Abstract
Secondary hyperparathyroidism (SHPT) is an early complication and a well-known factor negatively affecting cardiovascular mortality already in the late stages of chronic kidney disease (CKD). Negative effects can also be foreseen in early stages of CKD.
Aim: Set against this background, I performed detailed clinical review of the specific literature from 1975 on the various types of trials used to treat SHPT in order to assess their effect on uremic patients affected by CKD stage 3 stage 4.
Results: Out of the 1,820 papers reviewed, I identified 14 prospective controlled randomized trials involving in total 1,587 patients. Dietary approaches and the use of phosphorus chelating agents, either alone or in association, do not seem to be particularly promising for SHPT in uremic patients with CKD stage 3-4. Pending the publication of statistically wellstructured works on CKD stage 3-4, experience with calciummimetic agents in CKD stage 3-4 seems promising, even if there is a need to decrease the side effects most affecting medication compliance and as well safety calcium-mimetic agents seem to be more useful, especially in association with vitamin D derivatives. Further promising results seem to be provided by the latest generations of vitamin D derivatives such as paracalcitol which produces good SHPT control.
Keywords: secondary hyperparathyroidism, SHPT, chronic kidney disease stages 3-4, chronic renal failure stages 3-4.
Introduction
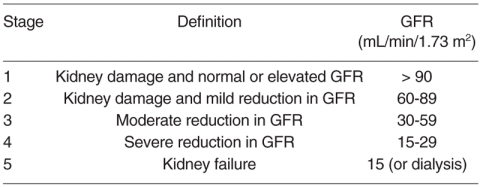
Secondary hyperparathyroidism (SHPT) is an early complication and a well known factor contributing to cardiovascular mortality already in the early stages of chronic kidney disease (CKD); the Kidney Disease Outcome Quality Initiative (K/DOQI) guidelines provide a 5-stage classification of CKD (Tab. I) (1) and a number of Authors strongly recommend the start of specific SHPT treatment already at CKD stage 3 (2-4), especially to prevent the onset of vascular calcifications, considering the potential for endothelial cells to undergo phenotypic transformation which triggers calcification within the vessels (5).
Table I.
Stages of Chronic Kidney Disease (CKD).
SHPT is a multifactor condition, in which a prominent role is played by reduced levels of 1,25(OH)2D3 due to the decreased activity of renal 1-alpha-hydroxylase induced by uremia. This enzyme performs a key function since it converts the 25-hydroxylated form into the active form; this situation is already observable in CKD stage 2 (6).
As kidney disease advances, apart from the progressive decrease in the enzyme’s activity, other factors also come into play: the negative influence of a number of uremic toxins, the direct inhibitory action of hyperphosphoremia (6, 7) and of hyperuricemia, metabolic acidosis, altered nutritional status, lower bone and parathyroid gland sensitivity to the action of active vitamin D3 even in the presence of higher serum levels of parathyroid hormone (PTHw) (4) and, last but not least, the direct stimulus on PTH synthesis by hypocalcemia and hyperphosphatemia, which play a key role in the most advanced stages of CKD (6) also due to the loss of kidney control in phosphate excretion and reduced intestinal re-absorption of ionized calcium (7); recently the role of FGF (fibroblast growth factor) 23 in the reduction of calcitriol synthesis has been indicated (8).
PTH levels may be high starting from the early stages of CKD and can continue to rise as GFR (glomerular filtration rate) worsens due to the combined action of hyperphosphatemia and/or hypocalcemia; all these factors contribute to creating a vicious circle also due to low production levels and failure to maintain the necessary serum concentrations of 1.25-OHD3 (9). According to a recent paper by Andress (10), about 40% of patients with CKD stage 3 and 80% of patients with CKD stage 4 are also affected by SHPT especially due to low levels of 1.25(OH)2D3. At cellular level, in the parathyroid glands actions unfold on several levels: on the glands’ surface, as CKD advances both the calcium receptors (Cars) and vitamin D receptors (VDRs) decrease (6); moreover, gland homeostasis and cellular function are negatively affected by some pro-inflammatory substances present in CKD (11). We can thus observe, already in patients affected by CKD stage 4, rather advanced SHPT stages, with scintigraphy and ultrasound findings of enlarged parathyroid gland.
Recent studies have revealed the influence in patients up to the most advanced stages of CKD of some genetic variants on the severity and development of an SHPT condition; these variations depend on difference in alleles of the genes of Vitamin D receptors which influence bone turnover, calcitriol and PTH levels, for instance the BsmI genotype; this negative influence may tend to decrease after some years from start of replacement therapy (12).
The K-DOQI guidelines recommend, in CKD stage 4, the maintenance of slightly increased PTH levels (70-110 ρg/mL) to guarantee good bone turnover (13).
In patients with different stages of CKD, excluding the by now rare cases of iatrogenic osteomalacia due to aluminum, only found in terminal forms of CKD, besides SHPT conditions there are also other bone situations including adynamic bone disease (up to 32%), mixed forms of osteodystrophy, and normal or almost normal hystomorphometric bone situations (14).
In recent years, treatment of SHPT in the various stages of CKD has been changing radically. While the literature has many detailed reports on SHPT treatment on CKD in stage 5, it provides fewer and fragmentary reports on SHPT treatment in the earlier stages of uremia.
For all the reasons outlined above, I focused my review on indepth perusal of the literature in order to carry out a comprehensive clinical review of the studies reported therein on SHPT treatment and assess the impact of the various treatment approaches only in stage 3 (GFR: 30-59 mL/min/1.73 m2) and stage 4 with some signs about the most important complications as calcemia and phosphatemia levels, Ca x P product, cardiovascular morbidities (GFR: 15-29 mL/min/1.73 m2) CKD (Tab. I).
Material and Methods
Study design
I used a number of search engines to identify any study, both prospective and retrospective with special regard for randomized trials on: Medline Library, Embase, Cochrane Renal Group and Registry, Cinahl, American College of Physicians database, and Google Internet search engine to identify any studies not found on the aforesaid search engines.
Setting and population and selection criteria for studies
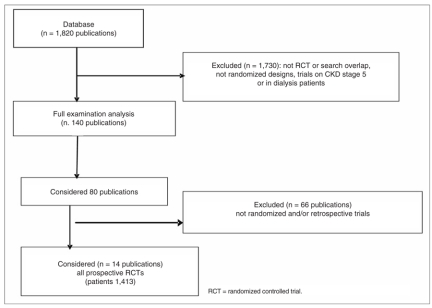
I selected 1,820 papers published from 1975 to the present day. Of these, I considered 80 studies involving 1,587 patients overall and spanning the various types of treatment mentioned below. Overall, 1,730 studies were statistically inadequate, or had been carried out on lab animals, or had been mostly performed on patients with CKD stage 5 or on patients already undergoing dialysis. I finally selected 14 studies concerning controlled randomized trials, compulsorily prospective and non retrospective involving an overall number of 1,413 patients. The search was conducted using the following keywords: chronic renal and/or kidney disease, secondary hyperparathyroidism, uremic/renal osteodystrophy management.
Results
Before starting review of all the therapeutic trials conducted from 1975 to the present day with the aim of reducing SHPT in patients suffering from CKD stage 3-4, I felt it best to start by illustrating the results of the 14 randomized trials considered. The studies and results are shown in Table II.
Table II.
Effects of various therapeutic trials on PTHi seric levels in patients affected by CKD on stages II-IV.
| Study, year (reference) | Patients total number & (treated/controls): | Trial design | CKD stage | Mean Difference treated patients (%) | Mean Difference controll patients (%) | P value | Side effects | Follow-up months | Tretment administered |
| Tougaard L et al., 1976 (75) | 24 (12/12) | DBRP | 4 | -40% | 0% | < 0.001 | Fewer hypercalcemia episodes in treated patients | 3 | 1-alpha-idroxycalciferol/ placebo |
| Christansen C et al., 1978 (62) | 16 (9/8) | RPCx | 3-4 | -1.89% | -1.2% | n.s. | Frequent hypercalcemia and < GFR in treated patients | 12 | 1,25(OH)2D3 + calcium/ placebo |
| Baker et al., 1989 (58) | 13 (7/6) | DBRP | 3-4 | -44,2% | -10,4% | < 0.001 | n.r. | 12 | 1,25(OH)2D3/placebo |
| Hamdy et al., 1995 (81) | 176 (87/89) | DBRP | 3-4 | 8% | 126% | < 0.001 | Hypercalcemic episodes in treated patients | 24 | Alpha-calcidol/placebo |
| Ritz et al., 1995 (61) | 45 (24/21) | DBRP | 3-4 | 10.9% | 49.6% | 0.05 | n.r. | 12 | Calcitriol low dose/placebo |
| Panichi et al., 1998 (78) | 31 (16/15) | RPCx | 3-4 | tw -50% sw -38.8% | qd 2% cg 9.2% | < 0.001 | n.r. | 3 | Calcitriol daily (qd), three/ weekly (tw), single weekly (sw) control group (cg) |
| Coburn et al., 2004 (85) | 55 (27/28) | DBRP | 3-4 | -46.3% | -2.3% | < 0.05 | n.r. | 8 | Doxecalciferol/placebo |
| Rix et al., 2004 (80) | 31 (16/15) | DBRP | 3-4 | -25% | 55% | < 0.001 | n.r. | 18 | Alpha-calcidol/placebo |
| Charytan et al., 2005 (99) | 54 (27/27) | DBRP | 3-4 | -39.5% | 4% | < 0.001 | Moderate adverse gastrointestinal events with calcimimetic | 3 | Calcimimetic/placebo |
| Coyne et al., 2006 (91) | 220 (107/113) | DBRP | 3-4 | -42 | 8.2% | < 0.001 | n.r. | 6 | Paricalcitol/placebo |
| Abboud H et al., 2006 (89) | 220 (107/113) | DBRP | 3-4 | tw -50% qd -33% | tw 7% qd 21% | < 0.001 | n.r. | 5 | Paricalcitol/placebo; intermittent dosing 3/per week (tw); daily dosing (qd) |
| Russo et al., 2007 (40) | 84 (29/28/27) | RP | 3-5 | lps -1.1% lpc 0.5% | lp -4.2% lp -4.2% | n.s | Higher total calcium score in lp & lpc | 24 | Low-phospate diet alone (lp), low phospate diet + sevelamer (lps); low phospate diet + CaCO3 (lpc) |
| Dogan et al., 2008 (71) | 40 (20/20) | RP | 3-4 | -24.1% | 5.5% | < 0.001 | n.r. | 1 | Cholecalciferol oral 300,000 IU monthly |
| Conchol M et al., 2008 (100) | 404 (303/101) | DBRP | 3-4 | -43.1% | -1.1% | < 0.001 | n.r. | 1 | Cinacalcet (pts ≤ 30% iPTH levels) |
| Total patient studied | 1,413 |
Legend: double-blind randomized prospective cotrolled trial (DBRP); randomized prospective controlled trial (RP), randomized prospective controlled study with cross-over (RPCx); n.r.: none reported.
Control of phosphate intake and/or gastrointestinal absorption
Role of diet or integrated dietary regimens
Besides their well-known beneficial effect in controlling uremia symptoms, personalized low-protein diets may help to prevent SHPT also in patients with initial stage CKD (15): reductions in hyperphosphatemia even PTH levels with a mix of amino acids and their ketoacid analogues and/or hypophosphoric diet compared with patients treated with calcium carbonate (16-18).
Further studies are needed to verify effects on bone turnover for CKD patients on long-term low-protein diet, since some Authors warn of a potential risk of slow-down in bone formation rate and ensuing potential risk of onset of adynamic bone disease (19) and suggest considering vitamin D supplementation especially in patients with low serum levels of vitamin D. Moreover, the patient must be constantly monitored and kept in a good nutritional state and kidney function outcome should be monitored avoiding episodes of hypercalcemia.
Role of phosphorus chelating agents
The increasing attention to and concern for controlling hyperphosphatemia has extended from ESRD (End Stage Renal Disease) patients, about 70% of whom is affected by manifest hyperphosphatemia, also to patients in much earlier CKD stages, because insufficient or bad control of phosphatemia is an important and commonly recognized factor of cardiovascular mortality (1, 20, 21). Treatable hyperphosphatemia occurs in about 8% in patient with CKD stage 4, in whom (22, 23) phosphatemia limits recommended by K-DOQI guidelines range from 2.7 to 4.6 mg/dl (24). A recent study by Vooormoolen (25) confirmed that hyperphosphatemia is an independent predictor of both rapid deterioration of renal function and higher mortality and underlined the crucial importance of controlling blood phosphorus levels and in a small subgroup of patients phosphatemia levels closely correlated with PTH levels independently of kidney function and calcemia.
The fundamental importance of controlling hyperphosphatemia has been reported, not only in order to halt the progress of SHPT but also to avoid a very damaging active process in which phosphate itself is directly involved by its entering into vessel walls; this mechanism is caused by a sodium-dependent cotransporter which induces the expression of a “master gene”: the Cbfα-1 gene, which triggers active vessel calcification causing a radical phenotypic change of the vascular cell into an almost typically osteogenic configuration (4, 5).
Role of calcium-based phosphate chelating agents: this category is mainly represented by calcium carbonate and calcium acetate. The two chelating agents act differently in the gastrointestinal tract, especially on the acquisition/absorption of elemental calcium (25.4 mmol/l calcium acetate versus 46.9 mmol/l calcium carbonate); calcium acetate has proven more useful in terms of lesser incidence of hypercalcemia episodes (26). Despite the well-known risks which have emerged over time with the use of these products, namely an increase in extra-osseal calcifications, Tsukamoto, Clark and Moriniere (27-29) reported beneficial effects on PTH, phosphorus, alkaline phosphatase, hence an overall improvement of SHPT without using vitamin D or its derivatives, but only calcium carbonate with an oral dose of 3 g/day in patients with CKD stage 2-4 obviously maintaining the patients in the range of calcium intake recommended by K/DOQI (30) guidelines. On the contrary, Aresté reported no beneficial effects on PTH or on calcitriol serum levels in patients treated with calcium carbonate alone (18). However, the use of calcium carbonate also in intermediate stage CKD in the presence of hyperphosphatemia should not be underestimated, in view of the well-known risk of vascular calcifications (31). Other far less widely used calcium-based chelating agents have been reported as exhibiting some effectiveness, such as calcium ketovalin and calcium citrate (32): the latter markedly increases the intestinal absorption of aluminum (33). Role of non-calcic phosphate chelating agents: the best known is Sevelamer hydrochloride, whose effectiveness on CKD stage 3-4 patients is difficult to gauge, due to some negative aspects such as iatrogenic acidosis and reports of gastro-enteric side effects; several trials are in progress of new preparations based on Sevelamer carbonate, with the aim of reducing these side effects (34). Undoubtedly, this product has already proven quite effective in preventing vascular calcifications in ESRD patients as shown by a number of studies (35, 36). Recently, two studies have performed systematic re-assessment on the effects of Sevelamer on mortality and calcemia levels (37, 38), but these studies, due to the limitations in use of the drug, which was reserved to patients undergoing hemodialysis, have not yielded clearly encouraging results on the outcome of SHPT in ESRD patients due to the lack of randomized clinical studies.
Thus, at the present time there are only sporadic reports on the positive effects of Sevelamer on SHPT of CKD stage 3-4 patients; in 2004 Suñer reported in 89 patients suffering from CKD stage 4 metabolic acidosis, but on the other hand, favorable effects on the control of phosphatemia and total cholesterol but no influence on PTH levels (39). In a recent work, Russo et Al. randomized 90 non-diabetic uremic patients with CKD stage 3-4 (initial GFR between 26.3 and 33.4 ml/min) into three treatment groups: hypophosphoric diet, hypophosphoric diet + calcium carbonate, hypophosphoric diet + Sevelamer: he found no influence on PTH levels or significant changes in lipidemic profile; the Author reported a reduction in phosphaturia in the two groups of patients treated with phosphorus chelating agents, a marked increase in calcemia in patients treated with calcium, but its decrease in patients treated with Sevelamer.
This study wishes to stress the importance of treating pre-dialysis CKD patients, especially in order to prevent vascular calcifications (40).
Use of lanthanum carbonate, authorized in the U.S.A. in CKD stage 5 pre-dialysis patients, is so far inconclusive as to its effectiveness in controlling SHPT through the control of hyperphosphoremia (41), but it has shown beneficial effects on vascular calcifications. In rats rendered uremic, the control of hyperphosphoremia with lanthanum has shown good control of hyperphosphoremia and SHPT (42). At this point in time, it is difficult to gauge the type and quantity of accumulation associated with the use of lanthanum carbonate (43, 44) although trials lasting more than three years have demonstrated the lack of harmful effects on liver, bone and CNS (45). To date, there are no reports on CKD stage 3-4 patients providing data on the influence of this new non-calcic phosphochelating agent on the progress of SHPT.
Role of other types of phosphochelating agents
Aluminum hydroxide has always been considered a highly effective phosphochelating agent and in particular cases it is still used, but under strict dose and time control. Its side effects: osteomalacia, fractures, myopathy, microcytic anemia and encephalopathy, also fatal, strongly discourage its use, especially for long periods (46). Mak et al. (47), already in the mid 1980s recommended use of calcium carbonate, possibly with the addition of vitamin D in place of aluminum hydroxide, due to its already well-known harmful side effects in a population of 12 pediatric patients with non-advanced CKD. Magnesium carbonate has proven useful in controlling hyperphosphoremia in CKD patients, but it is only rarely used in non-dialyzed patients; moreover, its poor gastric tolerance impairs its positive effects in controlling hyperphosphoremia (48).
Sometime ago, in advanced CKD (>4) a certain interest was expressed for ferric citrate-based phosphate chelating agents, which are however undoubtedly less effective than calcium carbonate (49).
Treatment of secondary hyperparathyroidism with vitamin D and its derivatives
Vitamin D and its derivatives
For a number of years now, calcitriol and alfacalcidol have been considered the elective drugs for treating SHPT in ESRD (50, 51); however, experience has indicated to nephrologists some side effects largely underestimated at the start of use of these drugs: hypercalcemia and/or hyperphosphoremia (the latter may affect more than 30% of patients) as a consequence of increased intestinal absorption induced by these drugs, the danger of triggering and/or maintaining a state of adynamic bone disease (ABD), possible tachyphylaxis in about 30% of treated patients (52), their pro-inflammatory and anti-elastogenic action on blood vessel walls (53). The use in CKD patients of calcitriol and its derivatives, especially those acting on vitamin D receptors (VDRs) and the use of products such as alfacalcidol and doxercalciferol have however proven effective in reducing SHPT stages (54-59). I should also recall that treatment with vitamin D and its derivatives does not only act directly on VDRs but has its rationale also in actions not strictly linked to the treatment of SHPT such as: the possible reduction in parathyroid gland mass, decrease in renin and angiotensin II levels, reduction in proteinuria, improvement of immunocompetence and improvement of muscle performances (55). The latter class of compounds is made up of third-generation products with modified ring structure (paracalcitol and maxacalcitol).
In a recent meta-analysis, Palmer (60) considered the effects of different treatments based on vitamin D and its derivatives on various aspects of SHPT: overall mean risk of not treating SHPT in patients suffering from CKD stage 3-4 was 2.86 times higher than in treated patients; Ritz (61) also showed that already in initial stages of CKD calcitriol administered in low doses prevents the onset, worsening and complications of SHPT.
However, in my review, out of 1,809 papers I extrapolated only ten controlled randomized trials on the use of vitamin D in patients with CKD stage 3-4, for a total of 871 patients (46% of the overall number of patients); only 9 trials showed significant control on PTH levels, for instance through use of paracalcitol, doxercalciferol, calcitriol, with daily administration, also oral, or through use of 1α25(OH)D3. Three trials also reported a significant decrease in alkaline phosphatase with calcitriol and doxercalciferol. However I should not underestimate the possible side effects on renal function in pre-dialysis CKD patients, especially through use of calcitriol (62) which should be administered with extreme caution and with personalized doses. Reports on blood phosphorus levels when using these products are scarcely encouraging and contradictory; moreover, the positive results reported on bone histomorphometry are still not wholly convincing and require additional confirmation.
1) 25(OH)D3 (25-hydroxycholecalciferol) and 24,25(OH)D3
A recent study by Stavroulopoulos et Al. attempted to identify significant predictive factors in patients with CKD stage 3 and 4: the study yielded interesting results: indeed, in CKD stage 3- 4, 39% of patients had 25(OH)D deficiency, 33% significant insufficiency and only 6% severe insufficiency with variable consequences for bones in the form of osteopenia or osteoporosis due to the deleterious stimulus produced by PTH (63). 25-hydroxycholecalciferol and calcifediol levels in these stages of CKD can be normal or reduced in the presence of dysnutrition (64, 65). In a state of D deficiency with a possible risk of osteomalacia 25(OH)D3 it is possible for osteomalacia to arise. Despite their lower affinity for VDRs compared with calcitriol, 25(OH)D3 or 24,25(OH)D3 have been used to treat uremic osteodystrophy for a number of years (25-50 μg/day) also in paediatric age groups (66-67), in patients with various stages of CKD until the advent of its active hydroxylated form. Administration of 25(OH)D3, later 1-α-hydroxylated at the level of the kidneys in 1,25(OH)D3 or 24- hydroxylated in 24,25(OH)D3, proved effective in improving bone lesions. However, their use requires careful monitoring, above all to avoid the risk of intoxication from accumulation especially when using 25-hydroxycholecalciferol. The availability of active calcitriol reduced interest in prior medications, although dosing of 25(OH)D3 and its administration in the event of deficiency in CKD stage 3-4 patients has proven useful for supplementing serum levels. I should also consider that 25(OH)D3 is subject to considerable seasonal circadian fluctuations and correlations with the menopause, requiring in some cases wintertime vitamin D supplementation (68-70). A recent randomized study by Dogan et Al has shown significant effect on iPTH levels in patients treated with a single oral dose of 300,000 U of cholecalciferol compared to the control group (iPTH: -24.1% versus +5.5% respectively; p < 0.001) (71).
2) 1,25-OHD3, alfacalcidol, doxecalciferol
Christiansen (62) Massry et al. (72) as early as the 1980s recommended administration of vitamin D, especially the active form, already with GFR ranging between 30 and 51 ml/min especially in the presence of normophosphatemia, to combat the devastating effects of SHPT. Greater caution was required for patients with GFR < 25 ml/min especially due to more frequent hyperphosphatemia and the risk of vascular calcifications. K/DOQI guidelines also recommend, for CKD stage 3-4, treatment with calcitriol, alfacalcidol or doxecalciferol, especially on the basis of 25(OH)vitaminD serum levels, with PTH > 70 and 100 pg/ml for CKD stage 3 and 4 respectively, with calcemia levels < 9.5 mg/dl and blood phosphorus < 4.6 mg/dl. Monthly monitoring of calcemia and blood phosphorus is recommended especially in the early stages of treatment; PTH should be checked every 3-6 months; vitamin D doses should be determined on the basis of blood levels of the above mentioned parameters (1).
Low levels of 1,25(OH)2D3 play a crucial role already in earlystage CKD in the genesis of SHPT, both directly, increasing PTH levels, and indirectly, influencing intestinal absorption of calcium and serum levels of ionized calcium. I should never forget that oral administration of 1,25(OH)2D3 contributes to increasing the absorption of phosphates at intestinal level, partly offsetting its own beneficial effects (single-pass enteral effect).
I should also consider that in patients with CKD stage 3-4 about 50% of hypercalcemia episodes have been reported, requiring discontinuation of treatment even at low doses (> 0,5 μg/day) (72). This category of drugs belongs to VDR activators, also because the sensitivity of these receptors decreases as uremia progresses. Based on the above considerations, it is now common practice to subdivide vitamin D and its derivatives into two main classes of VDR stimulants (VDRAs) (73):
a) non selective and agonist activators (VDRAs);
b) selective VDRAs.
Non selective VDRAs – In this category, I shall only consider: calcitriol (1α,25(OH)2D3), alfacalcidol (1α,25(OH)D3) and doxercalciferol (1α,25OHD2). Vitamin D2 must be activated at position C25 and C1 in the liver and kidney; vitamin D3 in position C1 in the kidney in order to perform their function; the form 1α,25(OH)2D3 must go through both positions.
Some experiences in intermediate-stage CKD have been reported, such as Nordal’s (74) on 13 patients who received calcitriol in the dose of 0.36 μg/ml/day without particularly convincing results on bone histomorphometry, even less on PTH levels. Other Authors, such as Tougaard already in 1976 (75),
Watabane in 1998 and Christiansen (62) reported positive effects for SHPT from the use of calcitriol or 1-α(OH)2D3, but also expressed concern at the possible adverse impact of these drugs on renal function, especially in advanced-stage CKD.
Coen (76) and Baker (58) considered use of 1,25(OH)2D3 in low doses (0.25 μg/day) promising in controlling SHPT in the less advanced stages of CKD. Recently, oral administering of calcitriol has proven not only effective in reducing PTH levels, but also able to reduce diastolic dysfunction in advanced CKD patients (77). Panichi (78) also demonstrated the effectiveness of calcitriol on SHPT in 16 patients with CKD stage 3-4, administering oral pulses of calcitriol, in a very well planned crossover trial, dividing patient cohorts into three groups: daily oral administration (qd), oral administration thrice a week (tw) and oral administration once a week (sw); in 15 control patients (cg) PTH levels were monitored without administering any therapy (tr): the best results were obtained with thrice-weekly administration (Figure 1).
Figure 1.
- Study flow diagram.
The most encouraging results in the more advanced stages of CKD are those reported by Birkenhäger-Frenkel (79) in whose patients long-term alfacalcidol treatment (0.44 μg/day) seems effective in preserving bone mass or increasing mineralization of the osteoid tissue. Other works warned that the price paid in medium-moderate CKD was greater incidence of hypercalcemia (80, 81). The beneficial effects of 1α25(OH)D3 and 1α(OH)D3 on SHPT in medium-severe CKD were already known in the 1970s, though accompanied by a certain increase in hypercalcemia episodes (82, 83). Improvement of SHPT has been reported in pediatric patients with pre-dialysis stages of CKD (84) through administration of alfacalcidol in oral pulses (0.5-3.0 μg/thrice a week). Against these hypercalcemia inducing effects, we should point out that unfortunately 1α25(OH) D3 has lower affinity on VDRs compared with calcitriol.
Doxercalciferol administered to 55 patients with CKD stages 3-4 (85) also produced a decrease in PTH (-30% in 74% of treated patients in 24 weeks) and of alkaline phosphatases without changes in blood phosphorus levels and with 4% episodes of hypercalcemia associated with significant mean increase in calciuria of 42%. Currently, a multicenter trial is under way, comparing action of cholecalciferol versus doxercalciferol (86) in patients with CKD stage 3-4. The final results are still to be released. Finally, in patients with vitamin D deficiency suffering from intermediate-stage CKD use of ergocalciferol has been recently re-proposed, in amounts calculated on the basis of calcidiol plasma levels 25(OH)D3: however, only in CKD stage 3 patients has a moderate decrease in PTH (-13.1%) been proven. No significant result was obtained in CKD stage 4 patients, despite the correction of calcidiol deficiency. This could be influenced by decrease in the activity 1α-hydroxylase with worsening of GFR, and by inhibitory action stemming from changes in calcemia and/or blood phosphorus (87).
Selective VDRAs – Today a clear nosological distinction (73) has been drawn between vitamins D and their derivatives acting at the level of specific receptors; selective products (VDRAs), as compared with non selective, trigger fewer hypercalcemia and hyperphosphatemia episodes thanks to lesser action on intestinal calcium absorption and at the same time lower calcium and phosphorus removal from the bone, affording undoubted advantages also for early stage CKD patients, especially as concerns the onset and possible progression of vascular calcifications and acceleration of cardiovascular risk, hence having a positive incidence on mortality.
Paracalcitol is indeed a selective VDRA which exerts a specific effect on gene expression in various types of cells, especially vascular cells. Its action on VDRs is determined by its particular molecular stoichiometric structure. As already noticed for maxacalcitol, paracalcitol has a more pronounced transcriptional effect which is also exerted on the inducers of calbindin-D messenger RNA making the intestinal absorption of calcium 10 times less powerful with paracalcitol than with calcitriol due to the lesser stimulation on intestinal calcium transporter proteins.
Experience in the use of paracalcitol in CKD stage 2-4 patients while still limited is promising, in view of its potential lesser hypercalcemia-inducing effect, hence of possible improvement of vascular calcifications: there are already some preliminary experiences in this sense (88-90). In a recent randomized trial, Coyne (91) demonstrated the beneficial action of paracalcitol in oral capsules (1 μg/day or 2 μg/thrice weekly for PTH < 500 pg/ml; 2-4 μg/thrice weekly for PTH > 500 pg/ml) on PTH levels (mean drop of 42%, with 91% of patients obtaining a reduction in PTH > 30% in 24 weeks of observation) without substantial influences on calcemia levels (2% episodes without significant increase in calciuria); the same results were obtained both with daily administration and with oral weekly pulses in CKD stage 3-4 patients. In this study I should not underestimate the markedly less hyperphosphatemia inducing effects of paracalcitol in comparison with doxercalciferol with reduction in cardiovascular mortality already with modest levels of hyperphosphatemia.
Paricalcitol effectively suppresses PTH with minimal impact on serum calcium and phosphorus unlike calcitriol for this narrow therapeutic window at higher doses because it could cause hypercalcemia and hyperphosphatemia; also doxercalciferol is associated with significant increases in serum phosphorus requiring greater use of oral phosphate binders (92).
A brief note on 22-oxacalcitriol (maxacalcitol): it acts on VDR but its binding action is 1/8th that of calcitriol (93). The transcriptional effect occurs on calbindin-D messenger RNA inductors which make the action of maxacalcitol less powerful than calcitriol on the intestinal absorption of calcium; the half-life of this compound is also shorter than that of calcitriol. To date, there is no confirmation and little interest on the use of this product in CKD stage 3-4 patients.
Similarly to maxacalcitol, in Japan falecalcitriol has been developed and is used for ESRD patients (1,25(OH)2-26,27-F6D3) a chain integrated by 6 fluoride molecules, whose metabolism is slower than that of calcitriol. There are no reports in the literature on the use of these latter two products in patients suffering from CKD stage 3-4.
Treatment of secondary hyperparathyroidism with calcimimetics
The precise role and effectiveness of calcimimetics in intermediate- advanced CKD are still to be ascertained. There are various biochemical-pharmacological bases which seem to support the usefulness of this drug in the various stages of CKD given their increasingly proven effectiveness in dialysis patients, the only ones to whom they have so far been indicated and in whom they have already achieved encouraging results in terms of reduction in SHPT, fractures and calcifications (94-98). More marked hypocalcemia-inducing action has been reported in patients with CKD stage 3-4 leading to suspension of the drug also in low doses (30 mg/day); hyperphosphatemia and paradoxical hypercalciuria have also been reported. Administration of calcimimetic also in the earlier stages of CKD (15-50 mL/min/1.73sq) according to Charytan led, in a double-blind randomized study in 27 patients, to 32% reduction in PTH compared to 6% in the control group that received a placebo (99). In this study, no relevant effects have been reported on calcemia and blood phosphorus but we should consider that 28% of patients were also administered vitamin D and 43% phosphate chelating agents or calcium supplements; compliance was good. Recently accordind to Charytan study is reported an important double-blind randomized study from Chonchol (100) with ratio treated patients/placebo controls of 3:1; the decrease in iPTH levels from baseline (cinacalcet) corresponding to a 43.1% compared with placebo 1.1% increase (p < 0.001); the patient cohort was totally 404. Also according to Schlieper (101) it produces a decrease in PTH levels in children suffering from stage 3-4 CKD but additional studies are needed on the effects of the calcimimetic on bone histomorphometry in patients with CKD stage 3-4, pending final approval of their use also in pre-dialysis patients. According to Dong’s review (102) the results of the calcimimetic in patients with stage 3-4 CKD can be held in consideration, bearing in mind that long-term administration of these substances to predialysis patients is not as yet recommended.
Surgical treatment of secondary hyperparathyroidism
One of the treatment options which has always been considered for ESRD patients is recourse to surgery (103); it is impossible to estimate the prevalence of the requirement for parathyroidectomy (PTx) on patients with CKD stage 4-5 with SHPT not controllable through medication and/or diet. The annual rate of PTx is lower than ESRD stages and it is likely to be markedly lower than 4%. The choice to perform PTx in patients with CKD stage 3-4 should be duly pondered, however, in situations of PTH > 800 ρg/ml and very bad control of calcemia and/or hyperphosphoremia, the surgical approach is a reasonable option. It is also necessary to consider that PTx may result in possible hypothyroidism and considerable and lasting hypocalcemia secondary to hypoparathyroidism which will require careful monitoring for several months without sure guarantees of even partial GFR recovery (104).
Other attempted treatments of secondary hyperparathyroidism
Between the 1970s and 1980s, teams headed by Caro and Besarab reported favorable effects of propanolol, cimetidine on PTH; (105-107): however, these studies were not followed by further investigations into the usefulness of this compound and soon abandoned.
On the use of bisphosphonates and their most recent derivatives there is scant literature on dialysis and transplanted patients. The use of these products in CKD stages 3-4 does not seem to be currently under investigation. Also data on the use of bisphosphonates in these stages of CKD is so far inconclusive, also in view of limited experience in ESRD dialysis patients; these drugs (108) act directly on osteoclasts as toxic analogues of ATP or by inhibiting the enzyme farnesyl-diphosphate- syntethase; in advanced SHPT, faster bone turnover induces greater efflux of bisphosphonate from bone: effects on calcemia, blood phosphorus and PTH levels in these stages of CKD are not predictable and there are no reports on this subject. Undoubtedly, their ability to inhibit bone mass loss is interesting especially in post-menopausal uremic women, but effects can be unpredictable or even harmful in CKD stage 3-4 patients with decreased bone turnover.
Discussion
While in the third millennium the attention of nephrologists has been targeting, especially in ESRD patients the prevention or treatment of cardiovascular calcifications (109), I cannot neglect the importance of monitoring SHPT in its various stages using the most effective types of treatment already in the early stages of CKD (85-87). Dietary approaches, also when very strict or supplemented by amino acids with their respective ketoanalogues with or without calcium carbonate do not seem able, in these stages of CKD, to positively influence the prevention, slowing down or improvement of SHPT. It is however useful to always use a dietary regimen to exercise optimum and more careful control of serum calcium and/or phosphorus levels, not only in order to prevent or control an SHPT condition. Reports on calcic or non-calcic phosphochelating agents do not seem to indicate any decisive influence on the outcome of SHPT; however, these treatments play a major complementary role in patients with CKD stage 3-4 in controlling phosphorus- calcium intake, in combination with a personalized diet (31).
Interesting results have been produced by the use of vitamin D and its derivatives, in particular as concerns the use of selective VDRAs due to their stronger action on parathyroid gland receptors accompanied by lesser influence on intestinal receptors with clear signs of less calcium and phosphorus absorption.
There is increasing evidence in the literature of their positive influence in the SHPT of terminal stage CKD which leads us to hope that the more effective treatments described above may also be effective in the intermediate phases of CKD; preliminary data show in particular that some uremic toxins are recognized as regulatory key factors in preventing vascular calcification in uremic conditions such as fetuin-A (alfa2-Heremans-Schmid glycoprotein), gamma carboxyglutamic acid protein, pyrophosphate, osteoprotegerin and bone morphogenetic proteins (110, 111). Also experience with calcimimetics in CKD stage 3-4 seems promising; however, it is necessary to address the side effects most affecting compliance and raising drug safety issues, namely hypocalcemia and tolerability, already reported in ESRD stages (112).
The significance of fibroblast growth factor 23 (FGF23) in CKD phase 3-4 remains to be defined regarding bone-kidney axis coordination and its role as diagnostic marker in these CKD phases (8). In conclusion pending statistically well structured studies on CKD stage 3-4, demonstrated promising results calcimimetics, very probably if in association with vitamin D, as well latest generations of vitamin D derivatives such as paracalcitol. For provide a good control of SHPT in CKD stages 3-4 must be backed up by further, more numerous studies.
References
- 1.NKF K/DOQI. Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification Guideline and 4.1. Active Vitamin D Therapy in Patients With Stages 3 and 4 CKD. 2002.
- 2.Eknoyan G, Levin A, Levin NW. Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;43(suppl 3):S1–S201. [PubMed] [Google Scholar]
- 3.Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol. 2005;16:507–513. doi: 10.1681/ASN.2004070610. [DOI] [PubMed] [Google Scholar]
- 4.Reichel H, Drueke T, Ritz E. Sleletal disorders. In: Davison AM, Cameron JS, Grunfeld JP, Kerr DNS, Ritz E, Winearls C, eds.Oxford Textbook of Clinical Nephrology. 2nd edition, Oxford: Oxford University Press. 1997:1954–1981. [Google Scholar]
- 5.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89(12):1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 6.Mehrothra R. Disordered mineral metabolism and vascular calcification in nondialyzed chronic kidney disease patients. J Ren Nutrition. 2006 Apr;16(2):100–118. doi: 10.1053/j.jrn.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Slatopolsky E, Brown A, Dusso A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 2001 Jan;37(1) 2:S54–7. doi: 10.1053/ajkd.2001.20740. [DOI] [PubMed] [Google Scholar]
- 8.Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial. 2007 Jul-Aug;20(4):302–8. doi: 10.1111/j.1525-139X.2007.00308.x. Review. [DOI] [PubMed] [Google Scholar]
- 9.Llach F, Yudd M. The importance of hyperphosphataemia in the severity of hyperparathyroidism and its treatment in patients with chronic renal failure. Nephrol Dial Transplant. 1998;13(3):57–61. doi: 10.1093/ndt/13.suppl_3.57. [DOI] [PubMed] [Google Scholar]
- 10.Andress DL, Coyne DW, Kalantar-Zadeh K, Molitch ME, Zangeneh F, Sprague SM. Management of secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Endocr Pract. 2008 Jan-Feb;14(1):18–27. doi: 10.4158/EP.14.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006 Jan;69(1):33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 12.Marco MP, Martínez I, Amoedo ML, Borràs M, Saracho R, Almirall J, Fibla J, Fernández E. Vitamin D receptor genotype influences parathyroid hormone and calcitriol levels in predialysis patients. Kidney Int. 1999 Oct;56(4):1349–53. doi: 10.1046/j.1523-1755.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 13.Quarles LD, Lobaugh B, Murphy G. Intact parathyroid hormone overestimates the presence and severity of parathyroid mediated osseous abnormalities in uremia. J Clin Endocrinol Metab. 1992;75:145–150. doi: 10.1210/jcem.75.1.1619003. [DOI] [PubMed] [Google Scholar]
- 14.Coen G, Mazzaferro S, Ballanti P, Sardella D, Chicca S, Manni M, Bonucci E, Taggi F. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: a cross-sectional study. Nephrol Dial Transplant. 1996;11:813–819. doi: 10.1093/oxfordjournals.ndt.a027404. [DOI] [PubMed] [Google Scholar]
- 15.Barsotti G, Cupisti A. The role of dietary phosphorus restriction in the conservative management of chronic renal disease. J Ren Nutr. 2005 Jan;15(1):189–92. doi: 10.1053/j.jrn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer K, Von Herrath D, Asmus G, Umlauf E. The beneficial effect of ketoacids on serum phosphate and pharathyroid hormone in patients with chonic uremia. Clin Nephrol. 1998 Aug;30(2):93–96. [PubMed] [Google Scholar]
- 17.Barsotti G, Cupisti A, Morelli E, Meola M, Cozza V, Barsotti M, Giovannetti S. Secondary hyperparathyroidism in severe chronic renal failure is corrected by very-low dietary phosphate intake and calcium carbonate supplementation. Nephron. 1998;79(2):137–141. doi: 10.1159/000045015. [DOI] [PubMed] [Google Scholar]
- 18.Areste N, Amor J, Cambil T, Salgueira M, Sanchez-Palencia R, Paez C, Gomez O, Palma A. Early treatment of secondary hyperparathyroidism in moderate renal insufficiency: low-phosphorus diet versus calcium carbonate. Nefrologia. 2003;23(2):64–68. [PubMed] [Google Scholar]
- 19.Lafage-Proust M-H, Combe C, Barthe N, Aparicio M. Bone Mass and Dynamic Parathyroid Function According to Bone Histology in Nondialyzed Uremic Patients after Long-Term Protein and Phosphorus Restriction. The Journal of Clinical Endocrinology & Metabolism. 1999;84((2)):512–519. doi: 10.1210/jcem.84.2.5485. [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 21.NKF K/DOQI. Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Guideline and 4.1. Active Vitamin D Therapy in Patients With Stages 3 and 4 CKD . Am J of Kidney Disease. 2002;39(2) february 2002. [PubMed] [Google Scholar]
- 22.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 23.Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group. Am J Kidney Dis. 2005 Nov;46(5):925–32. doi: 10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 24.NKF K/DOQI. Therapy in Patients With Stages 3 and 4 CKD. Evaluation of serum phosphorus levels. Guideline 3.1. 2002 [Google Scholar]
- 25.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW. PREPARE study group. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007 Nov;22(10):2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 26.Savica V, Calò AL, Monardo P, Santoro D, Bellinghieri G. Phosphate binders and management of hyperphosphatemia in endstage renal disease. Nephrol Dial Transpl. 2006;21:2065–2068. doi: 10.1093/ndt/gfl289. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto Y, Moriya R, Nagaba Y, Morishita T, Izumida I, Okubo M. Effect of administering calcium carbonate to treat secondary hyperparathyroidism in nondialyzed patients with chronic renal failure. Am J Kidney Dis. 1995 Jun;25(6):879–886. doi: 10.1016/0272-6386(95)90570-7. [DOI] [PubMed] [Google Scholar]
- 28.Clark AGB, Oner A, Ward G, Turner C, Rigden SPA, Haycock GB, Chantler C. Safety and Efficacy of Calcium Carbonate in Children with Chronic Renal Failure. Nephrol Dial Transplant. 1989;4:539–544. [PubMed] [Google Scholar]
- 29.Moriniere P, Roussel A, Tahiri Y, de Fremont JF, Maurel G, Jaudon MC, Gueris J, Fournier A. Substitution of aluminium hydroxide by high doses of calcium carbonate in patients on chronic haemodialysis: disappearance of hyperaluminaemia and equal control of hyperparathyroidism. Proc Eur Dial Transplant Assoc. 1983;19:784–7. [PubMed] [Google Scholar]
- 30.NKF K/DOQI. Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Guideline 5.5 Active Vitamin D Therapy in Patients With Stages 3 and 4. CKD. 2002 [Google Scholar]
- 31.Locatelli F, Cannata-Andía JB, Drüeke TB, Hörl WH, Fouque D, Heimburger O, Ritz E. Management of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemia. Nephrol Dial Transplant. 2002;17:723–731. doi: 10.1093/ndt/17.5.723. [DOI] [PubMed] [Google Scholar]
- 32.Saha H, Pietilä K, Mustonen J, Pasternack A, Mörsky P, Seppälä E, Reinikainen P. Acute effects of calcium carbonate and citrate on secondary hyperparathyroidism in chronic renal failure. Am J Nephrol. 1991;11(6):465–469. doi: 10.1159/000168360. [DOI] [PubMed] [Google Scholar]
- 33.Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int. 1990;38:937–941. doi: 10.1038/ki.1990.294. [DOI] [PubMed] [Google Scholar]
- 34.Duggal A, Martin H, Zhorov E, Dagher R, Plone MA, Goldberg J, Burke SH. Novel dosage forms and regimens for Sevelamerbased phosphate binders. J Ren Nutr. 2006 Jul;16(3):248–52. doi: 10.1053/j.jrn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 36.Russo D, Corrao S, Miranda I, et al. Progression of coronary artery calcification in pre-dialysis patients. Am J Nephrol. 2007;27:152–158. doi: 10.1159/000100044. [DOI] [PubMed] [Google Scholar]
- 37.Manns B, Stevens L, Miskulin D, Owen Wf Jr, Winkelmayer WC, Tonelli M. A systematic review of sevelamer in ESRD and an analysis of its potential economic impact in Canada and the United States. Kidney Int. 2004 Sep;66(3):1239–47. doi: 10.1111/j.1523-1755.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- 38.Tonelli M, Wiebe N, Culleton B, Lee H, Klarenbach S, Shrive F, Manns B. Alberta Kidney Disease Network. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrol Dial Transplant. 2007 Oct;22(10):2856–66. doi: 10.1093/ndt/gfm421. [DOI] [PubMed] [Google Scholar]
- 39.Suñer M, Guerrero A, Montes R, Rivera M, Ruiz A, Martínez-García M, Pérez-Valdivia MA, Mateos J. Tratamiento de la hiperfosfatemia con sevelamer en pacientes con insuficiencia renal crónica avanzada. Nefrologia. 2004;24:2. [PubMed] [Google Scholar]
- 40.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney International advance online publication. 2007 doi: 10.1038/sj.ki.5002518. doi 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 41.Freemont AJ, Hoyland JA, Denton J. The effects of lhantanum carbonate and calcium carbonate on bone abnormalities in patients with end-stage renal disease. Clin Nephrol. 12;54(6):428–437. [PubMed] [Google Scholar]
- 42.Ben-Dov IZ, Pappo O, Sklair-Levy M, Galitzer H, Ilan Y, Naveh-Many T, Silver J. Lanthanum carbonate decreases PTH gene expression with no hepatotoxicity in uraemic rats. Nephrol Dial Transplant. 2007 Feb;22(2):362–8. doi: 10.1093/ndt/gfl623. [DOI] [PubMed] [Google Scholar]
- 43.Behets GJ, Dams G, Vercauteren SR, et al. Does the phosphate binder lanthanum carbonate affect bone in rats with chronic renal failure? J Am Soc Nephrol. 2004;15:2219–2228. doi: 10.1097/01.ASN.0000133022.32912.95. [DOI] [PubMed] [Google Scholar]
- 44.Lacour B, Lucas A, Auchere D, et al. Chronic renal failure is associated with increased tissue deposition of lanthanum after 28 day oral administration. Kidney Int. 2005;67:1062–1069. doi: 10.1111/j.1523-1755.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 45.Webster I, Gill M. Lanthanum carbonate, a new phosphate binder, is well tolerated over a 3 year period in dialysis patients; EDTA; Lisbon, Portugal. Abstract SP268 2004. [Google Scholar]
- 46.Alfrey A. Aluminum intoxication. N Engl J Med. 1984;310:1113–1115. doi: 10.1056/NEJM198404263101709. [DOI] [PubMed] [Google Scholar]
- 47.Mak RH, Turner C, Thompson T, Powell H, Hayocock GB, Chantler C. Suppression of secondary hyperparathyroidism in children with chronic renal failure by high dose of phosphate binders: calcium carbonate versus aluminum hydroxide. Br Med J (Clin Res Ed) 09 Jul 1985;291(6496):623–627. doi: 10.1136/bmj.291.6496.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer K, von Herrath D, Erley CM. Treatment of uremic hyperphosphatemi: is there still a need for aluminum salts? Am J Nephrol. 1988;8(3):173–8. doi: 10.1159/000167578. [DOI] [PubMed] [Google Scholar]
- 49.Hergesell O, Ritz E. Phosphate binders in uraemia: pharmacodynamics, pharmacoeconomics, pharmacoethics. Nephrol Dial Transplant. 2002;17:14–17. doi: 10.1093/ndt/17.1.14. [DOI] [PubMed] [Google Scholar]
- 50.Berl T, Berns AS, Hufer WE, et al. 1,25 dihydroxycholecalciferol effects in chronic dialysis. A double-blind controlled study. Ann Intern Med. 1978;88:774–780. doi: 10.7326/0003-4819-88-6-774. [DOI] [PubMed] [Google Scholar]
- 51.Memmos DE, Eastwood JB, Talner LB, et al. Double-blind trial of oral 1,25-dihydroxy vitamin D3 versus placebo in asymptomatic hyperparathyroidism in patients receiving maintenance haemodialysis. Br Med J (Clin Res Ed) 282:1919–1924. doi: 10.1136/bmj.282.6280.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quarles LD, Yohay DA, Carroll BA, et al. Prospective trial of pulse oral versus intravenous calcitriol treatment of hyperparathyroidism in ESRD . Kidney Int. 1994;45:1710–1721. doi: 10.1038/ki.1994.223. [DOI] [PubMed] [Google Scholar]
- 53.Norman PE, Powell JT. Vitamin D shedding light on the development of disease in peripheral arteries. Arteriosler Thromb Vasc Biol. 2005;25:39–46. doi: 10.1161/01.ATV.0000148450.56697.4a. [DOI] [PubMed] [Google Scholar]
- 54.Andress D. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 55.Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitaminD3. Semin Dial. 2007 Jul-Aug;20((4)):316–24. doi: 10.1111/j.1525-139X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 56.Marco MP, Martínez I, Amoedo ML, Borràs M, Saracho R, Almirall J, Fibla J, Fernández E. Vitamin D receptor genotype influences parathyroid hormone and calcitriol levels in predialysis patients. Kidney Int. 10;56(4):1349–53. doi: 10.1046/j.1523-1755.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- 57.Healy MD, Malluche HH, Goldstein DA, et al. Effects of long-term therapy with calcitriol in patients with moderate renal failure. Arch Intern Med. 1980;140:1030–1033. [PubMed] [Google Scholar]
- 58.Baker LR, Abrams L, Roe CJ, et al. 1,25(OH)2D3 administration inmoderate renal failure: a prospective double-blind trial. Kidney Int. 1989;35:661–669. doi: 10.1038/ki.1989.36. [DOI] [PubMed] [Google Scholar]
- 59.Coburn JW, Maung HM, Elangovan L, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GFM. Meta-analysis: vitamin D compounds in chronic kidney disease. Annal Int Med. 2007 Dec;147(12):840–861. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- 61.Ritz E, Küster S, Schmidt-Gayk H, Stein G, Scholz C, Kraatz G, Heidland A. Low-dose calcitriol prevents the rise in 1,84-iPTH without affecting serum calcium and phosphate in patients with moderate renal failure (prospective placebo-controlled multicentre trial) Nephrol Dial Transplant. 1995 Dec;10(12):2228–34. doi: 10.1093/ndt/10.12.2228. [DOI] [PubMed] [Google Scholar]
- 62.Christiansen C, Rødbro P, Christensen MS, Hartnack B, Transbøl I. Deterioration of renal function during treatment of chronic renal failure with 1,25-dihydroxycholecalciferol. Lancet. 1978 Sep 30;2(8092 Pt 1):700–703. doi: 10.1016/s0140-6736(78)92702-2. [DOI] [PubMed] [Google Scholar]
- 63.Stavroulopoulos A, Porter CJ, Roe SD, Hosking DJ, Cassidy MJ. Relationship between vitamin D status, parathyroid hormone levels and bone mineral density in patients with chronic kidney disease stages 3 and 4. Nephrology (Carlton) 2008 Feb;13(1):63–67. doi: 10.1111/j.1440-1797.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 64.Eastwood JB, de Wardener HE, Gray RW, Lemann JL, Jr. Jr. Normal plasma 1,25(OH)D2 vitamin D concentrations in nutritional osteomalacia. Lancet. 1979;314:1377–1378. doi: 10.1016/s0140-6736(79)92012-9. [DOI] [PubMed] [Google Scholar]
- 65.Couttenye MM, D'Haese PC, Verschoren WJ, Behets GJ, Schrooten I, De Broe ME. Low bone turnover in patients with renal failure. Kidney Int Suppl. 1999 Dec;73:S70–6. doi: 10.1046/j.1523-1755.1999.07308.x. [DOI] [PubMed] [Google Scholar]
- 66.Witmer G, Margolis A, Fontaine O, Fritsch J, Lenoir G, Broyer M, Balsan S. Effects of 25-hydroxycholecalciferol on bone lesions of children with terminal renal failure. Kidney Int. 1976 Nov;10(5):395–408. doi: 10.1038/ki.1976.125. [DOI] [PubMed] [Google Scholar]
- 67.Teitelbaum SL, Bone JM, Stein PM, Gilden JJ, Bates M, Boisseau VC, Avioli LV. Calcifedol in chronic renal insufficiency. Skeletal response. JAMA. 1976 Jan;235(2):164–167. [PubMed] [Google Scholar]
- 68.Woitge HW, Knothe A, Witte K, Schmidt-Gayk H, Ziegler R, Lemmer B, Seibel MJ. Circaannual rhythms and interactions of vitamin D metabolites, parathyroid hormone, and biochemical markers of skeletal homeostasis: a prospective study. J Bone Miner Res. 2000 Dec;15(12):2443–50. doi: 10.1359/jbmr.2000.15.12.2443. [DOI] [PubMed] [Google Scholar]
- 69.Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open-label prospective trial. J Bone Miner Res. 2004 Aug;19(8):1221–30. doi: 10.1359/JBMR.040511. [DOI] [PubMed] [Google Scholar]
- 70.Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin D synthesis and metabolism after ultraviolet irradiation of normal and vitamin D deficient subjects. N Engl J Med. 1982;306:722–725. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 71.Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail. 2008;30(4):407–10. doi: 10.1080/08860220801964210. [DOI] [PubMed] [Google Scholar]
- 72.Massry SG, Goldstein DA, Malluche HH. Current status of the use of 1,25(OH)2D3 in the management of renal osteodystrophy. Kidney Int. 1980 Oct;18(4):409–18R. doi: 10.1038/ki.1980.154. Review. [DOI] [PubMed] [Google Scholar]
- 73.Brancaccio D, Bommer J, Coyne D. Vitamin D receptor Activator selectivity in the treatment of secondary hyperparathyroidism; understanding the difference among therapies. Drugs. 2007;67(14):1981–1998. doi: 10.2165/00003495-200767140-00002. [DOI] [PubMed] [Google Scholar]
- 74.Nordal KP, Dahl E, Halse J, Attramadal A, Flatmark A. Long-term low-dose calcitriol treatment in preanalysis chronic renalfailure: can it prevent hyperparathyroid bone disease? Nephrol Dial Transplant. 1995;10:203–206. [PubMed] [Google Scholar]
- 75.Tougaard L, Sorensen E, Brochner-Mortensen J, Christensen MS, Rodbro P, Sorensen AW. Controlled trial of 1apha-hydroxycholecalciferol in chronic renal failure. Lancet. 1976 May 15;1(7968):1044–7. doi: 10.1016/s0140-6736(76)92220-0. [DOI] [PubMed] [Google Scholar]
- 76.Coen G, Mazzaferro S, Bonucci E, Ballanti F, Massimetti C, Donato G, Landi A, Smacchi A, Della Rocca C, Cinotti GA. Treatment of secondary hyperparathyroidism of prediaysis chronic renal faillure with low doses of 1,25(OH)2D3: humoral an histomorphometric results. Min Electrolyte Metab. 1986;12(5-6):375–382. [PubMed] [Google Scholar]
- 77.Singh NP, Sahni V, Garg D, Nair M. Effect of pharmacological suppression of secondary hyperparathyroidism on cardiovascular hemodynamics in predialysis CKD patients: a preliminary observation. Hemodial Int. 2007 Oct;11(4):417–423. doi: 10.1111/j.1542-4758.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 78.Panichi V, Andreini B, De Pietro S, Migliori M, Taccola D, Giovannini L, et al. Calcitriol oral therapy for the prevention of secondary hyperparathyroidism in patients with predialitic renal failure. Clin Nephrol. 1998;49:245–250. [PubMed] [Google Scholar]
- 79.Birkenhäger-Frenkel DH, Pois H, Zeelenberg J, Eijgelsheim JJ, Kortz RA, Hǜpscher EA, Van Geelen J, Van Berkum FN, Birgenhäger JC. Effects of 1 alpha-hydroxyvitaminD3 on various stages of predialysis renal bone disease. Bone Miner. 1989 Jul;6(3):311–322. doi: 10.1016/0169-6009(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 80.Rix M, Eskilden P, Olgaard K. Effect of 18 months of treatment with alfacalcidol on bone in patients with mild to moderate chronic renal failure. Nephrol Dial Transplant. 2004;19:870–876. doi: 10.1093/ndt/gfg595. [DOI] [PubMed] [Google Scholar]
- 81.Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310:358–363. doi: 10.1136/bmj.310.6976.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bordier P, Zingraff J, Gueris J, Jungers P, Marie P, Pechet M, Rasmussen H. The effect of 1alpha(OH)D3 and 1alpha(OH)2D3 on the bone in patients with renal osteodystrophy. The. Am J Med. 1978 Jan;64(1):101–107. doi: 10.1016/0002-9343(78)90184-5. [DOI] [PubMed] [Google Scholar]
- 83.Pietrek J, Kokot F, Kuska J. Effect of 1alpha-hydroxyvitamin D3 on serum calcium and immunoreactive parathyroid hormone in patients with chronic renal insufficiency. Int Urol Nephrol. 1978;10(2):153–160. doi: 10.1007/BF02082136. [DOI] [PubMed] [Google Scholar]
- 84.Ala-Houhala M, Holmberg C, Ronnholm K, Paganus A, Laine J, Koskimies O. Alphacalcidol oral pulses normalize uremic hyperparathyroidism prior to dialysis. Pediatr Nephrol. Dec;9(6):737–741. doi: 10.1007/BF00868726. [DOI] [PubMed] [Google Scholar]
- 85.Coburn JW, Maung HM, Elangovan L, et al. Doxecalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 05;43(5):877–890. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 86.ClinicalTrials.gov registration number: NCT00285467 (clinical trials). Comparison of cholecalciferol versus doxecalciferol in the treatment of secondary hyperparathyroidism in chronic kidney disease stage three and four. Accessed at http//clinicaltrials.gov/ct/ show/ NCT00285467 on 10 October 2007.
- 87.Zisman AL, Hristova M, Tammy Ho L, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 88.Cheng S, Coyne D. Paricalcitol capsules for the control of secondary hyperparathyroidism in chronic kidney disease. Expert Opin Pharmacother. 04;7(5):617–2. doi: 10.1517/14656566.7.5.617. [DOI] [PubMed] [Google Scholar]
- 89.Abboud H, Coyne D, Smolenski O, Anger M, Lunde N, Qiu P, Hippensteel R, Pradhan RS, Palaparthy RV, Kavanaugh A, Melnick JZ, Williams LA, Batlle D). A comparison of dosing regimens of paricalcitol capsule for the treatment of secondary hyperparathyroidism in CKD stages 3 and 4. Am J Nephrol. 2006;26(1):105–14. doi: 10.1159/000092033. Epub 2006 Mar 14. [DOI] [PubMed] [Google Scholar]
- 90.Dobrez DG, Mathes A, Amdahl M, Marx SE, Melnick JZ, Sprague SM. Paricalcitol-treated patients experience improved hospitalization outcomes compared with calcitriol-treated patients in real-world clinical settings. Nephrol Dial Transplant. 2004;19:1174–1181. doi: 10.1093/ndt/gfh123. [DOI] [PubMed] [Google Scholar]
- 91.Coyne D. Paricalcitol Capsule for the Treatment of Secondary Hyperparathyroidism in Stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2(February)):263–276. doi: 10.1053/j.ajkd.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Brown AJ, Finch J, Grieff M, Ritter C, Kubodera N, Nishii Y, Slatopolsky E. The mechanism for the disparate actions of calcitriol and 22-oxacalcitriol on the intestine. Endocrinology. 1995;133:1158–1164. doi: 10.1210/endo.133.3.8396012. [DOI] [PubMed] [Google Scholar]
- 93.Brown AJ, Finch J, Grieff M, Ritter C, Kubodera N, Nishii Y, Slatopolsky E. The mechanism for the disparate actions of calcitriol and 22-oxacalcitriol on the intestine. Endocrinology. 1995;133:1158–1164. doi: 10.1210/endo.133.3.8396012. [DOI] [PubMed] [Google Scholar]
- 94.Moe SM, Chertow GM, Coburn JW, et al. Achieving NKFK/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67:760–771. doi: 10.1111/j.1523-1755.2005.67139.x. [DOI] [PubMed] [Google Scholar]
- 95.Andress DL, Coyne DW, Kalantar-Zadeh K, Molitch ME, Zangeneh F, Sprague SM. Management of Secondary Hyperparathyroidism in Stages 3 and 4. Chronic Kidney Disease. Endocrine Practice. 2008 Jan-Feb;14(1):18–27. doi: 10.4158/EP.14.1.18. [DOI] [PubMed] [Google Scholar]
- 96.Cunningham J, Urena P, Reichel H, et al. Long-term efficacy of cinacalcet in secondary hyperparathyroidism (HPT) of end-stage renal diesease (ESRD). XLII Congress of the ERA-EDTA, Abstract. 2005.
- 97.Cunningham J, Danese M, Olson K, Klassen P, Chertow G. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 10;68(4):1793–800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 98.Sensipar® (cinacalcet HCl). Full prescribing information. Thousand Oaks (CA) Amgen Inc. 2004 [Google Scholar]
- 99.Charytan C, Coburn JW, Chonchol M, Herman J, Lien HY, Liu WL, Klassen PS, McCary LC, Pichette V. Cinacalcet hydrochloride is an effective treatment for secondary hyperparathyroidism in patients with CKD not receiving dialysis. Am J Kidney Dis. 2005 Jul;46(1):58–67. doi: 10.1053/j.ajkd.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Chonchol M, Locatelli F, Abboud HE, Charytan C, de Francisco AL, Jolly S, Kaplan M, Roger SD, Sarkar S, Albizem MB, Mix TC, Kubo Y, Block GA. A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of Cinacalcet HCl in Participants With CKD Not Receiving Dialysis. Am J Kidney Dis. 2008 Dec 23; doi: 10.1053/j.ajkd.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 101.Schlieper G, Floege J. Calcimimetics in CKD-results from recent clinical studies. Pediatr Nephrol. 07 Feb 2008; doi: 10.1007/s00467-008-0900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong BJ. Cinacalcet: an oral calcimimetic agent for the management of hyperparathyroidism. Clinical Therapeutics. 2005;27(11):1725–1751. doi: 10.1016/j.clinthera.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 103.Peters BS, Moyses RM, Jorgetti V, Martini LA. Effects of parathyroidectomy on bone remodeling markers and vitamin D status in patients with chronic kidney disease-mineral and bone disorder. Int Urol Nephrol. 2007;39(4):1251–6. doi: 10.1007/s11255-007-9254-2. Epub 2007 Aug 7. [DOI] [PubMed] [Google Scholar]
- 104.Copley JB, Hui SL, Leapman S, Siemenda CW, Johnston CC. Longitudinal study of bone mass in end-stage renal disease patients: effects of parathyroidectomy for renal osteodystrophy. J Bone Miner Res. 1993 Apr;8(4):415–422. doi: 10.1002/jbmr.5650080405. [DOI] [PubMed] [Google Scholar]
- 105.Caro JF, Besarab J, Burke JF, Glennon JA. A possible role for propranolol in the treatment of renal osteodistrophy. Lancet. 1978 Aug;2(8087):451–454. doi: 10.1016/s0140-6736(78)91447-2. [DOI] [PubMed] [Google Scholar]
- 106.Besarab A, Caro JF, Ihle BU, Glennon JA, Fischer JA. Suppression of secondary hyperparathyroidism by propanolol in renal failure patients. Nephron. 1981;27(3):127–133. doi: 10.1159/000182038. [DOI] [PubMed] [Google Scholar]
- 107.Fiore CE, Lunetta M, Kanis JA. Long-term effects of histamine H2-receptor antagonists on serum parathyroid hormone in chronic renal failure. Clin Endocrinol (Oxf) 1985 Sep;23(3):277–282. doi: 10.1111/j.1365-2265.1985.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 108.Torres PU, Prié D, Beck L, Friedlander G. New Therapies for Uremic Secondary Hyperparathyroidism. Journal of Renal Nutrition. 2006;16(2):87–99. doi: 10.1053/j.jrn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 109.Levin A, Li YC. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005 Nov;68(5):1973–81. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 110.Cozzolino M, Brancaccio D. Is PTH a Risk Factor for Cardio-Vascular Calcifications in Haemodialysis? Nephrol Dial Transplant. 12 Sep 2007; doi: 10.1093/ndt/gfm687. [DOI] [PubMed] [Google Scholar]
- 111.Cozzolino M, Mazzaferro S, Pugliese F, Brancaccio D. Vascular calcification and uremia: what do we know? Am J Nephrol. 2008;28(2):339–46. doi: 10.1159/000111827. [DOI] [PubMed] [Google Scholar]
- 112.Torres PU. Cinacalcet HCl: a novel treatment for secondary hyperparathyroidism caused by chronic kidney disease. J of Renal Nutr. 2006;16(3):253–258. doi: 10.1053/j.jrn.2006.04.010. [DOI] [PubMed] [Google Scholar]