Abstract
Breathing is a vital behavior that is particularly amenable to experimental investigation. We review recent progress on three problems of broad interest. (i) Where and how is respiratory rhythm generated? The preBötzinger Complex is a critical site, whereas pacemaker neurons may not be essential. The possibility that coupled oscillators are involved is considered. (ii) What are the mechanisms that underlie the plasticity necessary for adaptive changes in breathing? Serotonin-dependent long-term facilitation following intermittent hypoxia is an important example of such plasticity, and a model that can account for this adaptive behavior is discussed. (iii) Where and how are the regulated variables CO2 and pH sensed? These sensors are essential if breathing is to be appropriate for metabolism. Neurons with appropriate chemosensitivity are spread throughout the brainstem; their individual properties and collective role are just beginning to be understood.
Keywords: preBötzinger, pacemaker, neurokinin, serotonin, raphe
Introduction
We breathe no matter what. Breathing movements start episodically in utero, are continuous at birth and, except for the briefest of pauses, continue without respite. The neural circuits underlying breathing must be wired correctly by birth and must change on the fly as the lungs and respiratory muscles mature and then age. These circuits must be stable yet responsive to challenges affecting O2, CO2, and pH levels in the body, such as exercise, sleep, and hypoxia. They must be well coordinated with other movements generating air flow, such as speech, and airway reflexes, such as cough or sneeze, as well as movements impacting the respiratory muscles and lungs, such as locomotion. Longer-lasting disturbances demand adaptive solutions; for example, breathing must be adjusted to accommodate physical changes associated with weight gain and loss, pregnancy, or disease. Breathing is also a special behavior from an experimental perspective because its systems level activity is present in reduced vertebrate preparations, even in vitro. Here we review recent progress in three critical aspects of breathing that should be of particular interest to neuroscientists (see also Funk & Ramirez 2002, Richter & Spyer 2001):
Rhythmicity: Where and how is the rhythm of breathing generated? Rhythmic movements like breathing are fundamental, complex behaviors. The neural mechanisms underlying generation of respiratory rhythm in mammals are particularly accessible to experimental investigation.
Plasticity: What are the characteristics and underlying mechanisms of long-term changes in breathing? The onset of a respiratory infection and changing environmental conditions (e.g., ascent to altitude, development and aging of the lung and chest wall, a change of weight, or pregnancy) require substantial changes in respiratory motor output to maintain blood gas homeostasis. Given the exceptional experimental models, breathing is a useful adaptive behavior to study.
Chemosensitivity: Where and how are breathing-regulated variables CO2/pH sensed and processed in the brain? Critical sensory information is provided by CO2-sensitive central chemoreceptors, which affect synaptic drive necessary for rhythm generation and modulate respiratory pattern to protect the brain from changes in CO2 and pH. Neurons throughout the brain are local environmental sensors, e.g., for temperature, osmolality, and CO2/pH. Central chemoreception is an excellent model for complex, distributed CNS environmental sensory feedback systems.
Rhythmicity
A previous Annual Review on this topic (Rekling & Feldman 1998) focused on two hypotheses: (i) The preBötzinger Complex (preBötC) contains the kernel for generating respiratory rhythm; (ii) neurons with voltage-dependent pacemaker properties underlie the generation of respiratory rhythm. While considerable data support these hypotheses, recent work suggests that a reevaluation is needed.
PreBötC as a Site for Rhythm Generation
Respiratory rhythm is generated by brainstem neurons (Figure 1). A ventrolateral column extending from the facial nucleus to the spinal cord contains respiratory premotor neurons projecting to spinal and cranial respiratory motoneurons and propriobulbar neurons. A small portion near the rostral end of this column, necessary for in vitro neonatal rodent medullary slice preparations to generate respiratory-related motor nerve output, was designated the preBötC (Smith et al. 1991). Perturbations of neuronal function in and around this area severely disrupt breathing in adult mammals (Bongianni et al. 2002, Fung et al. 1994, Hsieh et al. 1998, Mutolo et al. 2002, Ramirez et al. 1998, Solomon et al. 1999), but the absence of a clear anatomical marker for preBötC neurons hindered understanding of the sometimes contradictory results. Gray and colleagues (1999) focused on peptides that change respiratory frequency when applied directly to the preBötC, i.e., Substance P (SP) and opioids, assuming their receptors might identify rhythm-generating neurons. Neurons expressing the neurokinin 1 receptor (NK1R; the receptor for SP) demarked a limited region of the rodent ventrolateral respiratory column (as well as other medullary regions, many of which are central chemoreceptor sites—see below), and their presence was proposed to identify the preBötC (Gray et al. 1999). These preBötC NK1R neurons rarely express the GABA synthetic enzyme GAD67, the glycine transporter GlyT2, tyrosine hydroxylase, or choline acetyltransferase (Wang et al. 2001a); express the glutamate transporter VGLUT2 (Guyenet et al. 2002a); and are immunoreactive for glutamate (Liu et al. 2001). This finding suggests that the preBötC NK1R neurons are excitatory (Gray et al. 1999). Preproenkephalin (PPE) further divides NK1R neurons into two groups rostrocaudally (Guyenet et al. 2002a): PPE+ neurons tend to be larger, more caudal, and bulbospinal; PPE- neurons are smaller, more rostral, and propriobulbar. Moreover, not all NK1R neurons at the rostral end of the ventrolateral respiratory column are propriobulbar; the caudal-most NK1R neurons are bulbospinal (Makeham et al. 2001, Wang et al. 2001a). Given the generally held view that bulbospinal respiratory neurons do not participate in rhythmogenesis, the preBötC should be defined as that portion of the NK1R population containing propriobulbar neurons.
Figure 1.
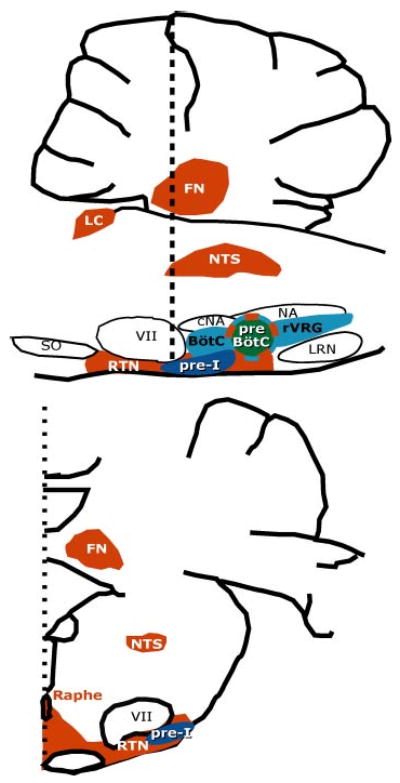
Respiratory premotor, putative rhythmogenic, and central chemoreceptor locations in the rat rostral medulla and cerebellum. (Top) Parasagittal view. (Bottom) Transverse view at the caudal level of the facial nucleus (vertical dotted line at top) (modified from Nattie 2000 and Gray et al. 1999). Blue areas in the ventrolateral medulla are the principal location of respiratory bulbospinal premotor neurons that project to phrenic, intercostal, and abdominal motoneurons, which drive muscles of the respiratory pump. The preBötzinger Complex plays a critical role in rhythm generation and central chemoreception. The pre-I neuron population, which may also play a role in rhythmogenesis, appears to be located ventral and rostral to the preBötC with many neurons close to the ventral surface. Red areas represent regions that play a role in central chemoreception. See text for details. Abbreviations: FN, fastigial nucleus; LC, locus ceruleus; NTS, nucleus of the solitary tract; VII, facial nucleus; rVRG, rostral ventral respiratory group; NA, nucleus ambiguus; cNA, compact division of the nucleus ambiguus; LRN, lateral reticular nucleus; RTN, retrotrapezoid nucleus; SO, superior olive; BötC, Bötzinger complex; preBötC, preBötzinger Complex.
Guyenet & Wang (2001) investigated the firing properties of preBötC NK1R neurons and made a remarkable finding. In anesthetized adult rats, all preBötC neurons that fired with a preinspiratory firing pattern expressed NK1R, whereas those with different respiratory-modulated patterns did not. Thus, a subset of the propriobulbar preBötC NK1R neurons constitute a well-defined class of neurons with many common properties that may underlie rhythm generation.
The rostral part of the ventrolateral respiratory column shows a distinct parcellation with respect to a number of additional markers. Subsets of NK1R neurons express μ-opiate (Gray et al. 1999) or somatostatin (P.A. Gray and J.L. Feldman, unpublished observations) receptors or somatostatin mRNA (Guyenet et al. 2002b). The Ca2+ binding proteins, parvalbumin, calretinin, and calbindin, label distinct parts of this region (Alheid et al. 2001). Parvalbumin neurons are caudal and rostral to the preBötC but are rarely within it. Calretinin colocalizes with neurons at the caudal end of the NK1R cluster, which may represent bulbospinal NK1R neurons and may overlap with the PPE+ bulbospinal neurons (Guyenet et al. 2002a). The significance of these phenotypic differences remains to be determined.
Lesioning NK1R PreBötC Neurons Profoundly Affects Breathing
Bilateral injections of the conjugate of the toxin saporin and Substance P (SP-SAP), which is specific to neurons expressing NK1R (Mantyh et al. 1995, 1997), into the preBötC of adult rats can produce a striking transformation in breathing pattern (Gray et al. 2001, Wang et al. 2002). Affected (preBötCNK1R−) rats breathe with an ataxic pattern in wakefulness, characterized by an irregular sequence of inspiratory efforts of near-normal amplitude interspersed with prolonged periods of low amplitude inspiration (Figure 2). Some lesioned rats were unaffected by the toxin, the principal difference between affected and unaffected lesioned rats being the degree of preBötC NK1R neuron loss; affected rats lost at least 75% of these neurons bilaterally.
Figure 2.
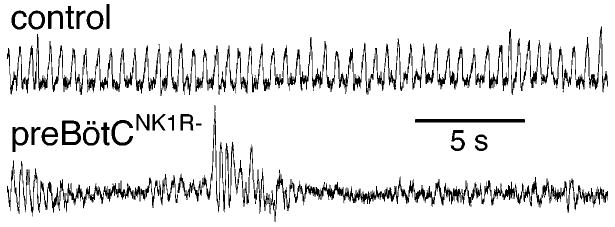
Near complete lesion of preBötC NK1R neurons causes an ataxic breathing pattern. (Top) Changes in pressure inside a plethysmograph (which are proportional to tidal volume; inspiration up) of a normal awake adult rat in room air. (Bottom) Similar measure in an awake adult preBötCNK1R− rat in room air. Redrawn from Gray et al. 2001. Used with permission from Nature (http://www.nature.com/).
Ventilatory responses of preBötCNK1R− rats to changes in inspired CO2 are markedly depressed. They respond to hypoxic levels well tolerated by control rats with apneas of increasing, eventually fatal duration. When breathing pure O2, ventilation in preBötCNK1R− rats is further depressed, with some rats developing fatal apneas. This response is presumably due to hyperoxic inhibition of peripheral chemoreceptor activity.
Because mice lacking either the NK1R or SP continue to breathe (Cao et al. 1998, De Felipe et al. 1998, Ptak & Hilaire 1999, Telgkamp et al. 2002), NK1Rs per se are not critical for breathing. Rather, NK1Rs mark preBötC neurons that are necessary for normal rhythm generation and are required for normal chemoreceptor responses. In the absence of preBötC NK1R neurons, the ataxic breathing pattern could be generated by other structures that may or may not normally contribute to breathing rhythm, including: (i) nonNK1R preBötC neurons, ventral respiratory group or other respiratory neurons (Dobbins & Feldman 1994) including the preinspiratory network (see below); (ii) vestigial brainstem rhythmogenic networks (Champagnat & Fortin 1997, Straus et al. 2000); or (iii) suprapontine structures normally underlying voluntary or emotional control of the respiratory muscles.
Pacemaker Neurons May Not Be the Kernel for Normal Rhythm Generation
Respiratory-related rhythm in vitro persists after blockade of Cl−-mediated synaptic inhibition (Feldman & Smith 1989, Onimaru et al. 1989), which suggests that pacemaker neurons present in the preBötC generate the rhythm (Del Negro et al. 2001, Koshiya & Smith 1999, Rekling & Feldman 1998, Smith et al. 1991). Attention has focused on the persistent sodium current (INaP) as driving the pacemaker potential, leading to periodic bursting in these neurons (Del Negro et al. 2002a, Thoby-Brisson & Ramirez 2000). If the pacemaker hypothesis is correct, then abolishing pacemaker neuron behavior in the preBötC should severely perturb or stop respiratory rhythm. When voltage-dependent pacemaker activity was blocked with the INaP antagonist riluzole in 50/50 preBötC neurons studied in slices from neonatal rodents (<P5), respiratory-related rhythm was unaffected (Del Negro et al. 2002b). These negative results must be interpreted with caution. Pacemaker neurons with different properties are present in slices from older mice and in more intact en bloc preparations. In P7 mice, there are two distinct populations of pacemaker neurons: Cd2+-sensitive/hypoxia-sensitive/riluzole-insensitive and Cd2+-insensitive/hypoxia-insensitive/riluzole-sensitive (Thoby-Brisson & Ramirez 2000), (F. Pena, M. Parkis, and J.-M. Ramirez, unpublished observations). These latter pacemaker neurons are presumably similar to those seen by Del Negro et al. (2002b) and may underlie gasping (Lieske et al. 2000, Ramirez et al. 2002). In en bloc preparations, preinspiratory neurons with pacemaker properties are found rostral to the preBötC (Ballayni et al. 1999) and could therefore play a role in rhythmogenesis in more intact preparations (see below). Nonetheless, the facts that rhythm remains in thin slices with no obvious sign of pacemaker activity and that a causal relationship between pacemaker activity and rhythm generation remains to be demonstrated warrant the consideration of mechanisms for respiratory rhythm generation that do not depend on endogenous bursting properties. An emergent network-based mechanism (“group pacemaker”), in which ensembles of neurons become rhythmically active through chemical and electrotonic excitatory synaptic interactions (Rekling et al. 2000), represents one alternative (Rekling & Feldman 1998).
Are Different Breathing Patterns Generated by PreBötC?
Mammals generate different breathing patterns, including eupnea (the normal resting pattern), sighing (where larger inspiratory efforts ride on top of eupneic breaths), and gasping (large amplitude, short inspiratory efforts interspersed with long expiratory pauses during anoxia). Correlates of these patterns can be elicited in brainstem slice preparations (Lieske et al. 2000) and can be differentiated pharmacologically. Glycine decouples augmented sigh-like from eupneic-like inspiratory bursts, whereas very low concentrations of Cd2+ abolishes the sigh-like pattern without affecting the eupneic-like rhythm. Anoxia abolishes both the eupneic-like and sigh-like patterns and induces a gasp-like one. These rhythms appear to originate in the preBötC, with overlapping sets of neurons active during each rhythm. Different patterns of output may result from modulation of synaptic or intrinsic properties of, or the coupling between, preBötC neurons (Lieske et al. 2000). Such an interpretation builds on observations in invertebrates that different motor patterns can be produced by reconfiguration of an existing network via neuromodulation (Marder & Calabrese 1996). Because reconfiguration in invertebrates is thought to be the consequence of their limited number of neurons, vertebrate breathing may rely on essential circuits with relatively few neurons; for example, there are only ∼600 preBötC NK1R neurons in rats (Gray et al. 2001, Guyenet et al. 2002a, Liu et al. 2001, Wang et al. 2001a).
Other Brainstem Loci as Sites for Rhythm Generation
There are respiratory neurons in the brain stem that are not captured in thin slice preparations, and some of these populations may contribute to rhythm generation in en bloc preparations or in vivo. Neurons with preinspiratory (pre-I) discharge patterns, some with pacemaker-like properties, exist in the medulla mostly ventral and rostral to the preBötC (Onimaru et al. 1989, 1997), with many neurons quite close to the ventral medullary surface (Figure 1). These neurons may be the kernel for rhythm generation (Ballayni et al. 1999). Pre-I neurons are distinguished from putative rhythm-generating inspiratory neurons in the preBötC because pre-I neurons are opiate-insensitive (Takeda et al. 2001), whereas preBötC inspiratory neurons are opiate-sensitive (Gray et al. 1999, Takeda et al. 2001). If preBötC opiate-sensitive inspiratory neurons are the sole source of respiratory rhythm, then opioids should cause continuous respiratory slowing in all experimental preparations (Mellen et al. 2003). This is not the case, however. In en bloc preparations and in neonatal rats in vivo, opioids cause the periods of inspiratory bursting to jump between control period and integer multiples of control period. This quantal slowing may result from failure of phasic drive from pre-I neurons to produce bursting in preBötC inspiratory neurons, whose rhythmogenic function is depressed or eliminated by opioids (Figure 3). In vivo, opiates cause inspiratory but not expiratory motor activity to skip breaths (Janczewski & Feldman 2002, Janczewski et al. 2002), suggesting that preBötC drives inspiratory activity, whereas the preinspiratory network drives expiratory activity (Figure 3).
Figure 3.
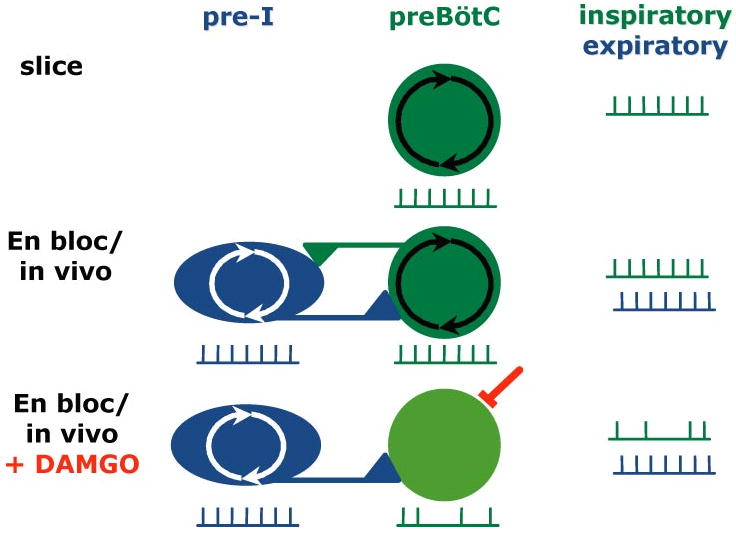
Two rhythmogenic networks, the preBötC and the pre-I network, may underlie generation of respiratory rhythm. In slices (top), only the preBötC is present, driving inspiratory activity. In en bloc preparations and in vivo (middle), the two populations interact seamlessly, producing a coordinated respiratory pattern of inspiratory and expiratory activity. When μ-opiates are added (bottom), preBötC neurons are hyperpolarized, whereas pre-I neurons are unaffected. Expiratory activity therefore continues uninterrupted, but inspiratory activity skips cycles because the preBötC rhythmogenicity is lost and its responsiveness to rhythmic drive from the pre-I network is depressed, leading to transmission failure. Based on Janczewski et al. 2002, Mellen et al. 2002.
The concept of coupled respiratory rhythmogenic networks in mammals is consonant with results from nonmammalian vertebrates. After blockade of synaptic inhibition in an in vitro turtle brainstem, respiratory-related activity is predominantly expiratory (Johnson & Mitchell 2002). In frog, discrete but synaptically coupled brainstem sites separately generate buccal and pulmonary rhythms (Wilson et al. 2002). The latter rhythm-generating network lies complete but dormant owing to GABAergic inhibition until the tadpole undergoes metamorphosis and begins air breathing (Straus et al. 2000). Similar differentiation between upper airway or expiratory and inspiratory pump rhythm-generating networks may be reflected in the anatomical organization of the preBötC and preinspiratory circuits. This organization may reflect distinctly different respiratory mechanics in mammals compared to nonmammalian vertebrates: Mammals are the only class of vertebrates with a diaphragm. The preBötC could underlie inspiration, i.e., the inspiratory-dominated diaphragmatic-oriented breathing pattern, whereas the pre-I neurons could be the principal source for breathing in nonmammalian vertebrates and still play a role in generation of breathing patterns in mammals. Under normal conditions in mammals, these two oscillators are well coordinated but may be dominated by the preBötC.
Developmental Changes in Neuronal Properties Underlying Rhythm Generation
We do not yet understand how closely mechanisms of rhythm generation in vitro represent those in intact, behaving mammals at any age (Ramirez et al. 2002, Rekling & Feldman 1998). Postnatal developmental changes in networks generating respiratory rhythm may occur, but they must be seamless because they cannot significantly interrupt breathing. Intracellular Cl− concentration changes have been reported in preBötC neurons in early postnatal rodent development (P0–P5) (Ritter & Zhang 2000), possibly causing depolarizing instead of hyperpolarizing post-synaptic potentials with synaptic GABA or glycine release during the first days of life. Whether these changes occur, and whether they affect rhythm-generating neurons is controversial (Brockhaus & Ballanyi 1998, Shao & Feldman 1997). Postnatal changes in Ca2+ channel expression also occur, possibly explaining the emergence of the Cd2+-sensitive pacemaker neurons after P6 in mice (Thoby-Brisson & Ramirez 2000).
Genetically Altered Mice
Many mice with single gene deletions are born live but die shortly thereafter. Although the specific cause of death is uncertain, the proximate cause of death often appears to be respiratory failure (Katz & Balkowiec 1997). Interpreting the mechanisms underlying presumed disruptions in respiratory rhythm is a significant challenge because many contributory pathologies must be evaluated. Respiratory patterns are disrupted in mice with restricted expression of genes that regulate rhombomeric development including krox-20 (Borday et al. 1997a, Borday et al. 1997b, Jacquin et al. 1996), kreisler (Chatonnet et al. 2002), and the homeobox gene Rnx (Shirasawa et al. 2000). Such disruptions are also seen in mice deficient in (i) neurotransmitter receptors, e.g., NMDAR1 (Funk et al. 1997, Sprengel & Single 1999); (ii) channels, e.g., a class of voltage-gated Na+ channels (Planells-Cases et al. 2000); (iii) trophic factors, e.g., brain-derived neurotrophic factor (BDNF) (Balkowiec & Katz 1998, Erickson et al. 1996, Katz & Balkowiec 1997); (iv) enzymes associated with neuromodulator function, e.g., monoamine oxidase-A (Bou-Flores et al. 2000); or (v) transcription factors associated with myelin formation (Bermingham et al. 1996).
Certain gene deletions are associated with alterations in chemosensory pathways. For example, mutations in both BDNF and glial cell line-derived neurotrophic factor (GDNF) profoundly affect the survival of carotid chemosensory neurons in the petrosal ganglion (Erickson et al. 2001). Other mutations can alter time-dependent ventilatory responses to hypoxia (Powell et al. 1998). Mutations involving endothelin-converting enzyme-1 (Renolleau et al. 2001), nitric oxide synthase-3 (NOS-3) (Kline et al. 2000), and monoamine oxidase-A (Burnet et al. 2001) alter the short-term hypoxic ventilatory response, whereas mutations in γ-glutamyl transpeptidase (Lipton et al. 2001) and NOS-1 (Kline et al. 2002a) alter short-term potentiation. In longer time domains, mutations in NOS-1 impair respiratory long-term facilitation (Kline et al. 2002a), and dopamine D-2 receptor knockouts are unable to express ventilatory acclimatization to chronic hypoxia (Huey et al. 2000b). Central CO2 chemosensory mechanisms are attenuated in mice deficient in the RET proto-oncogene or MASH-1, presumably via effects on cholinergic (Burton et al. 1997) or noradrenergic brainstem neurons (Dauger et al. 1999, 2001).
A major limitation of studies using genomic manipulations is the uncertainty as to whether a developmental anomaly or the postnatal alteration of a specific gene product causes breathing pathologies and whether such pathologies are unique to respiratory neurons or represent a generalized disruption of neuronal function, as would be the case for example with abnormal myelination. However, the future is bright. As marker molecules for key neurons in the central respiratory circuits are identified, they can be used as targets for genetic manipulation. In cases where the targets may be expressed in other parts of the nervous system, e.g., NK1Rs or μORs, strategies involving spatial, e.g., rhombomeric, targeting may prove invaluable. Other techniques, such as targeting neurons at specific sites with toxins or other molecules (Gray et al. 2001, Nattie & Li 2002b) may be more useful, particularly in the short term.
Optical Recording
The remarkable fact that the kernel of as basic a behavior as breathing continues in vitro is ripe for exploitation by optical recording techniques. Koshiya & Smith used a Ca2+-sensitive dye to visualize neuronal activity in the preBötC, finding neurons that continue to burst rhythmically following synaptic isolation (1999). Onimaru and colleagues recorded population activity from en bloc preparation using voltage-sensitive dyes (Onimaru et al. 1996, Tokumasu et al. 2001). Further improvements in the technology will allow experimenters to address questions of cellular properties and network organization that are otherwise impossible to study.
Plasticity
Breathing exhibits considerable plasticity (Mitchell & Johnson 2003) elicited by (i) hypoxia or chemoafferent neuron activation (Eldridge & Millhorn 1986, Mitchell et al. 2001a, Powell et al. 1998); (ii) abnormal O2 levels or stress during development (Carroll 2003, Gozal & Gozal 2001, Mitchell & Johnson 2003); (iii) exercise (McCrimmon et al. 1995, Turner et al. 1997); (iv) hypercapnia (Baker et al. 2001); (v) sensory denervation (Forster 2003, Forster et al. 2000, Kinkead et al. 1998); (vi) neural injury (Cheng et al. 2002, Golder et al. 2001, Goshgarian 2003); and (vii) conditioning (Gallego et al. 2001, Lukowiak & Syed 1999). Respiratory neuroplasticity helps to maintain regulatory function in the face of normal development and aging, altered environmental conditions, and cardiopulmonary or brain injury or disease.
While the respiratory control system exhibits a degree of activity-dependent synaptic potentiation and depression over relatively short time domains (Johnson & Mitchell 2002, McCrimmon et al. 1997, Zhou et al. 1997), neuromodulators are necessary for the induction and/or maintenance of most forms of respiratory neuroplasticity, particularly in adults (Mitchell & Johnson 2003). Several models of adult respiratory plasticity require serotonergic (Baker et al. 2001, Fuller et al. 2000, McCrimmon et al. 1995, Mitchell et al. 2001a), dopaminergic (Huey et al. 2000a,b), or noradrenergic receptor activation (Kinkead et al. 2001, Mitchell & Johnson 2003). Here we focus on serotonin-dependent respiratory plasticity because there has been more progress in understanding its cellular and synaptic mechanisms.
Serotonin-Dependent Respiratory Neuroplasticity
A classic model of serotonin-dependent plasticity is of a respiratory defense reflex, the facilitation of the sensorimotor synapse mediating the gill withdrawal reflex in Aplysia (Kandel 2001, Sutton et al. 2001). Serotonin is a key element in several other models of respiratory plasticity (McCrimmon et al. 1995, Mitchell & Johnson 2003) elicited by (i) intermittent hypoxia (Ling et al. 2001, Mitchell et al. 2001a, Prabhakar 2001), (ii) hypercapnic exercise (McCrimmon et al. 1995, Johnson & Mitchell 2001), (iii) cervical spinal sensory denervation (Kinkead et al. 1998), (iv) chemoafferent denervation (Forster et al. 2000, Forster 2003), (v) spinal cord injury (Golder et al. 2001; Hadley et al. 1999a,b), (vi) serotonin-induced burst frequency enhancement in in vitro turtle brainstems (Johnson et al. 2001), and (vii) conditioned responses to pH changes in Aplysia (Levy & Susswein 1999). Because recent progress has been made in understanding the mechanisms and manifestations of serotonin-dependent respiratory long-term facilitation following intermittent hypoxia, we focus on this model.
Respiratory Long-Term Facilitation (LTF)
Respiratory LTF is a long-lasting increase (>1 h) in ventilation (or respiratory motor nerve activity) that can be produced by hypoxia (Bach & Mitchell 1996) or the physiological surrogate, electrical stimulation of the carotid sinus nerves (Hayashi et al. 1993, Millhorn et al. 1980b). LTF is a serotonin-dependent central neural mechanism because it is abolished by serotonin receptor antagonists, serotonin depletion, or serotonergic neurotoxicity (Bach & Mitchell 1996, Millhorn et al. 1980a). LTF is elicited by hypoxia only when presented in an intermittent or episodic pattern (Baker & Mitchell 2000), demonstrating profound pattern sensitivity similar to other models of plasticity (Baker et al. 2001, Sutton et al. 2002).
LTF can be assessed by measuring integrated phrenic and hypoglossal nerve activity in anesthetized, vagotomized, and ventilated rats exposed to three five-minute episodes of hypoxia (Figure 4A). Mitchell and colleagues identified several factors that influence LTF in this model, such as age and gender. Compared with 3–4-month-old male Sprague Dawley rats (Fuller et al. 2000), LTF is attenuated in the phrenic nerve and nearly abolished in the hypoglossal nerve of 12–14-month-old male rats (Zabka et al. 2001a). In striking contrast, LTF increases with age in female rats and is influenced by the estrus cycle (Zabka et al. 2001b). The age and gender dependence of LTF resembles the age and gender dependence of obstructive sleep apnea in humans (Bixler et al. 2001), suggesting a possible link. Although intermittent hypoxia elicits unique forms of respiratory plasticity in neonates (Gozal & Gozal 2001, Moss 2000), LTF has not been studied in this age group. The study of LTF during development is a critical area of research, given the possibility that serotonin-dependent plasticity is involved in respiratory disorders of the newborn (Gaultier 2001, Kinney et al. 2001).
Figure 4.
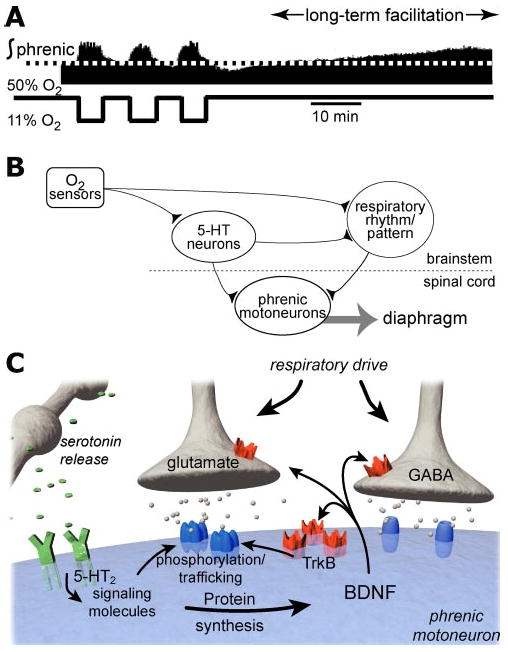
Working models of phrenic long-term facilitation (LTF). In the upper trace (A) integrated bursts of phrenic neural activity are shown before, during, and after episodic hypoxia (3 episodes, 5 min of 11% O2 with 5 min interval) in an anesthetized, paralyzed, vagotomized, pump-ventilated 3–4-month-old male rat (tracing modified from Zabka et al. 2001a). In (B), a network model of respiratory LTF is illustrated with a simple schematic indicating the relevant anatomical projections (after Bach et al. 1996). In the network model, raphe serotonergic neurons are activated during intermittent hypoxia, releasing serotonin in the vicinity of brainstem respiratory neurons as well as spinal motoneurons. The relevant serotonin receptors for LTF are proposed to be within the respective motor nuclei, i.e., within the spinal cord for phrenic LTF. A schematic of the cellular and synaptic consequences of serotonin release within the phrenic motor nucleus during intermittent hypoxia is shown in (C). Serotonin release in the vicinity of phrenic dendrites activates 5-HT2A receptors, thereby activating intracellular signaling molecules, e.g., kinases. The relevant signaling molecules initiate BDNF protein synthesis, presumably from existing BDNF mRNA. Subsequent BDNF release from the phrenic dendrites may activate pre- or postsynaptic tyrosine kinase B (TrkB) receptors, leading to enhanced function at glutamatergic synapses that transmit respiratory drive, or by inhibiting synaptic (GABA) inhibition.
Genetics is another important determinant of respiratory LTF because different substrains of Sprague Dawley rats exhibit different behaviors. Sprague Dawley rats from Harlan Colony 236 exhibit greater phrenic LTF than rats from Harlan Colony 205 (Fuller et al. 2000). Neither Harlan Colony exhibits much LTF in hypoglossal motor output in contrast with Sprague Dawley rats from Charles River/Sasco Colony K62 (Fuller et al. 2001a). There appear to be substantial differences in the magnitude of phrenic LTF between four inbred rat strains; Brown Norway and PVG strains have lower LTF than either the Lewis or Fischer strains (Bavis et al. 2003).
Respiratory LTF is also modified by prior experience, i.e., it exhibits metaplasticity. For example, bilateral cervical dorsal rhizotomy increases both hypoxia-induced phrenic LTF and serotonin terminal density near phrenic motoneurons (Kinkead et al. 1998). Chronic intermittent hypoxia also augments phrenic LTF (Ling et al. 2001). Although both chronic spinal sensory denervation and chronic intermittent hypoxia elicit functional, morphological, and neurochemical plasticity in breathing, their respective mechanisms of action on respiratory LTF may differ (Mitchell et al. 2001a).
LTF following episodic hypoxia in unanesthetized humans and other mammalian species presents a complex picture. In general, LTF is more difficult to detect in awake, unanesthetized animals or humans (Mitchell et al. 2001a). Whereas ventilatory LTF has been reported in awake dogs, goats, rats, and ducks (Cao et al. 1992, Mitchell et al. 2001b, Olson et al. 2001, Turner et al. 1997), it has not been found in awake humans (McEvoy et al. 1996). Yet, LTF is observed in sleeping humans with airway flow limitation (Babcock & Badr 1998), which suggests an effect specific to upper airway motor output (Aboubakr et al. 2001, Shkoukani et al. 2002). Differences between unanesthetized and anesthetized experimental preparations in the expression of LTF may relate to genetics (species or strain) because awake and anesthetized rats exhibit similar degrees of LTF once differences in CO2 regulation are accounted for (Olson et al. 2001); data are not available to compare any other species. Other factors may obscure LTF, such as inhibitory feedback from intact vagus nerves (Mateika & Fregosi 1997), or because raphe serotonergic neurons discharge at or near maximal levels in awake animals thereby limiting their dynamic range (Mitchell et al. 2001a). One report indicates that anesthetized, spontaneously breathing rats do not exhibit ventilatory LTF (Janssen & Fregosi 2000), although this observation may result from the relatively high levels of arterial CO2 characteristic of this preparation.
A Working Model of LTF
Network (Figure 4B) and cellular/synaptic (Figure 4C) models of respiratory LTF have been proposed (Bach & Mitchell 1996, McCrimmon et al. 1995, Mitchell et al. 2001a, Powell et al. 1998). The response elements are postulated to be raphe serotonergic neurons, which are activated by chemoafferent neurons or by CNS hypoxia and project to phrenic and hypoglossal motoneurons, as well as premoto-neurons and propriobulbar neurons of the ventral respiratory group. Persistent (poststimulation) raphe neuron activation and serotonin release may contribute to LTF for brief periods (Morris et al. 2002, Morris et al. 2000). However, because serotonin receptor antagonists block LTF when administered before but not after episodic hypoxia, continued receptor activation is not necessary 15 to 60 min posthypoxia (Fuller et al. 2001b). Thus, serotonin receptor activation is necessary for the induction, but not the maintenance, of respiratory LTF.
In the model illustrated in Figure 4, serotonin released by raphe neurons acts predominantly within respiratory motor nuclei. In the case of phrenic LTF, serotonin is hypothesized to act at the bulbospinal inspiratory premotor synapse onto phrenic motoneurons. In support of this hypothesis, intrathecal administration of a serotonin receptor antagonist to the cervical spinal cord attenuates phrenic but not hypoglossal motoneuron LTF (Baker-Herman & Mitchell 2002). Thus, the serotonin receptors underlying phrenic LTF are located within the spinal cord. Episodic hypoxia also increases the amplitude of short-latency phrenic potentials evoked by stimulation of the lateral funiculus, an effect highly correlated with phrenic LTF (Fuller et al. 2002), which further supports the idea that spinal cord neuroplasticity contributes to phrenic LTF.
In the scheme illustrated in Figure 4, raphe neuron activation during episodic hypoxia releases serotonin near respiratory motoneurons, initiating pattern-sensitive cellular and synaptic events that underlie LTF (Bach & Mitchell 1996, Fuller et al. 2000, Mitchell et al. 2001a). Since the main site of plasticity is at the level of respiratory motoneurons, the predominant manifestation of LTF is a persistent increase in respiratory burst amplitude. However, there is a small and variable LTF of frequency that must arise from effects at other sites (Baker-Herman & Mitchell 2002).
Because spinal serotonin receptor activation is necessary for the induction but not maintenance of LTF (Fuller et al. 2001b), repetitive serotonin, presumably 5-HT2A, receptor activation is hypothesized to initiate intracellular signaling events within respiratory motoneurons leading to synaptic facilitation and LTF (Baker et al. 2001, Fuller et al. 2000, Mitchell et al. 2001a). In this cellular/synaptic model (Figure 4C), signaling molecules activated by 5-HT2A receptors stimulate translation from existing mRNA, leading to the rapid synthesis of proteins necessary to maintain long-term facilitation (Baker-Herman & Mitchell 2002, Mitchell et al. 2001a). Although the necessary protein(s) is not yet known, a critical role for brain-derived neurotrophic factor (BDNF) is suggested by the observations that (i) intermittent hypoxia increases BDNF concentration in regions near the phrenic motor nucleus; (ii) the magnitude of phrenic LTF correlates with ventral spinal BDNF concentration in individual rats; (iii) spinal administration of K-252a, a tyrosine kinase inhibitor, blocks phrenic LTF; and (iv) intrathecal BDNF administration elicits phrenic, but not hypoglossal, facilitation (T.L. Baker-Herman and G.S. Mitchell, unpublished results). Since BDNF is highly concentrated within phrenic motoneurons (C.B. Mantilla and G.C. Sieck, personal communication), BDNF may be released from phrenic motoneuronal dendrites, strengthening bulbospinal synaptic inputs to phrenic motoneurons leading to LTF. BDNF synthesis (Mitchell et al. 2001) and release (Balkowiec & Katz 2000) exhibit pattern sensitivity appropriate for an involvement in respiratory LTF. Consequences of BDNF signaling could include (i) increasing glutamate receptor insertion on the postsynaptic membrane (Grosshans et al. 2002, Malinow & Malenka 2002); (ii) phosphorylating glutamate receptors (Czapla et al. 1999); (iii) increasing glutamate release (Schinder & Poo 2000); and/or (v) decreasing GABA-mediated neurotransmission (Henneberger et al. 2002) onto phrenic motoneurons.
There may be a role for nitric oxide in ventilatory LTF because it is not observed in mutant mice deficient in NOS-1 (Kline et al. 2002b). Although nitric oxide offsets hypoxia-induced respiratory depression during intermittent hypoxia in neonates (Gozal & Gozal 1999), it inhibits 5-HT2 receptor function (Nozik-Grayck et al. 2002), decreases BDNF secretion in the hippocampus (Canossa et al. 2002), and increases with 5-HT2 receptor activation (Tian et al. 2002). Thus, NOS knockout mice may lack ventilatory LTF because key elements in the mechanism have been disrupted.
Chemosensitivity
Breathing depends on input from chemoreceptors (Ballantyne & Scheid 2001; Nattie 1998, 1999), which provide the essential information about O2, CO2, and pH. O2-sensitive chemoreceptors are external to the brain in the carotid bodies. CO2/pH-sensitive chemoreceptors are in the carotid bodies, but major sites are also within the brain (Figure 1) and are referred to as central chemoreceptors. CO2/pH signals are related to the acid-base status of the blood and brain and reflect the adequacy of breathing relative to metabolism. Small changes in CO2/pH can affect breathing. For example, in awake humans at rest, a 1-mm Hg increase in PCO2 increases ventilation by ∼20%–30%. In vitro, neurons from many brain locations are excited or inhibited by CO2/pH (see below), but it has been difficult to link this neuronal chemosensitivity to breathing, i.e., chemoreception, in vivo. Central chemoreceptor sites contain chemosensitive neurons and participate in the control of breathing. They may also contain neurons, or overlap with adjacent sites, that are integrative in function or involved in rhythm and pattern generation, making their experimental study difficult.
What is Sensed and How?
Both intra- and extracellular pH appear to serve as the proximate stimulus for central chemoreception. Extracellular pH may be sensed by respiratory-modulated neurons (Bayliss et al. 2001), while intracellular pH is sensed in neurons of the locus ceruleus and medullary raphe (Filosa et al. 2002, Wang et al. 2001b, Washburn et al. 2002). pH is sensed by many proteins, likely via histidine (Jiang et al. 2001), a finding predicted by considerations of acid-base balance in heterotherms (Nattie 1999). Examples of candidate pH-sensitive proteins present at many chemoreceptor locations include (i) low resistance gap junctions (Dean et al. 2001, Solomon et al. 2001); (ii) TASK channels (Bayliss et al. 2001, Washburn et al. 2002); (iii) inward rectifier K+ channels (Jiang et al. 2001); and (iv) pH-sensitive membrane ion transport proteins, e.g., the Na+/H+ exchange protein subtype 3 (NHE3), which may affect chemosensitivity by altering the degree and timing of intracellular pH changes (Putnam 2001, Wiemann & Bingmann 2001). More than one pH-sensing function may operate simultaneously. For example, in the pulmonate snail, intracellular pH seems to be the key determinant of chemoreception, but there are at least two pH-sensitive K+ channels: one affected by extracellular pH, the other by both intra- and extracellular pH (J. Denton, F. McCann, & J. Leiter, unpublished observations). There is no evidence that CO2 itself can be sensed, although this is an intriguing possibility.
Where Does Central Chemoreception Occur?
Central chemoreceptor sites are widely distributed within the lower brain (Figure 1) (Ballantyne & Scheid 2001; Bernard et al. 1996; Coates et al. 1993; Li et al. 1999; Loeschcke 1982; Nattie 1998, 1999, 2001; Nattie & Li 2001, 2002a; Solomon et al. 2000). These sites have been identified based on several criteria:
c-fos expression after exposure to high CO2 levels (Haxhiu et al. 2001, Haxhiu et al. 1996, Larnicol et al. 1994, Okada et al. 2002, Sato et al. 1992, Teppema et al. 1997). Although c-fos expression occurs in many neurons activated by increased CO2, its presence alone is not conclusive data that these neurons are chemosensitive, as some activated neurons could be part of the network response, nor does it necessarily label all chemosensitive neurons, as all activated neurons need not express c-fos.
presence of CO2-sensitive neurons in vitro (Bayliss et al. 2001, Dean et al. 1989, Filosa et al. 2002, Kawai et al. 1996, Okada et al. 1993, Oyamada et al. 1998, Richerson et al. 2001, Wang et al. 2001b). Chemosensitivity observed in vitro is not sufficient to demonstrate involvement in the control of breathing but suggests specific experiments in vivo.
focal acidification at these but not other sites stimulates breathing (Bernard et al. 1996, Coates et al. 1993, Nattie & Li 1996, Solomon et al. 2000), suggesting functional chemoreception. Initial studies in anesthetized animals used focal acidification produced by acetazolamide injections, an effect comparable to an increase in arterial PCO2 from 40 to 63 mm Hg—a large stimulus (Coates et al. 1993). Stimulation at any one site increases ventilatory output by 20%–40% of the increase seen with an equivalent increase in arterial PCO2.
The above criteria identify central chemoreceptor sites (Figure 1) at (i) nucleus tractus solitarius (NTS); (ii) locus ceruleus; (iii) the midline medullary raphe; (iv) rostral aspect of the ventral respiratory group or the preBötC; (v) the fastigal nucleus; and (vi) regions lying just beneath the ventral medullary surface, especially the retrotrapezoid nucleus (RTN).
Recent efforts to identify sites of central chemoreception have focused on using localized elevations in CO2 as the stimulus (with smaller focal pH changes) in freely behaving rodents during wakefulness and sleep. Microdialysis of a CO2-rich solution produces a focal pH change equivalent to a 6.6 mmHg increase in arterial PCO2 (Li & Nattie 2002). These studies have revealed a remarkable degree of site and state dependence in the responses. Local acidification in the retrotrapezoid nucleus increases breathing by 24% in wakefulness but has no affect during sleep (Li et al. 1999). In the medullary raphe, breathing increases by 15%–20% during NREM sleep only (Nattie & Li 2001). In the caudal NTS, local acidification increases breathing by 16% in sleep and 28% in wakefulness (Nattie & Li 2002a). These results suggest that different sets of central chemoreceptor sites are effective in sleep and wakefulness, at least at these low stimulus intensities. This is unexpected because the lower overall CO2 sensitivity observed in sleep as compared to wakefulness has been attributed to sleep-related effects at all chemoreceptor sites. Since a 6.6-mm Hg increase in systemic CO2 increases ventilation by 120%, a response much greater than produced by any of the single sites examined so far (Li & Nattie 2002), no one site appears to account for the high overall sensitivity of breathing to small changes in systemic CO2, the CO2 response.
Focal Disruption
A complementary approach to understanding chemoreception is by examination of the systemic CO2 response, i.e., the change in ventilation produced by an increase in systemic CO2, after inhibiting or destroying neurons at putative central chemoreceptor sites. The CO2 response of intact animals is reduced by (i) cooling the ventrolateral medullary surface near the region of the retrotrapezoid nucleus (Forster et al. 1997); (ii) neurotoxin-induced lesions of the NTS (Berger & Cooney 1982); the medullary raphe (Dreshaj et al. 1998) or the retrotrapezoid nucleus (Akilesh et al. 1997); and (iii) muscimol inhibition of the retrotrapezoid nucleus (Curran et al. 2001) and medullary raphe (Messier et al. 2002). However, disruption of any single site does not abolish the CO2 response.
Cell-specific lesioning using SAP-conjugated toxins, as described above, is a useful extension of these techniques. NK1Rs, useful in demarking the preBötC within the ventral respiratory column, are also present at all central chemoreceptor sites (Nattie 2001, Nattie & Li 2002b). An SP-SAP-induced reduction of NK1R immunoreactivity by 44% in the RTN/parapyramidal region decreases the CO2 response by 28%–30% in both sleep and wakefulness (Figure 5). Destruction of >75% of NK1R neurons by SP-SAP injection in the preBötC decreases the CO2 response substantially (Gray et al. 2001). Substantial destruction of medullary raphe serotonergic neurons by injection of SERT-SAP (a saporin conjugate with a serotonin transport protein antibody) decreases the CO2 response by 18%–24% in sleep and wakefulness (Nattie et al. 2002). A complicating factor in the interpretation of these experiments is the difficulty in separating chemosensitivity per se from integrative and rhythm- or pattern-generating functions, which may be performed by the same or adjacent neurons. Focal stimulation by CO2 produces a response initiated by chemosensitive neurons. Lesions destroy these neurons but likely also affect neurons involved with other aspects of the control of breathing. In fact, chemosensitive neurons may themselves serve integrative or rhythm- and pattern-generating functions. Better phenotypic description of chemosensitive neurons in vitro will lead to more specific tools for use in vivo, which will allow differentiation of chemosensitive from other neuronal functions. Of interest is that bilateral lesions of NK1R neurons in the preBötC (Gray et al. 2001) and in the RTN/parapyramidal region (Nattie & Li 2002b) cause hypoventilation, with a rise in arterial CO2 in wakefulness. The fact that the remaining chemoreceptor sites cannot restore CO2 to normal levels reinforces the inference that multiple chemoreceptor sites maintain normal thresholds and sensitivities to CO2 (Forster et al. 1997).
Figure 5.
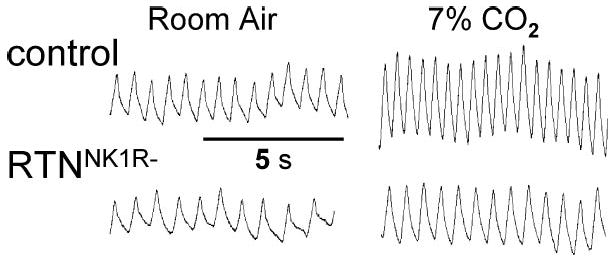
Lesions of NK1R neurons in the retrotrapezoid nucleus blunts ventilatory response to increased PCO2. (Top) Breathing (inspiration up) in a normal rat in room air and 7% CO2. (Bottom) Breathing in a rat with significant loss of NK1R neurons in the retrotrapezoid nucleus (RTNNK1R−) (Nattie & Li 2002b; E. Nattie and A. Li, unpublished data).
Perivascular Role
Central chemosensitive neurons may be responsive to brain vascular CO2 and pH. In the neonatal rat brainstem, processes from dye-filled chemosensitive neurons project to pial blood vessels (Kawai et al. 1996). In CO2-exposed rats, c-fos positive neurons are in close proximity to large vessels (Haxhiu et al. 2001, Okada et al. 2002), as are serotonergic neurons (Bradley et al. 2002), which are chemosensitive in vitro (Bradley et al. 2002, Richerson et al. 2001, Wang et al. 2001b). Given the high diffusivity of CO2, this proximity to large vessels suggests that these chemoreceptors are responsive to changes in blood pH or perhaps to rapid changes in CO2 produced in a breath-to-breath manner (Millhorn et al. 1984).
Is Chemosensitivity a Unique Property?
When first discovered, central chemoreception was considered a unique property of specialized neurons with tonic firing patterns at the ventral medullary surface. Recent work indicates that neurons at other brainstem sites directly linked to the control of breathing are involved in chemosensitivity. In the rostral ventral medulla, inhibition of glutamate and muscarinic receptors (Nattie 1999), or P2X2 receptors (Thomas et al. 1999), or stimulation of GABAA receptors (Curran et al. 2001) all decrease the CO2 response. Focal stimulation by CO2 provides evidence for chemoreception in the retrotrapezoid nucleus (Nattie & Li 2002b), NTS (Nattie & Li 2002a), rostral ventral respiratory group (Nattie 1999), and preBötC (Solomon et al. 2000)—regions that contain respiratory premotor and motor neurons.
Serotonergic and noradrenergic medullary neurons are involved in state-dependent modulation of sensorimotor and autonomic function as well as neuronal plasticity (see above). Serotonergic neurons are important in normal growth and development of respiratory motoneurons (Edagawa et al. 2001, Hilaire & Duron 1999). These monoamine-containing neurons are also chemosensitive and involved with chemoreception. In the caudal medullary raphe (i) single neurons are excited by CO2 in vitro (Wang et al. 2001b), (ii) focal acidification of the raphe stimulates breathing in vivo (Bernard et al. 1996) but only during sleep (Nattie & Li 2001), and (iii) nonspecific lesion or focal inhibition of the medullary raphe (Dreshaj et al. 1998, Messier et al. 2002) and specific killing of medullary serotonergic neurons by injection of SERT-SAP in the rat (Nattie et al. 2002) all decrease the CO2 response. In the locus ceruleus, norepinephrine-containing neurons express c-fos after systemic CO2 stimulation (Haxhiu et al. 2001) and are excited by CO2 in vitro (Filosa et al. 2002). Stimulation of the locus ceruleus by focal acidosis increases breathing in anesthetized rats (Coates et al. 1993). We do not know the relative importance in central chemoreception of the medullary raphe and locus ceruleus in comparison to that of chemoreceptor regions that contain respiratory premotor and motor neurons.
Why then are there so many chemoreceptor sites? Central chemoreception may be organized in a hierarchical manner like that proposed for temperature regulation (Nattie 2001, Satinoff 1978). In this concept, in evolution, as the nervous system becomes progressively more complex and capable, additional central chemoreceptor sites provide a more sophisticated control system. Presumably, new sites developed in parallel with or as part of major steps in vertebrate evolution, e.g., air breathing, homeothermy, and sleep. Although there are widely distributed central chemoreceptor sites, we do not know how they function relative to each other, whether there is some type of top-down organization, and, if so, which sites are dominant.
Respiratory Rhythmicity, Plasticity, and Chemosensitivity: Biological and Clinical Significance
There are few systemic brain functions more fundamental to life than breathing. Defects in the neural mechanisms of respiratory rhythm generation, central chemoreception, or respiratory plasticity may play critical roles in the etiology of multiple respiratory control disorders.
Sudden Infant Death Syndrome (SIDS), the greatest cause of human death in the first 12 months of life, is commonly suspected to result from respiratory failure during sleep. A large subset of children who have died from SIDS exhibit abnormalities in kainate, muscarinic, and serotonergic receptor binding in brainstem regions homologous with known central chemoreceptor locations in nonprimate mammals (Kinney et al. 1995, Panigrahy et al. 1997). Current emphasis has been placed on defects in serotonergic neurons as a causative factor in SIDS (Kinney et al. 2001). These neurons are chemosensitive in vitro (Bradley et al. 2002, Richerson et al. 2001, Wang et al. 2001b), and in newborn piglets, their inhibition depresses CO2 responses and disrupts sleep (Curran et al. 2001, Darnall et al. 2001, Messier et al. 2002). Defects in serotonergic neurons may also suggest inadequate serotonin-dependent developmental plasticity in the respiratory control system because serotonin modulates development in many regions of the nervous system (Edagawa et al. 2001, Hilaire & Duron 1999). Multiple abnormalities in autonomic and respiratory control, including defects in central chemoreception and serotonin-dependent plasticity, may emerge with disruption of medullary serotonergic neurons. These abnormalities may make an infant less able to respond to physiological stresses that are encountered during sleep, such as increased inspired PCO2 when breathing face down in soft bedding, or decreased arterial PO2 produced by sleep-related airway obstruction.
Sleep-disordered breathing or sleep apnea is the periodic cessation of breathing (apnea) during sleep. Approximately 5% of middle-aged males (Bixler et al. 2001) and 2% of children have sleep-disordered breathing (Gaultier 2001, O'Brien & Gozal 2002). Sleep apnea is associated with significant comorbidity including hypertension, insomnia, depression, an increased incidence of automobile accidents (Young et al. 2002), as well as hyperactivity and learning deficits (Gozal & Gozal 2001, Row et al. 2002). Sleep apnea is associated with age-related defects in the serotonergic nervous system (Kubin et al. 1998, Nakano et al. 2001, Veasey et al. 2001), which could underlie deficits in upper airway tone during sleep.
Central chemoreception is absent or reduced in children with Congenital Central Hypoventilation Syndrome (CCHS) (Spengler et al. 2001), a rare condition with about 200 cases recognized today. These children function relatively normally during wakefulness but require ventilatory support during sleep to avoid very high CO2 and low O2 levels secondary to inadequate breathing. The abnormality is unknown. This experiment of Nature suggests a special importance for central chemoreception during sleep.
Respiratory insufficiency is the major cause of morbidity and mortality in spinally injured patients (Ramer et al. 2000). Serotonin-dependent plasticity may play critical roles in spontaneous and induced functional recovery following spinal cord injury (Golder et al. 2001, Hadley et al. 1999a,b). Additional respiratory disorders have implicated serotonin in their pathophysiological mechanism, including panic anxiety hyperventilation disorders (Klein 1996) and ventilatory instability in Rett Syndrome (Dunn & MacLeod 2001). By harnessing or augmenting serotonin-dependent plasticity, more effective therapeutic strategies for these devastating respiratory control disorders may be possible.
Conclusion
Around 350 B.C. Aristotle (1995) wrote “A few of the earlier philosophers have dealt with respiration; some of them have offered no explanation…; others have discussed it without much insight and with insufficient experience of the facts.” We are confident that we have gone beyond this. Considerable data continue to point to the preBötC as an essential element of the system generating respiratory rhythm. Whether it is the principal site for rhythm generation, one of multiple sites, or just a part of a much larger complex remains to be established. Specific patterns of receptor expression in the preBötC will provide a basis for understanding its function. As yet, we do not understand the neural mechanisms that underlie rhythm generation—they are up for grabs. Eupnea, sighing, and gasping are breathing patterns that may be generated by a single neural network that undergoes reconfiguration to meet different behavioral needs. The notion that pacemaker neurons drive the normal rhythm remains unsubstantiated, and there are data suggesting that it is not the case. These issues remain rich areas for investigation.
Respiratory neuroplasticity triggered by physiological perturbations such as episodic hypoxia, repeated exercise, or neural injury has only recently been appreciated. In adult mammals, serotonin-dependent plasticity appears to play a prominent role. We now have a model that allows detailed studies of the cellular and synaptic mechanisms, which gives rise to system plasticity, i.e., respiratory long-term facilitation. Extensive research on LTF and other forms of respiratory plasticity is needed, to advance both our understanding of the respiratory system and neuroplasticity in general.
A new view of central chemoreception as a widely distributed system has emerged. Discrete sites, sensitive to small pH changes in a state-dependent manner, act in concert to establish system sensitivity. Different combinations of chemosensitive sites operate in wakefulness and sleep, providing adequate chemoreception in the face of changing arousal state. Many cell types and molecular mechanisms are involved in the process of chemoreception. The unexpected complexity of this chemosensory system suggests that there remains much to learn about its function.
A thorough understanding of respiratory rhythm generation, plasticity, and chemoreception is essential before it will be possible to understand the etiology of devastating respiratory disorders such as SIDS and obstructive sleep apnea. Such understanding may provide the rationale for new therapeutic strategies for these and other, seemingly unrelated disorders that cause respiratory insufficiency, such as Rett Syndrome, spinal cord injury, or anxiety/hyperventilation disorders.
Acknowledgments
The authors' work was supported by the National Institutes of Health (HL 37941, 40959, 65383, 69064, 28066, 53319, NICHD 36379, and NS 24742) and the Parker B. Francis Foundation.
Contributor Information
Jack L. Feldman, Email: feldman@ucla.edu.
Gordon S. Mitchell, Email: mitchell@svm.vetmed.wisc.edu.
Eugene E. Nattie, Email: eugene.nattie@dartmouth.edu.
Literature Cited
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–57. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–79. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Gray PA, Habtemarkos R, Feldman JL, McCrimmon DR. Calcium binding proteins, NK1 receptors, and compartments in the ventral respiratory group of the rat. Soc Neurosci Abstr. 2001;27:1667. [Google Scholar]
- Aristotle . In: Aristotle VIII. Goold G, editor. Cambridge, MA: Harvard Univ. Press; 1995. p. 431. [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–16. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–60. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol. 2001;129:25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–19. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–46. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J Physiol (Lond) 1998;510:527–33. doi: 10.1111/j.1469-7793.1998.527bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–23. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Scheid P. Central chemosensitivity of respiration: a brief overview. Respir Physiol. 2001;129:5–12. doi: 10.1016/s0034-5687(01)00297-3. [DOI] [PubMed] [Google Scholar]
- Ballayni K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–684. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Baker-Herman TL, Zabka AG, Golder FJ, Fuller DD, Mitchell GS. Respiratory long-term facilitation differs among inbred rat strains. FASEB J. 2003 In press. [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–74. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Cooney KA. Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol: Respir Environ Exerc Physiol. 1982;52:131–40. doi: 10.1152/jappl.1982.52.1.131. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Scherer SS, O'Connell S, Arroyo E, Kalla KA, et al. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–62. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol. 1996;80:108–15. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Carfi M, Pantaleo T. Respiratory responses to ionotropic glutamate receptor antagonists in the ventral respiratory group of the rabbit. Pflugers Arch. 2002;444:602–9. doi: 10.1007/s00424-002-0874-1. [DOI] [PubMed] [Google Scholar]
- Borday V, Fortin G, Champagnat J. Early ontogeny of rhythm generation and control of breathing. Respir Physiol. 1997a;110:245–49. doi: 10.1016/s0034-5687(97)00089-3. [DOI] [PubMed] [Google Scholar]
- Borday V, Kato F, Champagnat J. A ventral pontine pathway promotes rhythmic activity in the medulla of neonate mice. Neuroreport. 1997b;8:3679–83. doi: 10.1097/00001756-199712010-00005. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, et al. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–56. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–2. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–39. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Burnet H, Bevengut M, Chakri F, Bou-Flores C, Coulon P, et al. Altered respiratory activity and respiratory regulations in adult monoamine oxidase A-deficient mice. J Neurosci. 2001;21:5212–21. doi: 10.1523/JNEUROSCI.21-14-05212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Kawashima A, Brayer JA, Kazemi H, Shannon DC, et al. RET proto-oncogene is important for the development of respiratory CO2 sensitivity. J Auton Nerv Syst. 1997;63:137–43. doi: 10.1016/s0165-1838(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S. Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99:3282–87. doi: 10.1073/pnas.042504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–88. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–94. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–89. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Champagnat J, Fortin G. Primordial respiratory-like rhythm generation in the vertebrate embryo. Trends Neurosci. 1997;20:119–24. doi: 10.1016/s0166-2236(96)10078-3. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, del Toro ED, Voiculescu O, Charnay P, Champagnat J. Different respiratory control systems are affected in homozygous and heterozygous kreisler mutant mice. Eur J Neurosci. 2002;15:684–92. doi: 10.1046/j.1460-9568.2002.01909.x. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Guo SZ, Lipton AJ, Gozal D. Domoic acid lesions in nucleus of the solitary tract: time-dependent recovery of hypoxic ventilatory response and peripheral afferent axonal plasticity. J Neurosci. 2002;22:3215–26. doi: 10.1523/JNEUROSCI.22-08-03215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Curran AK, Darnall RA, Filiano JJ, Li A, Nattie EE. Muscimol dialysis in the rostral ventral medulla reduced the CO2 response in awake and sleeping piglets. J Appl Physiol. 2001;90:971–80. doi: 10.1152/jappl.2001.90.3.971. [DOI] [PubMed] [Google Scholar]
- Czapla MA, Simakajornboon N, Holt GA, Gozal D. Tyrosine kinase inhibitors modulate the ventilatory response to hypoxia in the conscious rat. J Appl Physiol. 1999;87:363–69. doi: 10.1152/jappl.1999.87.1.363. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Curran AK, Filiano JJ, Li A, Nattie EE. The effects of a GABAA agonist in the rostral ventral medulla on sleep and breathing in newborn piglets. Sleep. 2001;24:514–27. doi: 10.1093/sleep/24.5.514. [DOI] [PubMed] [Google Scholar]
- Dauger S, Guimiot F, Renolleau S, Levacher B, Boda B, et al. MASH-1/RET pathway involvement in development of brain stem control of respiratory frequency in newborn mice. Physiol Genom. 2001;7:149–57. doi: 10.1152/physiolgenomics.00056.2001. [DOI] [PubMed] [Google Scholar]
- Dauger S, Renolleau S, Vardon G, Nepote V, Mas C, et al. Ventilatory responses to hypercapnia and hypoxia in MASH-1 heterozygous newborn and adult mice. Pediatr Res. 1999;46:535–42. doi: 10.1203/00006450-199911000-00008. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–97. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Dean JB, Kinkade EA, Putnam RW. Cell-cell coupling in CO2/H+-excited neurons in brainstem slices. Respir Physiol. 2001;129:83–100. doi: 10.1016/s0034-5687(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–61. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Smith JC. Persistent sodium current, membrane properties, and bursting behaviors of pre-Bötzinger Complex inspiratory neurons in vitro. J Neurophysiol. 2002a;88:2242–50. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron. 2002b;34:821–30. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dreshaj IA, Haxhiu MA, Martin RJ. Role of the medullary raphe nuclei in the respiratory response to CO2. Respir Physiol. 1998;111:15–23. doi: 10.1016/s0034-5687(97)00110-2. [DOI] [PubMed] [Google Scholar]
- Dunn HG, MacLeod PM. Rett syndrome: review of biological abnormalities. Can J Neurol Sci. 2001;28:16–29. doi: 10.1017/s0317167100052513. [DOI] [PubMed] [Google Scholar]
- Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–37. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F, Millhorn D. Handbook of Physiology. Bethesda, MD: Am. Physiol. Soc.; 1986. The respiratory system; pp. 313–62. [Google Scholar]
- Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–89. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, et al. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–71. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann NY Acad Sci. 1989;563:114–30. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Filosa J, Dean J, Putnam R. Role of intracellular and extracellular pH in the chemosensitive response of rat locus ceruleus neurons. J Physiol (Lond) 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–94. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Forster HV, Ohtake PJ, Pan LG, Lowry TF. Effect on breathing of surface ventrolateral medullary cooling in awake, anesthetized and asleep goats. Respir Physiol. 1997;110:187–97. doi: 10.1016/s0034-5687(97)00083-2. [DOI] [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–46. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genom. 2001a;4:175–81. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Mitchell GS. Respiratory long term facilitation is associated with enhanced spinally evoked phrenic potentials. Soc Neurosci Abs. 2002:363.1. [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001b;90:2001–6. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fung ML, Wang W, St John WM. Medullary loci critical for expression of gasping in adult rats. J Physiol. 1994;480(Pt 3):597–611. doi: 10.1113/jphysiol.1994.sp020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J Neurophysiol. 1997;78:1414–20. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- Funk GD, Ramirez JM. Special issue: ‘Neural control of breathing’. Respir Physiol Neurobiol. 2002;131:1–3. doi: 10.1016/s1569-9048(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Gallego J, Nsegbe E, Durand E. Learning in respiratory control. Behav Modif. 2001;25:495–512. doi: 10.1177/0145445501254002. [DOI] [PubMed] [Google Scholar]
- Gaultier C. Abnormalities of the chemical control of breathing: clinical correlates in infants and children. Pediatr Pulmonol. 2001;23(Suppl):114–17. [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–89. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. Plasticity of the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E. Episodic hypoxia enhances late hypoxic ventilation in developing rat: putative role of neuronal NO synthase. Am J Physiol. 1999;276:R17–22. doi: 10.1152/ajpregu.1999.276.1.R17. [DOI] [PubMed] [Google Scholar]
- Gozal E, Gozal D. Respiratory plasticity following intermittent hypoxia: developmental interactions. J Appl Physiol. 2001;90:1995–99. doi: 10.1152/jappl.2001.90.5.1995. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger Complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–30. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–68. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002a;22:3806–16. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC. Somatostatin immunoreactivity is a diagnostic marker of the pre-Bötzinger Complex. Soc Neurosci Abs. 2002b;28:362.4. [Google Scholar]
- Guyenet PG, Wang H. Pre-Botzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–46. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999a;160:433–45. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of the serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4 h following C2 spinal cord hemisection. Exp Neurol. 1999b;160:479–88. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Tolentino-Silva F, Pete G, Kc P, Mack SO. Monoaminergic neurons, chemosensation and arousal. Respir Physiol. 2001;129:191–209. doi: 10.1016/s0034-5687(01)00290-0. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol. 1996;105:35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–19. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Juttner R, Rothe T, Grantyn R. Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J Neurophysiol. 2002;88:595–603. doi: 10.1152/jn.2002.88.2.595. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–60. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hsieh JH, Chang YC, Su CK, Hwang JC, Yen CT, Chai CY. A single minute lesion around the ventral respiratory group in medulla produces fatal apnea in cats. J Auton Nerv Syst. 1998;73:7–18. doi: 10.1016/s0165-1838(98)00117-9. [DOI] [PubMed] [Google Scholar]
- Huey KA, Brown IP, Jordan MC, Powell FL. Changes in dopamine D(2)-receptor modulation of the hypoxic ventilatory response with chronic hypoxia. Respir Physiol. 2000a;123:177–87. doi: 10.1016/s0034-5687(00)00175-4. [DOI] [PubMed] [Google Scholar]
- Huey KA, Low MJ, Kelly MA, Juarez R, Szewczak JM, Powell FL. Ventilatory responses to acute and chronic hypoxia in mice: effects of dopamine D(2) receptors. J Appl Physiol. 2000b;89:1142–50. doi: 10.1152/jappl.2000.89.3.1142. [DOI] [PubMed] [Google Scholar]
- Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, et al. Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–58. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. μ-opioid receptor agonist suppresses rhythmic inspiratory but neither preinspiratory nor expiratory activity in the newborn rat. Soc Neurosci Abstr. 2002;28:221.11. [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–26. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol. 2000;89:1345–51. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu H, Cui N, Wu J. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol. 2001;129:141–57. doi: 10.1016/s0034-5687(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity in descending synaptic inputs to respiratory spinal motoneurons. Respir Physiol Neurobiol. 2002;131:79–90. doi: 10.1016/s1569-9048(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JE, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro. J Appl Physiol. 2001;91:2703–12. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–38. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Katz DM, Balkowiec A. New insights into the ontogeny of breathing from genetically engineered mice. Curr Opin Pulm Med. 1997;3:433–39. doi: 10.1097/00063198-199711000-00009. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–92. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:207–18. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–43. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–50. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–47. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- Klein DF. Panic disorder and agoraphobia: hypothesis hothouse. J Clin Psychiatry. 1996;57(Suppl 6):21–27. [PubMed] [Google Scholar]
- Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol. 2002a;539:309–15. doi: 10.1113/jphysiol.2001.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA. 2002b;99:821–26. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Yang T, Premkumar DR, Thomas AJ, Prabhakar NR. Blunted respiratory responses to hypoxia in mutant mice deficient in nitric oxide synthase-3. J Appl Physiol. 2000;88:1496–508. doi: 10.1152/jappl.2000.88.4.1496. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–63. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. J Physiol-Paris. 1994;88:81–88. doi: 10.1016/0928-4257(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Levy M, Susswein AJ. Separate effects of a classical conditioning procedure on respiratory pumping, swimming, and inking in Aplysia fasciata. Learn Mem. 1999;6:21–36. [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–19. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–7. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–88. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–74. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- Liu YY, Ju G, Wong-Riley MT. Distribution and colocalization of neurotransmitters and receptors in the pre-Botzinger Complex of rats. J Appl Physiol. 2001;91:1387–95. doi: 10.1152/jappl.2001.91.3.1387. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowiak K, Syed N. Learning, memory and a respiratory central pattern generator. Comp Biochem Physiol A Mol Integr Physiol. 1999;124:265–74. doi: 10.1016/s1095-6433(99)00114-2. [DOI] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Pilowsky PM. NK1 receptor and the ventral medulla of the rat: bulbospinal and catecholaminergic neurons. Neuroreport. 2001;12:3663–67. doi: 10.1097/00001756-200112040-00012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–32. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–79. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–25. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Dekin M, Mitchell GS. Glutamate, GABA and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing: Central Nervous Control. New York: Marcel-Dekker; 1995. pp. 151–218. [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, et al. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–76. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol. 1996;81:866–75. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–26. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier M, Li A, Nattie E. Muscimol inhibition of medullary raphe neurons decreases the CO2 response and alters sleep in newborn piglets. Respir Physiol Neurobiol. 2002;133:197–214. doi: 10.1016/s1569-9048(02)00168-4. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Kiley JP. Oscillations of medullary extracellular fluid pH caused by breathing. Respir Physiol. 1984;55:193–203. doi: 10.1016/0034-5687(84)90022-7. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980b;42:171–88. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, et al. Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001a;90:2466–75. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–74. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001b;124:117–28. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Nuding S, Dick TE, Shannon R, Lindsey BG. Neural network plasticity in respiratory motor control. J Appl Physiol. 2003 doi: 10.1152/japplphysiol.00715.2002. In press. [DOI] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Shannon R, Lindsey BG. Respiratory neural activity during long-term facilitation. Respir Physiol. 2000;121:119–33. doi: 10.1016/s0034-5687(00)00123-7. [DOI] [PubMed] [Google Scholar]
- Moss IR. Respiratory responses to single and episodic hypoxia during development: mechanisms of adaptation. Respir Physiol. 2000;121:185–97. doi: 10.1016/s0034-5687(00)00127-4. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Carfi M, Pantaleo T. Respiratory changes induced by kainic acid lesions in rostral ventral respiratory group of rabbits. Am J Physiol Regul Integr Comp Physiol. 2002;283:R227–42. doi: 10.1152/ajpregu.00579.2001. [DOI] [PubMed] [Google Scholar]
- Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med. 2001;163:1191–97. doi: 10.1164/ajrccm.163.5.2004230. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A, Richerson G, Lappi D. Specific killing of rat medullary raphe 5-HT neurons by a serotonin transporter antibody-saporin conjugate reduces the ventilatory response to increased CO2 during sleep and wakefulness. Soc Neurosci Abs. 2002;28 In press. [Google Scholar]
- Nattie EE. Central Chemoreceptors, pH, and Respiratory Control. New York: Wiley-Liss; 1998. p. 560. [Google Scholar]
- Nattie EE. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–35. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–68. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–95. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–57. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002a;92:2119–30. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Substance P-saporin lesions of neurons with NK1 receptors in one chemoreceptor site in rat decreases ventilation and chemosensitivity. J Physiol (Lond) 2002b;544(2):603–16. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozik-Grayck E, McMahon TJ, Huang YC, Dieterle CS, Stamler JS, Piantadosi CA. Pulmonary vasoconstriction by serotonin is inhibited by S-nitrosoglutathione. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1057–65. doi: 10.1152/ajplung.00081.2001. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Gozal D. Behavioural and neurocognitive implications of snoring and obstructive sleep apnoea in children: facts and theory. Paediatr Respir Rev. 2002;3:3–9. doi: 10.1053/prrv.2002.0177. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge F. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–39. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- Okada Y, Muckenhoff K, Scheid P. Hypercapnia and medullary neurons in the isolated brain stem-spinal cord of the rat. Respir Physiol. 1993;93:327–36. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, et al. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–16. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Firing properties of respiratory rhythm generating neurons in the absence of synaptic transmission in rat medulla in vitro. Exp Brain Res. 1989;76:530–36. doi: 10.1007/BF00248909. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol. 1997;47:385–403. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kanamaru A, Homma I. Optical imaging of respiratory burst activity in newborn rat medullary block preparations. Neurosci Res. 1996;25:183–90. doi: 10.1016/0168-0102(96)01048-6. [DOI] [PubMed] [Google Scholar]
- Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol. 1998;513:381–98. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, et al. Decreased kainate receptor binding in the arcuate nucleus of the sudden infant death syndrome. J Neuropathol Exp Neurol. 1997;56:1253–61. doi: 10.1097/00005072-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Caprini M, Zhang J, Rockenstein EM, Rivera RR, et al. Neuronal death and perinatal lethality in voltage-gated sodium channel alpha(II)-deficient mice. Biophys J. 2000;78:2878–91. doi: 10.1016/S0006-3495(00)76829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–34. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol. 2001;90:1986–94. doi: 10.1152/jappl.2001.90.5.1986. [DOI] [PubMed] [Google Scholar]
- Ptak K, Hilaire G. Central respiratory effects of substance P in neonatal mice: an in vitro study. Neurosci Lett. 1999;266:189–92. doi: 10.1016/s0304-3940(99)00289-x. [DOI] [PubMed] [Google Scholar]
- Putnam RW. Intracellular pH regulation of neurons in chemosensitive and nonchemosensitive areas of brain slices. Respir Physiol. 2001;129:37–56. doi: 10.1016/s0034-5687(01)00281-x. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research—a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449–72. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger Complex in vivo eliminates breathing but not gasping. J Physiol. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol. 2002;131:43–56. doi: 10.1016/s1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger Complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger Complex. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. RC113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Dauger S, Vardon G, Levacher B, Simonneau M, et al. Impaired ventilatory responses to hypoxia in mice deficient in endothelin-converting-enzyme-1. Pediatr Res. 2001;49:705–12. doi: 10.1203/00006450-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–89. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhyth-mogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–72. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–84. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res. 2002;52:449–53. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–45. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger Complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–60. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth KA, Brown GA, et al. Rnx deficiency results in congenital central hypoventilation. Nat Genet. 2000;24:287–90. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol. 2002;92:2565–70. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger Complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–29. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Botzinger Complex in vivo. J Neurophysiol. 1999;81:1150–61. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, O'Neal MH., III CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Halat TJ, El-Maghrabi MR, O'Neal MH., III Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol. 2001;129:101–21. doi: 10.1016/s0034-5687(01)00299-7. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Gozal D, Shea SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol. 2001;129:247–55. doi: 10.1016/s0034-5687(01)00294-8. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Single FN. Mice with genetically modified NMDA and AMPA receptors. Ann NY Acad Sci. 1999;868:494–501. doi: 10.1111/j.1749-6632.1999.tb11318.x. [DOI] [PubMed] [Google Scholar]
- Straus C, Wilson RJ, Remmers JE. Developmental disinhibition: turning off inhibition turns on breathing in vertebrates. J Neurobiol. 2000;45:75–83. doi: 10.1002/1097-4695(20001105)45:2<75::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ. Interaction between amount and pattern of training in the induction of intermediate-and long-term memory for sensitization in aplysia. Learn Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron. 2001;31:143–54. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Takeda S, Eriksson LI, Yamamoto Y, Joensen H, Onimaru H, Lindahl SG. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95:740–49. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–13. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–90. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci. 2000;20:5858–66. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian RX, Kimura S, Kondou N, Fujisawa Y, Zhou MS, et al. DOI, a 5-HT2 receptor agonist, induces renal vasodilation via nitric oxide in anesthetized dogs. Eur J Pharmacol. 2002;437:79–84. doi: 10.1016/s0014-2999(01)01616-8. [DOI] [PubMed] [Google Scholar]
- Tokumasu M, Nakazono Y, Ide H, Akagawa K, Onimaru H. Optical recording of spontaneous respiratory neuron activity in the rat brain stem. Jpn J Physiol. 2001;51:613–19. doi: 10.2170/jjphysiol.51.613. [DOI] [PubMed] [Google Scholar]
- Turner DL, Bach KB, Martin PA, Olsen EB, Brownfield M, et al. Modulation of ventilatory control during exercise. Respir Physiol. 1997;110:277–85. doi: 10.1016/s0034-5687(97)00093-5. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Chachkes J, Fenik P, Hendricks JC. The effects of ondansetron on sleep-disordered breathing in the English bulldog. Sleep. 2001;24:155–60. doi: 10.1093/sleep/24.2.155. [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci. 2002;22:3755–64. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J Comp Neurol. 2001a;434:128–46. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001b;85:2224–35. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–65. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann M, Bingmann D. Ventrolateral neurons of medullary organotypic cultures: intracellular pH regulation and bioelectric activity. Respir Physiol. 2001;129:57–70. doi: 10.1016/s0034-5687(01)00282-1. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol. 2002;540:557–70. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001a;531:509–14. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001b;91:2831–38. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Champagnat J, Poon CS. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptor desensitization. J Neurosci. 1997;17:5349–56. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


