Abstract
Vascular endothelial growth factor-receptors (VEGF-Rs) are pivotal regulators of vascular development, but a specific role for these receptors in the formation of heart valves has not been identified. We took advantage of small molecule inhibitors of VEGF-R signaling and showed that blocking VEGF-R signaling with receptor selective tyrosine kinase inhibitors, PTK 787 and AAC 787, from 17–21 hours post fertilization (hpf) in zebrafish embryos resulted in a functional and structural defect in cardiac valve development. Regurgitation of blood between the two chambers of the heart, as well as a loss of cell-restricted expression of the valve differentiation markers notch 1b and bone morphogenetic protein-4 (bmp-4), was readily apparent in treated embryos. In addition, microangiography revealed a loss of a definitve atrioventricular constriction in treated embryos. Taken together, these data demonstrate a novel function for VEGF-Rs in the endocardial endothelium of the developing cardiac valve.
Keywords: VEGF receptors, cardiac valves, endothelium, NFAT, zebrafish
Introduction
Development of heart valves begins around day 22 of gestation in humans, embryonic day (E) 8.5 day in mice, and 43 hpf in zebrafish (Harvey and Rosenthal, 1999). The nascent valves begin as swellings, known as endocardial cushions, which reside between the endocardial and myocardial layers at the atrioventricular (AV) canal and in the outflow tract. The cushions become cellularized as endothelial cells delaminate, migrate into the cushion matrix, and undergo an endothelial to mesenchymal transdifferentiation (EMT) to give rise to interstitial cells of the valve(Armstrong and Bischoff, 2004; Person et al., 2005).
A number of genes encoding cell surface receptors, glycosaminoglycan biosynthetic enzymes, signaling molecules, and transcription factors have been found to be essential for proper valve development. One notable example is the calcineurin-dependent transcription factor NFATc1. Knockout of NFATc1 in the mouse leads specifically to defective aortic and pulmonic valve development with subsequent death at E 14–15 due to congestive heart failure (de la Pompa et al., 1998; Ranger et al., 1998). The endocardial cushions in these mice are hypoplastic, suggesting that lack of NFATc1 leads to dysregulation in an early step in cushion formation. From recent studies, it appears that two waves of NFAT signaling, first in the myocardium and second in the endocardium, are involved in formation heart valve leaflets (Chang et al., 2004). A second mutant relevant to the present study is the zebrafish jekyll mutant in which the enzyme uridine 5’-diphosphate glucose dehydrogenase is disrupted (Walsh and Stainier, 2001). The lack of this biosynthetic enzyme results in regurgitation or “toggling” of blood between the atrium and ventricle of the zebrafish heart, as well as defects in cell differentiation at the AV boundary.
A possible function for VEGF in valve development has been suggested by studies of transgenic mice. First, VEGF was found expressed in the developing heart, including a subset of endothelial cells in the endocardial cushions in mice expressing a LacZ reporter cassette in the 3’untranslated region of VEGF(Miquerol et al., 1999). Second, premature induction of VEGF in the embryonic myocardium prevented formation of the endocardial cushions, suggesting VEGF may negatively regulate EMT in valve development (Dor et al., 2001; Dor et al., 2003) . New studies show that myocardial VEGF expression is repressed in the nascent cushion and that this is allows endocardial endothelial cells to initiate their differentiation into mesenchymal cells (Chang et al., 2004). The role of VEGF in EMT is likely to be complex, and depend on spatial and temporal regulation given that, in two other studies of mouse embryonic valve development, VEGF was found to be a positive regulator of EMT (Enciso et al., 2003; Hallaq et al., 2004). In our laboratory, we showed a functional link between VEGF and the transcription factor NFATc1 in human post-natal valve endothelial cells (HPVEC)(Johnson et al., 2003). This prompted us to test directly the role of VEGF-R in cardiac valve development.
In this study, we identified a narrow window of time when VEGF-R signaling is required for formation of a functional valve in zebrafish embryos. To do this, we used two different tyrosine kinase inhibitors, namely AAC 789 and PTK 787. These 1-anilino-(4-pyridylmethyl)phthalazine compounds exhibit selectivity for human VEGF-Rs over other structurally-related receptor tyrosine kinases (Wood et al., 2000; Bold et al., 2002). We chose zebrafish as a model system because heart development and blood flow can be visualized in real time in transparent embryos, and unlike mammals, zebrafish are not noticeably affected by the lack of normal blood circulation for a few days.
RESULTS
PTK 787 and AAC 789 inhibit VEGF-induced nuclear localization of NFATc1
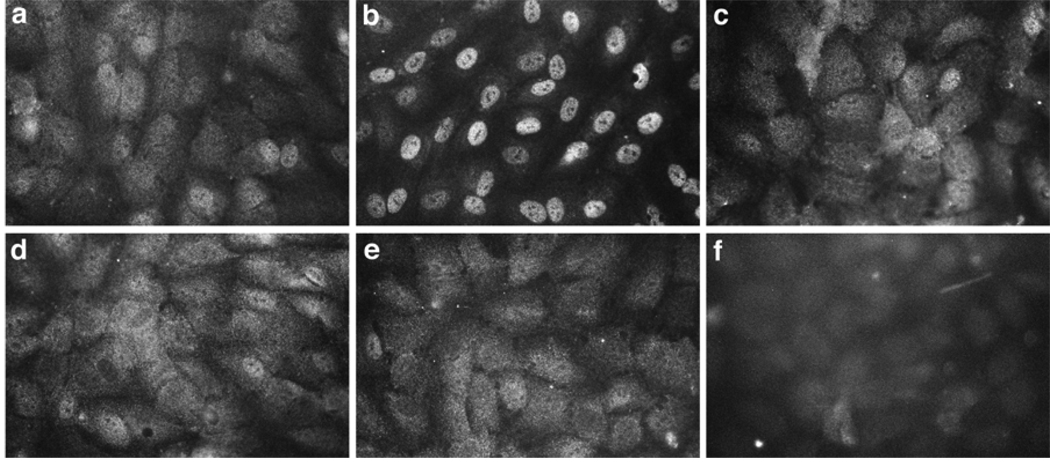
We demonstrated previously that VEGF-induced proliferation of HPVEC is mediated by VEGF-R2-induced NFATc1 translocation into the nucleus(Johnson et al., 2003). Therefore, we tested whether AAC 789 and PTK 787 could block VEGF-induced NFATc1 nuclear translocation in HPVEC. Pretreatment with either inhibitor, for one hour prior to VEGF stimulation, effectively blocked NFATc1 nuclear translocation (Fig. 1). These results showed that the receptor tyrosine kinase inhibitors disrupt VEGF-mediated NFATc1 nuclear translocation, and therefore would be useful for probing events downstream of VEGF-Rs. For comparison, FK506 was tested in parallel and, as expected, blocked VEGF-induced nuclear translocation of NFATc1 (Fig. 1). (FK506 is an immunosuppresant drug that inhibits the phosphatase activity of calcineurin, which in turn blocks de-phosphorylation and nuclear translocation of NFATs.) The tyrosine kinase domain between human and zebrafish VEGF-R2/KDR is highly conserved (Habeck et al., 2002). In addition, PTK 787 has been shown to block vascular development in zebrafish embryos (Chan et al., 2002; Lee et al., 2002) and VEGF-induced autophoshorylation of zebrafish VEGF-R2/flk-1(Chan et al., 2002). Hence, we used PTK 787, and additionally AAC 789, to test our hypothesis that signaling through VEGF-Rs is required for valve development.
Fig. 1.
VEGF-induced NFATc1 nuclear translocation was blocked by the VEGF-R tyrosine kinase inhibitors. HPVEC grown for 24 h in Endothelial Basal Media media with 10% FBS were either mock stimulated (panel a) or stimulated with 50 ng/ml VEGF165 (R&D Systems) for 30 min (panels b–f). Intracellular localization of NFATc1 was visualized using an anti-human NFATc1-specific mAb (panels a–e). NFATc1 expression in untreated HPVECs (a) was detected in the cytoplasm, as evident by the diffuse staining throughout the cells. Nuclear localization of NFATc1 was evident in HPVECs treated with VEGF165 (b). Pretreatment of HPVEC with 1 µM FK506 (c), 2µM AAC789 (d), or 2 µM PTK787 (e) inhibited VEGF-induced nuclear translocation of NFATc1. VEGF-treated HPVEC incubated with isotype-matched control IgG (f).
PTK 787 or AAC 789 cause toggling of blood within the hearts of zebrafish embryos
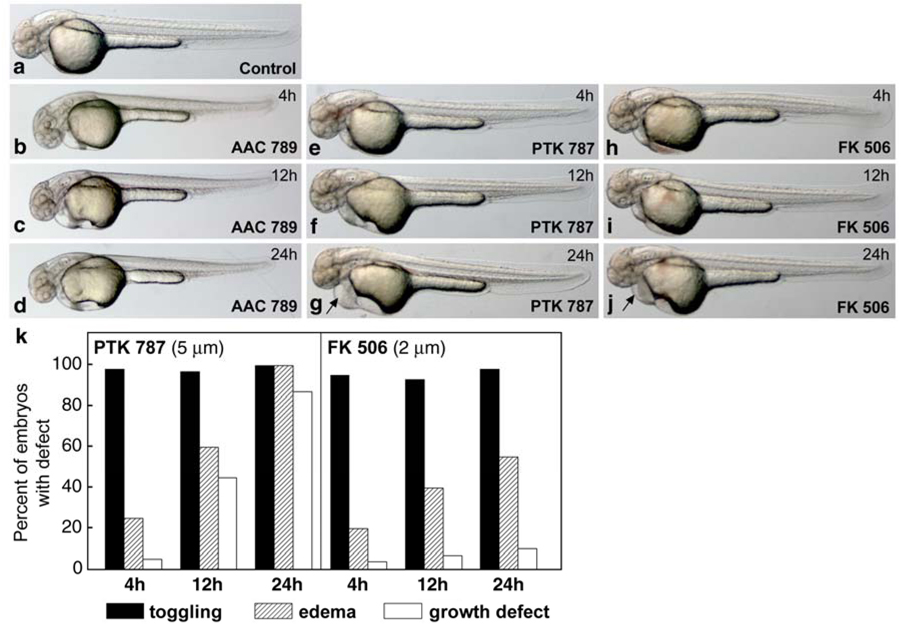
To avoid pleiotropic effects on vascular development, zebrafish were treated with 5 µM AAC 789 or 5 µM PTK 787 beginning at 17 hpf (15 somite stage) when heart development is already underway. The inhibitors were removed after 4, 12 or 24 h, and embryos were allowed to continue their growth until 48 hpf, a time when valve formation is normally complete (Walsh and Stainier, 2001). Treatment with either inhibitor, individually, for the three time periods resulted in toggling of blood between the atrial and ventricular chambers of the heart. To visualize this defect in real time, video microscopy was performed on live embryos (see on-line supplemental data: Movie S1, control embryo (note directional flow of blood through the heart and robust circulation); Movie S2, embryo treated for 4 h with AAC 789; Movie S3, embryo treated for 4 h with PTK 787). The toggling of blood seen in the VEGF-R–tyrosine kinase inhibitor-treated embryos represents ineffective and non-directional blood flow through the heart chambers. The phenotype of the AAC 789- and PTK 787-treated embryos was strongly reminiscent of the jekyll mutation in which the cardiac valve fails to develop (Walsh and Stainier, 2001). Not surprisingly, since NFATc1 is required for valve development in mice, FK506 (2 µM) also induced toggling of blood in the hearts of zebrafish embryos (on-line supplemental data; Movie S4, embryo treated for 4 hours with FK506). Delaying the addition of PTK787 until 22 hpf resulted in normal embryos without evident toggling in the heart (data not shown) suggesting that VEGF-R signaling is most critical for valve development at the 15–18 somite stage, corresponding to 17–19 hpf. A dose-response experiment in which PTK 787 was tested at 1, 2, 5, 7.5, and 10uM revealed that PTK 787 caused toggling at 1 and 2 uM, with nearly complete penetrance at 5uM. Hence, 5uM was used for all subsequent experiments, including those performed with AAC 789.
The morphology of zebrafish embryos at 48 hpf after treatment with AAC789, PTK 787 or FK506 for the specified periods of time is shown in Fig. 2. The gross morphology of embryos treated for 4 h with either AAC 789 (panel b) PTK 787 (panel e) or FK506 (panel h) was indistinguishable from control embryos (panel a), despite the easily observed toggling of blood within the hearts of embryos treated with these molecules (on-line supplemental data). Prolonged treatment with inhibitors for 12 or 24 h resulted in continued regurgitation of blood in the heart (not shown), but also some growth retardation in the head and tail and epicardial edema in PTK 787-treated embryos (panel g, arrow). Embryos treated with FK506 for 4 h (panel h), 12 h (panel i) or 24 h (panel j) showed almost the same morphology at 48 hpf as control embryos, with the exception of epicardial edema at 24 h (panel j, arrow). Edema and/or growth retardation was not observed in AAC 789-treated embryos (panels c, d). Panel k provides a quantitative summary of the defects observed. The percentage of embryos with growth defects increased with prolonged exposure to PTK 787 indicating that the wash-out at 4 h was effective in removing the inhibitor. Toggling was quantitated at 48 hpf in AAC 789-treated embryos as well: 93% (n= 42) of embryos in 4 h treatment group, 100% (n= 26) in 12 h treatment group, and 97% (n=35) in 24 h treatment group (data not shown). As a baseline comparison, no embryos in the untreated group showed any regurgitation of blood (n=178).
Fig. 2.
Morphology of AAC 789 -, PTK787-, and FK506-treated zebrafish embryos at 48 hpf. Zebrafish embryos staged at 15 somites (17 hpf) were treated with AAC 789 (5 µM), PTK 787 (5 µM) or FK506 (2 µM) for 4h, 12h, and 24h. At the end of each time period, embryos were washed several times with fresh medium to remove the inhibitor drugs and allowed to develop until 48hpf. Treatment for 4h with AAC 789 (b), PTK787 (e), or FK506 (h) resulted in toggling of blood within the heart (see on-line supplemental data), compared to control embryos, while no apparent effect on gross morphogenesis was visible. Treatment with PTK 787 for 12 h induced minor epicardial edema as well as minor retarded craniofacial development (f, and panel k). Treatment with PTK787 for 24 h resulted in severe epicardial edema (g, arrow), shortened tails, retarded brain growth, and blood toggling in the heart (panel k). Embryos treated with AAC 789 or FK506 for 12h (c, i) and 24h (d, j) showed almost the same morphology at 48hpf as control embryos (a), with the exception of minor epicardial edema in embryos treated for 24 h with FK506 (j, arrow). (k), The number of embryos with defects in each condition and at each time point (n= 160–500) is shown. No embryos in the untreated group showed any regurgitation of blood (n=178) (not shown).
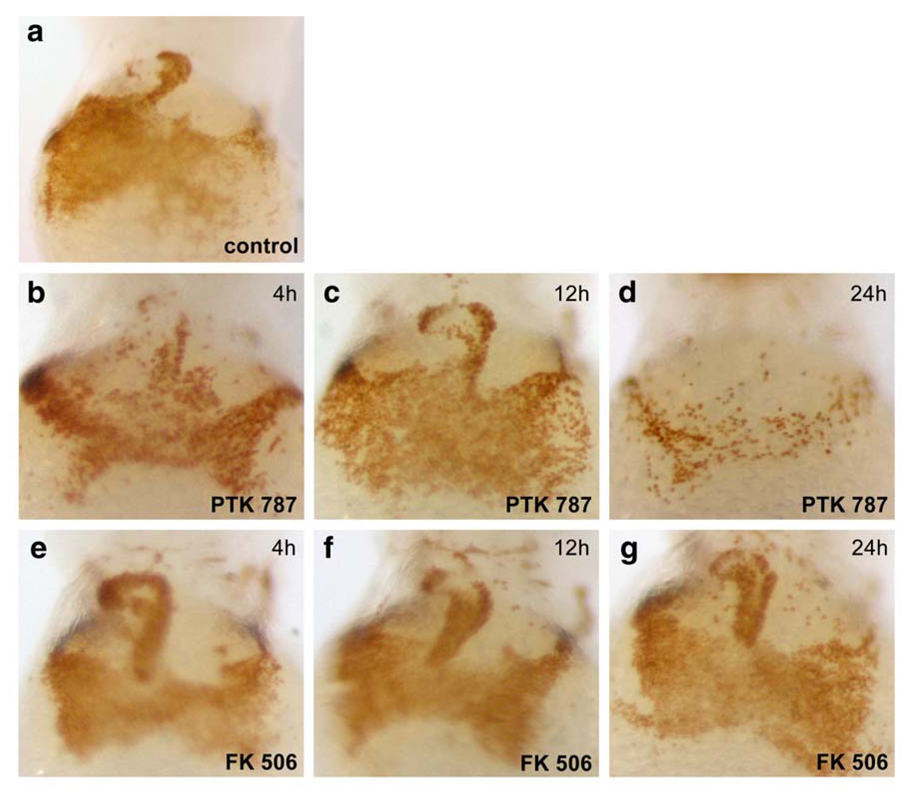
VEGF-R2/KDR is required for vasculogenesis and hematopoiesis (Eichmann et al., 1997; Shalaby et al., 1997). Thus, prolonged inhibition of VEGF-R signaling could inhibit hematopoiesis in zebrafish embryos, and thereby affect blood viscosity and shear forces in the developing vasculature. Intracardiac fluid forces have been shown to play an important epigenetic role in heart valve formation in zebrafish (Hove et al., 2003). To test whether exposure to PTK 787 decreased blood cell number in the circulation, and thus potentially altered hemodynamic forces in the developing heart, embryos were stained with o-dianisidine to detect hemoglobinized blood (Fig. 3). Normal circulation throughout the heart and the ducts of Cuvier on the yolk sac in a control embryo at 48 hpf is shown in panel a. At 17 hpf, embryos were treated with PTK-787 or FK506 for 4h, 12 h, and 24 h, washed thoroughly, and then allowed to develop until 48 hpf. Blood circulation and levels of hemoglobinized blood appeared normal except in embryos treated for 24 h with PTK 787. Hence, gross alterations in the blood viscosity are unlikely to have contributed to the cardiac valve defect seen in embryos treated for 4 h PTK 787 or with FK506.
Fig. 3.
Effects of PTK 787 and FK506 on hemoglobinized blood levels and circulation. Embryos treated with PTK 787 and FK506 as described in Fig. 2 were stained with o-dianisidine for circulating hemoglobinized red blood cells(Iuchi and Yamamoto, 1983) in the yolk sac and heart (30). (a) control embryos at 48 hpf. (b) PTK787 treatment for 4h had no effect on oxygenated blood levels and circulation. (c) 12 h and (d) 24 h treatment with PTK 787 resulted in decreased blood in yolk-sac and heart, especially in embryos treated for 24h (d). FK506 did not affect oxygenated blood levels or circulation, even after 24 h exposure. (4h, e; 12h, f; 24h, g).
PTK 787 and AAC 789 alter expression patterns of notch 1b and bmp-4 in the AV boundary region
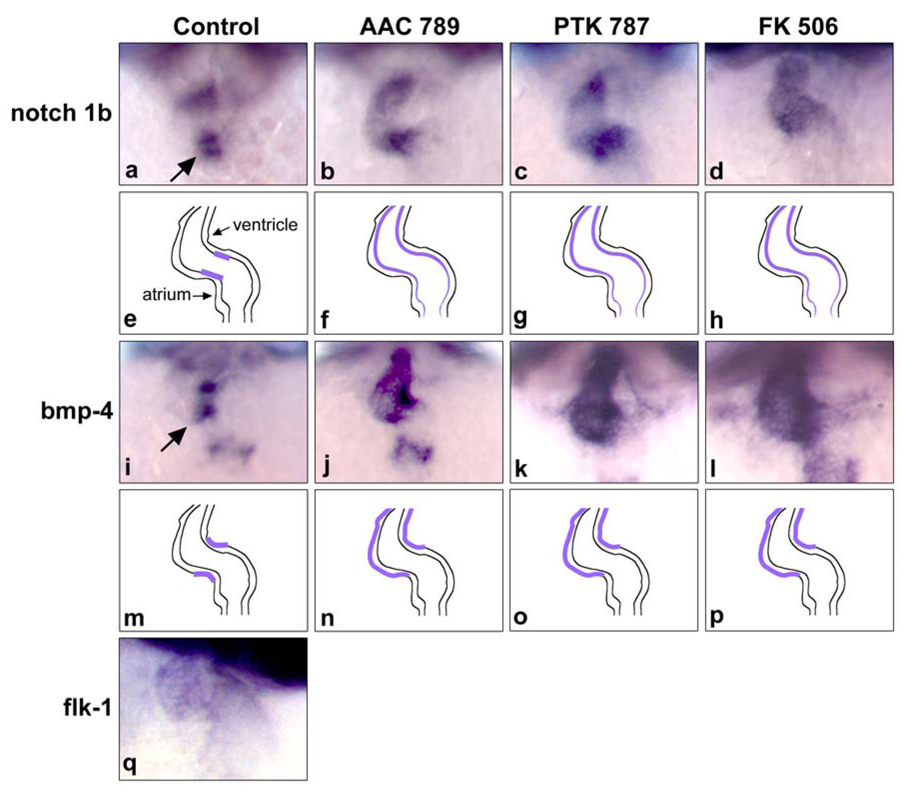
To investigate whether the toggling of blood in the heart is associated with defects in cell differentiation in the endocardial cushions, we examined expression of early markers of valve differentiation. The expression patterns of notch 1b (Westin and Lardelli, 1997) and bmp-4 (Nikaido et al., 1997) in the AV valve, and the endothelial marker, VEGFR2/flk-1, were examined by whole mount in situ hybridization. Notch 1b and bmp-4 were initially expressed throughout the anterio-posterior extent of the heart (data not shown), but then became restricted to the valve region (Fig 4a and i), as reported previously(Westin and Lardelli, 1997; Walsh and Stainier, 2001). Notch 1b at 48hpf was clearly restricted to the AV boundary of the endocardium in normal mock-treated embryos (Fig. 4a). In contrast, AAC789, PTK787 and FK506 induced dispersed and ectopic expression of Notch1b at the constriction and ventricular endocardium as well as weak atrial expression (Fig. 4b, c and d). FK506 caused a similar loss of cell-restricted expression of notch 1b in the valve region (Fig. 4d).
Fig. 4.
Disruption of VEGF or calcineurin signaling results in altered expression of notch 1b and bmp4 in cardiac valves. In situ hybridization analysis of the endocardial marker notch 1b (panels a–d), the myocardial marker bmp-4 (panels i–l), and the endothelial marker flk-1 (panel q) in the AV valve region. a, notch 1b was restricted to the AV region of the endocardium in control embryos (see arrow). b, notch 1b was ectopically expressed beyond the AV boundary into the ventricle and weakly in the atrium of embryos treated with AAC 789. c–d, similar ectopic expression of notch 1b in embryos treated with PTK 787 and FK506, respectively. e, f, g, h, schematic figures for the expression patterns seen in the panels above. i bmp-4 expression was prominent in the in myocardial region of AV valve at 48 hpf in control embryos (see arrow). j, AAC 789 treatment for 4 h resulted in strong ectopic expression of bmp-4, especially in the ventricle region. k–l, PTK787 and FK 506 induced similar mis-localized expression patterns m, n, o, p, schematic figures for expression patterns of bmp-4 seen in the panels above. q, expression of flk-1 in the endocardium of control embryos at 48 hpf.
Bmp-4 expression was restricted to the myocardium of the AV boundary at 48hpf zebrafish embryos (Fig. 4i), as reported previously (Walsh and Stainier, 2001). AAC 789- and PTK 787-treated embryos showed dispersed expression of bmp-4 in the ventricle and to a lesser extent in the atrium (Fig. 4j and k). FK506-treated embryos displayed a similar diffuse pattern of bmp-4 expression (Fig.4l). Expression of VEGF-R2/flk-1, the target of AAC 789 and PTK 787, is seen weakly throughout the endocardium of the developing heart at 48 hpf (panel q), consistent with previous reports (Thompson et al., 1998). The defects in endocardial and myocardial patterning, as indicated by ectopic expression of both notch 1b and bmp-4, may disrupt cell-cell communication within the developing valve region and thereby disrupt cell fate decisions during valve development.
AAC 789 and PTK 787 cause a morphological defect in the AV boundary region
We used three approaches to gain insight into the morphological defect caused by the small molecule inhibitors of VEGF-R signaling. The first approach was to use the signal generated by the notch1b in situ hybridization probe to highlight the AV boundary region in histological sections (Fig 5 A, C). In control embryos, notch 1b was detected in a constricted region at the base of the ventricle in a region consistent with the AV boundary (Panel A). The small size of the embryos at 48 hpf made it difficult to find sections that included the atrium. In contrast, the notch 1b signal was dispersed throughout the ventricular region of embryos treated with AAC789 for 4 hours beginning at the 15 somite stage (Panel C). This dispersed localization is consistent with results in Fig 4 and with dysregulated notch 1b expression observed in other published cases of morphological defects in zebrafish heart valve development (Walsh and Stainier, 2001).
Fig. 5.
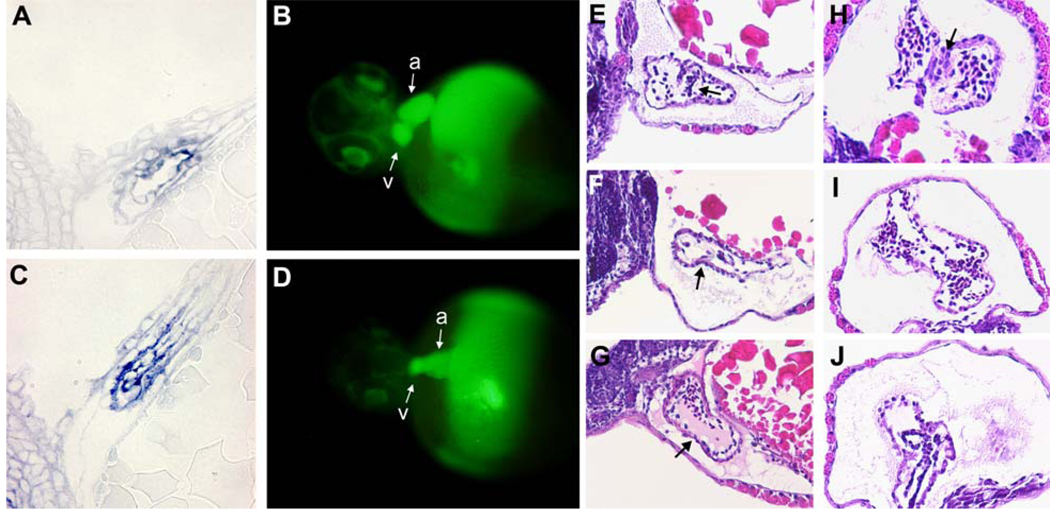
Defects in the AV boundary region of AAC 789-treated embryos. Control embryos (A, B) and embryos treated with 5uM AAC 789 for 4 hours beginning 17hpf (C, D) were analyzed by two methods. In situ hybridization for notch 1b was performed on whole mount embryos, which were then embedded in plastic resin, sectioned, and stained with eosin (A,C). Microangiography using FITC-Dextran was performed on embryos at 56 hpf (B, D). Fluorescence images were captured within 30 minutes of injection. a, atrium; v, ventricle Panels E–J are H&E stained histological sections of embryos untreated (E,H), or treated with 5uM PTK 787 for 4 hours beginning at 17 hpf (F, I) or with 2uM FK (G, J). Sagittal (E–G) or transverse (H–J) sections through embryos at 68 hpf are shown.
We also used micro-angiography to gain information about the morphological defect. Embryos were treated without or with AAC789 for 4 hours beginning at the 15 somite stage, washed to remove the inhibitor, and allowed to develop until approximately 50 hpf. The hearts of anesthetized embryos were micro-injected with FITC-dextran, and then observed and the images captured on a fluorescence microscope within 30 minutes. The FITC-dextran filled the heart and circulatory path of the embryo: this provided a striking view of the boundary between the atrial and ventricular chambers of the heart in control embryos (Fig 5, Panel B). In contrast, the demarcation between the two heart chambers was absent in embryos treated with AAC-789 for 4 hours beginning at the 15 somite stage.
We also examined the endocardial cushion by H&E staining paraffin sections from control and treated embryos (Fig 5, panels E–J). Panels E–G show sagittal sections and panels H–J show transverse sections through embryos sectioned at 68 hpf. The endocardial/myocardial cellular borders, as well as blood cells within the heart chamber, were seen in all embryos. In control embryos (panels E and H), cells with a distinct morphology were seen in the mid-region of the heart chamber perhaps indicating these are valvular cells. In PTK787- treated (panels F and I) and FK506- treated (panels G and J) embryos, there was a notable lack of cells in this region of the heart corresponding to the AV boundary; this was most apparent in the sagittal sections (see arrows). These histological findings are consistent with a lack of valvular development.seen by microangiography (panel D). In summary, the results from these three types of morphological analyses revealed defective valve development in the AV boundary, consistent with the functional toggling defect seen in live embryos.
Discussion
Chemical disruption of VEGF-R signaling in zebrafish embryos phenocopies the genetic disruption of UDP-glucose dehydrogenase in zebrafish (Walsh and Stainier, 2001). In both cases, toggling of blood, i.e., regurgitation, within the heart is evident in live embryos and loss of cell-restricted expression of notch-1b and bmp-4 is revealed by in situ hybridization analyses. Walsh and Stainier speculated that UDP-glucose dehyrogenase provides essential precursors for biosynthesis of hyaluronic acid because of the similar, jekyll-like defects observed in mice deficient for hyaluronan synthase-2 (Camenisch et al., 2000). Many studies have shown that VEGF modulates extracellular matrix biosynthesis and in turn, the ECM influences cell behavior during development (Ortega et al., 1998). Whether VEGF-R signaling and hyaluronic acid biosynthesis function in an integrated manner during valve development remains unknown.
The TGF-β family has been shown to play important roles in endocardial cushion morphogenesis (Harvey and Rosenthal, 1999). TGFβ3 is expressed in endocardium and contributes to cardiac valve remodeling after EMT(Camenisch et al., 2002). More recently, it has been shown that TGFβ1 induces EMT in clonal populations of valve endothelial cells (Paranya et al., 2001). TGFβ1 has been shown to increase endoglin expression (Sanchez-Elsner et al., 2002), an ancillary TGFβ receptor that is important for heart valve development (Arthur et al., 2000). The upregulation of endoglin by TGFβ1 is cooperative with hypoxia, which is an upstream regulator of VEGF, suggesting cross-talk between VEGF and TGFβ1 in valvulogenesis. Based on the results presented here and published studies, we speculate that VEGF and TGFβ1 may function together via cooperative interactions during development of cardiac valves.
Mice deficient in VEGF-R2/flk-1 die in utero because of lack of endothelial and blood cells (Shalaby et al., 1995). Recent analysis of the zebrafish flk-1 mutant reveals that flk-1 is not essential for vasculogenesis before 36 hpf (Habeck et al., 2002); instead, angiogenesis of the intersegmental vessels, central arteries and veins that vascularize the brain, gut, and pectoral fins are absent. Defects in heart valve formation were not reported. The milder phenotype of the zebrafish flk-1 mutant suggests the presence of a second VEGF receptor in zebrafish that partially compensates for the loss of the redundant isoform (Habeck et al., 2002). Hence, the use of molecule inhibitors such as AAC 789 and PTK787 highlights the unique advantage of ablating signaling through multiple, redundant VEGF receptors. Such inhibitors, in effect, allow one to easily perform a “conditional knockout” experiment, and thereby uncover novel and specific functions of the targeted proteins. Indeed, use of small cell permeable molecules to perturb function of specific proteins in zebrafish is emerging as an exciting area of chemical genomics (Pichler et al., 2003).
In summary, based on results presented here, as well as previous studies (Dor et al., 2001; Johnson et al., 2003), we speculate that by inducing nuclear translocation of NFATc1, zebrafish VEGF-R(s) regulate genes involved in proliferation, migration and differentiation of endocardial cells in the valve forming region of the developing heart valve in a precisely regulated spatio-temporal manner. This speculation is consistent with recent findings reported by Chang and colleagues (Chang et al., 2004). In their study of murine valvulogenesis, they showed that NFAT signaling in the myocardium at E9 suppresses VEGF expression, producing conditions that initiate EMT. At E11, NFAT signaling in the endocardium contributes to elongation of the valve leaflets and further remodeling, which is likely to involve endothelial proliferation, migration, and perhaps additional EMT. This scenario would be consistent with the positive role role for VEGF in EMT seen at E10.5 (Hallaq et al., 2004). In this elegant study, VEGFa signaling in AV explants from E10.5 embryos was shown promote morphological changes in endocardial cells, active celluar migration into the collagen gel, and the expression of smooth muscle alpha-actin, a marker for cells undergoing EMT. We postulate that in these later stages of EMT, VEGF-mediated endothelial cell proliferation is necessary to replenish the endothelial monolayer of the developing valve leaflet as previous endothelial cells have migrated into the cardiac jelly to become mesenchymal cells. Without sufficient VEGF signaling, EMT may come to a halt because of an insufficient number of endothelial cells. Hence, high levels of VEGF, especially at the onset of EMT, inhibit valve development, but too little VEGF signaling, especially at later points when cellular proliferation and migration are ongoing, will limit valve development. Our results presented here provide the first direct in vivo evidence that VEGF-R signaling is required for cardiac valve development. Further studies will be necessary to identify the genetic diversity of VEGF-R and NFAT isoforms in zebrafish and to correlate these homologs with their mammalian counterparts.
Experimental Procedures
Cell Culture and Immunofluorescence
HPVEC were isolated from human pulmonary valve leaflets as described (Johnson et al., 2003). To detect VEGF-induced nuclear-localization of NFATc1, HPVECs were fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and incubated with mouse anti-human NFATc1 monoclonal antibody (7A6 from Santa Cruz Biotechnology, Inc.) diluted 1:500 followed by FITC-conjugated anti-mouse IgG diluted 1:200.
Zebrafish strains and growth conditions
Standard AB strain zebrafish were maintained and used for the experiments in our study. Embryos were collected from natural matings, dechorionated with pronase at 15–18 somite developmental stage, and maintained in 0.2 mM 1-phenyl-2-thio-urea (PTU) (Sigma) to inhibit pigment formation. Dechorionated embryos were maintained in 2 milliliters of E3 medium with PTU in a 6 well dish.
Kinase inhibitor
The VEGFR-2 tyrosine kinase inhibitors, PTK787 and AAC 789(Bold et al., 2002), were kindly provided by Novartis Pharma AG. Embryos were treated with either dimethyl sulfoxide in PTU or inhibitors (AAC789, PTK787, FK506) in dimethyl sulfoxide and PTU. Care was taken to minimize exposure of AAC 789 to light and freeze-thaw cycles.
Whole mount in situ hybridisation and o-dianisidine staining
Expression of bmp4 and notch 1b were detected by whole mount in situ hybridzation as described (Thisse et al., 1994). Live embryos were stained with o-dianisidine (Sigma) as described (Iuchi and Yamamoto, 1983). For each probe and treatment condition, 10–15 embryos were analyzed. For higher resolution analysis, a set of whole mount embryos were hybridized with notch 1b, embedded in plastic resin, sectioned, counterstained with eosin, and examined by light microscopy. To examine tissue morphology, embryos were embedded in plastic resin, sectioned and stained with eosin to visualize tissue morphology.
Micro-angiography of zebrafish embryos
Circulation through the heart was visualized by injecting FITC-dextran (Sigma) into the sinus venosa as described (Lee et al., 2002). Untreated and AAC 789-treated embryos were injected approximately 50–56 hpf and visualized by fluorescence microscopy within 30 minutes of the injection.
On-line Supplemental Data
Movies S1, S2, S3 and S4 – Quick Time (QT) videos of zebrafish embryos. Movie S1, control embryos at 48 hpf. Movie S2, embryos treated with 5 µM AAC 789 for 4 hours from 17–21 hpf, washed several times to remove AAC 789, and then allowed to develop until 48 hpf. Movie S3, embryos treated in the same manner with 5 µM PTK 787 and analysed at 48 hpf. Movie S4, embryos were treated in the same manner with 2 µM FK506 and analyzed at 48 hpf. Live embryos were recorded by video imaging (100 frames in 10 secs). Similar results were seen in embryos at 72 hpf.
Supplementary Material
Acknowledgments
We thank Dr. Michael Klagsbrun, Vascular Biology Program and Department of Surgery, Children’s Hospital, Boston for insights about VEGF/VEGF-receptors in the zebrafish model system. Present address for YML is College of Natural Sciences, Department of Biotechnology, Kyungpook National University, South Korea.
Grant Support: NIH HL60490 to JB and funds from the William Randolph Hearst Foundations to BHP.
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGF-R
VEGF-receptor
- NFAT
nuclear factor in activated T cells
- HPVEC
human pulmonary valve endothelial cells
- EMT
endothelial to mesenchymal transdifferentiation
- PTU
1-phenyl-2-thio urea
References
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- Bold G, Frei J, Furet P, Manley P, Bruggen J, Cozens R, Ferrari S, Hofmann F, Martiny-Baron G, Mestan J, Meyer T, Wood J. CGP 79787D (PTK787/ZK22584), CGP 84738, NVP-AAC789, NVP-AAD777 and related 1-anilino-(4-pyridylmethyl)phthalazines as inhibitors of VEGF- and bFGF-induced angiogenesis. Drugs of the Future. 2002;27:43–55. [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- Dor Y, Klewer SE, McDonald JA, Keshet E, Camenisch TD. VEGF modulates early heart valve formation. Anat Rec. 2003;271A:202–208. doi: 10.1002/ar.a.10026. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Corbel C, Nataf V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci U S A. 1997;94:5141–5146. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol. 2003;160:605–615. doi: 10.1083/jcb.200209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12:1405–1412. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- Hallaq H, Pinter E, Enciso J, McGrath J, Zeiss C, Brueckner M, Madri J, Jacobs HC, Wilson CM, Vasavada H, Jiang X, Bogue CW. A null mutation of Hhex results in abnormal cardiac development, defective vasculogenesis and elevated Vegfa levels. Development. 2004;131:5197–5209. doi: 10.1242/dev.01393. [DOI] [PubMed] [Google Scholar]
- Harvey R, Rosenthal N, editors. Heart Development. San Diego: Academic Press; 1999. [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Iuchi I, Yamamoto M. Erythropoiesis in the developing rainbow trout, Salmo gairdneri irideus: histochemical and immunochemical detection of erythropoietic organs. J Exp Zool. 1983;226:409–417. doi: 10.1002/jez.1402260311. [DOI] [PubMed] [Google Scholar]
- Johnson EN, Lee YM, Sander TL, Rabkin E, Schoen FJ, Kaushal S, Bischoff J. NFATc1 mediates vascular endothelial growth factor-induced proliferation of human pulmonary valve endothelial cells. J Biol Chem. 2003;278:1686–1692. doi: 10.1074/jbc.M210250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, Klagsbrun M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci U S A. 2002;99:10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Saji T, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Ortega N, L'Faqihi FE, Plouet J. Control of vascular endothelial growth factor angiogenic activity by the extracellular matrix. Biol Cell. 1998;90:381–390. doi: 10.1111/j.1768-322x.1998.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Pichler FB, Laurenson S, Williams LC, Dodd A, Copp BR, Love DR. Chemical discovery and global gene expression analysis in zebrafish. Nat Biotechnol. 2003;21:879–883. doi: 10.1038/nbt852. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Halpern ME, Postlethwait JH. Goosecoid expression in neurectoderm and mesendoderm is disrupted in zebrafish cyclops gastrulas. Dev Biol. 1994;164:420–429. doi: 10.1006/dbio.1994.1212. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Westin J, Lardelli M. Three novel Notch genes in zebrafish: implications for vertebrate Notch gene evolution and function. Dev. Genes Evol. 1997;207:51–63. doi: 10.1007/s004270050091. [DOI] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.