Abstract
The delayed-rectifier K+ channel Kv2.1 exists in highly phosphorylated somatodendritic clusters. Ischemia induces rapid Kv2.1 dephosphorylation and a dispersal of these clusters, accompanied by a hyperpolarizing shift in their voltage-dependent activation kinetics. Transient modulation of Kv2.1 activity and localization following ischemia is dependent on a rise in intracellular Ca2+and the protein phosphatase calcineurin. Here, we show that neuronal free Zn2+also plays a critical role in the ischemic modulation of Kv2.1. We found that sub-lethal ischemia in cultured rat cortical neurons led to characteristic hyperpolarizing shifts in K+ current voltage dependency and pronounced dephosphorylation of Kv2.1. Zn2+chelation, similar to calcineurin inhibition, attenuated ischemic induced changes in K+ channel activation kinetics. Zn2+chelation during ischemia also blocked Kv2.1 declustering. Surprisingly, we found that the Zn2+rise following ischemia occurred in spite of calcineurin inhibition. Therefore, a calcineurin-independent rise in neuronal free Zn2+ is critical in altering Kv2.1 channel activity and localization following ischemia. The identification of Zn2+ in mediating ischemic modulation of Kv2.1 may lead to a better understanding of cellular adaptive responses to injury.
Keywords: calcineurin, neuroprotection, potassium channel, preconditioning
Introduction
Ischemia triggers accumulation of extracellular glutamate, a rise in intracellular Ca2+ and occurrence of repetitive waves of depolarization, leading to profound changes in neuronal excitability (Lee et al., 1999; Dietz et al., 2009). Delayed-rectifier voltage-dependent potassium channels (Kv) are important in regulating neuronal excitability (Du et al., 2000). Of these, Kv2.1 is a major component of delayed-rectifier potassium currents (IK) in cortical neurons (Murakoshi et al., 1997; Du et al., 2000; Malin & Nerbonne, 2002; Pal et al., 2003) and exists in large, highly phosphorylated clusters on the surface of soma and proximal dendrites (Scannevin et al., 1996). Mild ischemic injury is associated with dephosphorylation of Kv2.1, dispersal of somatodendritic Kv2.1 clusters and hyperpolarizing shifts in voltage dependency (Misonou et al., 2005). The latter has been proposed as a mechanism for limiting neuronal excitability and thus preventing or limiting widespread excitotoxic cell death (Surmeier & Foehring, 2004). Such changes in Kv2.1 following ischemia are transient, returning to baseline conditions within hours of stimulus cessation, and are mediated by a rise in intracellular Ca2+ and protein phosphatase 3 (calcineurin) activity (Misonou et al., 2005).
In addition to a rise in neuronal Ca2+, ischemic injury also leads to an accumulation of free Zn2+ in neurons (Sensi & Jeng, 2004; Galasso & Dyck, 2007; Hershfinkel et al., 2009). Recent evidence suggests that the Zn2+ rise may actually precede the rise in intracellular Ca2+, serving as a very early signal in the ischemic cascade (Medvedeva et al., 2009). This rise in neuronal Zn2+ following lethal ischemic insults has been associated with irreversible neuronal injury mediated by mitochondrial dysfunction (Medvedeva et al., 2009), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation (Suh et al., 2008; Brennan et al., 2009), generation of reactive oxygen species (Dineley et al., 2008) and activation of a p75NTR-mediated death executer (Park et al., 2000). In contrast to lethal injury, sub-lethal ischemia can activate endogenous signaling pathways that render neurons tolerant to subsequent ischemic damage that would otherwise have been irreversible (Kitagawa et al., 1990; Gidday, 2006). Recent evidence has shown sub-lethal ischemia leads to a transient, early rise in neuronal free Zn2+, which is both necessary and sufficient for neuronal tolerance (Lee et al., 2008; Aras et al., 2009). This rise in neuronal free Zn2+ largely originates from intracellular sources and triggers Zn2+-regulated gene expression (Aras et al., 2009).
In the present study, we found a critical role for Zn2+ in the rapid modulation of Kv2.1 following sub-lethal ischemia. We first confirmed that sub-lethal chemical ischemia leads to the transient modulation of Kv2.1 voltage dependency and phosphorylation state. The altered K+ channel activation kinetics, which limit neuronal excitability, are dependent on a rise in neuronal free Zn2+. Moreover, the ischemia-induced dispersal of Kv2.1 clusters is also Zn2+-dependent. We found that both altered kinetics and localization of Kv2.1 following chemical ischemia are dependent on calcineurin activity, but that the Zn2+ rise occurs independently of this phosphatase. Thus, Zn2+ may represent a novel early signal in the modulation of Kv2.1 channel activity and localization following sub-lethal chemical ischemia.
Materials and methods
Rat primary neuronal cultures and preconditioning
All experiments were performed in primary cortical cultures prepared from embryonic day 16 Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA). Harvesting of embryonic brain tissue was done with the approval of the University of Pittsburgh School of Medicine and in accordance with National Institutes of Health protocols. As previously described in detail (Hartnett et al., 1997), the animal is killed with 1–2 minutes of CO2 inhalation in a large plexiglass chamber. Cortices were dissociated with trypsin, and the resultant cell suspension was adjusted to 670 000 cells per well (six-well tissue culture plates containing five 12-mm poly-L-ornithine-treated coverslips per well). Cultures were maintained at 37°C in 5% CO2, in a growth medium composed of a volume-to-volume mixture of 80% Dulbecco’s modified minimal essential medium, 10% Ham’s F12-nutrients and 10% bovine calf serum (heat-inactivated, iron-supplemented) with 25 mM HEPES, 24 U/mL penicillin and 24 μg/mL streptomycin. Non-neuronal cell proliferation was inhibited after 2 weeks in culture with 1–2 μM cytosine arabinoside, after which the cultures were maintained in growth medium containing 2% serum and without F12-nutrients. Cultures were utilized at 18–22 days in vitro. An in vitro model of ischemic preconditioning was previously developed in our laboratory (McLaughlin et al., 2003; Aras et al., 2009). Briefly, cortical cultures were treated with 3 mM potassium cyanide (KCN) in a glucose-free balanced salt solution (NaCl, 150 mM; KCl, 2.8 mM; CaCl2, 1 mM; and HEPES, 10 mM; pH 7.2) for 90 min at 37°C. Preconditioning with KCN attenuates subsequent excitotoxic cell death by ~50% (McLaughlin et al., 2003; Aras et al., 2009).
Electrophysiology
All recordings were made using the whole-cell configuration of the patch-clamp technique as described previously (McLaughlin et al., 2001). The extracellular solution contained (in mM): NaCl, 115; KCl, 2.5; MgCl2, 2.0; HEPES, 10; and D-glucose, 10; pH was adjusted to 7.2 with concentrated KOH; 0.250 mM TTX was added to inhibit voltage-gated sodium channels. The intracellular (electrode) solution contained (in mM): K-Gluconate, 100; EGTA, 11; KCl, 10; MgCl2, 1; CaCl2 ×2H2O, 1; and HEPES, 10; pH was adjusted to 7.2 with concentrated KOH; 0.22 mM ATP was added and osmolarity was adjusted to 280 mOsm with sucrose. All measurements were obtained under voltage clamp with an Axopatch 1C amplifier (Molecular Devices, Sunnyvale, CA, USA) and pClamp software (Molecular Devices) using 2–3 MΩ electrodes. Partial compensation (80%) for series resistance was provided in all instances. Currents were filtered at 2 kHz and digitized at 10 kHz (Digidata; Axon Instruments). K+ currents were evoked with a series of 200-ms voltage steps from a holding potential of −50 mV to +80 mV in 10-mV increments. Before the start of the depolarization, a single prepulse to −10 mV was given for 30 ms to inactivate A-type K+ currents. Peak conductance (G) was calculated from peak steady-state current amplitudes (I) using the equation G = I/(V-EK), where EK is the Nerst K+ equilibrium potential. The conductance was plotted against the potential (V) and fitted to a single Boltzmann function G = Gmax/(1 + exp[-(V −V1/2)/k]), where Gmax is the maximum conductance, V1/2 is the potential at which the channel has half-maximal conductance, and k is the parameter that represents the slope of the activation curve.
Immunofluorescence
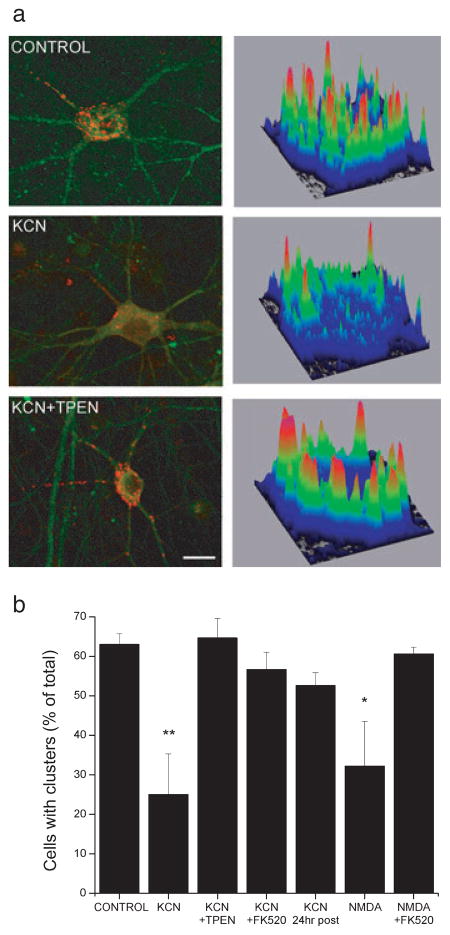
Kv2.1 labeling was performed essentially as described by Misonou et al. (2004). Immediately following chemical ischemia, neurons were washed three times in ice-cold PBS and fixed with 4% paraformaldehyde for 15 min. Following three washes with PBS, neurons were permeabilized for 5 min in PBS containing 0.3% Triton X-100. Neurons were washed three times in PBS and then incubated in PBS containing 1% bovine serum albumin (BSA) for 5 min. Following overnight incubation with anti-Kv2.1 rabbit polyclonal (1: 500; Alomone Labs, Jerusalem, Israel) and anti-microtubule-associated protein-2 (MAP2) mouse monoclonal (1: 500; Sigma-Aldrich, St Louis, MO, USA) antibodies, neurons were incubated in FITC anti-mouse (1: 1000; Sigma-Aldrich) and Cy5 antirabbit (1: 1000; Jackson Immunoresearch, West Grove, PA, USA) secondary antibodies at room temperature for 1 h. Coverslips containing neurons were then mounted onto glass slides and allowed to air-dry before imaging. Imaging of neurons was performed on an Olympus Fluoview FV1000 confocal unit fitted to an Olympus BX61 microscope at 60 × (PlanApo, NA 1.4 oil) using Fluoview software (Olympus Fluoview, Center Valley, PA, USA). Laser and detector settings were retained for all images collected. Multiple (5–10) optical sections (0.5 μm) were acquired to generate a collapsed image file. Control and treatment groups were always run in parallel within the same immunocytochemical procedure. Collapsed raw images were transferred to NIH image processing software (ImageJ; http://rsbweb.nih.gov/ij/) for analysis. Following background subtraction, neuronal somas were selected and a plot displaying a three-dimensional graph of pixel intensity over a region of interest was used to display Kv2.1 localization (Fig. 3a). Channel clusters on the plots appeared as orange–red peaks in pixel intensity, which corresponded to > 70% of maximal intensity (Fig. 3a). A cell showing primarily diffuse staining was identified as such when its associated surface map revealed < 5 orange–red peaks. Using these plots, 75–100 cells from three or four independent experiments were classified as either having clusters or not. Although infrequent (< 10% of total), neurons containing regions of both clustered and diffuse staining patterns were scored according to the predominant (> 50% of cell surface) staining pattern.
Fig. 3.
Zn2+ was required for the calcineurin-dependent ischemic declustering of Kv2.1 in cortical neurons. (a) Neurons exposed to control, 3 mM KCN, or KCN in the presence of 1 μM TPEN were fixed and immunostained with anti-Kv2.1 (1: 500; red) and anti-MAP2 (1: 500; green) antibodies. In KCN-treated groups, neurons were fixed immediately or 24 h following stimulus. Representative neurons and their associated surface maps from each treatment group from three or four independent experiments are shown. Background-subtracted neuronal surface maps show relative Kv2.1 staining intensity values plotted along the area of the cell body. (b) Neurons were exposed to control, 3 mM KCN (90 min), KCN in the presence of 1 μM TPEN or 5 μM FK520 (90 min), 100 μM NMDA with 10 μM glycine (10 min), or NMDA/glycine in the presence of 5 μM FK520 (10 min). Neurons were fixed and immunostained as in A. Fluorescent images were background-subtracted and cell surface maps were created to plot Kv2.1 staining intensity over the neuronal soma. Clusters on surface maps appeared as orange–red peaks in pixel intensity. Using these plots, 75–100 cells from three or four independent experiments were classified as either clustered or not. Data points represent the percentage of cells with clusters compared to total cell counts (mean ± SEM; *P < 0.05, **P < 0.01 compared to control; one-way ANOVA-Dunnett). Scale bar in A, 10 μm.
Immunoblotting
Samples for biochemical analysis were prepared from neuronal cultures immediately following chemical ischemia. Neurons were washed three times with PBS and then incubated in lysis buffer [Tris-HCl, pH 7.4, 50 mM; NaCl, 150 mM; deoxycholic acid, 0.25%; nonyl phenoxylpolyethoxylethanol (NP-40), 1%; and EDTA, 1 mM] supplemented with protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN, USA), 1 mM phenylmethylsulphonyl fluoride and 100 μM vanadate for 5 min on ice. Cell lysates were harvested and centrifuged at 10 000 × g for 10 min at 4°C. Cell lysate samples were combined in a 1: 1 ratio with sample preparation buffer (Tris-HCl, pH 6.8, 62.5 mM; SDS, 2%; glycerol, 25%; and Bromophenol Blue, 0.01%) and incubated for 5 min at 100°C to denature proteins before gel electrophoresis. SDS-PAGE was carried out by standard procedures using the Mini Protean 3 System (Bio-Rad, Hercules, CA, USA). Equal amounts of cell lysate (15 μg) were separated on 7.5% SDS-PAGE gels and transferred onto a 0.2-μm nitrocellulose membrane. The membranes were blocked with 1% BSA in PBS with 0.05% Tween 20 before probing with either an anti-Kv2.1 mouse monoclonal (clone K89/34; NeuroMab, Davis, CA, USA) or an anti-GAPDH mouse monoclonal (Novus Biologicals, Littleton, CO, USA). Blots were incubated with a goat antimouse secondary antibody conjugated to HRP and were visualized with a SuperSignal CL-HRP Substrate System (Pierce Biotechnology, Rockford, IL, USA).
Neuronal Zn2+ imaging
To assess the relative magnitude of intracellular free Zn2+ in neurons we utilized the Zn2+-sensitive fluorescent probe FluoZin-3 AM (Molecular Probes, Eugene, OR, USA). FluoZin-3 AM is a cell-permeant, non-ratiometric fluorescent dye that responds robustly to physiological changes in cellular free Zn2+ (Kd for Zn2+ 10–20 nM) and is highly selective for this metal (Devinney et al., 2005). The small-molecule probe fluoresces upon binding Zn2+ and is best suited for assessing the presence of free Zn2+ in cells rather than determining its precise intracellular concentration (Thompson et al., 2002; Kay, 2003). Following treatment with chemical ischemia, neurons were loaded with FluoZin-3 (30 min; 5 μM prepared in buffered solution containing 144 mM NaCl, 3 mM KCl, 10 mM HEPES, 5.5 mM glucose and 5 mg/mL BSA; pH 7.3). The culture-containing glass coverslips were then immediately transferred to a recording chamber (Warner, Hamden, CT, USA) mounted on an inverted epifluorescence microscope super-fused with phenol red-free minimal essential medium, supplemented with 25 mM HEPES and 0.01% BSA. Images were acquired by exciting the fluorescent dye with 490 nm light every 10 s for 5 min using a computer-controlled monochromator (Polychrome II; TILL photonics, Martinsried, Germany) and CCD camera (IMAGO; TILL photonics). Following acquisition of baseline metal levels (for ~100 s), any neuronal free Zn2+ was chelated by superfusing cells with the membrane-permeant Zn2+ chelator N,N,N′,N′-tetrakis (2-pyridalmethyl) ethylenediamine (TPEN; 20 μM). The magnitude of the Zn2+ fluorescence for all neuronal cell bodies in a single field (n = 5–20 neurons) was determined by subtracting the fluorescence signal after TPEN perfusion from the initial baseline signal (ΔFTPEN), as described earlier (Knoch et al., 2008; Aras et al., 2009). With this method, larger ΔFTPEN values correspond to higher amounts of pre-existing free intracellular Zn2+ in neurons (Knoch et al., 2008; Aras et al., 2009; Hershfinkel et al., 2009).
Calcineurin activity assay
Calcineurin activity was measured using a malachite green-based colorimetric cell-free assay (cat. no. 207005; Calbiochem, San Diego, CA, USA) which utilized human recombinant calcineurin, an RII phosphopeptide substrate and bovine brain calmodulin. Each test sample contained 5 μL calcineurin, 0.15 mM RII phosphopeptide and 0.5 μL calmodulin, as per the manufacturer’s recommended protocol, along with 10 μL of either Zn2+ or TPEN at various concentrations. Following 10 min incubation at 30°C, reactions were terminated with 100 μL malachite green reagent. Color was allowed to develop for 30 min at room temperature before measuring absorbance of each sample at 600 nm (Wallac 1420 Victor2 multilabel counter; Perkin-Elmer, Waltham, MA, USA). Phosphatase activity is expressed as the absorbance at 600 nm, reflecting the amount of free phosphate released and detected by malachite green. Results were obtained from three independent experiments, each performed in duplicate. A standard activity calibration curve was performed with each experiment using phosphate standards.
Statistical analysis
Throughout the text, data are expressed as mean ± SEM. Statistical analysis was performed on electrophysiology, clustered cell counts and Zn2+ imaging data using an ANOVA with post hoc comparisons, as indicated in figure legends (GraphPad InStat software, La Jolla, CA, USA). An α of P < 0.05 was considered statistically significant.
Results
Sub-lethal ischemia altered potassium channel activity and Kv2.1 phosphorylation
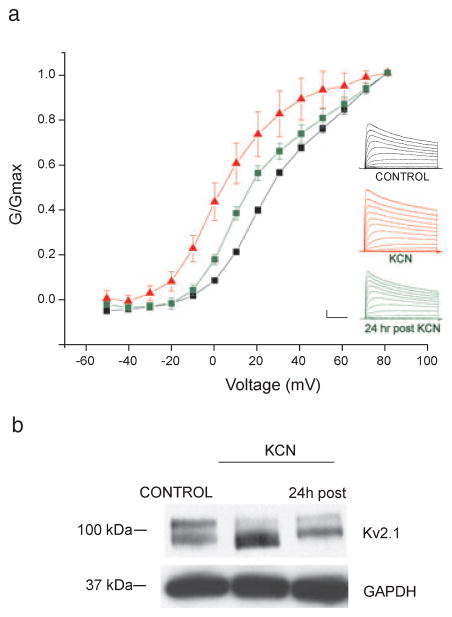
Transient hyperpolarizing shifts in the voltage-dependence of activation of neuronal IK dramatically limit neuronal excitability and are thus thought to be an adaptive cell response to ischemia (Surmeier & Foehring, 2004). Sub-lethal chemical ischemia, using KCN coupled with glucose-free conditions, reliably induces Zn2+-dependent excitotoxic tolerance in cortical neurons (Aras et al., 2009). To first determine whether sub-lethal ischemia could alter K+ channel activation in cortical neurons, whole-cell K+ currents were measured from control and KCN-preconditioned (3 mM, 90 min) neurons immediately following treatment. We found that sub-lethal ischemia indeed led to a hyperpolarizing shift in the voltage-dependency of K+ channel activation (control V1/2 26.8 ± 0.97 mV, KCN V1/2 8.88 ± 4.3 mV; F6,92 = 12.59, P < 0.001, ANOVA-Tukey; Fig. 1a), similar to those reported in hippocampal neurons (Misonou et al., 2005). These hyperpolarizing shifts were transient and partially returned to baseline conditions 24 h following the ischemic stimulus (V1/2 18.6 ± 2.1 mV; F6,92 = 12.59 P > 0.05 compared to control ANOVA-Tukey; Fig. 1a).
Fig. 1.
Chemical ischemia altered K+ channel activation properties and phosphorylation state. (a) Inset, representative IK currents in cortical neurons evoked with a series of 200-ms depolarizing steps from −50 to +80 mV recorded under whole-cell voltage clamp. A single 30-ms pre-pulse depolarization to −10 mV was given before each recording to minimize rapidly-inactivating K+ current. Whole-cell K+ currents were measured immediately following either control (black traces) or 3 mM KCN (red traces) exposure, or 24 h following 3 mM KCN (green traces). Main plot shows the conductance–voltage (G-V) relationship of peak potassium current recorded from neurons in each treatment group. Data points represent the mean ± SEM from 6–18 neurons. (b) Neurons were exposed to either vehicle or 3 mM KCN. In KCN-treated groups, cell lysates were harvested immediately or 24 h following stimulus. Lysates were separated and transferred to nitrocellulose membranes, which were probed with either an anti-Kv2.1 mouse monoclonal (1: 1000) or an anti-GAPDH mouse monoclonal (1: 1000) antibody. A blot representative of four independent experiments is shown. Calibration for insets in A, 2nA, 50 ms.
Hyperpolarizing shifts in the voltage-dependent activation of neuronal IK are associated with a dephosphorylation of the Kv2.1 channel (Misonou et al., 2004, 2005). To determine whether sub-lethal ischemia could dephosphorylate Kv2.1, we performed immunoblot experiments on cortical neurons exposed to either KCN or control conditions (Fig. 1b). The major forms of Kv2.1 in control-treated rat brain cultured neurons were found to exhibit a higher range of molecular weight bands (~95–105 kDa; Fig. 1b) than predicted from the deduced Kv2.1 primary sequence (95.3 kDa), reflecting its constitutively multi-phosphorylated state (Misonou et al., 2004). KCN led to a dramatic reduction in the molecular weight of Kv2.1, representing a dephosphorylation of the channel (Fig. 1b; Misonou et al., 2004). Changes in the phosphorylation state of Kv2.1 are reversible and are restored to control conditions hours after the stimulus (Misonou et al., 2004, 2005). Indeed, 24 h following KCN exposure, we found that Kv2.1 partially returned to its multi-phosphorylated state (Fig. 1b). Thus, reminiscent to the response following ischemia in hippocampal neurons (Misonou et al., 2005), sub-lethal ischemia led to a transient dephosphorylation of Kv2.1 in cortical neurons, accompanied by hyperpolarizing shifts in the voltage-dependence of neuronal IK.
Modulation of K+ channel activity was Zn2+- and calcineurin-dependent
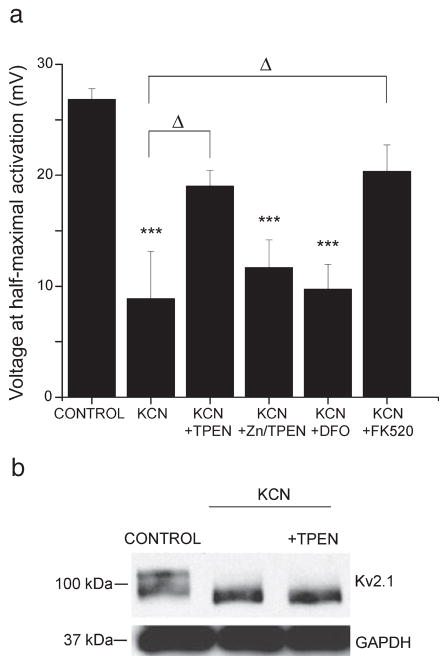
The hyperpolarizing shifts in K+ channel activation have been shown to be dependent on a rise in intracellular Ca2+ and the activation of calcineurin (Misonou et al., 2005). Recent evidence suggests that sub-lethal ischemia also triggers a transient rise in intracellular Zn2+, which is both necessary and sufficient for mediating long-term neuronal tolerance (Lee et al., 2008; Aras et al., 2009). Here, we investigated whether the chemical ischemia-induced Zn2+ rise also contributed to immediate changes in neuronal excitability. To test this, whole-cell K+ currents were measured from neurons preconditioned in the presence of the cell-permeant Zn2+-chelator TPEN (1 μM). It is noteworthy that under these conditions the neuroprotective effects of sub-lethal ischemia are severely attenuated (Aras et al., 2009). We found that TPEN almost completely blocked the hyperpolarizing shift in the voltage-dependence of activation (V1/2 19.0 ± 1.4 mV; F6,92 = 12.59, P > 0.05 compared to control, P < 0.05 compared to KCN, ANOVA-Tukey; Fig. 2a). In addition to binding Zn2+ with highest affinity (Kd 2.6 × 10−16), TPEN also binds iron (Kd 2.4 × 10−15). We thus confirmed the specificity of TPEN for Zn2+ using two different pharmacologic approaches. First, we recorded whole-cell K+ current from neurons exposed to KCN in the presence of 1 μM TPEN that was pre-bound to an equimolar concentration of Zn2+. By pre-binding TPEN to Zn2+, we can effectively abolish its ability to bind free Zn2+ elicited by KCN. We found that Zn2+–TPEN had no effect on the KCN-induced hyperpolarizing shift in voltage-dependency (V1/2 11.7 ± 2.5 mV; F6,92 = 12.59, P < 0.001 compared to control, P > 0.05 compared to KCN, ANOVA-Tukey; Fig. 2a). Moreover, we tested the effect of the iron chelator deferroxamine (100 μM; Kstability ~1031) on Kv2.1 voltage-dependency. We found that deferroxamine had no effect on the KCN-induced hyperpolarizing shift, indicating that iron probably does not mediate this process (V1/2 9.73 ± 2.3 mV; F6,92 = 12.59, P < 0.001 compared to control, P > 0.05 compared to KCN, ANOVA-Tukey; Fig. 2a). Together, these data confirm that Zn2+, rather than another heavy metal, is required for the changes in Kv2.1 voltage-dependence of activation following sub-lethal ischemia. However, when neurons were treated with exogenous Zn2+ and the Zn2+ ionophore pyrithione acutely (30 and 100 μM ZnCl2 with 1 μM pyrithione; 2–5 min), the voltage-dependence of activation of Kv2.1 channels was not shifted (not shown). However, exogenous Zn2+ exposure may not truly recreate the KCN-induced Zn2+ rise. While the increase in Zn2+ fluorescence in neurons following a 90-min KCN exposure was similar to that elicited by a short exposure to 1 μM Zn2+ with 1 μM pyrithione (Supporting information, Fig. S1), we have previously found and reported that extended exposure to 1–100 μM Zn2+ with 1 μM pyrithione leads to neuronal toxicity (Aras et al., 2009). Together, these data suggest that Zn2+ may be necessary but not sufficient for the ischemic-induced modification of neuronal IK.
Fig. 2.
Altered K+ channel activation properties were Zn2+- and calcineurin-dependent. (a) Whole-cell K+ currents were measured immediately following control, 3 mM KCN or 3 mM KCN in the presence of 1 μM TPEN, 1 μM TPEN prebound to 1 μM Zn2+, 100 μM deferroxamine or 5 μM FK520. Peak current for each neuron was converted to conductance and the conductance was plotted against each potential and fit to a Boltzmann distribution. Data points represent mean ± SEM half-maximal activation voltage (mV) for K+ currents obtained from 7 to 19 neurons (***P < 0.001 compared to control, ΔP < 0.05; one-way ANOVA-Tukey). (b) Neurons were exposed to either vehicle, 3 mM KCN or 3 mM KCN along with 1 μM TPEN for 90 min. Cell lysates were harvested immediately following exposure. Lysates were separated and transferred to nitrocellulose membranes, which were probed with either an anti-Kv2.1 mouse monoclonal (1: 1000) or an anti-GAPDH mouse monoclonal (1: 1000) antibody. A blot representative of three independent experiments is shown.
In order to confirm the role of calcineurin in this process, whole-cell K+ currents were measured from preconditioned neurons treated in the presence of the calcineurin inhibitor FK520 (5 μM). We found that, like TPEN, FK520 attenuated the preconditioning-induced hyper-polarizing shift in the voltage-dependence of K+ channel activation (V1/2, 20.3 ± 2.4 mV; F6,92 = 12.59, P > 0.05 compared to control, P < 0.05 compared to KCN, ANOVA-Tukey; Fig. 2a). Thus, both Zn2+ and calcineurin played a role in the modulation of the voltage-dependence of activation of neuronal IK following chemical ischemia. However, in contrast to the effect of either drug alone with KCN, we found that neurons exposed to KCN in the presence of 1 μM TPEN and 5 μM FK520 maintained leftward-shifted activation kinetics (V1/2, 8.26 ± 2.1 mV). This somewhat surprising result may be due to an interaction between the two chemicals, as suggested by earlier work (Bonilla et al., 2002).
Next, we examined whether Zn2+ was required for the ischemia-induced dephosphorylation of Kv2.1 channel subunits. Immunoblot experiments were performed from neurons exposed to either 3 mM KCN, 3 mM KCN with 1 μM TPEN, or control conditions (Fig. 2b). Surprisingly, despite the requirement for Zn2+ in mediating the hyperpolarizing shift in voltage-dependency, the metal chelator did not block the dephosphorylation of Kv2.1 following chemical ischemia (Fig. 2b). These data suggest that the effect of Zn2+ on changes in Kv2.1 may not require dephosphorylation. It should be noted, however, that not all of the seven potential calcineurin-sensitive phosphorylation sites on Kv2.1 (Park et al., 2006) may be dephosphorylated following chemical ischemia. Thus, a change in the phosphorylation state of one or a minority of potential calcineurin sites on Kv2.1 may not have been detected by our assay.
Chemical ischemia induced Zn 2+- and calcineurin-dependent declustering of Kv2.1
Hyperpolarizing shifts in K+ channel activation and dephosphorylation of Kv2.1 are accompanied by a dispersal of surface Kv2.1 clusters to a more uniform localization (Misonou et al., 2005). To determine whether the ischemic dispersal of Kv2.1 clusters is also dependent on a rise in free Zn2+, cortical neurons were exposed to KCN in the absence and presence of TPEN. Immediately following exposure, neurons were fixed and immunostained using antibodies specific for Kv2.1 and the neuronal marker microtubule-associated protein-2 (MAP2). Maps plotting the distribution of Kv2.1 within individual neuronal somata were used to determine whether a cell contained clusters (Fig. 3a). We found that sub-lethal ischemia led to a dispersal of somatic Kv2.1 clusters (control cells with clusters, 63%; KCN cells with clusters, 25%; F6,16 = 5.1, P < 0.01, ANOVA-Dunnett; Fig. 3a and b). Kv2.1 dispersal partially returned to a clustered localization 24 h following ischemia (cell with clusters, 53%; F6,16 = 5.1, P > 0.05 compared to control, ANOVA-Dunnett; Fig. 3b). When neurons were exposed to KCN in the presence of TPEN, declustering of Kv2.1 was blocked (cells with clusters, 65%; F6,16 = 5.1, P > 0.05 compared to control; Fig. 3a and b). As a control, we verified that both ischemia- and NMDA-mediated declustering was dependent on calcineurin by using FK520 (KCN+FK520 cells with clusters, 57%; F6,16 = 5.1 P > 0.05 compared to control, ANOVA-Dunnett; NMDA cells with clusters, 32%; F6,16 = 5.1 P < 0.05 compared to control, ANOVA-Dunnett; NMDA+FK520 cells with clusters, 61%; F6,16 = 5.1, P > 0.05 comapred to control, ANOVA-Dunnett; Fig. 3a and b; Misonou et al., 2005; Mulholland et al., 2008). Thus, in addition to calcineurin, ischemic declustering of Kv2.1 required an increase in neuronal free Zn2+.
Zinc accumulation was not calcineurin-dependent
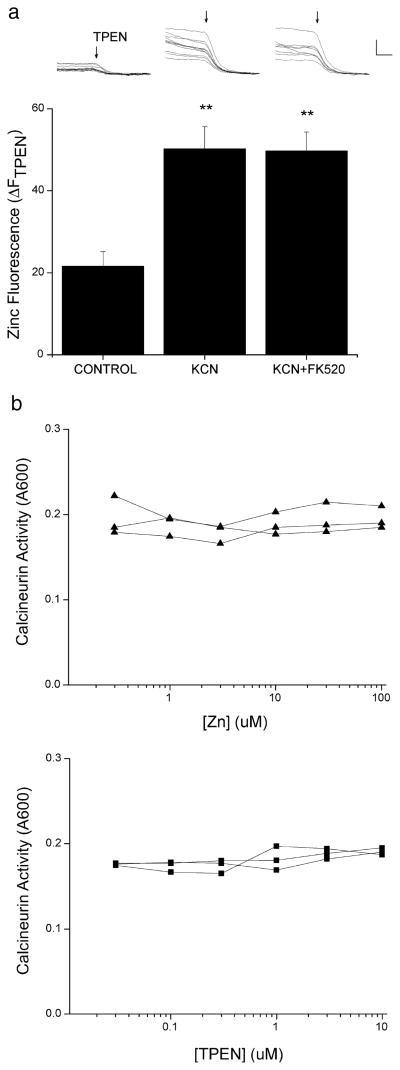
Both a rise in neuronal free Zn2+ and activation of calcineurin are required for the modulation of Kv2.1 following sub-lethal ischemia (Figs 2 and 3). To determine the interdependence of these two signaling events, we measured the preconditioning-induced Zn2+ rise in the absence and presence of the calcineurin inhibitor. Cortical neurons were loaded with the Zn2+-selective indicator FluoZin-3 (5 μM, 30 min) and imaged using live-cell wide-field microscopy. Intracellular TPEN-sensitive Zn2+ fluorescence rose significantly in neurons exposed to sub-lethal chemical ischemia (control ΔFTPEN, 21.6 ± 3.5; KCN ΔFTPEN, 50.2 ± 5.4; F2,29 = 13.186, P < 0.01, ANOVA-Dunnett; Fig. 4a) as previously reported (Aras et al., 2009). The increase in neuronal Zn2+ fluorescence following a 90-min 3 mM KCN exposure was similar to that elicited by a short exposure to 1 μM Zn2+ with 1 μM pyrithione (supporting Fig. S1). This KCN-induced increase in neuronal Zn2+ remained relatively unchanged in the presence of the calcineurin inhibitor FK520 (5 μM; KCN+FK520 ΔFTPEN, 49.7 ± 4.6; F2,29 = 13.186, P < 0.01 compared to control, ANOVA-Dunnett; Fig. 4a). These data suggest that the rise in neuronal Zn2+ occurred independently from, or was upstream of, calcineurin activity following preconditioning.
Fig. 4.
Chemical ischemia induced a calcineurin-independent Zn2+ rise. (a) Cortical neurons were exposed to control or 3 mM KCN in the absence or presence of 5 μM FK520. Representative fluorescence traces of several neurons in a single coverslip from each treatment group are shown above the corresponding bar from the bar graph. Arrow depicts the beginning of superfusion with 20 μM TPEN to chelate Zn2+ and quench fluorescence. Bar graph represents mean (+ SEM) ΔFTPEN measurements from 8 to 15 coverslips, each containing 10–25 neurons (**P < 0.01 compared to control; one-way ANOVA-Dunnett). (b) Calcineurin activity was measured using a cell-free colorimetric assay kit, in which RII phosphopeptide served as a substrate for human recombinant calcineurin. Recombinant calcineurin and bovine brain calmodulin were combined with increasing concentrations of Zn2+ (top, triangles) or TPEN (bottom, squares). Plots show the absorbance at 600 nm, indicating the amount of free phosphate released into the sample that can be detected by the malachite green reagent. Each line segment represents an independent experiment performed in duplicate. Calibration in A, 50 arbitrary fluorescence units, 50 s.
Finally, to determine whether Zn2+ can directly modulate calcineurin activity, we performed a cell-free calcineurin activity assay in which the RII phosphopeptide was used as a substrate for human recombinant calcineurin. We found that, when the reaction was performed in the presence of increasing concentrations of Zn2+, phosphatase activity remained relatively unchanged (Fig. 4b). To determine whether TPEN could modulate calcineurin activity, the reaction was performed in the presence of increasing concentrations of the metal chelator. Again, no change in calcineurin activity was observed (Fig. 4b). Thus, it is likely that Zn2+ may indirectly modulate calcineurin activity and, indeed, it may act in parallel to calcineurin in modulating Kv2.1.
Discussion
Previous reports have shown that ischemic modulation of Kv2.1 critically depends on a rise in intracellular Ca2+ and on the protein serine/threonine phosphatase calcineurin (Misonou et al., 2005). In the present study we found that Zn2+ also participates in the modulation of Kv2.1 channel activity following sub-lethal ischemic injury. We first confirmed that sub-lethal chemical ischemia in cortical neurons leads to a hyperpolarizing shift in the voltage-dependent activation of neuronal IK and an associated reduction in molecular weight of total Kv2.1 protein, reflecting a dephosphorylation of the channel. Changes in the voltage-dependency and phosphorylation state of Kv2.1 following sub-lethal ischemia in hippocampal neurons are transient, and recover shortly following stimulus cessation (Misonou et al., 2005). In cortical neurons, we found that the ischemia-induced changes only partially recovered to baseline conditions 24 h following KCN cessation. The difference in recovery between the two studies may simply be due to the differences in cellular model system (hippocampal neurons vs. mixed cortical culture) or stimulus duration (15 min vs. 90 min chemical ischemia). Importantly, however, KCN-induced sub-lethal ischemia led to characteristic changes in Kv2.1 activity and phosphorylation previously described following ischemia (Misonou et al., 2005).
An accumulation of neuronal free Zn2+, probably mediated by a combination of Zn2+ translocation from presynaptic vesicles (Koh et al., 1996) and Zn2+ liberation from intracellular stores (Aizenman et al., 2000), can trigger neurodegenerative signaling following lethal ischemia (Frederickson et al., 2005). An early rise in neuronal free Zn2+ has also been reported following sub-lethal injury, and this rise is required for neuroprotection in both in vivo and in vitro models (Lee et al., 2008; Aras et al., 2009). We previously reported that exposure to sub-lethal chemical ischemia (3 mM KCN for 90 min), an identical ischemic insult to that used in the present study, led to an increase in neuronal free Zn2+ in cortical neurons (Aras et al., 2009). This transient Zn2+ rise, measured using two different highly sensitive and Zn2+-specific assays, largely originated from intracellular sources and activated Zn2+-regulated gene expression (Aras et al., 2009). Indeed, neuronal tolerance conferred by sub-lethal chemical ischemia requires 24 h to develop and is critically dependent on new protein synthesis (McLaughlin et al., 2003). In the present study we suggest that, in addition to providing long-term tolerance via the activation of gene expression, an increase in free Zn2+ is also involved in rapid homeostatic excitability responses following ischemia via changes in Kv2.1. The delayed-rectifier Kv2.1 channel, similar to other K+ channels that are modulated in response to changes in the metabolic state (KATP channels) or [Ca]i (Ca2+-activated BK channels), can play an important role in limiting excitability following ischemia (Du et al., 2000; Misonou et al., 2005). While a role for Ca2+ and calcineurin in the modulation of Kv2.1 channel activation following ischemia has been described (Misonou et al., 2005), recent evidence has shown that a Zn2+ rise occurs immediately upon onset of ischemic injury and precedes, and may even contribute to, Ca2+ deregulation (Stork & Li, 2006; Medvedeva et al., 2009). Indeed, we found that the cell-permeable Zn2+ chelator TPEN significantly attenuated the hyperpolarizing shift in the voltage-dependent activation of IK to the same extent as calcineurin inhibition. Further, we found that the Zn2+ rise was not altered by calcineurin activity and that phosphatase activity was not affected by Zn2+. Together, these data suggest that the Zn2+ signaling may indirectly modulate calcineurin activation or perhaps occur concurrently. Thus, Zn2+ plays a critical early role in the modulation of K+ channel kinetics following ischemia.
Kv2.1 channels are localized to large clusters found over the soma and proximal dendrites of cortical neurons (Trimmer, 1991). The assembly of Kv2.1 into large surface clusters restricted to the cell body and proximal dendrites of neurons is probably mediated by a proximal restriction and clustering (PRC) domain located within the last 318 amino acids of the intracellular C terminus (Lim et al., 2000; Mohapatra & Trimmer, 2006). The cellular components involved in cluster maintenance are currently an intense line of investigation. For example, an actin cytoskeleton-based dynamic perimeter fence may regulate cluster size and localization (O’Connell et al., 2006). Remarkably, although clusters themselves exhibit little lateral mobility, single Kv2.1 channels are mobile within and even between clusters (Tamkun et al., 2007), arguing against the presence of a sustained physical association between Kv2.1 and a scaffolding protein. Tamkun and co-workers propose a model in which retention proteins interact with the Kv2.1 C terminus, perhaps in a phospho-dependent manner, to prevent individual channels from crossing the perimeter fence (O’Connell et al., 2006). While the role of Zn2+ in individual Kv2.1 channel mobility is not clear, the liberation of neuronal Zn2+ from intracellular stores has been shown to be an important upstream signaling event in the phospho-dependent surface delivery of new Kv2.1 channels in apoptosis (McLaughlin et al., 2001; Redman et al., 2007, 2009). The results presented here suggest that neuronal Zn2+ may also regulate existing Kv2.1 cluster activity and localization following ischemia. The functional significance of maintaining Kv2.1 channels in clusters also remains unclear. A recent report by Misonou and colleagues suggest that Kv2.1 clusters are strategically situated at junctions between astrocytic and neuronal membranes to achieve rapid modulation following ischemia (Misonou et al., 2008). The authors speculate that glutamate accumulation in the extracellular space following ischemia may activate ionotropic glutamate receptors, leading to elevated [Ca2+]i, calcineurin activation, and modulation of Kv2.1 (Misonou et al., 2008). Here, we provide evidence for an additional signaling event, the calcineurin-independent transient rise in neuronal Zn2+, in mediating the dispersal of channel clusters following ischemia. Thus, a rise in Zn2+ along with Ca2+ may be necessary for modulation of Kv2.1 following ischemia.
Finally, we examined the relationship between the two signaling events involved in modulation of Kv2.1 following ischemia, the rise in neuronal free Zn2+ and calcineurin activation. We first examined the temporal order of signaling events and observed that the Zn2+ rise occurred even in the presence of calcineurin inhibition. Calcineurin is a protein serine/threonine phosphatase that is regulated by Ca2+ and calmodulin binding (King & Huang, 1984). In addition, calcineurin is an Fe2+ and Zn2+ metalloenzyme containing both metals in its catalytic domain, and both are required for full phosphatase activity (Goldberg et al., 1995). It may be possible that Zn2+ could directly enhance, or that Zn2+ chelation by TPEN may prevent, full calcineurin activation following ischemia. However, we found evidence against direct modulation of calcineurin activity by Zn2+ or TPEN. Instead, Zn2+ may either be indirectly affecting calcineurin activity following ischemia, or may mediate a parallel signaling pathway leading to Kv2.1 modulation.
The data presented here strongly implicate a rise in free neuronal Zn2+ in the modulation of Kv2.1 channel activity and localization following ischemia. This study represents an intersection of emerging evidence implicating Zn2+ in triggering neuroprotective mechanisms and Kv2.1 modulation in limiting neuronal excitability following ischemia.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant NS043277 (to E.A.) and American Heart Association Pre-doctoral Fellowship 0715176U (to M.A.A.). We thank Karl Kandler for allowing us to use his imaging equipment, Edwin Levitan and Patrick Redman for helpful discussions, and Karen Hartnett for performing the calcineurin assays.
Abbreviations
- BSA
bovine serum albumin
- calcineurin
protein phosphatase 3
- IK
delayed-rectifier potassium current
- KCN
potassium cyanide
- Kv
delayed-rectifier voltage-dependent potassium channels
- TPEN
N,N,N′,N′-tetrakis (2-pyridalmethyl)ethylenediamine
References
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Aras MA, Hara H, Hartnett KA, Kandler K, Aizenman E. Protein kinase C regulation of neuronal zinc signaling mediates survival during preconditioning. J Neurochem. 2009;110:106–117. doi: 10.1111/j.1471-4159.2009.06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Won Suh S, Joon Won S, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, 2nd, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium. 2005;37:225–232. doi: 10.1016/j.ceca.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J Neurochem. 2009;109(Suppl 1):145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KE, Devinney MJ, 2nd, Zeak JA, Rintoul GL, Reynolds IJ. Glutamate mobilizes [Zn2+] through Ca2+-dependent reactive oxygen species accumulation. J Neurochem. 2008;106:2184–2193. doi: 10.1111/j.1471-4159.2008.05536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol. 2000;522(Pt 1):19–31. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Galasso SL, Dyck RH. The role of zinc in cerebral ischemia. Mol Med. 2007;13:380–387. doi: 10.2119/2007-00044.Galasso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995;376:745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E. NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+-free solutions in rat cortical neurons in vitro. J Neurochem. 1997;68:1836–1845. doi: 10.1046/j.1471-4159.1997.68051836.x. [DOI] [PubMed] [Google Scholar]
- Hershfinkel M, Kandler K, Knoch ME, Dagan-Rabin M, Aras MA, Abramovitch-Dahan C, Sekler I, Aizenman E. Intracellular zinc inhibits KCC2 transporter activity. Nat Neurosci. 2009;12:725–727. doi: 10.1038/nn.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MM, Huang CY. The calmodulin-dependent activation and deactivation of the phosphoprotein phosphatase, calcineurin, and the effect of nucleotides, pyrophosphate, and divalent metal ions. Identification of calcineurin as a Zn and Fe metalloenzyme. J Biol Chem. 1984;259:8847–8856. [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, Kamada T. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Knoch ME, Hartnett KA, Hara H, Kandler K, Aizenman E. Microglia induce neurotoxicity via intraneuronal Zn(2+) release and a K(+) current surge. Glia. 2008;56:89–96. doi: 10.1002/glia.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim YJ, Kim TY, Koh JY, Kim YH. Essential role for zinc-triggered p75NTR activation in preconditioning neuroprotection. J Neurosci. 2008;28:10919–10927. doi: 10.1523/JNEUROSCI.3421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Antonucci DE, Scannevin RH, Trimmer JS. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron. 2000;25:385–397. doi: 10.1016/s0896-6273(00)80902-2. [DOI] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci. 2002;22:10094–10105. doi: 10.1523/JNEUROSCI.22-23-10094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin B, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin B, Hartnett KA, Erhardt JA, Legos JJ, White RF, Barone FC, Aizenman E. Caspase 3 activation is essential for neuroprotection in preconditioning. Proc Natl Acad Sci USA. 2003;100:715–720. doi: 10.1073/pnas.0232966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium-and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005;25:11184–11193. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Thompson SM, Cai X. Dynamic regulation of the Kv2.1 voltage-gated potassium channel during brain ischemia through neuroglial interaction. J Neurosci. 2008;28:8529–8538. doi: 10.1523/JNEUROSCI.1417-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006;26:685–695. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- O’Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci. 2006;26:9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JA, Lee JY, Sato TA, Koh JY. Co-induction of p75NTR and p75NTR-associated death executor in neurons after zinc exposure in cortical culture or transient ischemia in the rat. J Neurosci. 2000;20:9096–9103. doi: 10.1523/JNEUROSCI.20-24-09096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci USA. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J Physiol. 2009;587:4393–4404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannevin RH, Murakoshi H, Rhodes KJ, Trimmer JS. Identification of a cytoplasmic domain important in the polarized expression and clustering of the Kv2.1 K+ channel. J Cell Biol. 1996;135:1619–1632. doi: 10.1083/jcb.135.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Jeng JM. Rethinking the excitotoxic ionic milieu: the emerging role of Zn(2+) in ischemic neuronal injury. Curr Mol Med. 2004;4:87–111. doi: 10.2174/1566524043479211. [DOI] [PubMed] [Google Scholar]
- Stork CJ, Li YV. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: a question on calcium overload. J Neurosci. 2006;26:10430–10437. doi: 10.1523/JNEUROSCI.1588-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Foehring R. A mechanism for homeostatic plasticity. Nat Neurosci. 2004;7:691–692. doi: 10.1038/nn0704-691. [DOI] [PubMed] [Google Scholar]
- Tamkun MM, O’Connell KM, Rolig AS. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J Cell Sci. 2007;120:2413–2423. doi: 10.1242/jcs.007351. [DOI] [PubMed] [Google Scholar]
- Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW, Frederickson C, Fierke C, Herman P. Fluorescent zinc indicators for neurobiology. J Neurosci Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci USA. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.