Abstract
Purpose
To identify the pattern of IRAK-1 protein expression in non-small cell lung carcinoma (NSCLC) and corresponding preneoplastic lesions.
Experimental Design
Archived tissue from NSCLC (adenocarcinoma and squamous cell carcinoma; n = 306) and adjacent bronchial epithelial specimens (n = 315) were analyzed for the immunohistochemical expression of IRAK-1, and the findings were correlated with patients’ clinicopathologic features. Furthermore, we investigated the correlation between IRAK-1 expression and expression of NF-κB and IL-1α in tumor specimens.
Results
NSCLC tumors demonstrated significantly higher cytoplasmic and lower nuclear IRAK-1 expression than normal epithelium. Squamous dysplasias had significantly higher cytoplasmic IRAK-1 expression that normal epithelium. In tumors, a significant positive correlation was detected between IRAK-1 expression (nuclear and cytoplasmic; P = 0.011) and IL-1α cytoplasmic expression (P < 0.0001). The correlation between the expression of the markers and patients’ clinicopathologic features varied according to tumor histologic type and sex. High IRAK-1 cytoplasmic expression correlated with worse recurrence-free survival in women with NSCLC (HR, 2.204; P = 0.033), but not in men. In adenocarcinoma, combined low level of expression of nuclear IRAK-1 and NF-κB correlated significantly with worse overall (HR, 2.485; P = 0.007) and recurrence-free (HR, 3.058; P = 0.006) survivals in stage I/II patients.
Conclusions
IRAK-1 is frequently expressed in NSCLC tissue specimens, and this expression is an early phenomenon in the sequential development of lung cancer. IRAK-1 is a novel inflammation-related marker and a potential target for lung cancer chemopreventive strategies.
Keywords: lung cancer, inflammation, preneoplastic lesions, dysplasia
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States (1). Non-small cell lung cancer (NSCLC) represents nearly 80% of lung tumors; the two most common NSCLC histologic types are squamous cell carcinoma (SCC) and adenocarcinoma (ADCA) (2). Both NSCLC histologic types are believed to arise after a sequential progression of premalignant lesions, which include bronchial squamous metaplasias and dysplasias for squamous cell carcinoma, and peripheral atypical alveolar hyperplasias (AAH) for a subset of adenocarcinomas (3). The identification of novel molecular mechanisms involved in the pathogenesis and progression of premalignant lesions may provide with new strategies for risk assessment and early detection, chemoprevention, and treatment of lung cancer.
Accumulating evidence suggests that tumor progression is governed by intrinsic genetic factors as well as by epigenetic and environmental factors. It has been hypothesized that chronic inflammation is a major consequence of certain environmental factors eventually leading to increased proliferation, survival, and migration of epithelial cells, as well as angiogenesis in the adjacent stroma, thereby promoting epithelial tumor development (4–6). In addition, inflammation and related pathways have been implicated in the pathogenesis of lung cancer, particularly in the tobacco related damage of the respiratory epithelium (3, 7, 8). However, the mechanisms involved in these processes are not well understood.
Innate immune cells within the inflammatory microenvironment secrete pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-α, interleukins (IL)-1, -6, and -8. The IL-1 and Toll-like receptors (IL-1R and TLR, respectively) have been implicated in activation of the transcriptional factor Nuclear factor-kappa B (NF-κB), a key player in the inflammatory process, that also promotes a plethora of cancer-related molecular functions including epithelial cell proliferation, survival, and angiogenesis (6). The stimulation of the TIR domain occurs upon ligand binding and activation of the receptors and results in the relay of a signaling cascade mediated by the IL-1R –associated kinase-1 (IRAK-1) (9) which leads to NF-κB activation (9–11) . While the role of NF-κB has been abundantly investigated in human tumors, including lung cancer (12–14), the potential role of IRAK-1 in lung tumorigenesis has not been studied.
Recently, by using high-throughput power-blotting western array, we identified proteins that were differentially expressed among cells constituting a human in vitro lung carcinogenesis model, including IRAK-1 protein that was overexpressed in the 1170-I lung tumorigenic cells compared to normal human bronchial epithelial (NHBE) cells (15). Although it is thought that the primary function of IRAK-1 is to participate in cytoplasmic events that lead to the nuclear translocation of NF-κB, recent data suggest that IRAK-1 can be also present in the nucleus of cells (16, 17). Thus, in the present study, we evaluated in tissue specimens cytoplasmic and nuclear IRAK-1 protein expression in normal, preneoplastic and malignant cells. We then assessed the levels of IRAK-1 mRNA expression in published microarray data cohorts as well as the immunohistochemical expression of its protein in a large set of NSCLC and preneoplastic lesion tissues. In addition, we investigated the correlation between IRAK-1 expression and clinicopathologic features of lung cancer patients as well as the immunohistochemical expression of the IRAK-1-related molecules IL-1α (an IL-1R ligand) and NF-κB. Because the suggested different pathogenesis of squamous cell carcinoma and adenocarcinoma of the lung (3), and the potential effect of sex in the role of inflammation in the pathogenesis of tumors (18), we investigated the expression of all markers by tumor histology and patients’ sex.
Material and Methods
Cell lines and culture conditions
We used five normal, premalignant, and tumorigenic cell lines derived from NHBE cells and six NSCLC cell lines. The NHBE-derived cell lines represent an in vitro sequential lung carcinogenesis model (15), composed of NHBE, immortalized BEAS-2B (19), immortalized 1799, transformed 1198, and tumorigenic 1170-I (20) cells. The NSCLC cell lines H1792, SK-MES-1, A427, H1299, H596, and H460 were obtained from Dr. Adi Gazdar (University of Texas Southwestern, Dallas, TX). Immortalized BEAS-2B cells were obtained from Dr. Curtis Harris (National Cancer Institute, Bethesda, MD). Immortalized 1799, transformed 1198, and tumorigenic 1170-I bronchial epithelial cells were obtained from Dr. Jonathan Kurie (The University of Texas M. D. Anderson Cancer Center, Houston, TX) and Dr. Andres J. P. Klein-Szanto (Fox Chase Cancer Center, Philadelphia, PA). NHBE cells, the BEAS-2B cells, and the 1799 cells were grown in keratinocyte serum-free medium (Life Technologies, Inc., Rockville, MD) supplemented with bovine pituitary extract (50 µg/ml) and epidermal growth factor (5 ng/ml). The 1198 cells and the 1170-I cells were grown in keratinocyte serum-free medium supplemented with bovine pituitary extract (50 µg/ml) and with 3% FBS.
Western blot analysis of IRAK-1 expression
Cells were washed in PBS and lysed in a cold lysis buffer containing 150 mmol/L NaCl, 0.02% NaN3, 2% Igepal CA-630, 0.5% sodium deoxycholate, 0.2% SDS, and 50 mmol/L Tris-HCl (pH 8.0) supplemented with the protease inhibitors leupeptin (1 µg/mL), aprotinin (1 µg/mL), pepstatin (0.5 µg/mL), and phenylmethylsulfonyl fluoride (100 µg/mL), and phosphatase inhibitor cocktails 1 and 2 from Sigma-Aldrich (San Diego, CA). Total protein concentration was determined by the BCA protein assay Kit (Pierce Biotechnology, Inc., Rockford, IL) and samples containing 50 µg of total cell extract were resolved on 10%–12% SDS-containing PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) by electroblotting. The membranes were then incubated in blocking buffer (5% nonfat dried milk, 10 mM Tris [PH 7.5], 100 mM NaCl, and 0.1 % Tween 20) for 1 h at room temperature after which they were incubated with either rabbit polyclonal anti-IRAK-1 antibody (H-273;Santa Cruz Biotechnology, Santa Cruz, CA) or mouse monoclonal antibody against human beta-actin (Sigma-Aldrich, San Diego, CA) overnight at 4°C. Antibody binding was detected by the standard enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ).
Processing of pellets from cell lines for immunohistochemical analysis
To validate IRAK-1 immunostaining in formalin-fixed and paraffin-embedded (FFPE) tissues, we prepared histology sections of pellets from normal, premalignant, tumorigenic, and NSCLC cell lines. Briefly, approximately 5 × 106 cells were used to prepare a cell pellet, which was fixed for 30 min using 10% buffered formalin. Fixed cell pellets were then embedded in paraffin using routine histology methodology. Five-µm-thick sections were obtained from the FFPE blocks for immunohistochemical staining with anti-IRAK-1 antibody.
Tumor and respiratory epithelium case selection and tissue microarray construction
We obtained archival, FFPE material from lung cancer specimens (lobectomies and pneumonectomies) containing tumor and adjacent normal and abnormal epithelium tissues surgically resected from patients who had no prior chemotherapy or radiotherapy from the Thoracic Tissue Bank at M. D. Anderson Cancer Center (Houston, TX). Tumor tissue specimens collected between 1997 and 2001 from 306 NSCLCs, including 194 (63%) adenocarcinomas and 112 (37%) squamous cell carcinomas, were histologically examined, classified using the 2004 World Health Organization (WHO) classification system (2), and selected for tissue microarray (TMA) construction. This study was approved by the M. D. Anderson Cancer Center Institutional Review Board
Detailed clinicopathologic information, including demographic, smoking status (never- and ever-smokers) and history (never, former, and current smokers), TNM staging, time of recurrence, and overall survival (OS) were available in most cases (Table 1). Tumors were pathologic TNM stages I–IV according to the revised International System for Staging Lung Cancer (21). Patients who had smoked at least 100 cigarettes in their lifetime were defined as smokers, and smokers who quit smoking at least 12 months before lung cancer diagnosis were defined as former smokers. After histologic examination, tumor TMAs were prepared using triplicate 1-mm-diameter cores per tumor, obtaining tissue from central, intermediate, and peripheral tumor areas.
Table 1.
Summary of the clinicopathologic features of patients with NSCLC.
| Feature | NSCLC Histologic Type | ||
|---|---|---|---|
| Squamous Cell Carcinoma (n = 112) |
Adenocarcinoma (n = 194) |
Total (n = 306) |
|
| Mean age (range), y | 68.5 (44.1–90.3) | 65.3 (33.5–88.6) | 66.5 (33.5–90.3) |
| Sex | |||
| Male | 68 | 76 | 144 |
| Female | 44 | 118 | 162 |
| Smoking status* | |||
| Never | 4 | 49 | 53 |
| Ever | 107 | 145 | 252 |
| TNM stage | |||
| I | 60 | 127 | 187 |
| II | 35 | 25 | 60 |
| III | 14 | 36 | 50 |
| IV | 3 | 6 | 9 |
Smoking status and history was not available in one patient with squamous cell carcinoma.
To assess the immunohistochemical expression of IRAK-1 in the early pathogenesis of NSCLC, we studied FFPE material from 315 specimens of bronchial and alveolar epithelium surgically resected from 87 patients with NSCLC (mean, 3.5 specimens per patient; range, 1–17 specimens). We histologically classified epithelial lesions by using the 2004 WHO classification system for preneoplastic lung lesions (2). We examined normal epithelium (n = 55), basal cell hyperplasia (n = 86), squamous metaplasia (n = 20), and squamous dysplasia (n = 77). The squamous dysplasias were arranged into two groups: low-grade (mild and moderate dysplasias; n = 16) and high-grade (severe dysplasia and carcinoma in situ; n = 51). We also examined 77 AAH, which are generally considered to be precursor lesions of lung adenocarinomas (2). Epithelial foci TMAs were constructed with single 2-mm-diameter cores.
Immunohistochemical staining and evaluation
The following primary antibodies were used for immunohistochemical staining: rabbit polyclonal anti-human C-terminus IRAK-1 antibody (H-273; Santa Cruz Biotechnology; dilution 1:100); rabbit polyclonal anti-human (amino acids 113–271) IL-1α antibody (H-159; Santa Cruz Biotechnology; dilution 1:50), and mouse anti-human monoclonal NF-κB p65 antibody (BD Biosciences PharMingen, San Diego, CA; dilution 1:250). FFPE tissue histology sections (5-µm-thick) were deparaffinized, hydrated, heated in a steamer for 20 min with 10 mM sodium citrate (pH 6.0) for antigen retrieval, and washed in Tris buffer Peroxide blocking was performed with 3% H2O2 in methanol at room temperature for 15 min, followed by 10% FBS in TBS-t for 30 min at room temperature. Primary antibody incubation was done for 2 h at room temperature. Secondary antibody incubation with Envision Plus Dual Link-labeled polymer (DAKO, Carpinteria, CA) was performed for 30 min, followed by application of diaminobenzidine chromogen for 5 min. The slides were then counterstained with hematoxylin and topped with a cover slip. FFPE pellets from lung cancer cell lines that had IRAK-1, NF-κB, and IL-1α expression as determined by Western blotting were used as positive controls. For a negative control, we used FFPE pellets from lung cancer cell lines that had been immunostained for IRAK-1 replacing the primary antibody with PBS.
Tumor and epithelial lesions were evaluated using the same methodology. Briefly, for each marker, an experienced lung cancer pathologist (I.I.W.) examined both the intensity and extent of immunostaining by light microscopy using a ×20 magnification objective. IRAK-1 immunoreactivity was detected in the cytoplasm and nucleus of epithelial cells, and IL-1α immunoreactivity was detected only in the cytoplasm. Although NF-κB immunoreactivity was detected in the cytoplasm and nucleus, only distinct nuclear immunostaining for NF-κB p65, which is considered activated NF-κB (14, 22), was quantified. Cytoplasmic expression was quantified using a four-value intensity score (0, none; 1+, weak; 2+, moderate; and 3+, strong) and the percentage (0–100%) of the extent of reactivity. A final cytoplasmic expression score was obtained by multiplying the intensity and reactivity extension values (range, 0–300). Nuclear expression of IRAK-1 and NF-κB was quantified using a score (range, 0–100) according to the percentage of positive nuclei present in 200 tumor and epithelial cells. Cytoplasmic and nuclear expression scores were used to determine the various levels of each biomarker’s expression
Assessment of IRAK-1 expression in microarray datasets
The cancer microarray database and integrated data-mining platform Oncomine (23)was utilized to analyze the expression of IRAK-1 in microarray databases of NSCLC available on-line. The statistical significances in IRAK-1 expression differences were provided by Oncomine and confirmed by a two-tailed t-test with random variance.
Statistical analysis
The biomarkers were dichotomized into low and high level groups as follows: cytoplasmic IRAK-1: low (score ≤ 200), high (score > 200); nuclear IRAK-1: low (score ≤ 35), high (score > 35); cytoplasmic IL-1α: low (score < 165), high (score ≥ 165); and nuclear NF-κB: low (score < 20.75), high (score ≥ 20.75). The Classification and Regression Tree (CART) method was used to identify the appropriate cutoff points of biomarker scores with respect to the overall survival. If no cutoff point was identified using the CART method, then the median was used as the cutoff point.
Associations between biomarker expression scores and patients’ clinicopathologic data were assessed using the Wilcoxon’s rank sum test or Kruskal-Wallis test, as appropriate, for continuous variables and the χ2 test for categorical variables. Survival curves were generated using the Kaplan-Meier method. The log-rank test was used to evaluate the difference in survival among biomarker expression levels. Cox proportional hazard models were fitted to assess the effects of covariates on overall survival and recurrence free survival. Repeated measures analysis of variance models were fitted using histology as the covariate to test the difference in biomarker expression among epithelial lesions. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant. Statistical analysis was performed using SAS (v 9.1, Cary, NC) and S-plus (v 8.0, Seattle, WA).
Results
IRAK-1 protein expression in NHBE-derived and NSCLC cell lines, and validation of immunohistochemical method
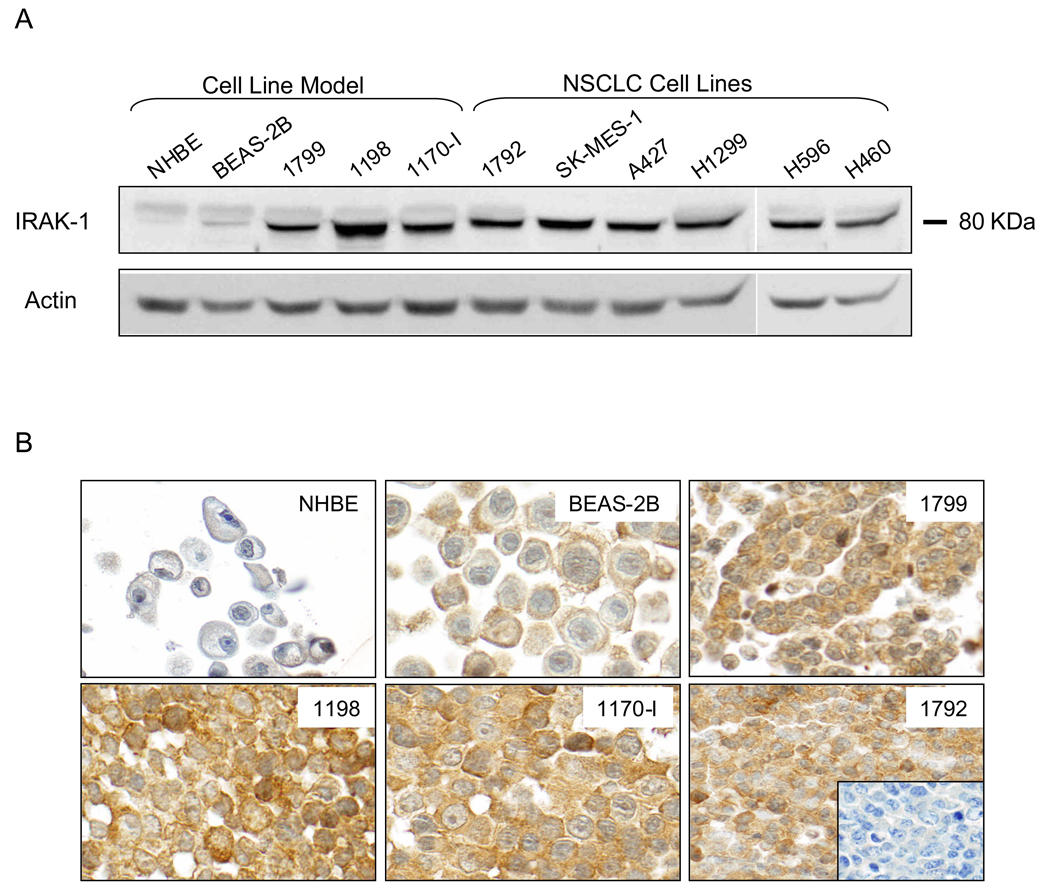
By Western-blot we found that IRAK-1 protein increased in bronchial immortalized 1799, transformed 1198 and tumorigenic 1170-I cells compared to the low levels detected in normal NHBE and immortalized BEAS-2B cells (Fig. 1A). In addition, higher levels of IRAK-1 protein were detected in the six NSCLC cell lines (A427, H460, H1299, H569, H1792, and SK-MES-1) when compared to the NHBE cells (Fig. 1A).
Fig. 1.
A, Western blot analysis of IRAK-1 expression in the in vitro cell line model, which includes normal human bronchial epithelium (NHBE), premalignant (BEAS-2B, 1799, and 1198) and malignant (1170-I) cells, and in six NSCLC cell lines (H1792, SK-MES-1, A427, H1299, H596, and H460). B, IRAK-1 immunostaining using formalin-fixed and paraffin-embedded cell line pellets of the in vitro cell line model and the 1792 cell line. Original magnification, ×400.
We validated the IRAK-1 immunohistochemistry methodology applied to FFPE material using histology sections from cell lines analyzed for IRAK-1 expression by immunoblotting. IRAK-1 immunohistochemical expression in the cytoplasm increased similarly with its protein levels highest in the malignant and transformed lung cells relative to the immortalized and normal cells (Fig. 1B).
IRAK-1 mRNA expression in normal and tumor tissue microarray sets
Our findings on the increase of IRAK-1 protein levels in the cells constituting the in vitro lung tumorigenesis model prompted us to analyze mRNA IRAK-1 expression levels in published microarray datasets of clinical NSCLC specimens. In accordance with our in vitro findings, IRAK-1 expression was significantly higher (all P < 0.001) in lung adenocarcinomas (normalized and centered median expression 1.15 to 1.36) compared to adjacent normal lung tissue (normalized and centered median expression 0.57 to 1.05) in four published cohorts analyzed (Supplementary Figure 1) (24–27). Additionally, IRAK-1 expression levels were significantly higher (P < 0.001) in squamous cell carcinomas (normalized and centered median expression 0.49) (SCC) relative to adjacent normal lung (normalized and centered median expression −0.01) from microarray data available in the report by Bhattacharjee et al (24).
Immunohistochemical expression of IRAK-1 in normal and malignant tissues
IRAK-1 expression was detected in the cytoplasm and nucleus of malignant and normal epithelial cells. Tumors with adenocarcinoma and squamous cell carcinoma histology showed significantly (P < 0.0001) higher cytoplasmic expression of IRAK-1 compared with normal epithelium. In contrast, the nuclear expression of IRAK-1 was significantly lower in tumors than normal epithelia for both tumor histologies (adenocarcinoma, P = 0.002, and squamous cell carcinoma, P = 0.0005). Both nuclear and cytoplasmic IRAK-1 expression in tumors was homogeneous by comparing the three tumor cores examined in the TMAs (data not shown).
Immunohistochemical expression of IRAK-1 in the sequential pathogenesis of lung cancer
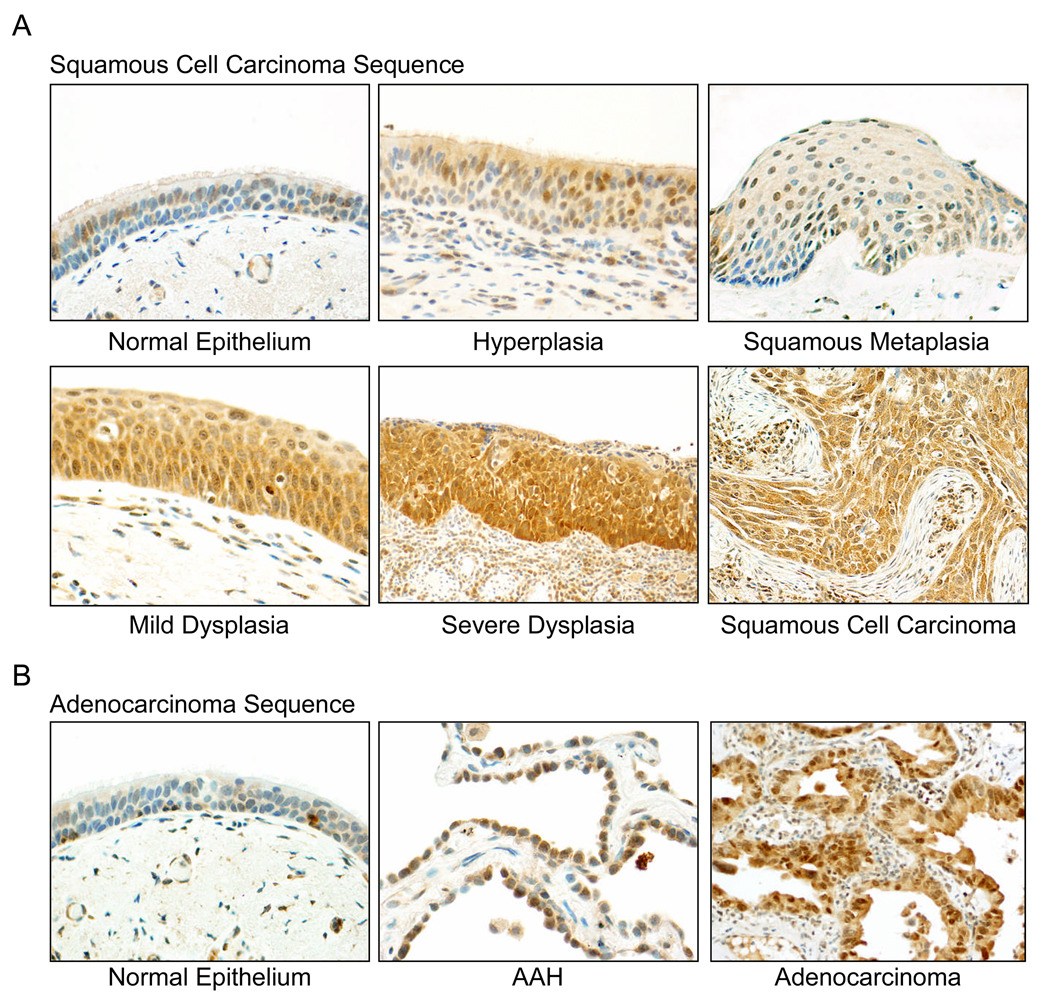
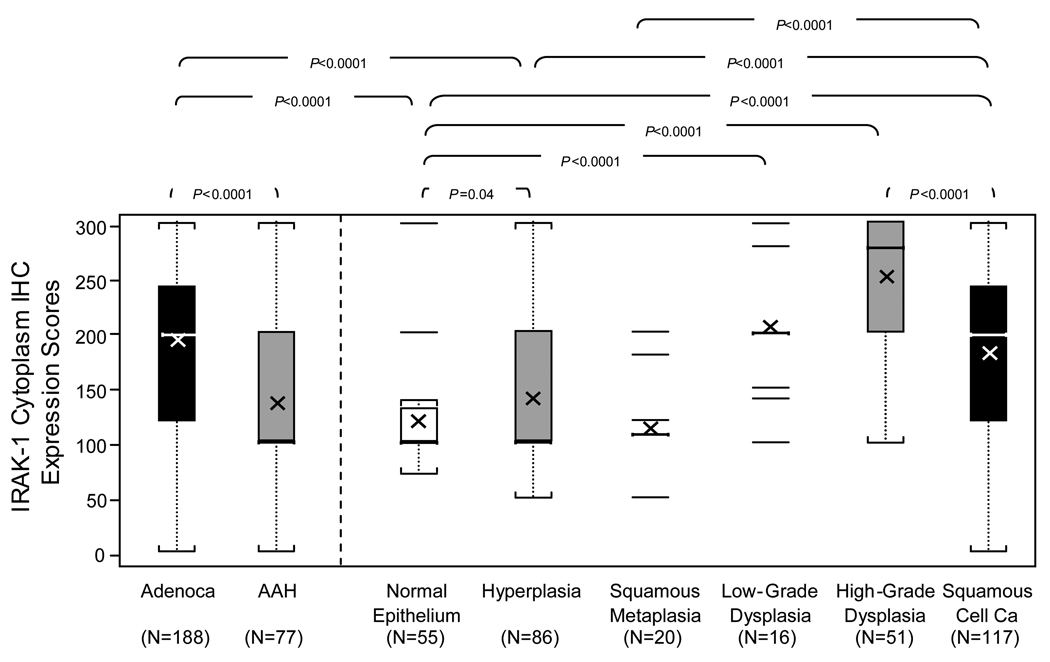
Similar IRAK-1 cytoplasmic immunostaining was detected in histologically normal and abnormal (hyperplasia and squamous metaplasia) bronchial epithelium specimens from lung tumor and adjacent epithelium tissues (Fig. 2 and 3). Of interest, both low-grade and high-grade squamous dysplasias had a significantly higher (P < 0.0001) IRAK-1 cytoplasmic expression than normal bronchial epithelium (Fig. 3). Somewhat surprising, invasive squamous cell carcinoma had a significantly lower (P < 0.0001) IRAK-1 cytoplasmic expression score than high-grade squamous dysplasias, and a significantly higher (P < 0.0001) IRAK-1 cytoplasmic expression score than normal and mildly abnormal bronchial epithelium. AAH, a putative precursor for a subset of lung adenocarcinomas, had a similar IRAK-1 cytoplasmic expression score to that of histologically normal bronchial epithelium and significantly lower (P < 0.0001) than that of lung adenocarcinomas (Fig. 3). In both tumor types, overlap on the levels of cytoplasmic IRAK-1 expression was detected between tumor specimens and corresponding preneoplastic lesions (high-grade dysplasia and AAH).
Fig. 2.
Immunohistochemical expression of IRAK-1 in the sequential pathogenesis of squamous cell carcinoma (Panel A) and adenocarcinoma (Panel B) sequences. Photomicrographs showing cytoplasmic and nuclear IRAK-1 immunostaining in normal and mildlyabnormal epithelia and NSCLC tumors. Original magnification, ×200
Fig. 3.
IRAK-1 cytoplasmic expression scores by epithelial and tumor specimen histology. In the box-plots, white bar in tumors and black bar in epithelial samples indicate median scores, and x indicates mean scores.
For nuclear IRAK-1, we observed a slightly different pattern of expression in the sequential preneoplastic lesions of lung cancer, especially for adenocarcinomas. Normal and mildly abnormal epithelia showed similar levels of IRAK-1 nuclear expression. Both low-grade and high-grade squamous dysplasias had a significantly higher (P < 0.0001) IRAK-1 nuclear expression score than normal bronchial epithelium (data not shown). Like IRAK-1 cytoplasmic expression, invasive squamous cell carcinoma exhibited a significantly lower (P < 0.0001) IRAK-1 nuclear expression score than high-grade squamous dysplasias (data not shown). AAH had a significantly lower (P < 0.0001) IRAK-1 nuclear expression score than lung adenocarcinomas (data not shown).
Correlation of immunohistochemical expression of IRAK-1 with clinical-pathologic features of patients with NSCLC
Both NSCLC histologic types, adenocarcinoma and squamous cell carcinoma, had similar cytoplasmic (Fig. 3) and nuclear IRAK-1 expression scores. No correlation was detected between IRAK-1 expression and patients’ sex, age at time of surgery, and smoking status and history. For all NSCLCs, IRAK-1 cytoplasmic expression tended to be lower in more advanced pathologic TNM stages (mean IRAK-1 cytoplasmic expression scores: stage I, 194.4; stage II, 177.7; stage III, 185.4; and stage IV, 144.4; P = 0.083). In particular, IRAK-1 cytoplasmic expression was generally lower in larger tumors (T; mean IRAK-1 cytoplasmic expression scores: T1, 200.1; T2, 183.8; T3, 163.9; and T4, 178.0; P = 0.065) and when distant metastasis was present (M; mean IRAK-1 cytoplasmic expression scores: M0, 189.5; and M1, 144.0; P = 0.040). Interestingly, the nine M1 and stage IV tumors showed a non-significant slightly higher expression of cytoplasmic IRAK-1 compared with normal epithelium. All these correlations exhibited a similar trend when adenocarcinoma and squamous cell carcinoma were examined separately, but they were found to be statistically significant only for pathologic TNM stage and metastasis in adenocarcinoma (data not shown).
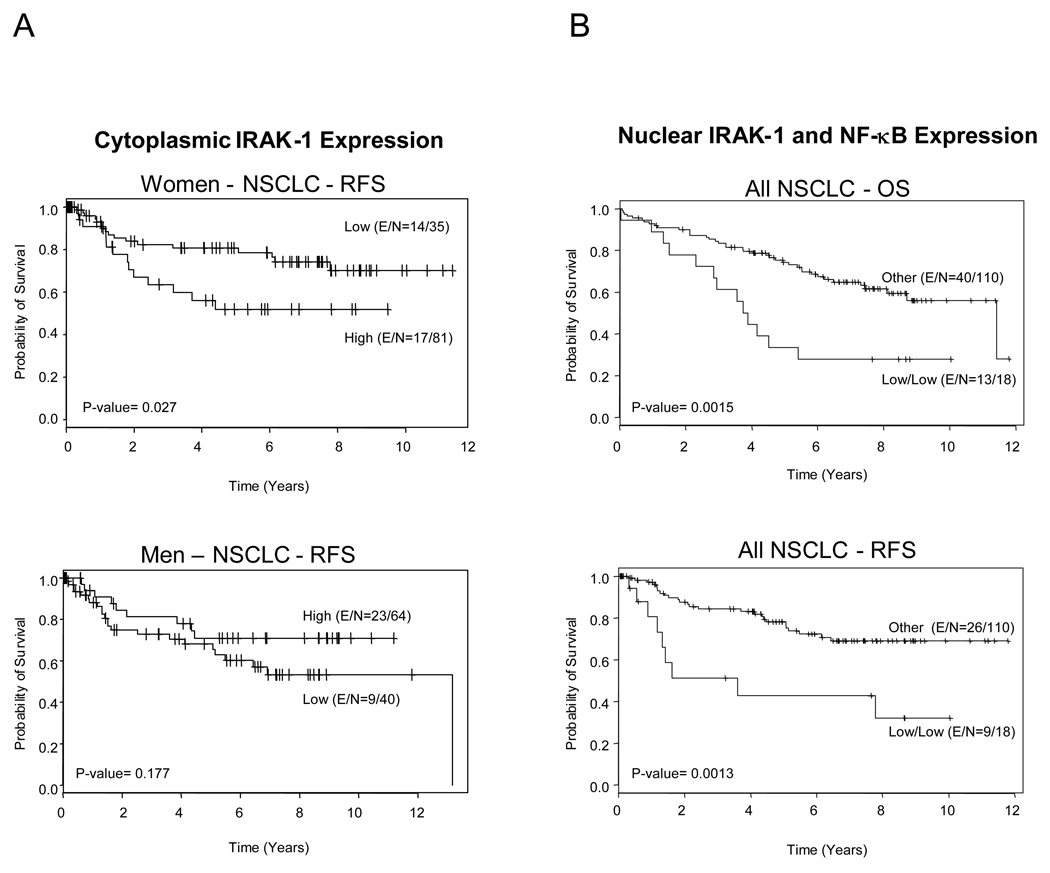
We correlated cytoplasmic and nuclear IRAK-1 expression with OS and RFS in patients with NSCLC at stages I and II who had not received either neoadjuvant or adjuvant treatments (n = 221). In patients with adenocarcinoma, we found that low IRAK-1 nuclear expression (score ≤ 35) correlated with worse RFS in the univariate analysis (P = 0.030); however, this association was not significant in the multivariate analysis (P = 0.086; hazard ratio [HR], 0.555; 95% confidence interval [95% CI], 0.284–1.09). By contrast, for all NSCLCs and each NSCLC histologic type, in the univariate analysis, we found that higher IRAK-1 cytoplasmic expression correlated with worse RFS in women and better RFS in men (Fig. 4A); however, in the multivariate analysis, this association was significant in women with NSCLC (P = 0.033; HR, 2.204; 95% CI, 1.067–4.555) but not in men with NSCLC (P = 0.18; HR, 0.585; 95% CI, 0.269–1.273) (Table 2).
Fig. 4.
A, Kaplan-Meier curves showing IRAK-1 cytoplasmic expression and recurrence-free survival (RFS) in patients with NSCLC by sex. IRAK-1 cytoplasmic expression score: high > 200, and low ≤ 200. B, Kaplan-Meier curves illustrating the effect of combined nuclear IRAK-1 and NF-κB expression levels on overall survival (OS) and RFS in patients with NSCLC. IRAK-1 nuclear expression score: low ≤ 35; and NF-κB expression score: low < 20.75.
Table 2.
Multivariate recurrence-free survival (RFS) and overall survival (OS) analyses using Cox regression model in patients with NSCLC.
| 95% CI of HR | ||||
|---|---|---|---|---|
| Variable | HR | Lower limit | Upper limit | P value |
| RFS in Women with NSCLC | ||||
| Age at time of surgery | 1.032 | 0.993 | 1.072 | 0.11 |
| Smoking history: Yes vs. No | 2.004 | 0.714 | 5.627 | 0.19 |
| Histology: ADC vs. SCC | 0.767 | 0.351 | 1.677 | 0.51 |
| Stage II vs. I | 3.108 | 1.354 | 7.134 | 0.008 |
| Cytoplasmic IRAK-1expression: High vs. Low* | 2.204 | 1.067 | 4.555 | 0.033 |
| RFS in Patients with Adenocarcinoma | ||||
| Age at time of surgery | 1.056 | 1.018 | 1.095 | 0.004 |
| Smoking history: Yes vs. No | 3.558 | 1.072 | 11.808 | 0.038 |
| Stage II vs. I | 2.608 | 1.055 | 6.449 | 0.038 |
| Nuclear IRAK-1/NF-κB expression : Low/Low vs. Others† | 3.058 | 1.386 | 6.748 | 0.006 |
| OS in Patients with Adenocarcinoma | ||||
| Age at time of surgery | 1.076 | 1.042 | 1.111 | <0.0001 |
| Smoking history: Yes vs. No | 2.570 | 1.070 | 6.169 | 0.035 |
| Stage II vs. I | 2.507 | 1.225 | 5.130 | 0.012 |
| Nuclear IRAK-1/NF-κB expression: Low/Low vs. Others2 | 2.485 | 1.281 | 4.819 | 0.007 |
Abbreviations: NSCLC, non-small cell lung cancer; CI, confidence interval; HR, hazard ratio.
IRAK-1 Cytoplasm: High score >200; Low score ≤200.
IRAK-1 Nuclear: Low score ≤35; NF-κB Low score <20.75.
Association between immunohistochemcial expression of IRAK-1 and that of IL-1α and NF-κB
Because of the intermediate role of IRAK-1 in the activation of NF-κB after IL-1R stimulation by IL-1α, (9–11), we examined the correlation between the immunohistochemical expression of IRAK-1 with that of IL-1 α (an IL- 1R ligand) and NF-κB using the 306 NSCLCs placed in TMAs. Relatively high levels of IL-1α cytoplasmic expression were detected in NSCLC tissue specimens: they were significantly higher (P < 0.0001) in adenocarcinomas (mean expression score: 130.5) than in squamous cell carcinomas (mean expression score: 113.6). We previously reported that NSCLC tissue specimens frequently exhibit NF-κB nuclear expression in malignant cells (15). In this current study, we found that adenocarcinomas (mean NF-κB nuclear expression score: 56.0) had significantly higher (P = 0.0001) levels of NF-κB nuclear expression than squamous cell carcinomas (mean NF-κB nuclear expression score: 40.9). Of interest, in NSCLC tissue specimens, we detected a significant correlation between IRAK-1 expression (nuclear and cytoplasmic) and IL-1α cytoplasmic expression (P = 0.011, r = 0.15 and P < 0.0001, r = 0.28, respectively). Remarkably, when both NSCLC histologic types were analyzed separately, only for adenocarcinomas the correlation between IRAK-1 expression (nuclear and cytoplasmic) and IL-1α cytoplasmic expression remained (P = 0.036, r = 0.15 and P < 0.0001, r = 0.32, respectively). No correlations were detected between IRAK-1 and NF-κB nuclear expression.
Correlation between the expression of combined inflammation-related markers and disease outcome in patients with NSCLC
Because of the biological interaction between IRAK-1, IL-1α, and NF-κB, we explored the effect of their combined expression on the disease outcome of patients with NSCLC at stages I and II who had not received either neoadjuvant treatments or adjuvant treatments (n = 221).
First, we examined the effect of the combined expression of NF-κB and IL-1α. In the multivariate analysis, we found that the immunohistochemical overexpression of NF-κB (NF-κB nuclear expression score ≥ 20.75) significantly correlated with better RFS (HR, 0.538, 95% CI, 0.316–0.918; P = 0.023) and OS (HR, 0.468, 95% CI, 0.312–0.702; P = 0.0002) in all NSCLC patients, and with better OS in patients with adenocarcinoma (HR, 0.448, 95% CI, 0.248–0.809; P = 0.008) and squamous cell carcinoma (HR, 0.482, 95% CI, 0.272–0.854; P = 0.012). No correlation between IL-1α cytoplasmic expression and RFS and OS was detected. Of the various combinations of biomarker expression tested, we found that a combined low level of expression of nuclear IRAK-1 (IRAK-1 nuclear expression score ≤ 35) and low level of NF-κB (NF-κB nuclear expression score < 20.75) correlated significantly with worse RFS and OS in patients with adenocarcinoma in both univariate (Fig. 4B) and multivariate analyses (Table 2). In the multivariate analysis, patients with adenocarcinoma had a HR of 3.058 (95% CI, 1.386–6.748; P = 0.006) for RFS, and HR of 2.485 (95% CI, 1.281–4.819; P = 0.007) for OS (Table 2). Interestingly, the prognostic effect of this combined low expression of nuclear IRAK-1 and NF-κB was not observed in patients with squamous cell carcinoma.
Discussion
In this study, we found that IRAK-1 is frequently overexpressed in NSCLC tissue specimens and that this overexpression occurs early in the sequential preneoplastic evolution of the most common NSCLC histologic types, adenocarcinoma and squamous cell carcinoma. IRAK-1 is a relatively novel molecule, which has not been previously associated with lung tumor development. In fact, only two reports have linked up-regulation of IRAK-1 with cancer (28, 29). Using rapid subtraction hybridization method, IRAK-1 was one of the six genes known to be found displaying elevated gene expression in metastatic melanoma cell lines compared with normal immortal melanocytes (28). In addition, in silico analysis of genes overexpressed in several solid tumors (including lung tumors) identified IRAK-1 as one of the genes up-regulated in tumors (29). To the best of our knowledge, this study is the first report on the expression of the IRAK-1 protein in lung cancer and its preneoplastic lesions.
Current evidence suggests that chronic inflammation is associated with tumor development and progression; however, the cellular and molecular mechanisms involved in this process are not fully understood (5). It has been shown that cancer cells acquire many properties that are characteristic of immune cells, which allow them to communicate with each other and, more importantly, to regulate the immune system for their own survival and growth (30). TIR domain containing receptors activates IRAK-1, and their intracellular signaling components, constitute an important cellular pathway mediating this tumor cell-microenvironment interaction that promotes inflammation and tumor cell growth (30). Neither IL-1Rs nor TLRs possess intrinsic kinase activity, so they rely on the recruitment and activation of adapter molecules and kinases, such as IRAKs, to transduce their signals (31). Thus, IRAK-1 activation constitutes an important signaling pathway in both TLR and IL-1R signal transduction (32). Although the four IRAK members are categorized as serine/threonine kinases and all contain a kinase-like domain, only IRAK-1 and IRAK-4 exhibit kinase activity (32). Many key functions seem to involve the ability of IRAK-1 to form or trigger the dissolution of membrane and cytoplasmic macromolecular signaling complexes in a temporal and special manner.
In the present study, using immunohistochemical methods, we have confirmed our previous findings that cytoplasmic IRAK-1 protein was significantly overexpressed in the in vitro sequential progression lung cancer cell model (15) comparing in vitro premalignant, tumorigenic and malignant cells with NHBE cells. In addition, we have validated the immunohistochemical technique performed in FFPE specimens by comparing IRAK-1 expression in FFPE cell pellets with protein levels obtained from fresh cells by Western blotting. Then, we showed that malignant tissue specimens obtained from lung adenocarcinoma and squamous cell carcinoma demonstrated a significantly higher level of IRAK-1 cytoplasmic expression compared with normal epithelial cells adjacent to tumors. Interestingly, our in silico analysis using published (24–27) gene expression microarray datasets of NSCLC tissue specimens showed that IRAK-1 RNA expression was significantly higher in both lung adenocarcinomas and squamous cell carcinomas compared to adjacent normal lung tissue. These results emphasize the potential important implication of IRAK-1 in NSCLC. These findings are consistent with the postulated mitogenic effect of the TLR/IL-1R signaling pathway activation in human tumors. It has been shown that IRAK-1 activation and formation of a macromolecular adaptor complex activates NF-κB and mitogen-activated protein kinases, and results, among other things, in nuclear translocation and transcriptional activation of inflammatory target genes (32).
We also identified IRAK-1 nuclear expression in normal, premalignant, and malignant epithelial cells. Both NSCLC histologic types, adenocarcinoma and squamous cell carcinoma, demonstrated lower levels of IRAK-1 nuclear expression than normal epithelium. Although it is known that the primary function of IRAK-1 is to participate in cytoplasmic events following TLR engagement that leads to activation of the IKK complex, degradation of IκB-α, and translocation of the NF-κB p65 subunit to the nucleus, published data suggest that IRAK-1 can be present in the nucleus (16, 17). Recently, it has been shown that transient overexpression of IRAK-1 results in enhanced NF-κB transcriptional activity even without concurrent IκB-α degradation or increased nuclear translocation of p65 (10). However, we did not find correlation between the expression of nuclear IRAK-1 and NF-κB. All these data suggest a novel role for IRAK-1 in which it may participate directly in a NF-κB-associated transcriptional event. To the best of our knowledge, our report is the first to show IRAK-1 nuclear expression in human tumors.
Lung cancers are believed to arise after a series of progressive pathologic changes (preneoplastic or precursor lesions) in the respiratory mucosa. Whereas the sequential preneoplastic changes have been defined for centrally arising squamous carcinomas, they have been poorly documented for adenocarcinomas (33). Mucosal changes in the large airways that may precede invasive squamous cell carcinoma include squamous dysplasia and carcinoma in situ in the central bronchial airway (33). Adenocarcinomas may be preceded by morphological changes, including AAH in peripheral airway cells (33). For the centrally located squamous cell carcinoma sequence, both high- and low-grade squamous dysplasias demonstrated significantly higher IRAK-1 cytoplasmic expression compared with histologically normal epithelium. Although with a high level of overlap, invasive squamous cell carcinoma showed a significantly lower IRAK-1 cytoplasmic expression score compared with high-grade dysplasias, suggesting a downregulation of IRAK-1 expression at the invasive stage. Conversely, lung peripheral AAHs demonstrated similar levels of IRAK-1 cytoplasmic expression as normal epithelium from bronchial and bronchiolar origin; however, adenocarcinomas demonstrated significantly higher levels of IRAK-1 protein expression, indicating that IRAK-1 cytoplasmic overexpression also occurs in early in the development of lung adenocarcinomas. A slightly similar pattern of expression of nuclear IRAK-1 was observed in lung cancer preneoplastic lesions sequence, for both squamous cell carcinoma and adenocarcinoma.
Currently available information suggests that preneoplastic lesions and molecularly abnormal epithelial foci are frequently extensive and multifocal throughout the lung, indicating a field effect or a field cancerization phenomenon by which much of the respiratory epithelium has been molecularly altered, presumably from exposure to carcinogens, including components of tobacco smoke (33). A common factor responsible for such a phenomenon in the lung airway could be the development of inflammation and the activation of inflammation-related pathways in the respiratory mucosa microenvironment, resulting in the activation of NF-κB in the respiratory epithelial cells. This is supported by our previous finding (14) that NF-κB p65 nuclear expression is frequent at early stages in the development of both major types of NSCLC. Our present findings suggest that IRAK-1 may also play an important role in the early pathogenesis of lung cancer, although its overexpression may be also an epiphenomenon associated to the progression of preneoplastic lesions to invasive carcinoma.
IRAK-1 plays a role as an intermediate factor between IL-1α receptor and NF-κB activation (9–11). Thus, we examined the correlation between the immunohistochemical expression of IRAK-1 and that of IL-1α and NF-κB using 306 NSCLCs placed in TMAs. We found that lung adenocarcinomas had significantly higher cytoplasmic IL-1α expression than squamous cell carcinomas. Consistent with our previous findings (14), lung adenocarcinomas exhibited significantly higher expression of nuclear NF-κB than squamous cell carcinomas. In tumors, we detected a significant correlation between both nuclear and cytoplasmic IRAK-1 expression and IL-1α cytoplasmic expression; however, no such correlations were detected comparing nuclear IRAK-1 and NF-κB expressions. These findings suggest the presence of an autocrine loop in the IL-R activation by IL-1α in malignant lung cancer cells. Stimulation of TLRs activates the adaptive immune system through the production of proinflammatory cytokines, including IL-1, and the induction of key cell-surface molecules that drive T cell activation (34). Activation of IL-1 receptor mediates a very complex pathway, involving a cascade of kinases organized by multiple adapter molecules into signaling complexes, leading to NF-κB activation (34). Although the stimulation of every member of the TLR family leads to the activation and nuclear localization of NF-κB (34), it has been suggested that IRAK-1 is dispensable for NF-κB activation (35). The fact that IRAK-1 knock-out mice have still shown NF-κB activation upon lipopolysaccharide challenge suggests that IRAK-1 participation is not always involved in NF-κB activation, which could explain the lack of correlation between both biomarkers in our study. Of interest, it has been shown that in human epithelial cells, Helicobacter pylori activates NF-κB without requiring IRAK-1 involvement, suggesting a mode of Myd88 activation different from the receptor-mediated mechanism (36).
In our multivariate survival analysis, nuclear and cytoplasmic IRAK-1 expression did not correlate with RFS and OS, except in women with NSCLC in whom higher IRAK-1 cytoplasmic expression was significantly correlated with worse RFS. Interestingly, this association was not observed in men with NSCLC. This unexpected sex difference in the role of IRAK-1 expression in the outcome of NSCLC highlights the potential effect of sex in the role of inflammation in the early development and progression of tumors (18). In liver cancer pathogenesis, it has been shown that estrogens suppress IL-6 mediated by MyD88, an adapter molecule that binds to IRAK-1, and, therefore, inhibit chemically induced liver carcinogenesis. It is possible that in women, cytoplasmic IRAK-1 overexpression correlates with different levels of estrogen and, thus, influences NSCLC recurrence.
Because of the intermediate role of IRAK-1 in the activation of NF-κB after IL-1R stimulation by IL-1α, we examined the influence of the combined expression of these biomarkers on disease outcomes of patients with NSCLC. Somewhat surprising, we found that combined low levels of expression of nuclear IRAK-1 and NF-κB correlated significantly with worse OS and RFS in patients with adenocarcinoma in both univariate and multivariate analyses. Independent analysis of each biomarker showed that low level of nuclear IRAK-1 expression showed a trend to worse RFS in patients with adenocarcinoma, and NF-κB expression was significantly correlated with RFS and OS in all NSCLC patients. Although somewhat contradictory, these findings can be explained by a better understanding the role of inflammation in cancer development and progression. It has been established that NF-κB activation is important for tumor development and progression by the production of inflammatory cytokines; however, these cytokines are also responsible of stimulating a tumor-induced immune suppression by host inflammatory cells (4–6). Therefore, the balance between the stimulation of the survival and proliferation of tumor cells and the induction of the mechanism to evade a host’s response is what defines the role of inflammation–related pathways in cancer progression. In our study, low NF-κB expression may be responsible for the lower tumor-induced inflammatory response, and low nuclear IRAK-1 expression may be associated with the low induction of NF-κB expression, as well as with other still uncharacterized functions. This relatively unknown function could be related to the suggested role of decreased IRAK-1 expression in the endotoxin tolerance phenomenon (37), by which decreased inflammatory pathways activation occurs in cells or organisms after pre-exposure to TLR/IL-1R ligands (32).
Statement of Translational Relevance
The inflammatory process seems to play an important role in the pathogenesis and progression of lung cancer. We report that the overexpression of IRAK-1 protein, a mediator in the activation of NF-κB, is frequently detected in a large series of adenocarcinomas and squamous cell carcinoma of the lung, as well as in lung cancer preneoplastic lesions. Our findings suggest that IRAK-1 is a novel inflammation-related marker and a potential target for lung cancer chemopreventive strategies.
Supplementary Material
mRNA IRAK-1 expression levels in published microarray datasets of clinical NSCLC specimens (24–27) showing significantly higher level of expression in adenocarcinomas (ADC; three datasets) and squamous cell carcinomas (SCC; one dataset) compared with normal lung tissues.
Acknowledgments
Grant support: NIH grant 1P01 CA91844-03 and Department of Defense W81XWH-04-1-0142.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Tumours of the lung. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer (IARC); 2004. pp. 9–124. [Google Scholar]
- 3.Wistuba I. Genetics of preneoplasia: Lessons from lung cancer. Curr Mol Med. 2007;7:3–14. doi: 10.2174/156652407779940468. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci. 2003;24:71–76. doi: 10.1016/S0165-6147(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 8.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 9.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK) -1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 11.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 13.Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004;173:2011–2022. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Liu D, Shishodia S, et al. Nuclear factor-kB (NF-kB) is frequenlty activated in lung cancer and preneoplastic lesions. Cancer. 2006 doi: 10.1002/cncr.22315. in press. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Behrens C, Wistuba, et al. Identification and validation of differences in protein levels in normal, premalignant, and malignant lung cells and tissues using high-throughput Western Array and immunohistochemistry. Cancer research. 2006;66:11194–11206. doi: 10.1158/0008-5472.CAN-04-1444. [DOI] [PubMed] [Google Scholar]
- 16.Bol G, Kreuzer OJ, Brigelius-Flohe R. Translocation of the interleukin-1 receptor-associated kinase-1 (IRAK-1) into the nucleus. FEBS Lett. 2000;477:73–78. doi: 10.1016/s0014-5793(00)01759-2. [DOI] [PubMed] [Google Scholar]
- 17.Su J, Richter K, Zhang C, Gu Q, Li L. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol. 2007;44:900–905. doi: 10.1016/j.molimm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science (New York, NY. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 19.Reddel RR, Ke Y, Gerwin BI, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer research. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 20.Klein-Szanto AJ, Iizasa T, Momiki S, et al. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6693–6697. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 22.Duffey DC, Chen Z, Dong G, et al. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer research. 1999;59:3468–3474. [PubMed] [Google Scholar]
- 23.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia (New York, NY. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stearman RS, Dwyer-Nield L, Zerbe L, et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. The American journal of pathology. 2005;167:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su LJ, Chang CW, Wu YC, et al. Selection of DDX5 as a novel internal control for Q-RT-PCR from microarray data using a block bootstrap re-sampling scheme. BMC genomics. 2007;8:140. doi: 10.1186/1471-2164-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nature medicine. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukerche H, Su ZZ, Kang DC, Fisher PB. Identification and cloning of genes displaying elevated expression as a consequence of metastatic progression in human melanoma cells by rapid subtraction hybridization. Gene. 2004;343:191–201. doi: 10.1016/j.gene.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia (New York, NY. 2004;6:744–750. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll--the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27:225–233. doi: 10.1038/sj.onc.1210907. [DOI] [PubMed] [Google Scholar]
- 31.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 32.Gottipati S, Rao NL, Fung-Leung WP. IRAK1: a critical signaling mediator of innate immunity. Cell Signal. 2008;20:269–276. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Wistuba Genetics of preneoplasia: lessons from lung cancer. Curr Mol Med. 2007;7:3–14. doi: 10.2174/156652407779940468. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. J Mol Med. 2005;83:258–266. doi: 10.1007/s00109-004-0622-4. [DOI] [PubMed] [Google Scholar]
- 35.Uematsu S, Sato S, Yamamoto M, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirata Y, Ohmae T, Shibata W, et al. MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori-infected human epithelial cells. J Immunol. 2006;176:3796–3803. doi: 10.4049/jimmunol.176.6.3796. [DOI] [PubMed] [Google Scholar]
- 37.Li CH, Wang JH, Redmond HP. Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J Leukoc Biol. 2006;79:867–875. doi: 10.1189/jlb.0905505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mRNA IRAK-1 expression levels in published microarray datasets of clinical NSCLC specimens (24–27) showing significantly higher level of expression in adenocarcinomas (ADC; three datasets) and squamous cell carcinomas (SCC; one dataset) compared with normal lung tissues.