Abstract
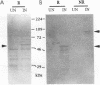
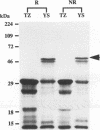
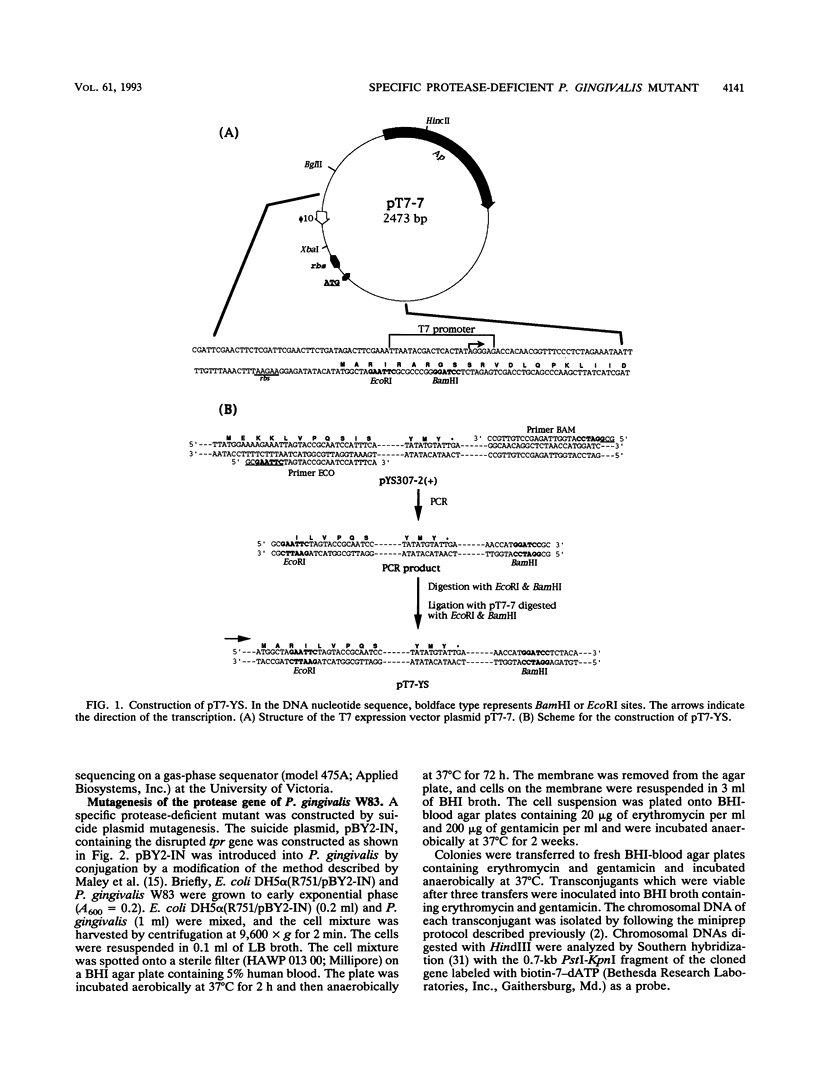
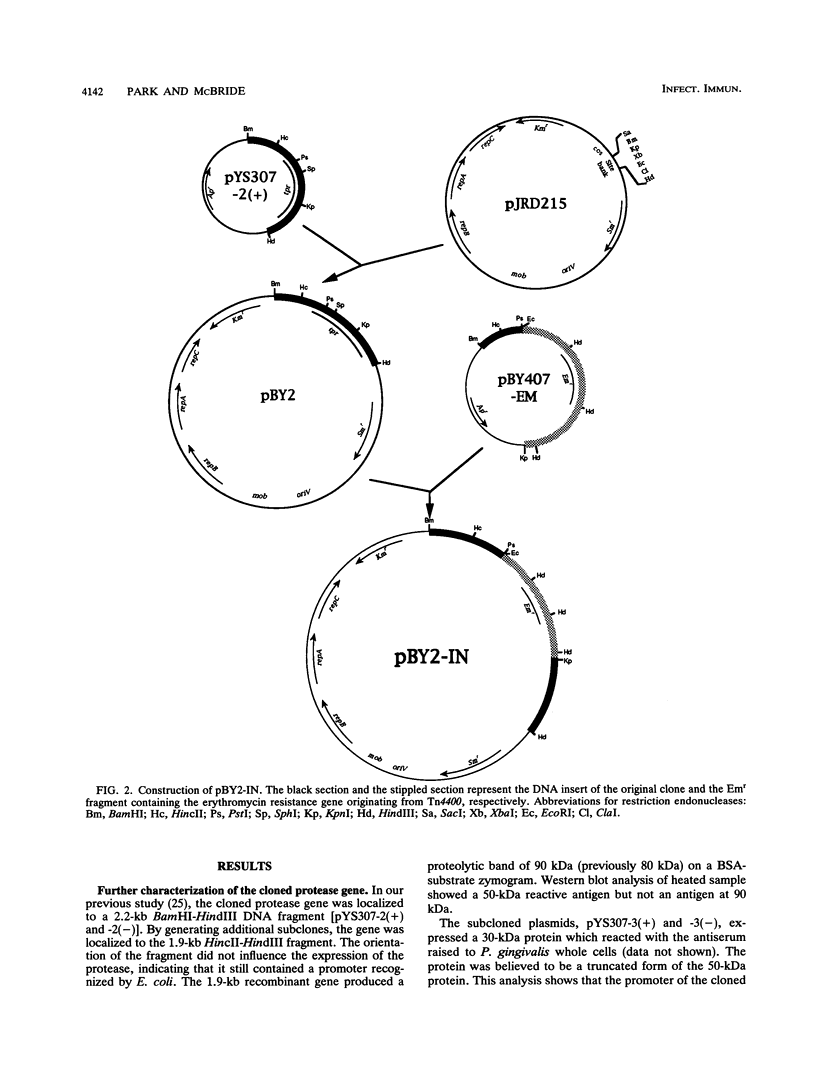
The previously described protease gene (tpr) of Porphyromonas gingivalis W83 was shown by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the recombinant protein and in vitro translation to encode a 50-kDa protein whose active form migrates with an apparent molecular mass of 90 kDa. The 50-kDa protein was expressed at high levels by using a T7 RNA polymerase/promoter system. The NH2-terminal sequence of the protein was identical to the amino acid sequence deduced from the DNA sequence of the protease gene. Affinity-purified antibody to the 90-kDa recombinant protease reacted with an 80-kDa P. gingivalis protein. A specific protease (Tpr)-deficient isogenic mutant of P. gingivalis was generated by homologous recombination between P. gingivalis chromosomal DNA and a suicide plasmid carrying the cloned gene disrupted by insertion of an erythromycin resistance gene. Gelatin substrate zymography showed that cell extracts of the mutant lacked a protease band that migrated with an apparent molecular mass of 80 kDa. Western immunoblots of the cell extracts indicated the loss of an antigen with a similar mass.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott M. A., Rigg G., Shah H., Williams D., Wallace A., Roberts I. S. Cloning and expression of a Porphyromonas (Bacteroides) gingivalis protease gene in Escherichia coli. Arch Oral Biol. 1990;35 (Suppl):97S–99S. doi: 10.1016/0003-9969(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Werb Z., McKerrow J. H. Elastin degradation. Methods Enzymol. 1987;144:288–305. doi: 10.1016/0076-6879(87)44184-0. [DOI] [PubMed] [Google Scholar]
- Bourgeau G., Lapointe H., Péloquin P., Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992 Aug;60(8):3186–3192. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Höfling J. F., Sundqvist G. K. Degradation of albumin, haemopexin, haptoglobin and transferrin, by black-pigmented Bacteroides species. J Med Microbiol. 1984 Aug;18(1):39–46. doi: 10.1099/00222615-18-1-39. [DOI] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Song M., Buivids I. A. Inhibition of Actinomyces viscosus--Porphyromonas gingivalis coadhesion by trypsin and other proteins. Oral Microbiol Immunol. 1992 Aug;7(4):198–203. doi: 10.1111/j.1399-302x.1992.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Mayrand D., McBride B. C. Further studies on the degradation of immunoglobulins by black-pigmented Bacteroides. Oral Microbiol Immunol. 1989 Mar;4(1):12–18. doi: 10.1111/j.1399-302x.1989.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Hoover C. I., Abarbarchuk E., Ng C. Y., Felton J. R. Transposition of Tn4351 in Porphyromonas gingivalis. Plasmid. 1992 May;27(3):246–250. doi: 10.1016/0147-619x(92)90028-9. [DOI] [PubMed] [Google Scholar]
- Kato T., Takahashi N., Kuramitsu H. K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992 Jun;174(12):3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J., Ellen R. P., Hoover C. I., Felton J. R. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991 Feb;70(2):82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Maley J., Shoemaker N. B., Roberts I. S. The introduction of colonic-Bacteroides shuttle plasmids into Porphyromonas gingivalis: identification of a putative P. gingivalis insertion-sequence element. FEMS Microbiol Lett. 1992 May 15;72(1):75–81. doi: 10.1016/0378-1097(92)90492-7. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Carlsson J., Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985 Nov;50(2):467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F. Characterization of Porphyromonas (bacteroides) gingivalis hemagglutinin as a protease. Biochem Biophys Res Commun. 1991 Jul 15;178(1):336–342. doi: 10.1016/0006-291x(91)91819-x. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F., Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33(1):75–80. doi: 10.1111/j.1348-0421.1989.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Odelson D. A., Rasmussen J. L., Smith C. J., Macrina F. L. Extrachromosomal systems and gene transmission in anaerobic bacteria. Plasmid. 1987 Mar;17(2):87–109. doi: 10.1016/0147-619x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Otogoto J., Kuramitsu H. K. Isolation and characterization of the Porphyromonas gingivalis prtT gene, coding for protease activity. Infect Immun. 1993 Jan;61(1):117–123. doi: 10.1128/iai.61.1.117-123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., McBride B. C. Cloning of a Porphyromonas (Bacteroides) gingivalis protease gene and characterization of its product. FEMS Microbiol Lett. 1992 May 1;71(3):273–278. doi: 10.1016/0378-1097(92)90721-y. [DOI] [PubMed] [Google Scholar]
- Pratt J. M., Boulnois G. J., Darby V., Orr E., Wahle E., Holland I. B. Identification of gene products programmed by restriction endonuclease DNA fragments using an E. coli in vitro system. Nucleic Acids Res. 1981 Sep 25;9(18):4459–4474. doi: 10.1093/nar/9.18.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progulske-Fox A., Oberste A., Drummond C., McArthur W. P. Transfer of plasmid pE5-2 from Escherichia coli to Bacteroides gingivalis and B. intermedius. Oral Microbiol Immunol. 1989 Sep;4(3):132–134. doi: 10.1111/j.1399-302x.1989.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Renart J., Sandoval I. V. Western blots. Methods Enzymol. 1984;104:455–460. doi: 10.1016/s0076-6879(84)04114-8. [DOI] [PubMed] [Google Scholar]
- Robillard N. J., Tally F. P., Malamy M. H. Tn4400, a compound transposon isolated from Bacteroides fragilis, functions in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1248–1255. doi: 10.1128/jb.164.3.1248-1255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Shoemaker N. B., Guthrie E. P. Recent advances in Bacteroides genetics. Crit Rev Microbiol. 1987;14(1):49–71. doi: 10.3109/10408418709104435. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. Complement factor D-like activity of Porphyromonas gingivalis W83. Oral Microbiol Immunol. 1991 Aug;6(4):216–220. doi: 10.1111/j.1399-302x.1991.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. The effect of periodontal proteolytic Bacteroides species on proteins of the human complement system. J Periodontal Res. 1988 May;23(3):187–192. doi: 10.1111/j.1600-0765.1988.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J., Shuttleworth C. A. The degradation of type I collagen and human plasma fibronectin by the trypsin-like enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch Oral Biol. 1988;33(5):323–329. doi: 10.1016/0003-9969(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. K., Carlsson J., Herrmann B. F., Höfling J. F., Vätäinen A. Degradation in vivo of the C3 protein of guinea-pig complement by a pathogenic strain of Bacteroides gingivalis. Scand J Dent Res. 1984 Feb;92(1):14–24. doi: 10.1111/j.1600-0722.1984.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Herrmann B., Tärnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985 Feb;19(1):85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Hänström L. Collagenolytic activity of black-pigmented Bacteroides species. J Periodontal Res. 1987 Jul;22(4):300–306. doi: 10.1111/j.1600-0765.1987.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Kato T., Kuramitsu H. K. Isolation and preliminary characterization of the Porphyromonas gingivalis prtC gene expressing collagenase activity. FEMS Microbiol Lett. 1991 Nov 15;68(2):135–138. doi: 10.1016/0378-1097(91)90116-r. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Snydman D. R., Shimell M. J., Malamy M. H. Characterization of pBFTM10, a clindamycin-erythromycin resistance transfer factor from Bacteroides fragilis. J Bacteriol. 1982 Aug;151(2):686–691. doi: 10.1128/jb.151.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., de Graaff J. The role of black-pigmented Bacteroides in human oral infections. J Clin Periodontol. 1988 Mar;15(3):145–155. doi: 10.1111/j.1600-051x.1988.tb01561.x. [DOI] [PubMed] [Google Scholar]