Abstract
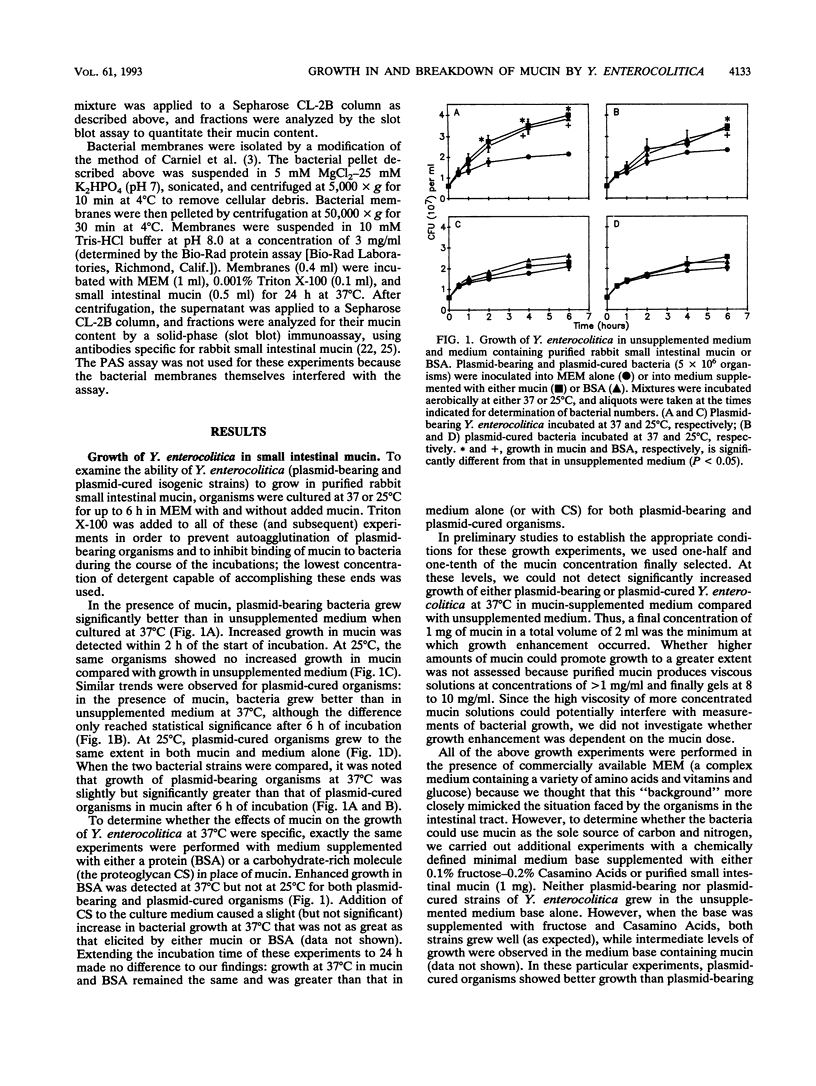
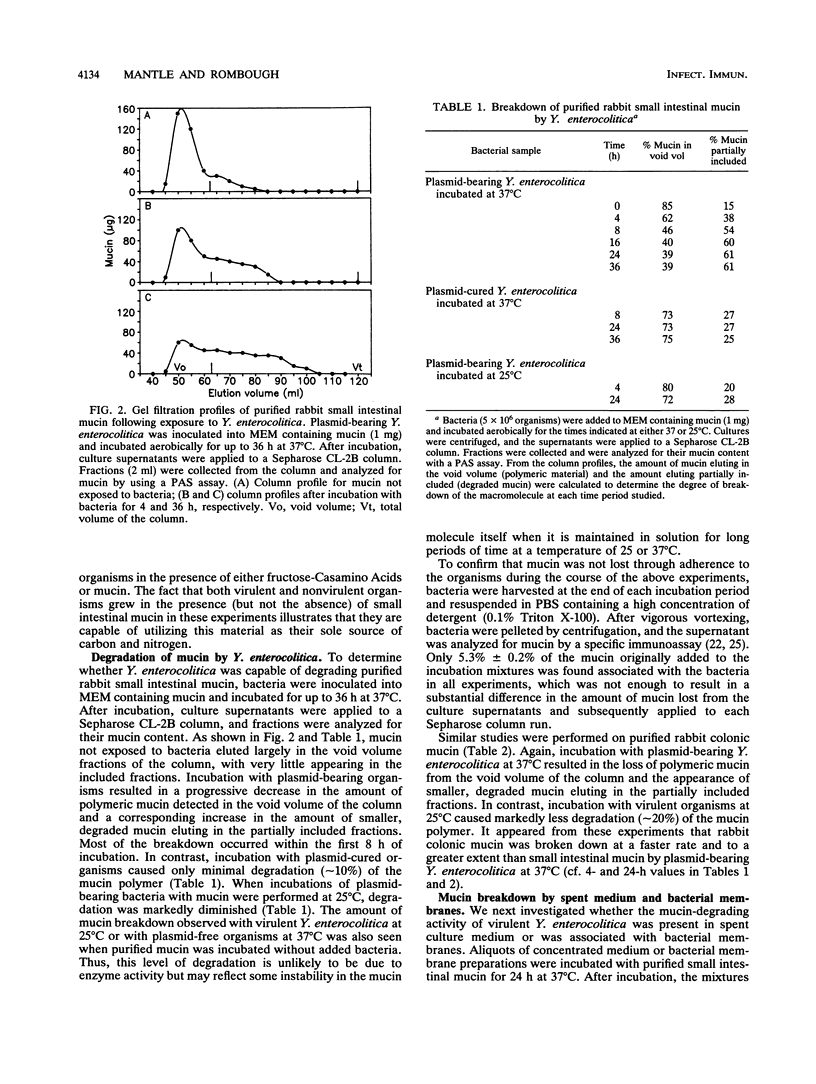
The mucus lining of the gastrointestinal tract serves as a protective barrier over the epithelial surface that must be crossed by invading bacteria seeking entry into the mucosa. The gel-forming component of mucus is mucin, a large polymeric glycoprotein. The present study examined the growth of Yersinia enterocolitica (with and without its virulence plasmid) in purified rabbit small intestinal mucin and the ability of bacteria to degrade mucin. Both virulent and nonvirulent organisms showed enhanced growth in mucin-supplemented media compared with unsupplemented media, but only at 37 degrees C and not at 25 degrees C. The effects of mucin were not specific because medium supplemented with bovine serum albumin also enhanced bacterial growth at 37 degrees C. Purified mucin was broken down into lower-molecular-weight components (assessed by monitoring its elution profile on a Sepharose CL-2B column) by plasmid-bearing Y. enterocolitica but not by plasmid-cured organisms. Culturing virulent Y. enterocolitica at 25 degrees C completely suppressed its capacity to degrade mucin, suggesting that this activity depends on plasmid expression. These results were confirmed in similar studies with purified rabbit colonic mucin. Mucin-degrading activity could be demonstrated in spent culture media from virulent Y. enterocolitica incubated at 37 degrees C but not in bacterial membrane preparations. Changes in the elution profiles of small intestinal and colonic mucins exposed to plasmid-bearing Y. enterocolitica at 37 degrees C were consistent with proteolytic depolymerization. The ability to grow well in mucin may help Y. enterocolitica to colonize the intestine, while the production of a mucin-degrading enzyme(s) by plasmid-bearing organisms may assist pathogenic strains to solubilize and penetrate the mucus gel layer.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A. E., Sellers L. A., Allen A., Cunliffe W. J., Morris E. R., Ross-Murphy S. B. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985 Jan;88(1 Pt 2):269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- Carniel E., Antoine J. C., Guiyoule A., Guiso N., Mollaret H. H. Purification, location, and immunological characterization of the iron-regulated high-molecular-weight proteins of the highly pathogenic yersiniae. Infect Immun. 1989 Feb;57(2):540–545. doi: 10.1128/iai.57.2.540-545.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Wagner S. A., Clamp J. R., Kriaris M. S., Hoskins L. C. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992 Oct;60(10):3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Aber R. C. Yersinia enterocolitica. N Engl J Med. 1989 Jul 6;321(1):16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- Crowther R. S., Roomi N. W., Fahim R. E., Forstner J. F. Vibrio cholerae metalloproteinase degrades intestinal mucin and facilitates enterotoxin-induced secretion from rat intestine. Biochim Biophys Acta. 1987 Jun 22;924(3):393–402. doi: 10.1016/0304-4165(87)90153-x. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Agustines M., McKee W. B., Boulding E. T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985 Mar;75(3):944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D. A., Pearson J. P., Allen A., Foster S. N. Mucolysis of the colonic mucus barrier by faecal proteinases: inhibition by interacting polyacrylate. Clin Sci (Lond) 1990 Mar;78(3):265–271. doi: 10.1042/cs0780265. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L. Plasmid-associated cell surface charge and hydrophobicity of Yersinia enterocolitica. Infect Immun. 1984 May;44(2):540–543. doi: 10.1128/iai.44.2.540-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Kelly J. K., Pai C. H. Invasiveness of Yersinia enterocolitica lacking the virulence plasmid: an in-vivo study. J Med Microbiol. 1987 Nov;24(3):219–226. doi: 10.1099/00222615-24-3-219. [DOI] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Basaraba L., Peacock S. C., Gall D. G. Binding of Yersinia enterocolitica to rabbit intestinal brush border membranes, mucus, and mucin. Infect Immun. 1989 Nov;57(11):3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool D. J., Marcon M. A., Forstner J. F., Forstner G. G. The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J. 1990 Apr 15;267(2):491–500. doi: 10.1042/bj2670491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Stocker B. A., Laux D. C., Cohen P. S. Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect Immun. 1988 Sep;56(9):2209–2217. doi: 10.1128/iai.56.9.2209-2217.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- Paerregaard A., Espersen F., Jensen O. M., Skurnik M. Interactions between Yersinia enterocolitica and rabbit ileal mucus: growth, adhesion, penetration, and subsequent changes in surface hydrophobicity and ability to adhere to ileal brush border membrane vesicles. Infect Immun. 1991 Jan;59(1):253–260. doi: 10.1128/iai.59.1.253-260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V., Seemayer T. A. Experimental Yersinia enterocolitica enteritis in rabbits. Infect Immun. 1980 Apr;28(1):238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz L., Jentoft N., Ho M. C., Dearborn D. G. Kinetics of proteolysis of hog gastric mucin by human neutrophil elastase and by Pseudomonas aeruginosa elastase. Infect Immun. 1988 Mar;56(3):703–704. doi: 10.1128/iai.56.3.703-704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizont R., Reed W. P. Differences in blood group B-specific mucinase activity between virulent and avirulent Shigella flexneri 2a strains. Microb Pathog. 1991 Aug;11(2):129–135. doi: 10.1016/0882-4010(91)90006-v. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Stanley R. A. In vitro utilization of mucin by Bacteroides fragilis. Appl Environ Microbiol. 1982 Feb;43(2):325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek J., Bilski J., Murty V. L., Slomiany A., Slomiany B. L. Colloidal bismuth subcitrate (De-Nol) inhibits degradation of gastric mucus by Campylobacter pylori protease. Am J Gastroenterol. 1989 May;84(5):506–510. [PubMed] [Google Scholar]
- Schulze-Koops H., Burkhardt H., Heesemann J., von der Mark K., Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992 Jun;60(6):2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mechanical characterization and properties of gastrointestinal mucus gel. Biorheology. 1987;24(6):615–623. doi: 10.3233/bir-1987-24614. [DOI] [PubMed] [Google Scholar]
- Skurnik M. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect Immun. 1985 Jan;47(1):183–190. doi: 10.1128/iai.47.1.183-190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertti R., Skurnik M., Vartio T., Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992 Jul;60(7):3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Holmes D. F., Sheehan J. K., Carlstedt I. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal Biochem. 1989 Oct;182(1):160–164. doi: 10.1016/0003-2697(89)90735-5. [DOI] [PubMed] [Google Scholar]
- Wadolkowski E. A., Laux D. C., Cohen P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect Immun. 1988 May;56(5):1030–1035. doi: 10.1128/iai.56.5.1030-1035.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



