel M, Gersell DJ, Karseladze AI, Hauptmann S, Rutgers J. Surface epithelial-stromal tumors. In: Tavassoli FA, Devilee P, editors. Pathology & Genetics, Tumors of the breast and female genital organs. Lyon, France: IARC press; 2003. pp. 117–145. [Google Scholar]
- 15.Vang R, Gown AM, Barry TS, Wheelen DT, Yemelyanova A, Seidman JD, Ronnett BM. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130–1139. doi: 10.1097/01.pas.0000213281.43036.bb. [DOI] [PubMed] [Google Scholar]
- 16.Ji H, Isacson C, Seidman JD, Ronnett BM. Cytokeratins 7 and 20, Dpc4 and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Vang R, Gown AM, Wu LS, Barry TS, Sheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006;19:1421–1428. doi: 10.1038/modpathol.3800698. [DOI] [PubMed] [Google Scholar]