Abstract
The aim of this study is to evaluate the clinical feature and pregnancy outcome in patients with ovarian cancer diagnosed during pregnancy. We retrospectively analyzed the medical records of 27 patients diagnosed with ovarian cancer during pregnancy at Cheil General Hospital & Women's Healthcare Center from January 1996 to December 2006. Mean age of the patients was 29.1 yr (range 23-40), and a mean follow-up period was 57 months (range 7-112 months). Of 27 patients, 15 (55.5%) had borderline malignancies, 7 (25.9%) had epithelial malignancies and 5 (18.6%) had germ cell tumors. A total of 26 patients received a conservative surgery preserving pregnancy. The mean time for surgical intervention during pregnancy was 20 weeks of gestational age. Of the 27 patients, 26 had full term delivery of a healthy baby without any congenital malformation. Only one patient with epithelial ovarian cancer had a relapse at 19 months after the first conservative operation with adjuvant chemotherapy. There were few data for managing patients with ovarian cancer diagnosed during pregnancy. This study results could help establish a guideline for management of ovarian malignancy complicating pregnancy.
Keywords: Ovarian Neoplasms, Pregnancy, Conservative Surgery
INTRODUCTION
The overall incidence of ovarian tumors in pregnancy is 2.4-5.7% (1). Of these tumors, approximately 5% are malignant (2, 3). Currently, an ultrasound is routinely used early in pregnancy, and this has led to an early diagnosis and management of asymptomatic ovarian tumors.
In contrast to previous guidelines for managing gynecological malignancies in pregnancy, today, most women with ovarian cancer complicating pregnancy hope to maintain the pregnancy and preserve fertility, which has been encouraged by late maternity and a low birthrate.
In the absence of large prospective randomized trials and cohort studies, it is difficult to know how to manage patients best and make standard guidelines for management of these tumors during pregnancy. To establish standard a guideline for management of ovarian cancer complicating pregnancy, it is necessary to identify surgical strategies with or without adjuvant intrapartum chemotherapy that results in safe oncologic and fetal outcomes. This study was undertaken to help establish a guideline for management of ovarian malignancy complicating pregnancy.
MATERIALS AND METHODS
We retrospectively analyzed the medical records of patients (n=27) with ovarian cancer complicating pregnancy who were treated at Cheil General Hospital and Women's Healthcare Center between January 1996 and November 2006. In these patients, the ovarian cancer was detected during gestation.
Twenty-five of these patients were diagnosed in our institute, and two were transferred from other hospitals. Conservative surgical management was defined as cystectomy, or unilateral salpingo-oophorectomy (USO), or USO with omentectomy and multiple biopsies. Specialized pathologists at our institution reported findings of intra-operative frozen biopsies and post-operatively confirmed pathology specimens for all 27 patients. The 1988 FIGO Staging System was retrospectively used for each case after reviewing the operative reports and pathologic findings. Patients were followed up with clinical, serum tumor marker and radiologic assessments. Follow-up information was recorded up to the date of last contact or death.
RESULTS
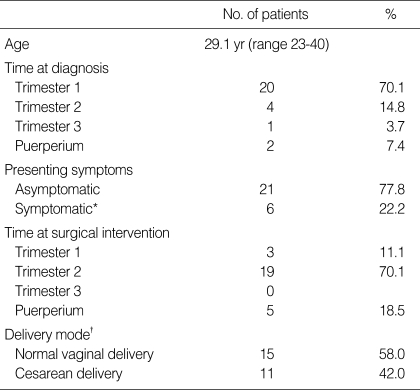
Mean age of the patients was 29.1 yr (range 23-40). Of 27 patients with ovarian tumor complicating pregnancy, 25 were diagnosed during pregnancy and the others were incidentally diagnosed at cesarean section. Mean time of diagnosis of ovarian tumor was 12.5 weeks of gestational age (GA) (range 5.0-41.0 weeks of GA). In the patients diagnosed during pregnancy, 6 had the presenting symptoms such as abnormally distended abdomen due to huge ovarian tumor. Most patients without symptoms (n=21) were diagnosed incidentally at early GA by a routine ultrasound examination (Table 1).
Table 1.
Characteristics of patients with ovarian cancers during pregnancy
*symptomatic, the presenting symptoms as abnormally distended abdomen due to huge ovarian tumor; †all patients had succeeded full term deliveries.
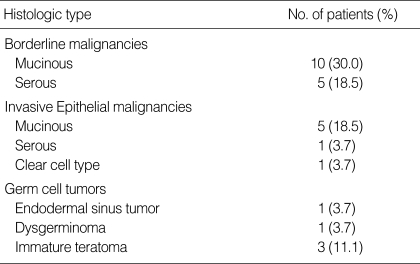
Histopathologic characteristics included ovarian borderline malignancy (n=15), epithelial ovarian cancers (EOC) (n=7) and malignant germ cell tumors (GCT) (n=5). The clinical and pathologic profiles of the 27 patients are shown in Table 2. The mean size of the ovarian tumors measured by ultrasound sonogram was 11.4 cm (range, 4-22 cm) and three of 27 patients had an ovarian tumor less than 6 cm in diameter.
Table 2.
Histopathologic characteristics of ovarian cancer (n=27)
All patients underwent surgical management for the initial treatment. The initial operation included a conservative operation (n=26) and a cytoreductive operation with hysterectomy (n=1). The conservative surgical management included cystectomy (n=10), USO (n=13) and USO with omentectomy and multiple biopsy (n=3). Patients with ovarian borderline malignancies underwent cystectomy (n=7), USO (n=6) or USO with omentectomy and multiple biopsy (n=2). No recurrence occurred in any patient with the borderline malignancy. The patients with germ cell tumor underwent cystectomy (n=2), USO (n=2) or USO with omentectomy and multiple biopsy (n=1), with no recurrence in any patient with malignant germ cell tumor. In the patients with EOC, the surgical approaches were cystectomy (n=1), USO (n=5) or cytoreductive operation with hysterectomy (n=1). In this group, one patient recurred (Table 3).
Table 3.
Surgical management by histologic type (n=27)
Invasive epithelial malignancies, Six in unilateral ovarian malignancy with an intact capsule, One is clear cell type, FIGO stage IIIC.
*one patient has recurred after 19 months after initial surgical management.
USO, unilateral salpingo-oophorectomy.
Of the 22 patients who underwent surgical interventions during pregnancy, there were no definite feto-maternal complications after surgical management. Of these 22 patients, nineteen (86.4%) received surgical intervention during second trimester and three (13.6%) during the first trimester.
A total of 8 patients received adjuvant chemotherapy; 3 patients received chemotherapy with fetus in utero, and 5 patients received chemotherapy just after delivery. One patient with EOC complicating ectopic pregnancy received postoperative chemotherapy. Of five patients with germ cell tumor, 3 patients received it with the fetus in utero (range, 22.8-30.6 weeks of GA) and two patients at 2 weeks after cesarean delivery with 4 courses of bleomycin, etoposide and cisplatin (BEP). Three patients with EOC received 6 courses of paclitaxel plus carboplatin at 2 weeks after cesarean delivery. All 26 patients with ovarian cancer complicating pregnancy were successful in having a full term delivery. Of the 26 healthy full-term infants, 15 were delivered by normal vaginal delivery and eleven were by cesarean section. No congenital malformations were detected in any of the 26 newborns. Neither dystocia nor tumor metastasis to the placenta or fetus was recorded.
DISCUSSION
Most patients with ovarian cancer have no specific symptoms. This asymptomatic character of ovarian cancer makes early diagnosis difficult. An ultrasound sonogram is a routine method for evaluating fetal status in women with pregnancy and it can also be used for early detection of an incidental ovarian tumor. With the wide use of routine prenatal ultrasound, finding of an adnexal mass in pregnancy is increasing (4). In contrast to past delivery modes, cesarean delivery is now more frequent, and this can also contribute to the early incidental diagnosis of ovarian tumor complicating pregnancy.
The incidence of ovarian tumor detected during pregnancy is 1/300 to 1/556 pregnancies (5-7). Of the ovarian tumors detected during pregnancy, the incidence of ovarian malignancy is 1/15,000 to 1/32,000 in the most reports (4, 8). In our series, ovarian cancer during pregnancy occurred in 0.29/1,000 deliveries (from 1996 to 2006, from 92,370 deliveries at Cheil General Hospital and Women's Healthcare Center). This high incidence could be explained by recent wide use of ultrasound examination as a routine antenatal evaluation in our hospital.
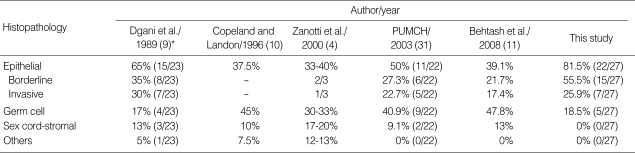
Several studies have reported that the histologic types of ovarian cancers during pregnancy are similar to those for nonpregnant women in the corresponding reproductive-age group (4, 9-11). In our series, borderline malignancy and invasive epithelial ovarian cancer were more dominant than other types of malignancies (Table 4).
Table 4.
Distribution of ovarian cancers in pregnancy by histology of tumor in literature
*Number in parenthesis, reference number.
In white women, dysgerminoma is the most common ovarian germ cell tumor coexisting with pregnancy, and constitutes 25-35% of all reported ovarian cancers (9, 12, 13). However, the most common subtype of germ cell tumor in our study was immature teratoma.
Surgical management of an adnexal mass, diagnosed during pregnancy, creates a dilemma to gynecologists. It is difficult to discriminate ovarian malignancies from functional cysts or benign ovarian tumors. If an adnexal mass, larger than 6 cm, has a complex structure, or ascites or persists for 16 gestational weeks, surgical management is critical for obtaining a final histologic diagnosis and ruling out malignancy (14).
When any surgical intervention is needed for an adnexal mass during pregnancy, the surgery should be performed during the safest period of pregnancy. Elective surgery for tumors with low suspicion of malignancy should be delayed until the second trimester (16-18 weeks of gestation), when risk for spontaneous abortion, hormonal dependence of the corpus luteum of pregnancy is reduced, and when functional cysts are resolved in the vast majority of cases (15). In the first trimester, spontaneous abortion rate after surgery has been documented to be 10%, while 76.3% patients progress to full-term delivery (16). A mass, first noticed in the third trimester, is best managed by awaiting fetal maturity as long as the clinical suspicion of malignancy is low. Premature labor is more likely to occur and pregnancy outcome is poor if surgical exploration is attempted during the third trimester (17, 18).
The principles of management in ovarian cancer complicating pregnancy include surgery with adequate staging. For advanced disease, the principles of adequate staging and debulking surgery should be similar to those used for the treatment of nonpregnant women. For most early-stage ovarian cancers, the surgical principles should be unilateral oophorectomy or adnexectomy with appropriate staging. Especially, in borderline ovarian malignancies and germ cell tumors, it is imperative to use conservative surgical strategies to maintain pregnancy. In our series, of 20 patients with borderline malignancies (n=15) and germ cell tumors (n=5), 9 patients received cystectomy, 8 patients received USO and 3 patients received USO with omentectomy and multiple biopsy as the initial surgical management. And all 5 patients with germ cell tumor received BEP (cisplatin, bleomycin, and etoposide) chemotherapy without any feto-maternal complications. Most malignant ovarian germ cell tumors could be treated by conservative surgery without compromising survival since these tumors are very sensitive to standard combinational chemotherapy (19). And borderline malignancies differ from invasive epithelial ovarian cancer in their indolent behavior and good prognosis. All six cases in Gotlieb's report (20) with immediate conservative surgery preserving pregnancy had satisfactory outcomes. Since there was no established benefit from postoperative therapy for ovarian borderline malignancy, even in late-stage diseases (21), conservative surgery is safe for these tumors (20, 22). Conservative surgical management for most malignant ovarian germ cell tumors and borderline malignancies diagnosed during pregnancy should be considered as the proper initial treatment.
Invasive epithelial cancer has the worst prognosis of all types of ovarian cancers. The standard management of EOC is based on the primary surgery, including hysterectomy and bilateral salpingo-oophorectomy with peritoneal sampling (peritoneal washing, omentectomy, multiple peritoneal biopsies and the removal of peritoneal implants) with lymph-node biopsy. Recently, with the help of several developed diagnostic tools, and an increase in self-health monitoring among women, early detection of ovarian cancer has increased and with the widespread use of routine prenatal ultrasound, the finding of an adnexal mass during pregnancy is an increasingly common occurrence. In contrast to past strategies for managing EOC, most young patients with malignancies desire to be managed focused on the quality of life after treatment, and to maintain the pregnancy if possible. However, frequent relapse of EOC makes conservative treatment strictly limited to patients with early stage EOC and low risks. In our study, 6 of 7 patients with EOC received comprehensive rather than complete staging operations (1 cystectomy and 5 USO). One of these six (16.6%) patients recurred. Our study demonstrates that in EOC complicating pregnancy, conservative management with adequate staging procedures should be considered for oncologic safety.
Generally, chemotherapy is compatible with the second or third trimester, when the risk of congenital malformation for fetuses exposed to chemotherapy is no greater than in the general population (23, 24). In our study, three patients with germ cell tumors received BEP chemotherapy during pregnancy, all of which had healthy babies without congenital malformations. The literature contains numerous reports of BEP regimen and adjuvant cisplatin and cyclophosphamide used in pregnancy with no untoward effects (8, 25-28). There were a few case reports describing the combined use of paclitaxel and carboplatin in human pregnancy, and there seems to be no significant fetal toxicity when these are administered during the second or third trimester (29, 30). Recently, no studies have evaluated the long-term consequences for children exposed to intrauterine chemotherapy. To establish standard guidelines for treatment and improve the quality of life in patients with pregnancy complicating ovarian cancer with an oncologic safety, the further studies regarding the proper management of these patients should be advanced.
References
- 1.Farahmand SM, Marchetti DL, Asirwatham JE, Deway MR. Ovarian endodermal sinus tumor associated with pregnancy: review of the literature. Gynecol Oncol. 1991;41:156–160. doi: 10.1016/0090-8258(91)90277-c. [DOI] [PubMed] [Google Scholar]
- 2.Whitecar MP, Turner S, Higby MK. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181:19–24. doi: 10.1016/s0002-9378(99)70429-1. [DOI] [PubMed] [Google Scholar]
- 3.Jacob JH, Stringer CA. Diagnosis and management of cancer during pregnancy. Semin Perinatol. 1990;14:79–87. [PubMed] [Google Scholar]
- 4.Zanotti KS, Belinson JL, Kennedy AW. Treatment of gynecologic cancers in pregnancy. Semin Oncol. 2000;27:686–698. [PubMed] [Google Scholar]
- 5.Lavery J, Koontz WL, Layman L, Shaw L, Gumpel U. Sonographic evaluation of the adnexa during early pregnancy. Surg Gynecol Obstet. 1986;163:319–323. [PubMed] [Google Scholar]
- 6.Hogston P, Lilford RJ. Ultrasound study of ovarian cysts in pregnancy: prevalence and significance. Br J Obstet Gynaecol. 1986;93:625–628. doi: 10.1111/j.1471-0528.1986.tb08037.x. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins MP, Duchon MA. Adnexal surgery in pregnancy. J Reprod Med. 1986;31:1035–1037. [PubMed] [Google Scholar]
- 8.Elit L, Bocking A, Kenyon C, Natale R. An endodermal sinus tumor diagnosed in pregnancy: case report and review of the literature. Gynecol Oncol. 1999;72:123–127. doi: 10.1006/gyno.1998.5190. [DOI] [PubMed] [Google Scholar]
- 9.Dgani R, Shoham Z, Atar E, Zosmer A, Lancet M. Ovarian carcinoma during pregnancy: a study of 23 cases in Israel between the years 1960 and 1984. Gynecol Oncol. 1989;33:326–331. doi: 10.1016/0090-8258(89)90521-0. [DOI] [PubMed] [Google Scholar]
- 10.Copeland LJ, Landon MB. Malignant disease in pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics, normal and problem pregnancies. 3rd ed. New York, NY: Churchill Livingstone; 1996. pp. 1155–1181. [Google Scholar]
- 11.Behtash N, Karimi Zarchi M, Modares Gilani M, Ghaemmaghami F, Mousavi A, Ghotbizadeh F. Ovarian carcinoma associated with pregnancy: a clinicopathologic analysis of 23 cases and review of the literature. BMC Pregnancy Childbirth. 2008;8:3. doi: 10.1186/1471-2393-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakri YN, Ezzat A, Akhtar, Dohami, Zahrani Malignant germ cell tumors of the ovary. Pregnancy considerations. Eur J Obstet Gynecol Reprod Biol. 2000;90:87–91. doi: 10.1016/s0301-2115(99)00213-4. [DOI] [PubMed] [Google Scholar]
- 13.Williams SD, Gershenson DM, Horowitz CJ. Ovarian germ-cell tumors. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and practice of gynecologic oncology edition. Philadelphia: PA: Lippincot; 1996. pp. 987–1001. [Google Scholar]
- 14.Dudkiewicz J, Kowalski T, Grzonka D, Czarnecki M. Ovarian tumors in pregnancy. Ginekol Pol. 2002;73:342–345. [PubMed] [Google Scholar]
- 15.Pitynski K, Basta A, Szczudrawa A, Oplawski M. Ovarian tumors in pregnancy in the material of the Department of Gynecology and Oncology Collegium Medicum of Jagiellonian University in Cracow. Ginekol Pol. 2002;73:371–375. [PubMed] [Google Scholar]
- 16.Ueda M, Ueki M. Ovarian tumors associated with pregnancy. Int J Gynaecol Obstet. 1996;55:59–65. doi: 10.1016/0020-7292(96)02718-x. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal N, Parul, Kriplani A, Bhatla N, Gupta A. Management and outcome of pregnancies complicated with adnexal masses. Arch Gynecol Obstet. 2003;267:148–152. doi: 10.1007/s00404-001-0287-y. [DOI] [PubMed] [Google Scholar]
- 18.Grendys EC, Jr, Barnes WA. Ovarian cancer in pregnancy. Surg Clin North Am. 1995;75:1–14. doi: 10.1016/s0039-6109(16)46529-1. [DOI] [PubMed] [Google Scholar]
- 19.Tewari K, Cappuccini F, Disaia PJ, Berman ML, Manetta A, Kohler MF. Malignant germ cell tumors of the ovary. Obstet Gynecol. 2000;95:128–133. doi: 10.1016/s0029-7844(99)00470-6. [DOI] [PubMed] [Google Scholar]
- 20.Gotlieb WH, Flikker S, Davidson B, Korach Y, Kopolovic J, Ben-Baruch G. Borderline tumors of the ovary: fertility treatment, conservative management, and pregnancy outcome. Cancer. 1998;82:141–146. [PubMed] [Google Scholar]
- 21.Kennedy AW, Hart WR. Ovarian papillary serous tumors of low malignant potential (serous borderline tumors). A long-time follow-up study, including patients with microinvasion, lymph node metastasis, and transformation to invasive serous carcinoma. Cancer. 1996;78:278–286. doi: 10.1002/(SICI)1097-0142(19960715)78:2<278::AID-CNCR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Donnez J, Munschke A, Berliere M, Piarard C, Jadou P, Smets M, Squifflet J. Safety of conservative management and fertility outcome in women with borderline tumors of the ovary. Fertil Steril. 2003;79:1216–1221. doi: 10.1016/s0015-0282(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 23.Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Fetal outcome after in utero exposure to cancer chemotherapy. Arch Intern Med. 1992;152:573–576. [PubMed] [Google Scholar]
- 24.Doll DC, Ringenberg QS, Yarbro JW. Antineoplastic agents and pregnancy. Semin Oncol. 1989;16:337–346. [PubMed] [Google Scholar]
- 25.Arango HA, Kalter CS, Decesare SL, Fiorica JV, Lyman GH, Spellacy WN. Management of chemotherapy in a pregnancy complicated by a large neuroblastoma. Obstet Gynecol. 1994;84:665–668. [PubMed] [Google Scholar]
- 26.Horbelt D, Delmore J, Meisel R, Cho S, Roberts D, Logan D. Mixed germ cell malignancy of the ovary concurrent with pregnancy. Obstet Gynecol. 1994;84:662–664. [PubMed] [Google Scholar]
- 27.Huang HP, Fang CN, Kan YY. Chemotherapy for ovarian mucinous cystadenocarcinoma during pregnancy: a case report. Eur J Gynaecol Oncol. 2004;25:635–636. [PubMed] [Google Scholar]
- 28.Bayhan G, Aban M, Yayla M, Gul T, Yaldiz M, Erden AC. Cisplatinum combination chemotherapy during pregnancy for mucinous cystadenocarcinoma of the ovary. Case report. Eur J Gynaecol Oncol. 1999;20:231–232. [PubMed] [Google Scholar]
- 29.Mendez LE, Mueller A, Salom E, Gonzalez-Quintero VH. Paclitaxel and carboplatin chemotherapy administered during pregnancy for advanced epithelial ovarian cancer. Obstet Gynecol. 2003;102:1200–1202. [PubMed] [Google Scholar]
- 30.Sood AK, Shahin MS, Sorosky JI. Paclitaxel and platinum chemotherapy for ovarian carcinoma during pregnancy. Gynecol Oncol. 2001;83:599–600. doi: 10.1006/gyno.2001.6439. [DOI] [PubMed] [Google Scholar]
- 31.Zhao XY, Huang HF, Lian LJ, Lang JH. Ovarian cancer in pregnancy: a clinicopathologic analysis of 22 cases and review of the literature. Int J Gynecol Cancer. 2006;16:8–15. doi: 10.1111/j.1525-1438.2006.00422.x. [DOI] [PubMed] [Google Scholar]