Abstract
To more accurately assess the activity and role of epithelial-cell derived antimicrobial peptides in their native settings, it is essential to perform assays at the surfaces under relevant conditions. In order to carry this out, we utilize 3-dimensional cultures of airway and gingival epithelium, which are grown at an air-liquid interface. Under these conditions, the cultures can be subjected to challenge with a variety of factors known to cause an increase in antimicrobial peptide gene expression. The functional relevance of this induction can then be assessed by quantifying antibacterial activity either directly on the surface of the cells or using the fluid secreted onto the apical surface of the cultures. The relative contribution of the peptides can also be measured by pre-incubation of the secreted fluid with specific inhibitory antibodies. Thus, a relatively inexpensive in vitro model can be used to evaluate the role of antimicrobial peptides in mucosal epithelium.
Keywords: airway, oral epithelium, vitamin D, cathelicidin
1. Introduction
In general, most antimicrobial peptides have been identified by protein purification methods from tissues, followed by in vitro assays to identify the active fraction (see (1-3), for example). Alternatively, DNA sequences coding for homologous peptides are identified through computational or experimental methods (e.g., (4,5)). Subsequently, the purified natural peptides or synthetic peptides based on the DNA sequences are characterized by in vitro antimicrobial assays against target organisms, which provides important information regarding the potential for the peptide's use as a therapeutic, or give clues to its role in innate defense. Furthermore, the expression of many host defense peptide genes is induced by a variety of factors both in cell culture (e.g., (6,7)) and in vivo (8,9), supporting their role in innate host defense of mucosal epithelia such as the airway, the oral cavity and the intestinal tract. However, to accurately assess the relationship between the induction of a peptide gene (as measured by mRNA levels) and an increase in antibacterial activity in a tissue is difficult.
Recently, novel methods for cell culture which provide three-dimensional model systems that recapitulate the mucosal epithelium for different tissues have been described (10,11). The systems rely on culturing epithelial cells in an air-liquid interface (ALI) in serum-free medium, leading to well-differentiated cultures, often covered with a cell-derived secretion. This provides an environment that allows for quantification of antimicrobial activity in mucosal secretions under conditions that are more representative of the original tissue, and allows the investigator to modulate antimicrobial peptide gene expression with exogenous factors. Assay conditions can then be developed to determine the role of endogenous antimicrobial peptides on mucosal surfaces.
2. Materials
2.1 Airway epithelial cell culture (adapted from ref. 11)
Bronchial Epithelial Growth Media Basal Medium without growth factors, cytokines, or supplements (BEBM) (Lonza, Walkersville, MD).
Bronchial Epithelial Growth Media (BEGM) SingleQuots Kit Supplement and Growth Factors added directly to medium. For 500 mL medium, add Bovine pituitary extract (BPE) 2 mL, insulin 0.5 mL, hydrocortisol 0.5 mL, gentamicin sulfate and amphotericin-B 0.5 mL, retinoic acid 0.5 mL, transferring 0.5mL, triiodothyronine 0.5 mL, epinephrine 0.5 mL, and hEGF 0.5 mL (Lonza, Walkersville, MD). Antibiotics may be added, but must be removed prior to antimicrobial assays (see Note 2).
Costar Transwell Permeable Supports 12 mm insert, 12 well plate.
Collagen from Human Placenta, Type VI. 10mg is dissolved in 20 mL of dH2O and 40 μl of concentrated acetic acid is added. The collagen is then incubated at 37 °C for 15-30 minutes for the collagen to fully dissolve. The stock solution is diluted 1:10 with dH2O to coat the transwell inserts.
Normal Human Bronchial Epithelial (NHBE) are obtained from Lonza. Other primary cultures may be used.
2.2 Gingival epithelial cell culture (adapted from ref. 12)
Dulbecco's Modified Eagle's Medium with glucose and L-glutamine, supplemented with 10% bovine serum and penicillin-streptomycin.
Collagen, Type I (rat tail) at 1.1 mg/mL in water with 43 μl concentrated acetic acid (33%) per 5 mL of solution is incubated at 37°C for 30 minutes to dissolve the collagen.
10× DMEM (without sodium bicarbonate) powder is prepared in water at 13.48%, filter sterilized, and aliquots are stored at -20°C.
10× reconstitution buffer: 22 mg/mL sodium bicarbonate, 20 mM Hepes, 0.62 N NaOH. Aliquots are stored at -20°C.
Keratinocyte serum free medium (KSFM) supplemented with L-glutamine. Calcium chloride is added to 0.03 M. Bovine pituitary extract and epithelial growth factor are supplied with the medium and are added per the manufacturer's instructions.
Costar Transwell Permeable Supports: 24-mm insert, polyester membrane, 6 well polystyrene plate.
OKF6/TERT oral keratinocyte cells are obtained with material transfer agreement from the laboratory of Dr. James Rhinewald, Harvard University.
2.3 Antimicrobial Assays
10× Phosphate Buffered Saline Solution.
1,25-dihydroxyvitamin D3 10 μg is made to 10-5M concentration by dissolving it in 100% ethanol. Vitamin D is added to the BEGM medium to make a final concentration of 10-8M to induce the expression of LL-37. Ethanol is used as a control.
LB broth Miller used to grow Pseudomonas aeruginosa in liquid culture and agar plates.
Bordet-Genou Agar used to grow Bordetella bronchiseptica on agar plates.
Stainer-Scholte medium, used to grow B. bronchiseptica in liquid culture.
AAGM (30 g/L of trypticase soy broth or 40 g/L of trypticase soy agar, 6 g/L yeast plus 0.75% dextrose [filter-sterilized] and 0.4% sodium bicarbonate [filter-sterilized] added after autoclaving) used to grow Aggregatibacter actinomycetemcomitans in liquid culture and agar plates.
3. Methods
Beta-defensins and cathelicidins are antimicrobial peptides expressed in mucosal epithelial cells (reviewed in (13,14)). Their expression is induced in response to a variety of agents including bacterial Lipopolysaccharide (LPS), Interleukin (IL)-1β and the active form of vitamin D, 1,25(OH)2 D3 (reviewed in (15,16)).
To assess the activity of these peptides in airway epithelial cells, primary cultures of bronchial epithelial cells are grown in an air-liquid interface and are allowed to mature and differentiate for 20 days before any experiments are performed. The bronchial epithelial cells are then basolaterally treated with an inducing agent, such as IL-1β (100ng/ml) or vitamin D at a concentration of 10-8M. The airway surface fluid (ASF) is then collected by washing the cells with 50 μl of filter-sterilized 1× PBS. As a control for the poorly water soluble 1,25(OH)2 D3, control cells are treated with an equal volume of ethanol. The effect of the inducing agents on the bactericidal activity of ASF is studied using airway pathogens such as B. bronchiseptica or P. aeruginosa. To determine whether specific peptides are responsible for all or part of the activity, the ASF can be pre-treated with inhibitory antibodies prior to incubation with bacteria. A relative increase in the number of colonies relative to control (pre-treated with non-specific IgG) suggests a role for that peptide in killing.
To quantify the activity of peptides on the surface of gingival epithelial cells, a 3-D culture system is used whereby epithelial cells are cultured on transwells coated with a feeder layer of cells in collagen (10). Once the top layer is confluent, surface medium is removed and the epithelial cells are cultured similar to airway cells in an air-liquid interface. Activity against bacteria is measured using challenge with the periodontal pathogen A. actinomycetemcomitans.
3.1 Airway Epithelial Cell Culture
Normal human bronchial epithelial (NHBE) cells are initially grown in monolayer culture by standard tissue culture techniques in BEGM until they reach confluence.
To prepare the air-liquid interface system, 12mm transwell inserts (see Note 1) are coated with 200 μL of diluted type VI collagen. Coated inserts are dried in a laminar flow hood overnight. After the collagen dries the plates are exposed to 30 minutes of UV light in the hood.
Confluent cultures of NHBE cells are washed with 1× HBSS and trypsinized. The trypsin is neutralized with 10% serum-based medium. The cells are then centrifuged at low speed for 5 minutes and resuspended in BEGM. Cell suspensions are counted and seeded in the 12mm transwell plates with approximately 250,000 cells per well. Once the cells reach confluence in the 12mm transwell inserts the medium is removed from the apical surface of the cells. Cells are kept in a 37°C, humidified 5% CO2 incubator. The cells in the transwell inserts are allowed 20 days to fully mature and differentiate before any experiments are done. The medium in the basolateral chamber is changed every 2-3 days. The apical surface is washed with either PBS or 10 mM phosphate buffer when the medium is changed.
Two days before the experiment is carried out, the apical surface is washed twice with 1× PBS and the basolateral medium is replaced with antibiotic-free BEGM.
The cells are treated basolaterally with an inducing agent such as 1,25(OH)2 D3 at a final concentration of 10-8 M or with IL-1β at 100 ng/mL for up to 48 hours. The ASF is collected in 1.5 mL tubes after induction by washing the apical surface of the cells with 50 μL of filter-sterilized 1× PBS. ASF is stored in -80°C.
3.2 Airway Surface Fluid Antimicrobial Assay
P. aeruginosa is streaked from a frozen stock to a LB plate and grown in a 37°C incubator overnight. B. bronchiseptica is streaked from a frozen stock to a Bordet-Genou (BG) agar plate grown in a 37°C incubator overnight.
A single colony of bacteria is inoculated and grown in liquid culture in a 37°C shaking incubator overnight. Bacteria from the overnight liquid culture is diluted 1:10 in fresh medium in a new tube and incubated for 3-6 hours in a 37°C shaking incubator to obtain bacteria in the mid-log phase of bacterial growth. These bacteria are then diluted to 5×104 CFU/mL in 10 mM Phosphate buffer.
500 CFU of bacteria (10 μL) are mixed with 90 μL of the ASF in a 1.5 mL tube. The mixture is incubated in a 37°C water bath while shaking at 75 rpm. The contents of the tube are seeded on LB plates (for P. aeruginosa) or BG-agar (for B. bronchiseptica) overnight.
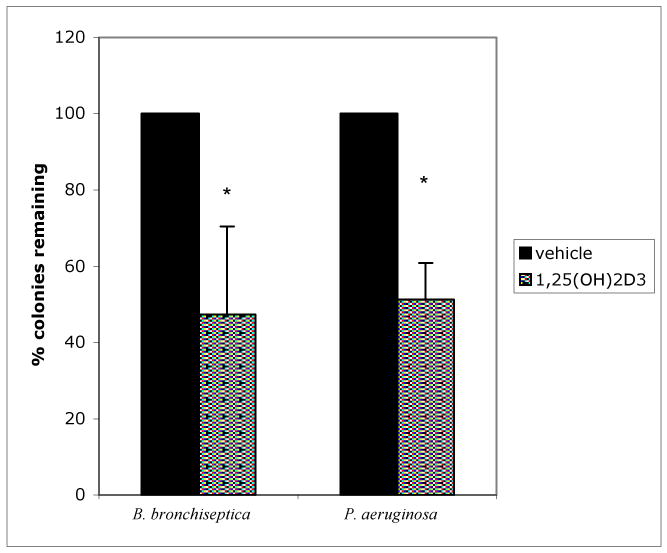
Bacterial colonies are counted to determine activity of the ASF against the bacteria. An example of the results is shown in Fig. 1.
Figure 1. Induction of antibiotic activity of airway surface fluid by 1,25(OH)2D3.
NHBE cells were grown on Transwell CM inserts and exposed to 10-8M 1,25(OH)2D3 basolaterally for 0, 24, and 48 hours. Antimicrobial activity of ASL against B. bronchiseptica and P. aeruginosa was determined by counting the number of colonies on 5% BG plates supplemented with 5% sheep blood and LB plates, respectively, after incubation with the ASL collected from the NHBE (Reproduced from ref. 7 with permission from Elsevier Science.)
3.3 Inhibition of activity by specific antibodies
Bacteria are prepared as above.
ASF (90 μL) is incubated with 1 μL anti-LL-37 or control serum for 1hr at 37°C.
Bacteria (in 10 μL) are added to the ASF mixture and incubated as above. An example of the results is shown in Fig. 2.
Figure 2. Inhibition of antimicrobial activity with LL-37 antibody.
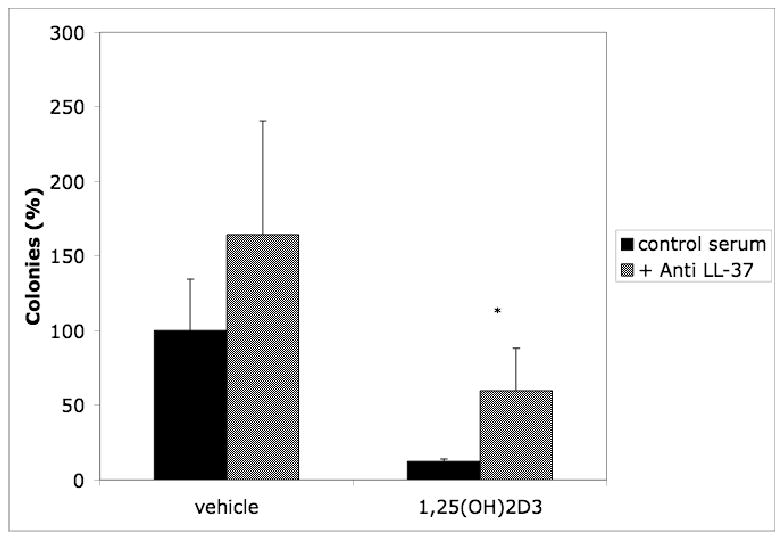
ASF was pretreated with 1 μl anti LL-37 antibody (gift of R. Gallo, UCSD) or control serum for 1 hr. prior to addition of P. aeruginosa in antimicrobial assays as described above. Results are reported as means ± SEM (*p<0.05) of at least three independent experiments. (Reproduced from ref. 7 with permission from Elsevier Science.)
3.4 Direct activity on airway epithelial cell surfaces
The bronchial epithelial cells in airway liquid interface are stimulated basolaterally for 24 hours.
P. aeruginosa is grown in liquid culture overnight (see Note 3) and then diluted 1:10 in new LB broth and incubated for 3-6 hours to obtain bacteria in mid-log phase.
The mid-log phase bacteria are diluted to 500 CFU in 0.5 μL and deposited on the apical surface of the bronchial epithelial cells. After 5 hours the apical surface of the cells is washed with 100 μL of PBS three times and plated to determine the number of viable colonies.
3.5 Gingival epithelial cell culture
Murine fibroblast cells NIH3T3 are grown as a monolayer in a 75 cm2 tissue culture flask at 37°C, 5% CO2 in DMEM/bovine serum to 90% confluence using standard tissue culture methods. Normal human gingival cells (OKF-6/TERT) are grown as a monolayer in 25 cm2 tissue culture flasks in KSFM to confluence at 37°C, 5% CO2 using standard tissue culture techniques.
To prepare the air-liquid interface, 24 mm Transwell inserts in a six-well plate are first coated with 0.8 mL/Transwell of an acellular collagen mixture (see Note 4). For 6 mL (one plate) combine in the following order: 0.6 mL 10× DMEM, 0.6 mL 10× reconstitution buffer, 0.54 mL FBS, 60 μl 200 mM L-glutamine, and 4.2 ml Collagen solution (to 0.77 mg/ml). Mix by gently swirling and inverting the tube. Try to avoid bubbles. Add 0.8 mL to each Transwell insert. Allow the collagen to solidify at room temperature for 30-60 minutes.
-
The cellular collagen mixture is then added to the Transwells. For each Transwell, 2.3 mL of the cellular (fibroblast) collagen mixture, containing 5-7 × 105 cells, is required. For one plate combine in the following order:
1.40 mL 10× DMEM
1.40 mL 10× reconstitution buffer
1.56 mL FBS
140 μl L-glutamine (200 mM)
9.5 mL Collagen solution (for a final concentration of 0.70 mg/mL)
b) Add 1.0 mL of 3T3 cells at 3-4 × 106/mL, for a final volume of 15 mL. The 3T3 cells are harvested using trypsin EDTA and standard tissue culture methods. After centrifugation, cells are resuspended in 1 mL DMEM/bovine serum, counted and diluted to 3-4 × 106/mL with medium. Mix the cellular collagen solution gently by inverting the tube.
c) Add 2.3 mL per Transwell on top of the acellular collagen layer and leave in the hood for 45-60 minutes without moving the plate. Then transfer to 37°C, 5%CO2 for 45-60 minutes, until the collagen gel solidifies.
d) Add DMEM/bovine serum medium: 1.5 mL each inside and outside of the Transwell insert. Continue incubation at 37°C, 5% CO2.
At 2-3 days slide a sterile spatula around the edge of the gels, to move them away from the sides of the Transwell inserts. After 5-7 days, the collagen gels should be completely contracted away from the sides of the inserts. Change the medium every 2-3 days.
-
Once the collagen gels have contracted, the epithelial (OKF6/TERT) cells are seeded on the top of the cellular (fibroblast) collagen gels.
a) OKF6/TERT cells are harvested using trypsin-EDTA and standard tissue culture techniques, counted and resuspended to 15 × 106 cells/mL in KSFM.
b) Remove the medium from inside the inserts. Remove about half the medium from outside the inserts. Leave enough so that the bottom of the insert rests completely on the medium, but not so much that the medium rises into the insert.
c) Add 60 mL of the OKF6/TERT suspension on top of the middle of each collagen gel. Confirm OKF6/TERT cell growth by also seeding the same amount of cells into 1 well of a 12 well plate.
d) Incubate at 37°C, 5% CO2 for 2 hours to allow the OKF6/TERT cells to attach. Then add 2 mL of KSFM to the inserts and 0.8 mL DMEM/bovine serum to the wells and continue incubation at 37°C, 5% CO2.
Replace the medium in the inserts with fresh KSFM on the next day and continue at 37°C, 5% CO2, replacing both media every 2 days.
-
After approximately 3-4 days (when the control well has grown to confluence) the inserts can be moved to the air-liquid interface plate containing differentiation medium (BEGM).
a) Add 0.7 mL per well BEGM with antibiotics to a new 6 well tissue culture plate.
b) Remove the medium from inside the Transwells and move each to the new plate. The bottom of the insert should be completely covered with medium, but the medium should not rise into the insert, allowing the cells to be fed from below and the apical surface of the epithelium to be exposed to air. Incubate the cells at 37°C, 5% CO2 for 15 days. Feed every 2-3 days by removing as much medium as possible and add 0.65 mL per well.
-
Equilibrate the cells in antibiotic-free medium.
a) Add 0.7 mL BEGM without antibiotics to each well of a new 6 well tissue culture plate.
b) Remove any medium from inside the Transwells and move each to the new plate.
c) Incubate the cells at 37°C, 5% CO2 for 6 days. Every 2-3 days remove as much medium as possible and add 0.65 mL per well BEGM without antibiotics.
3.6 Direct activity on gingival epithelial cell surfaces
Grow A. actinomycetemcomitans from frozen culture by streaking on an AAGM plate (see Note 3) and incubating at 37°C, 10% CO2 for 3-4 days.
Use a single colony to inoculate 30 mL of AAGM broth in a 75 cm2 tissue culture flask. Incubate at 37°C, 10% CO2 for 40-48 hours. The bacteria will grow as a biofilm on the bottom of the flask.
The next day, move the Transwell inserts containing the differentiated OKF6/TERT cells equilibrated in antibiotic-free medium to a new 6 well plate containing BEGM without antibiotics plus either 1,25-dihydroxyvitamin D3 at 10-8M or an equal volume of ethanol. Make at least duplicate wells for each treatment.
-
The following day, prepare a single-cell suspension of A. actinomycetemcomitans.
a) Remove the medium from the flask and wash three times with PBS.
b) Add 1 mL PBS, scrape the bacteria into the liquid and transfer to a microtube.
c) Vortex on the highest speed for 1 minute and then allow to settle for 10 minutes.
d) Transfer the top 750 μl, which is the single-cell suspension, to a new microtube.
e) Make a 1:10 dilution and find its absorbance at 595 nm.
f) An OD = 1 corresponds to 2.9 × 106 cfu/mL.
Dilute the single cell suspension to 1×103 cfu/μl in PBS.
At 18 hours after 1,25-dihydroxyvitaminD3 treatment of the Transwell insert cells, add 500 cfu (0.5 μl) to the center of the apical surface of each.
Incubate at 37°C, 10% CO2 for 1-3 hours.
Move each Transwell insert to an unused well. Wash the apical epithelial surface with 50 μl of PBS.
Remove the PBS wash and plate it on an AAGM agar plate. Repeat for each Transwell insert.
Incubate the plates at 37°C, 10% CO2 for 3-4 days.
Count the colonies present on each plate.
4. Notes
Some airway epithelial cells (such as NHBE) can be grown and differentiated in ALI on larger (e.g., 24 mm) inserts, while others require the smaller, 12 mm filters. More ASF can be obtained from the larger filters, so it is best to first determine which size is optimal. The number of cells seeded on the larger filters is increased accordingly.
Don't forget to change to antibiotic-free medium at least two days prior to the assay.
It helps to use bacteria that are resistant to at least one antibiotic. They can be streaked on antibiotic-containing plates, grown in medium with antibiotic, but the antibiotics should be washed out during processing prior to assays. Final plating on antibiotic-containing plates will then only identify the bacteria that were intentionally added to the assay.
-
For the gingival epithelial cell transwell preparation:
a) Bovine collagen Type I can also be used. If bought as a prepared solution (Organogenesis is one source), it is usually stored at 4°C. Test to see if it solidifies at room temperature. If it does, use pre-chilled pipettes and keep the solutions on ice. Do a trial run to see if this will cause the fibroblasts to lyse. If the collagen solution does not solidify at room temperature, warm at 37°C for 15 minutes before use and keep all reagents at room temperature during preparation of the collagen layers.
b) Adjust the amount of FBS in both the acellular and cellular collagen mixtures to maintain final collagen concentrations of 0.77 mg/mL and 0.70 mg/mL, respectively.
c) Observe the collagen as it is mixed into each solution to see that it mixes in completely.
d) A confluent 75 cm2 tissue culture flask of 3T3 cells (fibroblasts) contains more than enough cells for two transwell plates.
e) After 2-3 days, free up the edges of the collagen gels using either a sterile pipette tip or a sterile metal spatula.
f) For the epithelial layer (TERT cells), 2-3 confluent 25 cm2 flasks are needed for one transwell plate.
Acknowledgments
The authors would like to thank Drs. Leslie Fulcher and Scott Randell (University of North Carolina, Chapel Hill) and Helena Kashleva and Anna Dongari-Bagtzoglou (University of Connecticut) for assistance in growing the ALI cultures of airway and gingival cells, respectively. GD is supported by grants from the Cystic Fibrosis Foundation and the NIH (DE18781).
References
- 1.Cole AM, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci (USA) 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 4.Bals R, Goldman MJ, Wilson JM. Mouse β-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia JR, Krause A, Schulz S, Rodrigues-Jimenez FJ, Kluver E, Adermann K, Forssmann U, Frimpong-Boateng A, Bals R, Forssmann WG. Human beta-defensin 4: a novel inducible peptide with a salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–1821. [PubMed] [Google Scholar]
- 6.Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction Of Cathelicidin In Normal And CF Bronchial Epithelial Cells By 1,25-Dihydroxyvitamin D3. J Cystic Fibrosis. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caverly JM, Diamond G, Gallup JM, Brogden KA, Dixon RA, Ackermann MR. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect Immun. 2003;71:2950–2955. doi: 10.1128/IAI.71.5.2950-2955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dongari-Bagtzoglou A, Kashleva H. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nature Protocols. 2006;1:2012–2018. doi: 10.1038/nprot.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 12.Dongari-Bagtzoglou A, Kashleva H. Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog. 2006;40:271–8. doi: 10.1016/j.micpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 14.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 15.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond G, Laube D, Klein-Patel ME. In: Mammalian Host Defence Peptides. Hancock REW, Devine DA, editors. Vol. 6. Cambridge University Press; Cambridge, UK: 2004. pp. 111–38. [Google Scholar]