Summary
Altered ion channel expression and/or function may contribute to the development of certain human epilepsies. In rats, systemic administration of pilocarpine induces a model of human temporal lobe epilepsy, wherein a brief period of status epilepticus (SE) triggers development of spontaneous recurrent seizures that appear after a latency of two-three weeks. Here we investigate changes in expression of A-type voltage-gated potassium (Kv) channels, which control neuronal excitability and regulate action potential propagation and neurotransmitter release, in the pilocarpine model of epilepsy. Using immunohistochemistry, we examined the expression of component subunits of somatodendritic (Kv4.2, Kv4.3, KChIPl and KChIP2) and axonal (Kv1.4) A-type Kv channels in hippocampi of pilocarpine-treated rats that entered SE. We found that Kv4.2, Kv4.3 and KChIP2 staining in the molecular layer of the dentate gyrus changes from being uniformly distributed across the molecular layer to concentrated in just the outer two-thirds. We also observed a loss of KChIP1 immunoreactive interneurons, and a reduction of Kv4.2 and KChIP2 staining in stratum radiatum of CA1. These changes begin to appear 1 week after pilocarpine treatment and persist or are enhanced at 4 and 12 weeks. As such, these changes in Kv channel distribution parallel the acquisition of recurrent spontaneous seizures as observed in this model. We also found temporal changes in Kv1.4 immunoreactivity matching those in Timm's stain, being expanded in stratum lucidum of CA3 and in the inner third of the dentate molecular layer. Among pilocarpine-treated rats, changes were only observed in those that entered SE. These changes in A-type Kv channel expression may contribute to hyperexcitability of dendrites in the associated hippocampal circuits as observed in previous studies of the effects of pilocarpine-induced SE.
Keywords: epilepsy, IA, dentate gyrus, ion channels, CA1, immunohistochemistry
Introduction
Temporal lobe epilepsy (TLE) (i.e., spontaneous seizures involving the hippocampal formation) is the most prevalent refractory epilepsy (Chang and Lowenstein, 2003); mechanisms converting the normal hippocampus into an epileptic one are not fully understood. A rat model of human TLE (Turski et al., 1983) involves systemic administration of the muscarinic agonist pilocarpine to produce electrographic and behavioral limbic seizures, and status epilepticus (SE), that can last up to 12 h (acute phase). This is followed by a silent (seizure-free) period and, after a latency of two-three weeks, spontaneous recurrent seizures (Turski et al., 1983, Löscher, 2002). These behavioral changes are coincident with loss of inhibitory interneurons in CA1 stratum oriens (Andre et al., 2001, Cossart et al., 2001) and the dentate gyrus hilus (Buckmaster and Dudek, 1997), and by sprouting of recurrent mossy fiber axons from dentate granule cells into the inner molecular layer of the dentate gyrus and CA2 subfield (Wuarin and Dudek, 2001). However, the causative event(s) that form the link between initial injury, axonal reorganization, and later recurrent limbic seizures is not clear (Staley, 2004).
Changes in the abundance, distribution and activity of ion channels in hippocampal neurons, due to activity-dependent regulation (Zhang and Linden, 2003) or mutation (Mulley et al., 2003) may also contribute to development of hippocampal hyperexcitability and TLE. Expression of a number of different ion channels, including HCN channels (Jung et al., 2007), BK channels (Pacheco Otalora et al., 2008), GABA-A receptors (Fritschy et al., 1999), and Cav channels (Xu et al., 2007) is altered in response to pilocarpine-induced SE. Among ion channels, voltage-gated potassium (Kv) channels are critical regulators of neuronal excitability (Pongs, 1999). Transient or A-type Kv channels of the Kv1 channel family expressed in hippocampal presynaptic terminals play a key role in regulating neurotransmitter release (Dodson and Forsythe, 2004), while somatodendritic A-type Kv4 channels play a dynamic role in determining dendritic excitability (Johnston et al., 2003). In the mammalian hippocampus, Kv1.1 and Kv1.4 α subunits are highly expressed in nerve terminals of the perforant path, mossy fiber, and Schaffer collateral pathways (Sheng et al., 1992, Wang et al., 1993, Wang et al., 1994, Maletic-Savatic et al., 1995, Rhodes et al., 1997, Monaghan et al., 2001), while Kv4.2 α subunits and associated KChIPs are expressed at high levels in the dendrites of dentate granule cells, and of CA3 and CA1 pyramidal cells (Sheng et al., 1992, Maletic-Savatic et al., 1995, Varga et al., 2000, Rhodes et al., 2004). Pharmacological blockade of presynaptic Kv1 channels (Bagetta et al., 2004), or genetic ablation of Kv1.1 expression in mice (Smart et al., 1998) is epileptogenic, as is pharmacological blockade of somatodendritic Kv4 channels (Avoli, 2001). Importantly, a recent study shows that the amplitude of backpropagating action potentials is increased in CA1 pyramidal cell dendrites in pilocarpine-induced TLE, suggesting decreased functional expression of dendritic Kv4 channels (Bernard et al., 2004). However, as A-type Kv channels are targets of modulation by phosphorylation (Roeper et al., 1997, Hoffman and Johnston, 1999, Anderson et al., 2000), the observed changes in current amplitude could involve channel modulation or altered expression and/or localization of Kv4/KChIP subunits. Here we determined whether altered expression and/or localization of component subunits of presynaptic and somatodendritic A-type Kv channels occur following induction of SE in the pilocarpine experimental model of human TLE.
Material and Methods
Pilocarpine Treatment
Sprague-Dawley rats (200-300g) were housed individually or in pairs and maintained on food and water ad libitum. Subjects were pretreated with 1mg/kg dose i.p. of scopolamine methyl nitrate and 30 minutes later received an injection of 380 mg/kg of pilocarpine HCl (Turski et al., 1983). Subjects were observed for 1 hr and scored according to a modified Racine seizure scale (Racine, 1972): 1=facial movements, 2=head nodding and chewing, 3=unilateral forelimb clonus, 4=bilateral forelimb clonus, 5=bilateral forelimb clonus, rearing and loss of balance. A second observation and scoring period (30 min) began 2-3 hrs after the pilocarpine injection. At the conclusion of this period, all subjects were given diazepam (10 mg/kg) which essentially halted SE at ∼3hrs. Subjects that scored 3 and above in both observations periods were designated as pilocarpine-SE subjects and included in the experimental group. Subjects that scored 2 or below in one of the observation periods were designated as no-SE (NSE) subjects and excluded from the experimental group. Additional subject groups consisted of either drug treated with no apparent seizure (no score on both observation periods), or untreated age-matched individuals. Subjects were sacrificed at 1, 2, 4, 6, 8 and 12 weeks (or greater) following pilocarpine treatment.
Immunohistochemistry
Animals were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and then perfused through the ascending aorta with saline. Most subjects were prepared for Timm's stain (see method below) while others were directly perfused with fixative containing freshly depolymerized 4% paraformaldehyde in 0.1 M NaPO4 buffer at pH 7.4, to improve on the quality of preservation and subsequent immunohistochemical staining. The brain was removed and immersed in cryoprotectant, 10% sucrose for 18h, and then 30% sucrose for at least two days, both in 0.1 M NaPO4 buffer, and at 4 °C, then frozen and cut on sliding microtome in 40 μm thick horizontal sections for DAB staining (Rhodes et al., 1995) and sagittal sections for immunofluorescence staining (Menegola and Trimmer, 2006).
All procedures were designed for light microscope immunohistochemistry using subunit specific antibodies for Kv1.4 (Monaghan et al., 2001), and for Kv4.2, Kv4.3 and the Kv4 family associated proteins KChIP1, KChIP2 and KChIP3 (Rhodes et al., 2004). All free-floating sections were incubated overnight at 4 °C in antibody vehicle containing mouse monoclonal antibodies. Detection of antibody-antigen complexes was accomplished using the ABC Elite peroxidase reaction (Vector Laboratories, Burlingame, CA) and visualized using a nickel-enhanced diaminobenzidine procedure (Tago et al., 1986, Rhodes et al., 1995).
For immunofluorescence staining free floating sections were blocked in a 10% goat serum solution/0.1M PB + 0.3% Triton-x-100, then incubated overnight in vehicle containing 2 different mouse monoclonal antibodies of different IgG isotype (Menegola and Trimmer, 2006). The sections were then incubated in isotype-specific Alexa-conjugated secondary antibody (Invitrogen). To allow for a direct comparison of the signal intensity of the staining of sections from control and pilocarpine seized subjects, fluorescent images of each pair of sections (control, pilocarpine-SE) were taken using the same exposure time with a CCD camera installed on a Zeiss Axioskop-2 microscope, with Zeiss Fluar 10x/0.5numerical aperture lens, using Axiovision software. Intensity was measured using NIH ImageJ software by selecting a 100 μm × 100 μm area from the molecular layer of the DG adjacent to the hippocampal fissure and of the stratum radiatum of CA1 adjacent to the pyramidal cell layer. The fluorescence intensity measured on a comparable region of a section from the same animal but incubated with secondary AB alone was then subtracted (Menegola and Trimmer, 2006). For the 1-week time point sections from 2 control animals and 3 pilocarpine-SE animals were analyzed. For the 4-week time point sections from 3 control animals and 4 pilocarpine-SE animals were analyzed.
Timm's Stain
Animals were deeply anesthetized as described above and then perfused through the ascending aorta with 40-60 ml of ice-cold saline. The brains were extracted from the cranium and immersed in 0.1% sodium sulfide in a 0.1 M NaPO4 buffer, pH 7.3 at room temperature with gentle agitation for 90 minutes. Tissue was then immersed in the fixative described above and stored overnight at 4 °C and finally placed in a cryoprotectant for at least 48 hrs. Cut sections of 40 μm were mounted on a slide, air dried, and immersed in a physical developer in the dark and gently agitated in a water bath at 26 °C for 60-70 minutes. The composition of the physical developer consisted of 60 ml of 50% (w/v) gum arabic in deionized H2O, 10ml citrate buffer made up of 25.5%w/v citric acid and 23.5%w/v sodium citrate in deionized H2O, 30 ml 5.67% (w/v) hydroquinone in deionized H2O and 0.5 ml of 17% (w/v) silver nitrate, deionized H2O (Danscher and Zimmer, 1978). Slides were rinsed in water for ∼1hr at 40 °C, some counter-stained with thionin, and then dehydrated and coverslipped.
Sections stained with Timm's stain were rated on a scale of 0 to 3, similar to the rating system previously described (Tauck and Nadler, 1985), for the extent of supragranular mossy fiber staining. In brief, sections with virtually no supragranular staining were scored 0; sections with sparse or discontinuous staining were scored as 1; sections with continuous staining but of an intensity less than the hilus were scored as 2; and finally, continuous staining with intensity essentially equal to the hilus, were scored as 3.
Results
A-type Kv channel expression in control rat hippocampus
Kv channels exhibit diverse patterns of cellular expression and subcellular localization in the normal adult rat hippocampus (Trimmer and Rhodes, 2004). We and others have shown that Kv1 α subunits, such as Kv1.4 (Fig. 1), are preferentially expressed on presynaptic terminals of axons associated with perforant path, mossy fiber and Schaffer collateral pathways (Sheng et al., 1992, Monaghan et al., 2001, Trimmer and Rhodes, 2004). Kv4.2 and KChIP2 are found on dendrites of dentate granule cells and pyramidal neurons (Fig. 2), while KChIP1 (Fig. 3) is found in interneurons throughout the hippocampus (Rhodes et al., 2004). Kv4.3 is also present in these interneurons (Fig. 3), as well as in the dendrites of dentate granule cells, and CA3 but not CA1 pyramidal cells (Rhodes et al., 2004). These patterns of staining are reliably observed in all sets of control animals, those that were untreated age-matched animals, and those pilocarpine-treated animals that failed to enter SE (Fig. 1).
Figure 1.
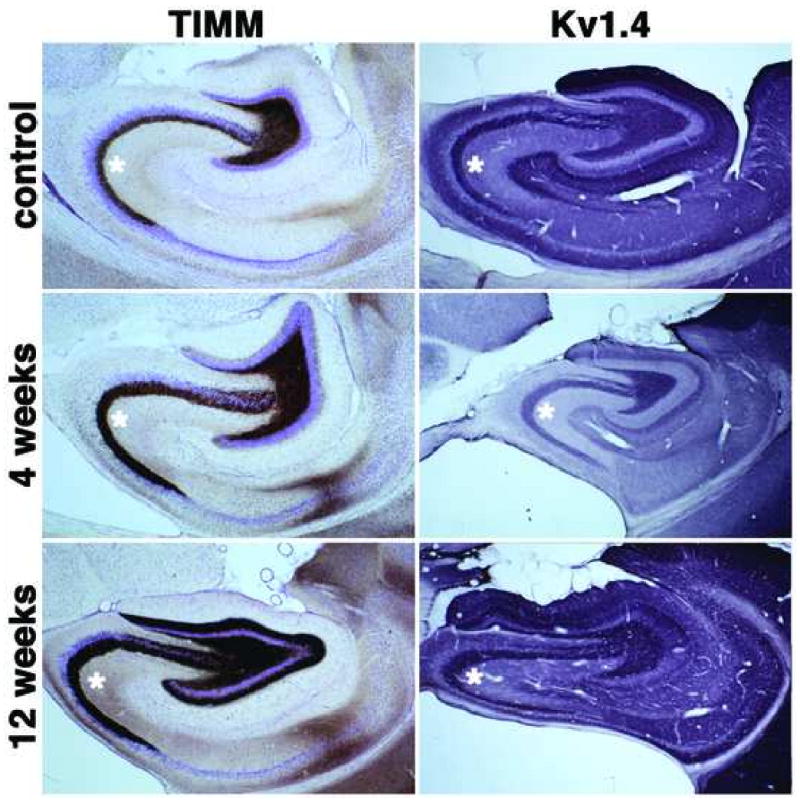
Timm's staining and immunohistochemical localization of Kv1.4 channel α subunits in the hippocampal formation of control and SE subjects. Photomicrographs of horizontal sections taken to show the areal and laminar distribution of Timm's stain and Kv1.4 in the hippocampus of a control rat, and rats 4 and 12 weeks post-SE. Immunoreactivity for Kv1.4 is concentrated in presynaptic terminals. Note the thickening of the mossy fiber layer (asterisks) in subjects 4 weeks after SE, and the dramatic increase in Timm's staining in stratum lucidum of CA3 and in the molecular layer of dentate gyrus due to mossy fiber sprouting 12 weeks post-SE. Kv1.4 immunoreactivity displays parallel increases (asterisks) and the appearance of strong Kv1.4 immunoreactivity in the inner third of the dentate molecular layer at 12 weeks post-SE (see Fig. 4 for higher magnification view).
Figure 2.
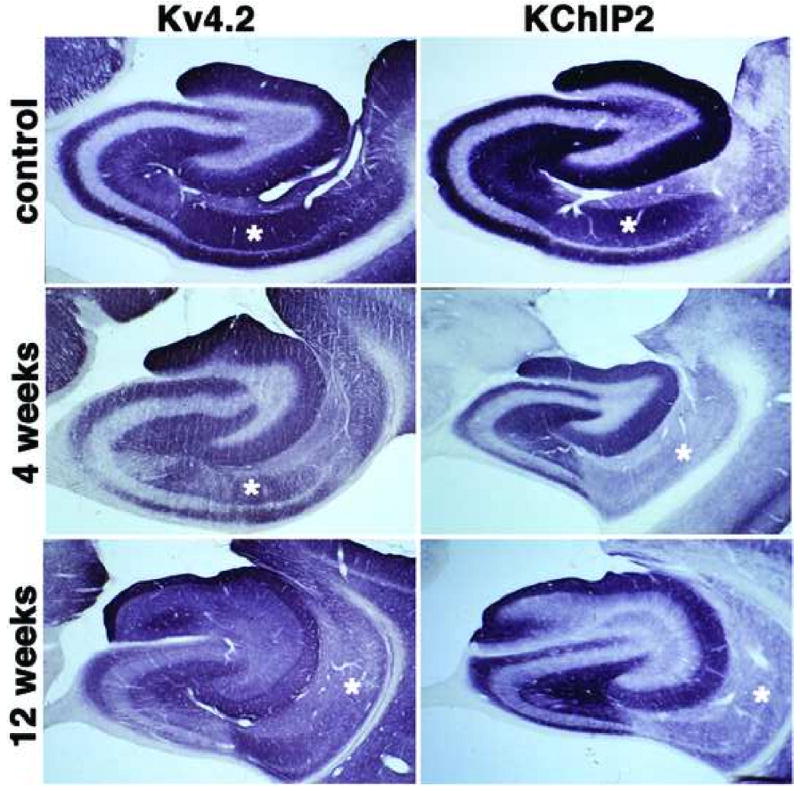
Immunohistochemical localization of Kv4.2 channel α subunits and KChIP2 in the hippocampal formation of control and SE subjects. Photomicrographs of horizontal sections taken to show the areal and laminar distribution of Kv4.2 and KChIP2 in the hippocampus of a control rat, and rats 4 and 12 weeks post-SE. Note the decrease in Kv4.2 and KChIP2 staining in CA1 stratum radiatum (asterisks) at 4 weeks post-SE, compared to that shown for the control animal. Note that at 12 weeks post-SE Kv4.2 and KChIP2 immunoreactivity changes in the molecular layer of the dentate gyrus, such that the staining is no longer uniformly distributed across the molecular layer but becomes concentrated in just the outer two-thirds (see Fig. 5 for higher magnification view). Kv4.2 and KChIP2 staining is also decreased in CA1 stratum radiatum at 12 weeks post-SE (asterisks).
Figure 3.
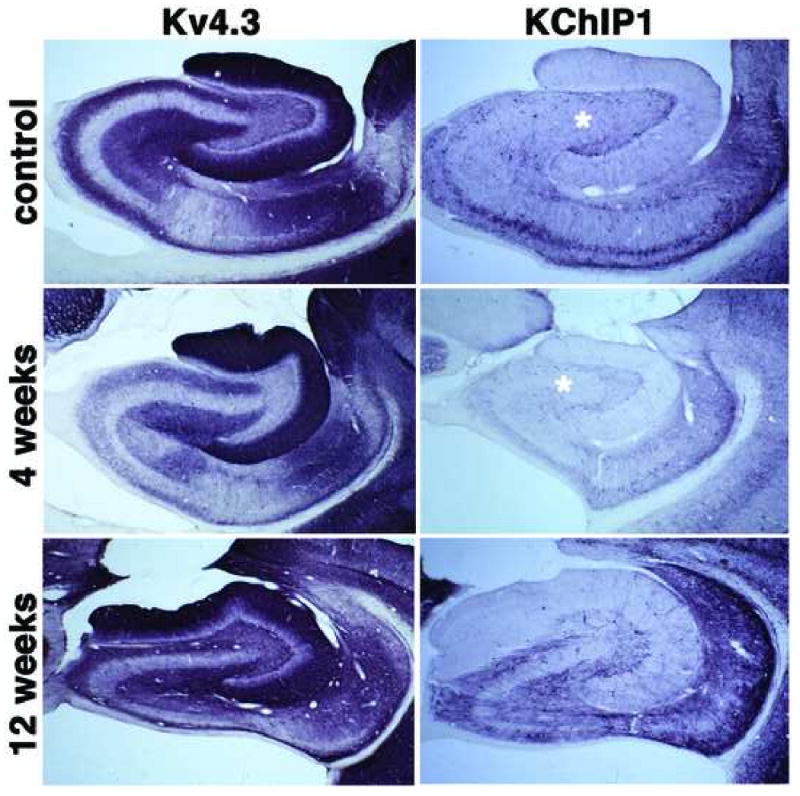
Immunohistochemical localization of Kv4.3 channel α subunits and KChIP1 in the hippocampal formation of control and SE subjects. Photomicrographs of horizontal sections taken to show the areal and laminar distribution of Kv4.3 and KChIP1 in the hippocampus of a control rat, and rats 4 and 12 weeks post-SE. Note the changes in Kv4.3 immunoreactivity in the molecular layer of the dentate gyrus at 12 weeks post-SE, similar to those seen for Kv4.2 and KChIP2 in Fig. 2 (see Fig. 5 for higher magnification view) and loss of KChIP1 immunoreactivity in interneurons throughout the dentate hilus (asterisk) at 4 weeks post-SE.
Changes in Timm's staining in the hippocampus of pilocarpine seized rats
One of the pronounced anatomical effects of TLE exhibited in the pilocarpine animal model is mossy fiber sprouting. Timm's staining reveals the presence of zinc, and because this metal is highly concentrated in mossy fiber boutons, the distribution of mossy fiber contacts can be observed as Timm's-stained granules using the light microscope (Tauck and Nadler, 1985, Patrylo and Dudek, 1998). Mossy fiber projections to the CA3 region regularly establish their synaptic contacts in the stratum lucidum located immediately superficial to the pyramidal cell layer. Strong Timm's staining is also normally observed in the dentate hilus, site of normal recurrent dentate granule cell synapses (Figs. 1, 4). Rats that had been subjected to pilocarpine-induced SE exhibited an expansion of Timm's staining in the stratum lucidum of CA3 four weeks after pilocarpine treatment. This is evident from comparing the narrow band of Timm's stain relative to the thionin-stained Nissl substance in stratum lucidum of CA3 in control animals to the much broader band of Timm's stain which obscures the thionin stain in sections from animals four and twelve weeks after pilocarpine-induced SE (Fig. 1).
Figure 4.
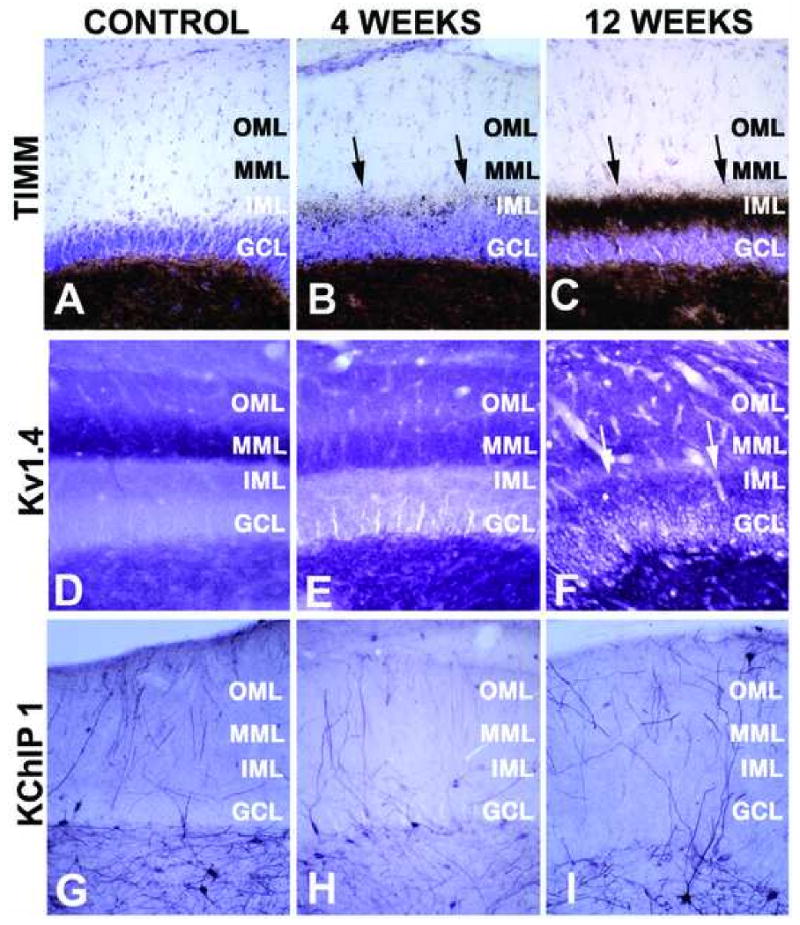
High magnification photomicrographs of Timm's staining, and Kv1.4 and KChIP1 immunoreactivity in the dentate gyrus of control and SE subjects. Photomicrographs of horizontal sections taken to show the areal and laminar distribution of Timm's stain (A-C), and Kv1.4 (D-F), and KChIP1 (G-I) immunoreactivity in the hippocampus of a control rat (A, D, G) and rats four (B, E, H) and twelve (C, F, I) weeks after pilocarpine treatment. Note the progressive increase in Timm's staining in the molecular layer of the dentate gyrus (arrows in panels B, C) due to mossy fiber sprouting, and the associated increase in Kv1.4 staining in the stratum lucidum of CA3 and the molecular layer of the dentate gyrus. The loss of KChIP1 immunoreactivity in interneurons throughout the dentate hilus is apparent, especially four weeks after pilocarpine treatment. GCL: granule cell layer; IML: inner molecular layer; MML” middle molecular layer; OML: outer molecular layer.
The molecular layer of the dentate gyrus normally receives very little recurrent mossy fiber input and as such exhibits little Timm's staining (Figs. 1, 4). While animals four weeks after pilocarpine-induced SE also have little molecular layer Timm's staining (Figs. 1, 4), there is a dramatic appearance of Timm's staining in the inner third of the molecular layer of the dentate gyrus in sections from animals at twelve weeks post-seizure (Figs. 1, 4). This staining represents the dramatic development of a recurrent excitatory dentate granule cell circuit due to mossy fiber sprouting. Such changes in Timm's staining were observed in every rat that entered SE in response to pilocarpine treatment, and never observed in rats that received pilocarpine treatment but did not enter SE, or in sham injected rats (data not shown). Thus, the pilocarpine-induced seizures led to changes in the hippocampus typical of human TLE and consistent with the use of this and other animal models of TLE (Tauck and Nadler, 1985, Patrylo and Dudek, 1998, Löscher, 2002).
Changes in expression of axonal A-type Kv channels in the hippocampus of pilocarpine seized rats
Long-term changes in hippocampal excitability in response to pilocarpine-induced seizures can result from altered hippocampal circuitry, altered excitability of individual neurons, or a combination of the two. To investigate the effects of pilocarpine treatment on the expression patterns of Kv1.4 α subunits critical in regulating neurotransmitter release from nerve terminals (Geiger and Jonas, 2000), brain sections obtained from rats four and twelve weeks after pilocarpine-induced SE were stained with anti-Kv1.4 monoclonal antibodies. Changes in Kv1.4 immunoreactivity were observed in both stratum lucidum of CA3 (Fig. 1), and in the molecular layer of the dentate gyrus (Figs. 1, 4). The changes in the staining pattern of Kv1.4 in stratum lucidum of CA3 matched those observed for Timm's staining of the zinc-rich mossy fiber terminals (Fig. 1). Samples from rats that entered pilocarpine-induced SE also exhibited staining for Kv1.4 in the inner third of the molecular layer of the dentate gyrus (arrows in Fig. 4F). This staining is in addition to the normal Kv1.4 staining in the middle third of the molecular layer, and again matches the appearance of Timm's staining in the inner third of the dentate molecular layer in SE subjects (Fig. 4C). Such changes in Kv1.4 staining were observed in every rat that entered SE in response to pilocarpine treatment, and never observed in rats that received pilocarpine treatment but did not enter SE, or in sham injected rats (data not shown). This suggests that Kv1.4 is expressed in mossy fiber nerve terminals that participate in both their normal synaptic targets on CA3 pyramidal neurons, and in the recurrent mossy fiber terminals that arise from mossy fiber sprouting and which form aberrant synapses on dentate granule cells.
Changes in expression of dendritic A-type Kv channels in the hippocampus of pilocarpine seized rats
Dendritic A-type Kv currents play a critical role in regulating the extent to which backpropagating action potentials invade the dendritic tree, and also impact the movement of synaptic potentials from the dendritic arbor to the soma (Jerng et al., 2004). As such the amplitude of dendritic A-type currents is thought to be a major determinant of the input-output relationships of neurons. The amplitude of A-type currents in the dendrites of hippocampal CA1 pyramidal neurons is reduced in response to pilocarpine-induced seizures (Bernard et al., 2004), although the underlying mechanisms (changes in expression and localization of component subunits versus modulation of the activity of existing channels) are not known. To determine if pilocarpine-induced seizures altered the expression and localization of component subunits of dendritic A-type Kv channels, we stained sections from control and pilocarpine-SE animals with monoclonal antibodies specific for the transmembrane Kv4.2 and Kv4.3 α subunits, and KChIP1 and KChIP2 auxiliary subunits. In sections from control animals, the staining patterns for Kv4.2 and KChIP2 exhibit extensive overlap, being present at high levels in strata oriens and radiatum of CA3 and CA1 (Fig. 2). In each case the staining is associated with dendrites of pyramidal neurons, with very little staining found in the pyramidal cell bodies (Rhodes et al., 2004). The pattern of staining in CA3 pyramidal cell dendrites is not significantly altered in pilocarpine-SE animals, either at four or twelve weeks post-seizure (Fig. 2). However, in strata oriens and radiatum of CA1 there is a sharp decrease in staining for KChIP2 at both four and twelve weeks post-seizure, relative to control (Fig. 2). Similar but less pronounced changes in Kv4.2 staining are also observed in pilocarpine-SE subjects versus controls (Fig. 2). Such changes were observed in every rat that entered SE in response to pilocarpine treatment, and never observed in rats that received pilocarpine treatment but did not enter SE, or in sham injected rats (data not shown).
Dendrites of dentate granule cells express high levels of Kv4.2, Kv4.3 and KChIP2, yielding strong staining for these subunits in the molecular layer of the dentate gyrus (Figs. 2, 5). Staining is present throughout the molecular layer, although it is more pronounced in distal portions of the granule cell dendrites in the middle to outer third of the molecular layer (Fig. 5). In pilocarpine-SE animals, but not pilocarpine-treated animals that did note enter sustained SE or controls, there is a pronounced change in Kv4.2, Kv4.3 and KChIP2 immunoreactivity in the molecular layer of the dentate gyrus, such that the staining is no longer uniformly distributed across the molecular layer but becomes concentrated in just the outer two-thirds. Moreover, at twelve weeks post-seizure a sharp band of intense Kv4.2, Kv4.3 and KChIP2 immunoreactivity is observed at the border of the inner and middle third of the molecular layer (Fig. 5C, F, I). In control subjects, Kv4.3 and KChIP1 are found coexpressed in interneurons throughout the hippocampus (Fig. 3). In pilocarpine-SE subjects, there is a loss of KChIP1 immunoreactive interneurons that is most prominent in the dentate hilus. These changes are already apparent one week after pilocarpine treatment (not shown) and fully established by four weeks after pilocarpine treatment, with some recovery apparent at twelve weeks following status epilepticus (Fig. 3). KChIP1 staining in interneurons scattered through the pyramidal cell layer of CA3 is also lost in the pilocarpine-SE animals (Fig. 3). Whether a similar reduction in Kv4.3 staining in interneurons is also seen in pilocarpine-SE subjects is difficult to determine due to the presence of Kv4.3 staining on dendrites of dentate granule and CA3 pyramidal cells. Such changes were observed in every rat that entered sustained SE, but never observed in rats that received pilocarpine treatment but did not enter sustained SE, or in sham injected rats (data not shown).
Figure 5.
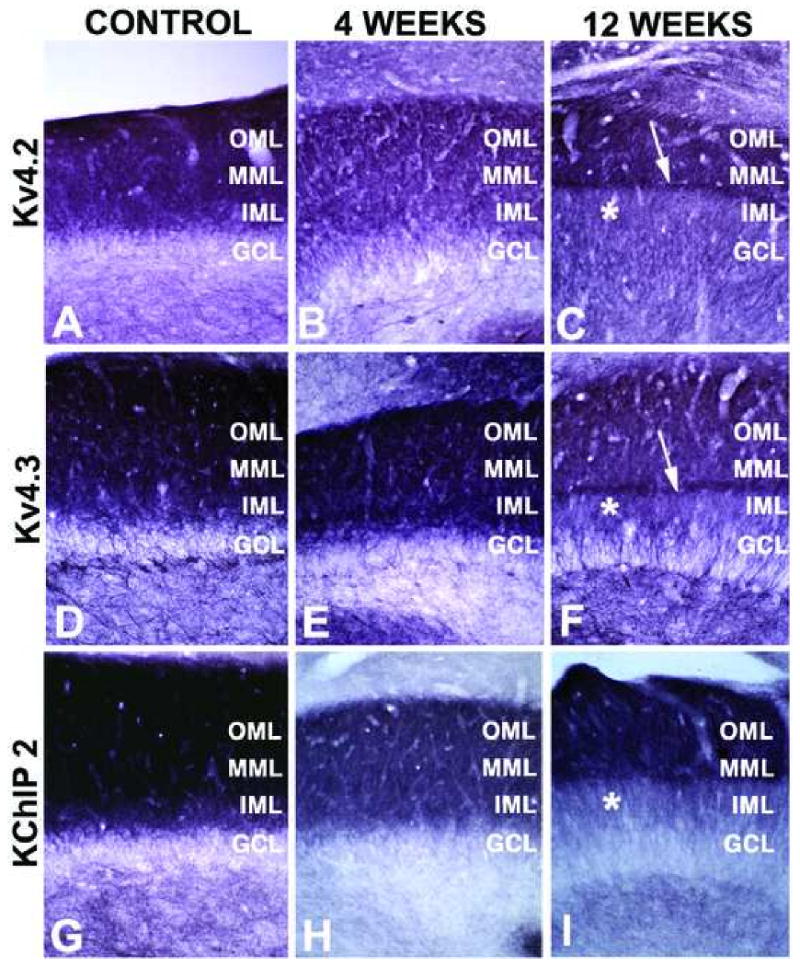
High magnification photomicrographs of Kv4 and KChIP2 immunoreactivity in the dentate gyrus of control and SE subjects. Photomicrographs of horizontal sections taken to show the areal and laminar distribution of Kv4.2 (A-C), and Kv4.3 (D-F), and KChIP2 (G-I) immunoreactivity in the hippocampus of a control rat (A, D, G) and rats four (B, E, H) and twelve (C, F, I) weeks after pilocarpine treatment. Note the progressive decrease in immunoreactivity for each of these channel subunits in the inner third of the molecular layer of the dentate gyrus, and the sharp boundary of staining at the boundary between the inner third and outer two-thirds of the molecular layer. Laminae marked as described in Figure 4 legend.
Quantitative analyses of changes in Kv4 and KChIP2 expression
We next used double label immunofluorescence staining to better quantify the changes in expression of Kv4.2 and KChIP2 in stratum radiatum of CA1 of pilocarpine-SE subjects. Our studies focused on pilocarpine-SE subjects one (n=3) and four (n=4) weeks after SE, during the period that has been established to lead to acquisition of the spontaneous, recurrent seizure phenotype, and used staining for the postsynaptic density protein PSD95 as both a marker for the overall density of dendrites/synapses, as well as an internal standard for the expected response to seizures (Cornejo et al., 2007). As with the DAB staining shown above, the effects of pilocarpine seizures on immunofluorescence staining for the A-type channel pore-forming Kv4.2 subunit were apparent in stratum radiatum of CA1, where a decrease in Kv4.2 staining was already apparent 1 week after the pilocarpine seizures (Fig. 6A-C). At this time point Kv4.2 staining decreased by 22.3 ±9.6% (n=3, p=0.56) in CA1 relative to that in control animals. At this time point staining for PSD95 had increased (144.8±9.7%; n=3, p= 0.1) in the seizure animals relative to that in controls, consistent with previous studies of seizure animals (Cornejo et al., 2007) and of human patients with epileptic cortical dysplasia (Ying et al., 2004). Decreased Kv4.2 staining was also apparent in the molecular layer of the dentate gyrus, although the changes here were minimal relative to that observed in CA1
Figure 6.
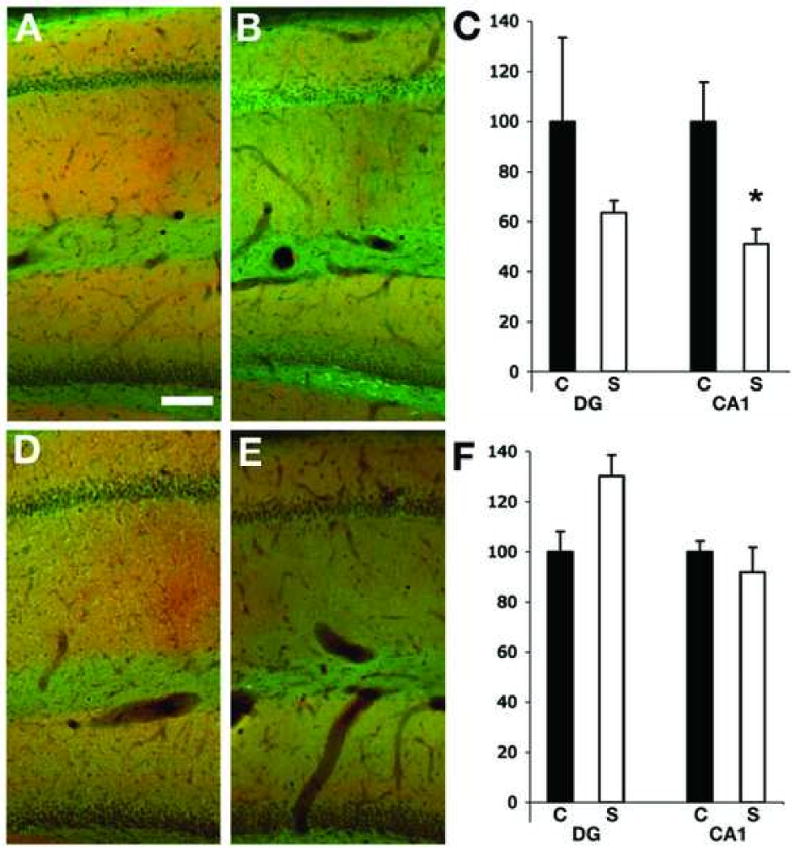
Expression of Kv4.2 and PSD 95 in the hippocampus of control and SE subjects. Double label immunofluorescence staining of sagittal hippocampal sections of sham- (A and D) and pilocarpine-injected rats 1 week (B) and 4 weeks (E) after pilocarpine treatment. Scale bar =100 μm. The fluorescence intensity for Kv4.2 and PSD95 staining was measured in the molecular layer of the DG and in the stratum radiatum of CA1, and the ratio Kv4.2/PSD95 was calculated for both regions in control and seizures animal at 1 week (C) and 4 weeks (F) post-SE. S= seizure animals (white column), C= control animals (black column). The decrease in Kv4.2 immunoreactivity was significant only in the CA1 region of seizure animals 1 week after the injection of pilocarpine (p=0.04, n=2 control and 3 seizure animals). In the DG the immunoreactivity for Kv4.2 showed a tendency to be decreased but that was not significant (p=0.5, n=3 controls and 4 seizure) at 4 weeks after pilocarpine treatment.
The decreases in Kv4.2 staining were even more apparent four weeks post-seizure (Fig. 6D-F), at which point Kv4.2 staining in stratum radiatum of CA1 had decreased by 30.8±6.6% (n=3, p=0.015) in seizure subjects relative to that in control subjects. However, at this time point PSD95 staining had also decreased by 19.2±9.5. Again, larger decreases were observed in stratum radiatum of CA1 than in the molecular layer of dentate gyrus, where little or no change in Kv4.2 staining was observed four week post-seizure. As with the DAB staining, changes in immunofluorescence staining for the A-type channel auxiliary KChIP2 subunit were more apparent than for Kv4.2. A decrease in KChIP2 staining in the CA1 region of the hippocampus is already apparent 1 week after seizures (Fig. 7A-C). At this time point KChIP2 staining decreased to 68.7±4.5% (p=0.01; n=3) in CA1 stratum radiatum relative to staining in control sections. At this time point staining for PSD95 increased in CA1 of the seizure subjects (150.7±19.5%, n=3) relative to control, similar to the value (144%) obtained in sections double stained for Kv4.2 and PSD95 (Fig 6). Decreased KChIP2 staining was also apparent in the molecular layer of the dentate gyrus, although the changes here (7.4±4.9% decrease) versus KChIP2 staining in control subjects were not significant. Increases in PSD95 staining in the molecular layer (145±11.9%, n=3) were again observed. The decrease in KChIP2 staining was even more apparent four weeks post-seizure (Fig. 7D-F), at which point KChIP2 staining in seizure subjects had decreased to 38.8 ±3.2% (n=3, p=0.001) versus control subjects. However, as in Fig. 6, PSD95 staining had also decreased at this time point, to 78.7±8.1% of control levels. Again, larger decreases were observed in stratum radiatum of CA1 at four weeks than were observed in the molecular later of dentate gyrus (73.0±7.4% of control values). However, in the molecular layer of the dentate gyrus the lower level of KChIP2 staining in seizure subjects versus controls was paralleled by changes in PSD95 staining (72.7±8.1%). These data support a specific change in the density of Kv4.2 and KChIP2 component subunits of dendritic A-type channels in the dendrites of CA1 pyramidal neurons, as opposed to a change in dendritic density per se.
Figure 7.
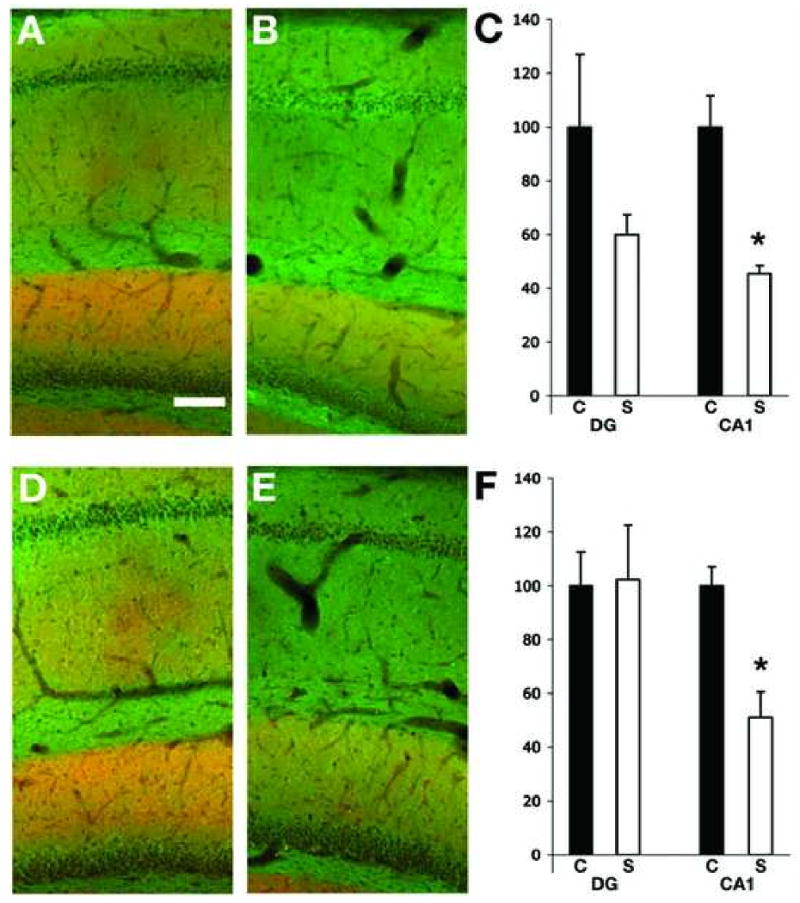
Expression of KChIP2 and PSD 95 in the hippocampus of control and SE subjects. Double label immunofluorescence staining of sagittal hippocampal sections of sham- (A and D) and pilocarpine-injected rats 1 week (B) and 4 weeks (E) after pilocarpine treatment. Scale bar =100 μm. The intensity for KChIP2 and PSD 95 was measured in the molecular layer of the DG and in the stratum radiatum of CA1, and the ratio KChIP2/PSD95 was calculated for both regions in control and seizures animal at 1 week (C) and 4 weeks (F) post-SE. S= seizure animals (white column), C= control animals (black column). A significant decrease in KChIP2 immunoreactivity in CA1 of pilocarpine treated animals was present at 1 week after pilocarpine treatment (p=0.01, n= 2 control and 3 seizure animals) and was also significant at 4 weeks after pilocarpine treatment (p=0.01, n= 3 control and 4 seizure animals). Changes in the molecular layer of the dentate gyrus were not significant.
Discussion
A-type Kv channels play critical roles in regulating neuronal excitability. Targeting of Kv1 family A-type channels to sites at or near presynaptic terminals, for example hippocampal mossy fiber boutons, confers upon these channels a prominent role in controlling transmitter release (Sheng et al., 1992, Maletic-Savatic et al., 1995, Debanne et al., 1997, Rhodes et al., 1997, Cooper et al., 1998, Pongs, 1999, Geiger and Jonas, 2000, Monaghan et al., 2001). Moreover, these channels can be reversibly modulated by activity-dependent phosphorylation (Roeper et al., 1997) suggesting that they can play a role in dynamic modulation of presynaptic function. The dramatic expansion of Kv1.4 staining in stratum lucidum of CA3 in pilocarpine-SE subjects suggests that expansion of the mossy fiber terminal field, due to SE-induced sprouting, is accompanied by parallel increases in targeting of Kv1.4 to the newly sprouted mossy fiber axons and terminals.
Increased Kv1.4 expression is also associated with the enhancement of recurrent mossy fiber terminals in the molecular layer of the dentate gyrus. Prominent Kv1.4 staining is observed in the middle third of the molecular layer of the dentate gyrus in control subjects (Sheng et al., 1992, Maletic-Savatic et al., 1995, Rhodes et al., 1997), due to its expression on axons and terminals of perforant path afferents from entorhinal cortex (Monaghan et al., 2001). However, in pilocarpine-SE subjects, a new band of prominent Kv1.4 staining was now observed in the inner third of the molecular layer, corresponding to the pilocarpine-SE-induced appearance of Timm's staining at this site. These data suggest that while the seizure-induced recurrent mossy fiber axons and terminals may be aberrant in their location, they have typical expression of presynaptic A-type Kv channel subunits. Presynaptic A-type channels on conventional mossy fiber boutons play a critical role in regulating glutamate release and as such regulate excitability within hippocampal circuits (Geiger and Jonas, 2000). Our data suggest that the function of the aberrant mossy fibers can be regulated by Kv1.4 subunits; enhancing activity of these channels through pharmacologic modulation may represent a potential therapeutic target for TLE.
Dendritic and somatodendritic A-type channels composed of Kv4.2, Kv4.3 and KChIP subunits have widespread expression in the hippocampus. However, their cellular and subcellular distribution varies across hippocampal subfields, such that different cells and subcellular domains may have characteristic complements of Kv4-containing A-type channels (Sheng et al., 1992, Serodio and Rudy, 1998, Rhodes et al., 2004). The precise subcellular localization of Kv4-containing A-type channels may dramatically impact the processing of synaptic input, levels of intracellular Ca2+, and the backpropagation of action potentials (Hoffman et al., 1997, Johnston et al., 2003). Moreover, Kv4-containing A-type channels are targets for modulation by reversible phosphorylation (Adams et al., 2000, Anderson et al., 2000, Jerng et al., 2004, Varga et al., 2004), such that these functions can be dynamically regulated. Kv4.2 is the predominant dendritic A-type channel on principal cells in the hippocampus, being expressed at high levels on the dendrites of dentate granule cells, and CA3 and CA1 pyramidal cells; this last group in the absence of detectable Kv4.3. Although the enhanced expression of Kv4.2 in the middle third of the molecular layer of the dentate gyrus seen here may reflect activity-dependent compensatory upregulation of Kv4.2 in response to mossy fiber sprouting and enhanced excitatory input to dentate granule cell dendrites, the dramatic reduction of Kv4.2 immunoreactivity in the inner third of the dentate molecular layer may reflect a fundamental change in excitability within the recurrent mossy fiber circuit. Loss of A-current density in this proximal region of the granule cell dendrite, a location that receives massive aberrant excitatory mossy fiber input following induction of SE and as spontaneous seizures develop, would lower the threshold for action potential firing and may directly contribute to the development of spontaneous recurrent seizures.
The mechanism underlying the altered compartmentalization of these A-type Kv channel subunits in dentate granule cell dendrites of SE subjects is not known. Presumably, the Kv4 and KChIP subunits at these sites are synthesized in the granule cell soma and transported to the dendrite (Rivera et al., 2003). Whether the loss of these subunits in the inner third of the molecular layer, and their concentration in the middle third involves alteration in this initial transport, or differences in clustering at specific sites, in the SE subjects is not yet known. Immunohistochemical studies in naïve mouse brain with phosphospecific antibodies reveal that Kv4.2 located on dentate granule cell dendrites exhibits a specific pattern of phosphorylation, with little immunoreactivity for antibodies specific for ERK/MAP kinase and PKA phosphorylation sites relative to other hippocampal areas (Varga et al., 2000). It is intriguing that the localization of Kv4.2 on cerebellar granule cell dendrites co-cultured with pontine grey nuclear neurons is dramatically altered by cerebellar mossy fiber synaptic input, by a mechanism that involves active transmission through ionotropic glutamate receptors and activation of downstream Ca2+-dependent signaling pathways (Shibasaki et al., 2004). The striking appearance of Kv4 and KChIP subunits in the middle third of the molecular layer in SE subjects resembles that of Arc mRNA that is locally translated at this site in response to medial perforant path activity (Steward et al., 1998). Which of these mechanisms (altered transport, changes in phosphorylation state associated with altered protein-protein interactions, local translation) or others that yield this prominent change in localization is not known. That these Kv4 channel subunits accumulate at a site of altered activity, in the form of increased recurrent mossy fiber input, does suggest activity-dependent regulation of subcellular localization. The altered localization of Kv4 channel subunits on dentate granule cell dendrites that is induced in this experimental model of TLE provides an opportunity for future studies aimed at identifying the mechanism underlying subcellular compartmentalization of A-type Kv4 channels on dendrites and its activity-dependent regulation.
We found that pilocarpine induces a decrease in the density of Kv4.2 and KChIP2 component subunits of A-type channels in stratum oriens and stratum radiatum of CA1. A recent study found increased dendritic excitability in CA1 pyramidal cell dendrites in response to the same pilocarpine model of TLE used here (Bernard et al., 2004). Decreased levels of Kv4.2 immunoreactivity on immunoblots of membrane fractions isolated from hippocampal CA1 of pilocarpine-SE rats relative to controls were also observed (Bernard et al., 2004). The authors proposed that a reduction in dendritic A-type Kv currents (as mediated by Kv4 channels) in CA1 pyramidal cells contributes to the acquisition of spontaneous seizures (Bernard et al., 2004). Our immunohistochemical findings support these electrophysiology and biochemistry data and suggest that decreased levels of A-type Kv currents and Kv4.2 protein, are based, at least in part, on the sustained decreased expression of Kv4.2/KChIP2 subunits in CA1 pyramidal cell dendrites in the pilocarpine model of TLE.
A previous study found that hippocampal nodular heterotopias, which are prone to hyperexcitability, also have reduced expression of Kv4.2 (Castro et al., 2001). In situ hybridization studies revealed that generalized seizures induced by pentylenetetrazole (Tsaur et al., 1992) or kainic acid (Francis et al., 1997) altered the regional hippocampal gene expression of rat Kv4.2. Moreover, a mutation resulting in expression of a truncated Kv4.2 subunit was recently described in a human patient with TLE (Singh et al., 2006). Together, these studies suggest that Kv4.2 expression, localization and function are in general highly responsive to changes in neuronal activity, especially under conditions of hyperexcitability associated with epileptic seizures, and that such changes may provide for the acquisition of more permanent seizures.
Recently, Schwartz and Jung (Guo et al., 2005) generated a Kv4.2 knockout mouse. These mice exhibit a complete loss of Kv4.2, and in the hippocampus as well as other brain areas do not exhibit any detectable compensatory upregulation of Kv4.3 (Menegola and Trimmer, 2006). In addition to the complete loss of Kv4.2, the expression of KChIP2 is reduced in stratum radiatum of CA1 and in the molecular layer of the dentate gyrus (Menegola and Trimmer, 2006) in a manner similar to what we observed here in the pilocarpine model of acquired TLE. As such it is intriguing that the Kv4.2 knock-out mice do not exhibit an overt seizure phenotype (Hu et al., 2006), perhaps due to posttranslational upregulation of other Kv currents (Nerbonne et al., 2008) in the absence of increased expression of Kv channels subunits (Chen et al., 2006, Menegola and Trimmer, 2006, Nerbonne et al., 2008). Whether this mode of compensation in response to prolonged loss of dendritic A current also occurs in response to the acute loss of Kv4.2 and KChIP2 in response to pilocarpine-induced SE is not known. Experiments analyzing the seizure phenotype of wild-type adult animals subjected to an acute (i.e. inducible) knockdown of Kv4.2 in CA1 pyramidal neurons and dentate granule cells will allow for insights into the specific contribution of the reduced Kv4.2 levels to the seizure phenotype of the pilocarpine subjects, versus other known consequences of pilocarpine treatment (mossy fiber sprouting, affects on interneurons, etc.).
Our studies show that the cellular and subcellular expression of multiple component subunits of dendritic/somatodendritic A-type channels in specific hippocampal subfields are altered by pilocarpine-induced seizures. Moreover, these changes occur in the time frame that corresponds to the acquisition of the permanent spontaneous seizure phenotype in these subjects. Changes in A-type Kv1.4-containing channels in mossy fiber presynaptic terminals, and Kv4.2-containing channels in dentate granule cell and CA1 dendrites, represent attractive targets for intervening in the acquisition of the permanent epileptic phenotype in this animal model of human TLE.
Acknowledgments
Supported by NIH grants NS42225 and NS34383 (to J.S.T.), and by Wyeth Research. M.M. acknowledges postdoctoral fellowship from the Epilepsy Foundation through the generous support of the American Epilepsy Society and the Milken Family Foundation. The authors thank Dr. Mark Bowlby and Mr. Robert Arias for their assistance with the pilocarpine seizure model, and Drs. Philip A. Schwartzkroin and H. Jürgen Wenzel for helpful comments on the manuscript. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275:5337–5346. doi: 10.1074/jbc.275.8.5337. [DOI] [PubMed] [Google Scholar]
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GAbaergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. doi: 10.1002/hipo.1060. [DOI] [PubMed] [Google Scholar]
- Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42(Suppl 3):2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Palma E, Piccirilli S, Del Duca C, Morrone AL, Nappi G, Corasaniti MT, Dolly JO. Involvement of a glutamergic mechanism in gamma-dendrotoxin-induced hippocampal neuronal cell loss in the rat. Basic Clin Pharmacol Toxicol. 2004;94:132–138. doi: 10.1111/j.1742-7843.2004.pto940306.x. [DOI] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Castro PA, Cooper EC, Lowenstein DH, Baraban SC. Hippocampal heterotopia lack functional Kv4.2 potassium channels in the methylazoxymethanol model of cortical malformations and epilepsy. J Neurosci. 2001;21:6626–6634. doi: 10.1523/JNEUROSCI.21-17-06626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–426. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Danscher G, Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry. 1978;55:27–40. doi: 10.1007/BF00496691. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–217. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Francis J, Jugloff DG, Mingo NS, Wallace MC, Jones OT, Burnham WM, Eubanks JH. Kainic acid-induced generalized seizures alter the regional hippocampal expression of the rat Kv4.2 potassium channel gene. Neurosci Lett. 1997;232:91–94. doi: 10.1016/s0304-3940(97)00593-4. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Kiener T, Bouilleret V, Loup F. GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–445. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol. 1999;81:408–411. doi: 10.1152/jn.1999.81.1.408. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal cells. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RWt. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D'Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola M, Trimmer JS. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci. 2006;26:12137–12142. doi: 10.1523/JNEUROSCI.2783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan MM, Trimmer JS, Rhodes KJ. Experimental localization of Kv1 family voltage-gated K+ channel alpha and beta subunits in rat hippocampal formation. J Neurosci. 2001;21:5973–5983. doi: 10.1523/JNEUROSCI.21-16-05973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Petrou S, Berkovic SF. Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol. 2003;16:171–176. doi: 10.1097/01.wco.0000063767.15877.c7. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Gerber BR, Norris A, Burkhalter A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol. 2008;586:1565–1579. doi: 10.1113/jphysiol.2007.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, Zarei M, Knaus HG, Garrido-Sanabria ER. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2008;1200C:116–131. doi: 10.1016/j.brainres.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol. 1998;79:418–429. doi: 10.1152/jn.1998.79.1.418. [DOI] [PubMed] [Google Scholar]
- Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999;452:31–35. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci. 1995;15:5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci. 2003;6:243–250. doi: 10.1038/nn1020. [DOI] [PubMed] [Google Scholar]
- Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel Kv1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+. A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Nakahira K, Trimmer JS, Shibata R, Akita M, Watanabe S, Ikenaka K. Mossy fibre contact triggers the targeting of Kv4.2 potassium channels to dendrites and synapses in developing cerebellar granule neurons. J Neurochem. 2004;89:897–907. doi: 10.1111/j.1471-4159.2004.02368.x. [DOI] [PubMed] [Google Scholar]
- Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, Matsuda K, Inoue Y, Yamakawa K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–253. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Staley K. Epileptic neurons go wireless. Science. 2004;305:482–483. doi: 10.1126/science.1101133. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986;34:1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Sheng M, Lowenstein DH, Jan YN, Jan LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn Mem. 2000;7:321–332. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- Xu JH, Long L, Tang YC, Hu HT, Tang FR. Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus. 2007;17:235–251. doi: 10.1002/hipo.20263. [DOI] [PubMed] [Google Scholar]
- Ying Z, Bingaman W, Najm IM. Increased numbers of coassembled PSD-95 to NMDA-receptor subunits NR2B and NR1 in human epileptic cortical dysplasia. Epilepsia. 2004;45:314–321. doi: 10.1111/j.0013-9580.2004.37703.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram:experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]


