Abstract
Hyaluronic acid (HA), a glycosaminoglycan located between keratinocytes in the epidermis, accumulates dramatically following skin wounding. To study inductive mechanisms, a rat keratinocyte organotypic culture model that faithfully mimics HA metabolism was used. Organotypic cultures were needle-punctured 100 times, incubated for up to 24h, and HA analyzed by histochemical and biochemical methods. By 15 min post-injury, HA levels were elevated 2 fold, increasing to 4-fold by 24 h. HA elevations far from the site of injury suggested the possible involvement of a soluble HA-inductive factor. Media transfer experiments (from wounded cultures to unwounded cultures) confirmed the existence of a soluble factor. From previous evidence, we hypothesized that an EGF-like growth factor might be responsible. This was confirmed as follows: (1), EGF receptor (EGFR) kinase inhibitor (AG1478) completely prevented wounding-induced HA accumulation. (2), Rapid tyrosine-phosphorylation of EGFR correlated nicely with the onset of increased HA synthesis. (3), A neutralizing antibody that recognizes HB-EGF blocked wounding-induced HA synthesis by ≥ 50%. (4), Western analyses demonstrated that release of activated HB-EGF (but not amphiregulin nor EGF) occurs after wounding. In summary, rapid HA accumulation after epidermal wounding occurs via a mechanism requiring cleavage of HB-EGF and activation of EGFR signaling.
INTRODUCTION
Hyaluronic acid (HA; also called hyaluronan) is a high molecular weight carbohydrate polymer that plays a role in tissue growth, development, wound healing, and inflammation. HA consists of a disaccharide unit (-β1,4-glucuronic acid-β1,3-N-acetylglucosamine-) repeated several thousand times. HA can be considered an “honorary proteoglycan,” i.e., a pure glycosaminoglycan without any protein component. Because of its tremendous size (over 2 million Daltons) and unusual physiochemical properties that allow it to retain large amounts of solvent, HA is an important molecule with space-filling, lubricating, and filtering functions in connective tissues. With no sulfate groups nor other charge modifications, HA might at first glance appear to be a passive molecule. However, HA is actually very dynamic, interacting with specific proteins in the matrix and with receptors on the cell surface to mediate physiological changes in cells and tissues (Day and Prestwich, 2002; Hascall and Laurent, 1997; Hascall et al., 2004; Toole et al., 2002; Turley et al., 2002).
The effects of HA upon living cells in tissues can be profound. A high level of HA in the extracellular matrix (ECM) promotes proliferation and migration of normal cells, as well as invasion and metastases of malignant cells (Toole et al., 2002). Low levels of HA, on the other hand, tend to correlate with cellular differentiation (Agren et al., 1997; Toole et al., 2002). In general, the ECM becomes enriched in HA during episodes of rapid cellular migration and proliferation, both in developing and in regenerating tissues. HA-rich matrices also have the capacity to bind growth factors, which in turn can influence both cell growth and differentiation by manipulating local concentrations of the factors. High levels of HA in embryonic and fetal tissues are essential during embryonic development. For example, mice lacking the gene for HA synthase 2 (Has2), a major synthetic enzyme for HA, show striking defects in cardiac development due to failure in the epithelial-mesenchymal transition (Camenisch et al., 2000).
An important question is whether or not HA is essential for the cellular migration and proliferation that occurs in a healing wound. Much evidence supports this notion. For example, large amounts of HA are found in healing skin, especially at early stages of tissue repair (West et al., 1997). The high levels of HA are found in both dermal and epidermal components of the skin. HA is a major component of early granulation tissue, and fibroblasts isolated from early wounds produce more HA than do fibroblasts from normal skin ((Clark, 1996), page 27). High levels of HA correlate with improved repair and scarless wound healing in both fetal sheep (West et al., 1997) and in mice (Mack et al., 2003). In the epithelial layer (epidermis), it has been shown that levels of HA are increased for many days following disruption of the epidermis by tape stripping (Tammi et al., 2005). Injury to the stratum corneum of the skin by friction and solvents (so-called “barrier injury”) leads to accumulations of HA nearly as large in magnitude as those seen after mechanical wounding (Maytin et al., 2004). Because wounding of the skin causes the release of proinflammatory cytokines (Clark, 1996; Wood et al., 1992), and because cytokines and other peptide factors are known to stimulate increased transcription of HA-synthetic enzymes (Karvinen et al., 2003; Pienimaki et al., 2001), it appears likely that wounding-stimulated production of such factors might participate in the increased levels of HA that one sees in the skin. Once produced, HA in its native form interacts with cell-surface receptors (CD44 and RHAMM) to regulate intracellular signaling (Turley et al., 2002), or the HA can be degraded into small fragments (by hyaluronidase enzymes) and interact with toll-like receptors (TLR4) on cell surfaces (Taylor et al., 2004). Changes in HA expression that occur in injured tissues can have profound effects upon the migration and activation of inflammatory cells including monocytes and macrophages (reviewed in (Hascall et al., 2004)). Therefore, injury-induced changes in HA are likely to be critical for regulating the influx of inflammatory cells that occurs immediately after wounding of the skin.
Despite the abundance and apparent importance of cutaneous HA, relatively little is actually known about the mechanisms that govern its synthesis and degradation in the skin, nor about the functional impact of changes in HA expression. Because the epidermis (outer epithelial layer) of the skin serves as the primary environmental barrier in vertebrates, with dire consequences in terms of severe infection and fluid loss when the epidermis is disrupted, we have been focusing our studies on understanding HA metabolism and function in this tissue. To avoid some of the uncontrollable variables associated with whole-animal studies, we employed an in vitro three-dimensional model (organotypic epidermal cultures) in which immortalized rat epidermal keratinocytes (REKs) are allowed to stratify on a collagen substrate in the absence of fibroblasts. This system generates a multi-layered epidermal tissue with the same morphological features, functional barrier properties, and responses to barrier injury as the native epidermis (Ajani et al., 2007; Pasonen-Seppanen et al., 2001; Passi et al., 2004; Tammi et al., 2000). Because our model epidermis has a fully-intact basement membrane beneath the keratinocytes and an impervious stratum corneum at the top (Tammi et al., 2000), any HA produced by the epidermal cells remains entirely within the epidermal compartment, mimicking the situation of the true epidermis in vivo (Passi et al., 2004). In this paper, we use the organotypic model to investigate mechanisms of HA induction in response to mechanical injury (puncture wounding) of epidermal tissue. Our data demonstrate significant changes in HA levels, and elucidate a response whereby mechanical injury leads to release of a soluble factor that mediates an increased synthesis of HA in distant cells. A major component of this soluble signal turns out to be the well-known growth factor, HB-EGF.
RESULTS
Induction of epidermal HA levels after skin wounding can be modeled in REK 3-D organotypic cultures
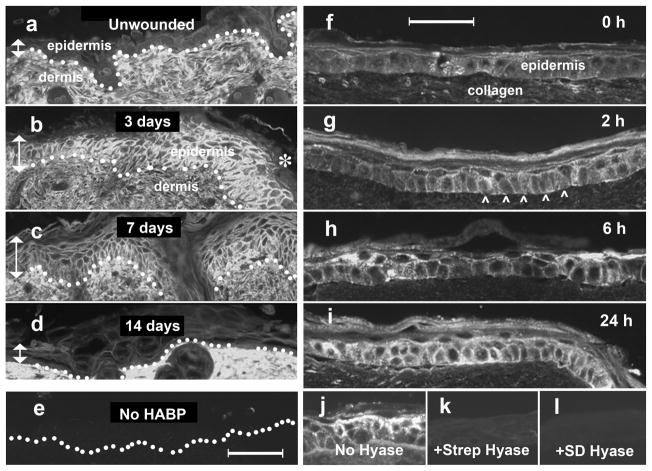
Scalpel injury of native skin causes a robust increase in epidermal HA, with highly elevated levels of HA seen at the edges of the wound (Fig. 1). In vivo, these increases are first detectable by 24 h (data not shown), reach a maximum by 3 d (Fig. 1b), start to decline by one week (Fig. 1c), and completely resolve by two weeks (Fig. 1d). To replicate this phenomenon in the organotypic model, REK lift cultures were injured by evenly-spaced needle punctures (100 stabs administered ~2 mm apart over the surface of the culture; see Materials and Methods) and then analyzed histologically using a “cigarette roll” method followed by immunochemical staining for HA. Increased HA levels were seen within 2 h (Fig. 1g), and rose steadily at 6 h and 24 h (Fig. 1h, i). Specificity of the HA-binding probe was demonstrated by pre-digesting the tissue specimens with two different bacterial hyaluronidases, each of which abrogated the signal (Fig. 1j – l).
Figure 1. Levels of HA are increased after epidermal wounding in the skin (in vivo) and in REK lift cultures (in vitro).
(a – e), Staining of HA in wounded skin. Anesthetized C57BL/6J mice were wounded with a scalpel, and at designated times post-wounding the skin was biopsied, fixed in paraffin, and processed for histochemical staining using biotinylated HA-binding protein (HABP) and avidin-conjugated Cy3 (bright signal). Photomicrographs were taken immediately lateral to the wound edge (e.g., asterisk in panel b). Locations of epidermis, and dermis are shown. Vertical arrows, height of epidermis. Dotted line, dermal-epidermal junction. Scale bar, 100 μm. Panels (f – i), staining of HA in REK organotypic cultures after puncture wounding. Following puncture injury with a 28-gauge needle, cultures were incubated for the times indicated, harvested, paraffin-embedded, and stained with biotinylated HABP/Cy3 (bright signal). Arrowheads, location of basement membrane. Scale bar, 50 μm. (j, k, l), Experiment to demonstrate HA-specificity of HABP staining. Three paraffin sections from the same needle-injured REK specimen were incubated overnight with (j) saline, (k) Streptomyces hyaluronidase, or (l) Strep. dysgalactiae hyaluronidase, then stained with HABP (see Materials and Methods for details).
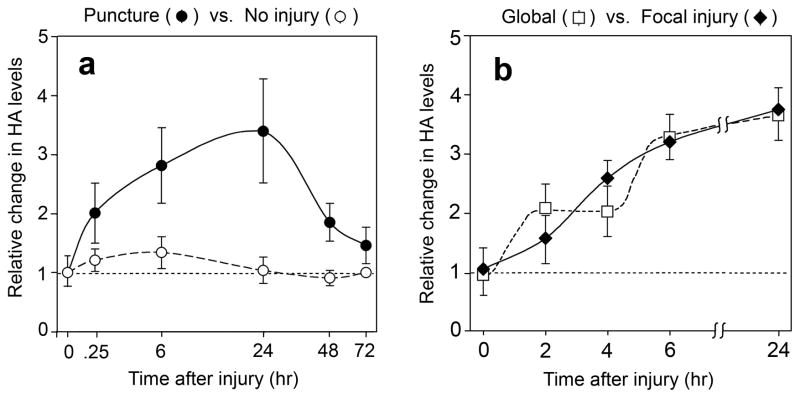
Following a peak at 24 h after injury, HA levels declined at 48 h and 72 h (Fig. 2a, closed circles). To rule out the possibility that increases in HA might be due to stress or changes in carbohydrate metabolism related to manipulation of the cultures during puncture, the experiment was conducted using time-matched sham controls that were handled identically except for receiving no needle injury; no significant induction of HA was observed in the control cultures (Fig. 2a, open circles).
Figure 2. Time course of induction of HA levels following puncture wounding of REK lift cultures.
(a), Change in HA signal in wounded cultures relative to time-matched uninjured control cultures, as a function of time (logarithmic scale) after injury. Data from wounded cultures (closed circles) or unwounded controls (open circles) are expressed relative to uninjured time zero controls. HA fluorescent stain intensity was quantified using imaging processing as described in Materials and Methods. Data are pooled from between 3 and 5 experiments per time point, and reported as the mean ± SD. (b), The magnitude and location of increases in HA are independent of the site of injury. A 28-gauge needle was used to deliver the punctures in either a random, global pattern (open squares) or in a focal (central linear) pattern (closed diamonds) over the surface of the culture. Cultures were harvested, stained with HABP/Cy3, and relative HA levels throughout the culture quantified by image processing. Bars represent the mean ± range of 2 experiments.
We wished to ask whether accumulation of HA in the lift cultures occurs only locally at the site of injury, or whether the effects of injury are more widespread. As an initial approach, we compared two patterns of injury, one in which 100 needle punctures were delivered evenly over the entire culture surface (Fig. 2b, open squares), versus a more localized pattern in which the punctures were delivered in a few closely-spaced rows down the center of the culture (Fig. 2b, closed diamonds). In both cases HA induction was observed throughout the culture, and in the cultures receiving localized injury, HA induction was observed at least 1 cm away from the nearest puncture site. Two formal possibilities to explain stimulation of HA accumulation at such a distance include: (i) the production and release of a soluble paracrine factor; (ii) a juxtacrine or biomechanical inductive effect between cells. In this paper, we have examined the first possibility.
The induction of epidermal HA is mediated by a soluble factor
To test the hypothesis that a soluble factor may be responsible for wounding-induced accumulation of HA, experiments were designed as in Fig. 3. Paired sets of “donor” and “recipient” organotypic cultures were used for these studies. Keratinocytes were seeded, grown submerged for 2 days to achieve confluency, then lifted for 5 d to the air interface to promote stratification. At the desired time prior to harvest, the donor cultures were injured with a needle (at the time labeled “puncture” in Fig. 3) and at the end of day 7, harvested for HA analysis. Concurrently, conditioned media from the donor cultures were transferred to corresponding recipient cultures (Fig. 3). Recipient cultures were incubated for a further 24 h and then harvested for HA analysis. To control for the effects of glucose depletion (knowing that rapid HA turnover is a major contributor to glucose consumption in REK lift cultures (Tammi et al., 2001), (Passi et al., 2004), culture media were changed daily and the experimental design (Fig. 3) insured that all cultures were in a given medium for identical periods of time.
Figure 3. Protocol for media transfer experiments.
Time line for experiments involving puncture injury of REK lift cultures, followed by transfer of the culture media to uninjured recipient cultures. (See text.)
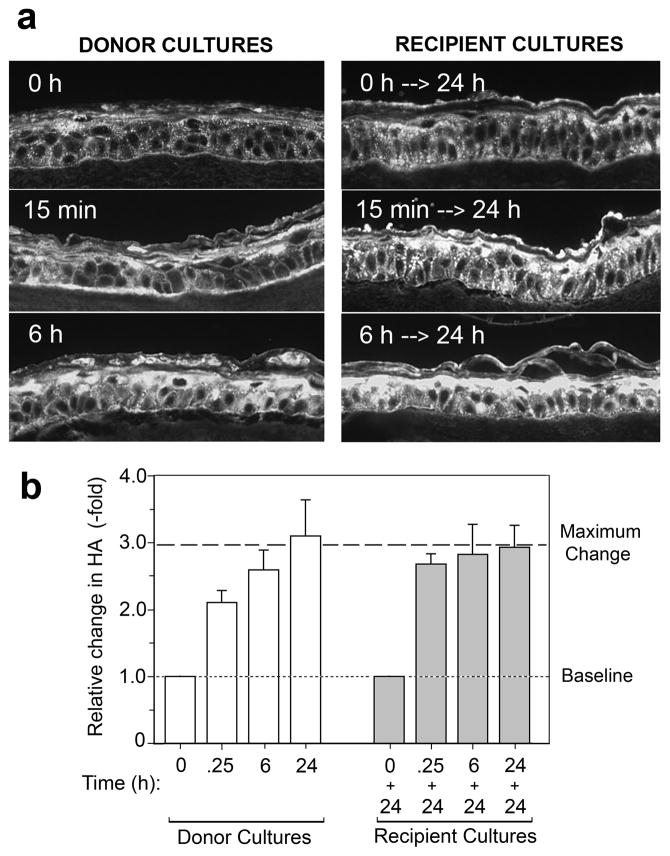
Results of media transfer experiments in which HA was detected immunochemically are illustrated in Fig. 4. In the punctured donor cultures, a significant increase in HA was observed at 15 min after injury, and HA levels continued to rise at 6 h and 24 h (Fig. 4a, left side, and Fig. 4b, open bars). In the uninjured recipient cultures, exposure to conditioned medium derived from the donor cultures caused a strong HA induction (Fig. 4a, right side), confirming our prediction of a bioactive factor in the media. HA levels achieved in the recipient cultures after 24 h exposure to conditioned medium (Fig. 4b, closed bars) were essentially identical to the HA level reached in donor cultures 24 h after injury (dotted line), suggesting that whatever factor(s) are released into the medium exert a similar HA-elevating effect in both sets of cultures.
Figure 4. HA production is increased in normal REK lift cultures treated with conditioned medium from injured cultures.
Donor cultures were punctured in a random pattern, and the medium was conditioned for various times (0 h, 15 min, 6 h, or 24 h) prior to transferring it to a second set of cultures on day 7. The donor cultures were harvested, while the recipient cultures were incubated for another 24 h prior to harvest (see Fig. 3 for protocol). (a), Immunofluorescent micrographs of 5 μm sections of paraffin-fixed REK cultures following staining with bHABP and Cy3-avidin for detection of HA (bright signal). (b), Quantitation of the relative increase in HA in the donor cultures as a function of time, determined by image processing of digital micrographs. Data are pooled from 2 experiments, 10 microscopic fields per experiment, mean ± range. Note that HA levels achieved in the recipient cultures (closed bars) after 24 h in conditioned medium are nearly identical to the maximum HA level (Max) reached in the donor cultures after 24 h (dotted line).
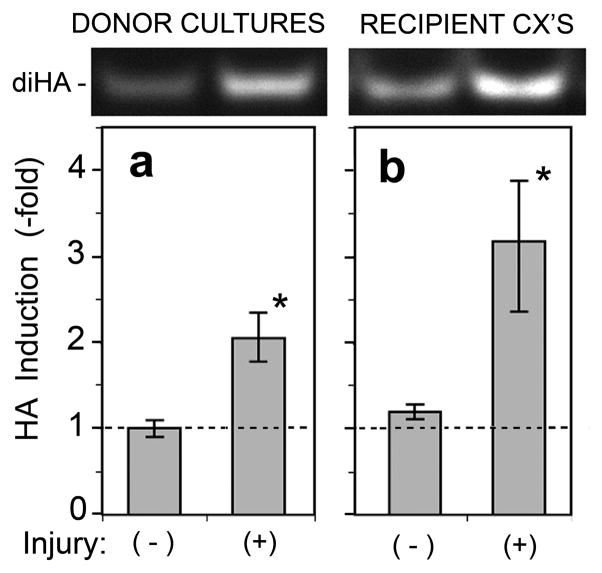
To confirm these apparent changes in HA by an independent method, we analyzed the organotypic cultures using a biochemical technique called FACE (fluorophore assisted carbohydrate electrophoresis). This method quantifies the total mass of HA in a sample by enzymatic degradation to the minimal disaccharide unit, followed by separation on an acrylamide gel (Fig. 5, top panels). During media transfer experiments, the total amount of HA in both the injured cultures (Fig. 5a) and the recipient cultures (Fig. 5b) was significantly increased as a result of puncture injury.
Figure 5. Biochemical confirmation of wounding-induced HA accumulation observed after direct injury, or following incubation with conditioned media from injured cultures.
REK lift cultures were injured by needle puncture, as per the protocol illustrated in Fig. 3. (a), Donor cultures were harvested at 6 h after injury; (b), the conditioned media was transferred to recipient cultures which were then harvested 24 h later. Cultures were lysed for carbohydrate analyses on acrylamide gels using the FACE technique (see Methods). The HA disaccharide bands (diHA) are shown at the top. Graphs show relative induction of HA measured densitometrically; data pooled from 5 independent experiments for (a), and 6 independent experiments for (b); mean ± SEM; (*), p < 0.05.
Wounding leads to increased expresssion of HA synthetic (Has) enzymes
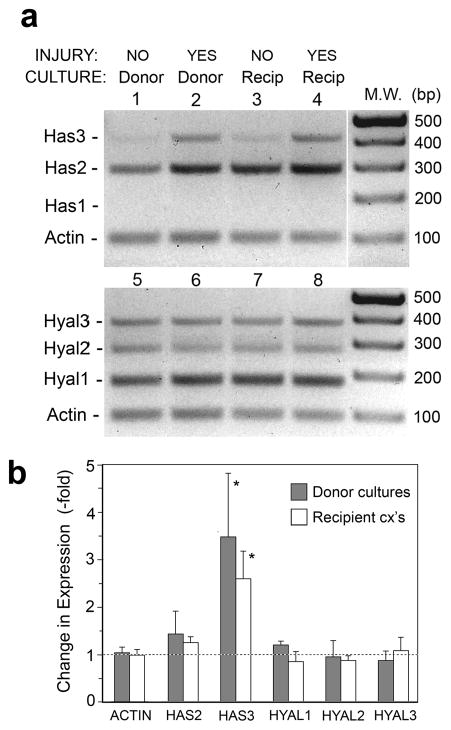
HA accumulation after wounding might be the net result of increased synthesis, decreased degradation, or both. To distinguish between these potential mechanisms, expression of all major mammalian HA synthetic enzymes and catabolic enzymes was measured by RT-PCR. In a slightly modified version of the protocol in Fig 3, donor cultures were harvested at 6 h after puncture and recipient cultures were harvested at 6 h after media transfer. The mRNA levels of Has enzymes (Has1, Has2, and Has3) and Hyal enzymes (Hyal1, Hyal2, and Hyal3) were measured using rat-specific primers designed to yield amplification products appropriately sized to allow simultaneous display on a single gel (see Fig. 6). Puncture injury led to increased levels of Has2 and Has3, both in the donor and recipient cultures (Fig. 6a), with a significant 3-fold increase for Has3 (Fig. 6b). Has1, as previously reported in REKs, was not detectable (Pienimaki et al., 2001). Expression of Hyal enzymes was essentially unchanged, suggesting that elevated HA levels after injury may result primarily from increased HA-synthetic activity rather than from decreased HA catabolism.
Figure 6. Increased HA production after injury coincides with an upregulation of the Has2 and Has3 genes.
Donor cultures were either not injured (NO) or injured (YES) as indicated, then incubated for 6 h and donor cultures harvested for RNA analysis. Conditioned medium was transferred from donor to recipient cultures and incubated for a further 6 h, then harvested for RT-PCR analysis. (a), Multiplex RT-PCR analyses of all the Has and Hyal genes, and β-actin (Actin) as an invariant control. Primer pairs were designed to yield well-separated product sizes. Each PCR reaction was run separately, and the four reactions were combined per lane to yield a convenient display. Note the apparent upregulation of Has2 and Has3 in lanes 2 and 4. (b), Densitometric scanning of bands. Data show changes relative to noninjured controls (mean ± SD), averaged from three independent culture experiments and normalized to actin. (*), Increase is significant at p <0.05 level.
Induction of HA synthesis after puncture wounding requires activation of the EGFR pathway
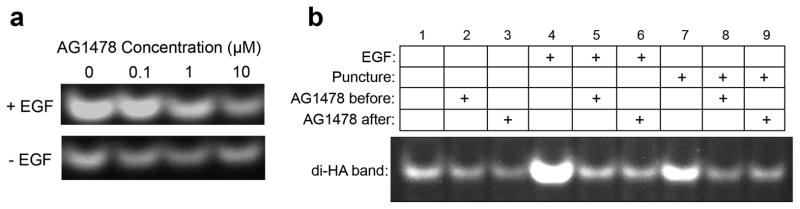
Several lines of evidence suggested that EGF growth factor receptors (synonyms include EGFR, ErbB1, and HER1 receptors; see (Harris et al., 2003; Pastore et al., 2008; Sahin et al., 2004) for reviews) are potentially involved in the HA response to injury. The Tammi laboratory had shown in REK cells that several growth factors, including exogenously-added EGF and KGF (FGF-7), are strong inducers of HA synthesis through enhanced expression of the Has2 gene (Karvinen et al., 2003; Pienimaki et al., 2001; Saavalainen et al., 2005). Elevated HA levels after retinoic acid treatment in the REK system requires EGFR activation (Pasonen-Seppanen et al., 2008). Interestingly, many other laboratories have shown that migration of keratinocytes and corneal epithelial cells after wounding is blocked by inhibitors of EGFR tyrosine kinase (Block et al., 2004; Xu et al., 2006) and involves the shedding of HER family ligands, including HB-EGF (Shirakata et al., 2005; Tokumaru et al., 2000). Thus, we hypothesized that EGFR and HER-family ligands might be linked to wounding-induced HA synthesis. AG1478 is an inhibitor of EGFR kinase. In an initial titration, increasing doses of AG1478 were tested for their ability to block induction of HA caused by exogenously-added EGF; a concentration of 10 μM AG1478 was maximally effective (Fig. 7a, top row) while not inhibiting HA levels in the control cells (Fig. 7a, bottom row). Using 10 μM AG1478, a puncture-transfer experiment was then performed to examine changes in HA (Fig. 7b). After puncture, the wounding-induced HA increase (Fig. 7b, compare lanes 1 and 7) was blocked when the inhibitor was added either before puncture (lanes 2 and 8), or at the time of media transfer (lanes 3 and 9). The inhibitor blocked HA induction by the positive control (exogenously-added EGF) as expected (lanes 4, 5 and 6).
Figure 7. Injury-induced HA production can be blocked by AG1478, an inhibitor of the epidermal growth factor receptor (EGFR).
(a), Titration experiment in which a positive control (addition of exogenous EGF, 20 ng/ml for 6 h) was applied in the presence of different doses of inhibitor as indicated. (b), Complete injury experiment in which lift cultures were punctured 6 h prior to transferring the medium to a second (recipient) culture. Lanes 1-3, negative controls (no injury). Lanes 4-6, positive controls (EGF). Lanes 7-9, injured cultures. The REK lift cultures were incubated with AG1478 (10 μM) for 1 hr before injury, or just after media transfer, as indicated above the figure. Lanes 1, 4, 7, no inhibitor added; Lanes 2, 5, 8, AG1478 added 1 h before injury; Lanes 3, 6, 9, AG1478 added at the point of media transfer. Recipient cultures were incubated for 24 h, harvested, and analyzed for HA content by FACE. Pre-incubation with AG1478 appeared to block EGF- or puncture injury-induced HA production in both the donor and recipient cultures. AG1478 added at the point of medium transfer had a similar effect in the recipient cultures.
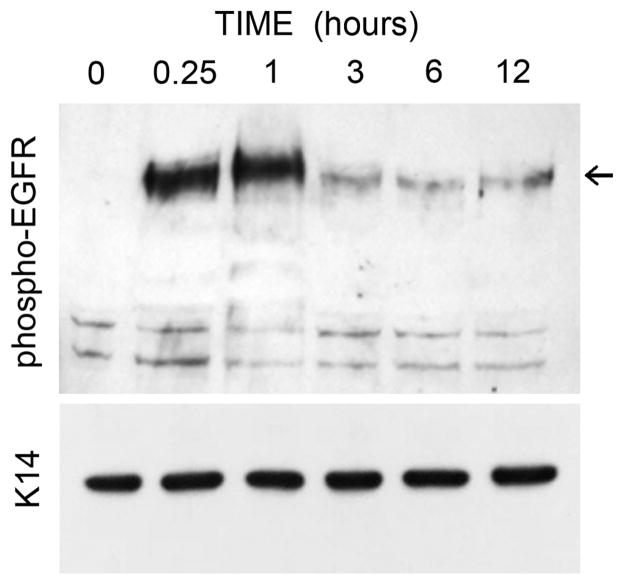
To more directly examine the status of the EGFR, lift cultures were punctured and the cell layer collected at various time points after injury to examine the autophosphorylation status of the receptor. EGFR was rapidly phosphorylated on tyrosine residues within 15 min, and remained activated at 1 hr but showed downregulation by 3 h (Fig. 8). Overall, these data support the idea that induction of HA after puncture injury requires activation of the EGFR receptor.
Figure 8. Activation (phosphorylation) of EGFR occurs rapidly after puncture injury.
REK lift cultures were harvested at the indicated times after needle puncture, and equal amounts of protein were separated on gels for Western analysis as described in Materials and Methods. Arrows, locations of p-EGFR (~178 kD). Keratin 14 (K14) served as a loading control (~55 kD).
Identification of HB-EGF as an EGFR ligand released after wounding of organotypic REK cultures
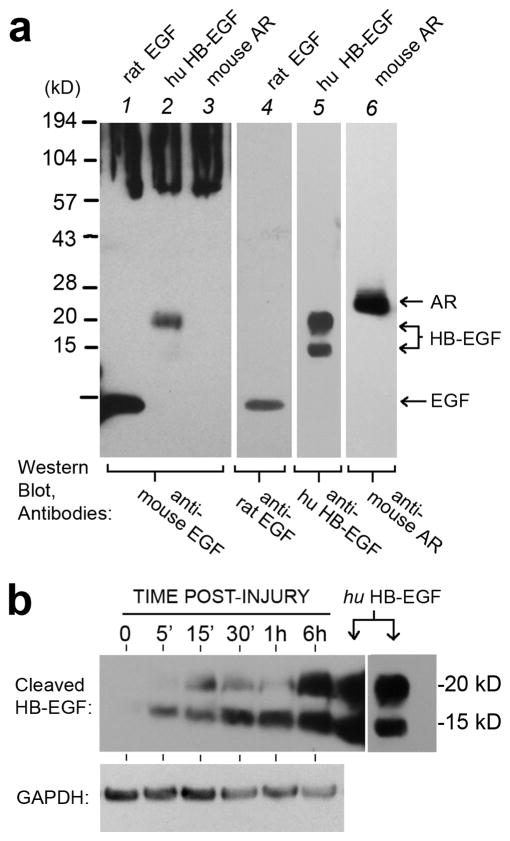
We next sought to identity EGF-family ligand(s) responsible for HA upregulation. An antibody against murine EGF, from Millipore/Upstate (#06-102), looked promising because it had been characterized by its ability to neutralize EGFR activity.1 (Henceforth, we refer to this antibody as the “neutralizing antibody”). However, despite strong evidence for EGF receptor activation after injury in skin (Shirakata et al., 2005; Stoscheck et al., 1992; Tokumaru et al., 2000), EGF itself is not normally present in keratinocytes (Rittie et al., 2006), a fact confirmed by Western analysis of our 3-D REK cultures (data not shown). Therefore, we tested the specificity of the neutralizing antibody against other factors likely to be expressed in REKs, including amphiregulin (AR) and HB-EGF (Fig. 9a). Using recombinant proteins, we found that the antibody cross-reacted with HB-EGF (lane 2), but not with AR (lane 3). To establish whether release of activated HB-EGF may be occurring, REKs were injured, harvested at various times, and soluble proteins (RIPA extracts) analyzed on Western blots using a different antibody specific for cleaved HB-EGF. No HB-EGF was detected in controls, neither the pro-HB-EGF precursor (> 27 kDa) nor the active, cleaved forms (Goishi et al., 1995). However, beginning within 5 min after injury and persisting at 6 h, peptides of the expected size for cleaved HB-EGF ( ~14 and~ 19 kDa; (Goishi et al., 1995)) were produced (Fig. 9b). Thus, HB-EGF is a major ligand released after injury.
Figure 9. Specificity of a potential HB-EGF neutralizing antibody, and release of cleaved HB- EGF after wounding.
(a), An anti-mouse EGF antibody from Millipore/Upstate (Cat No #06-102) was analyzed for its specificity of binding to the recombinant proteins listed above each lane (all from R&D Systems). Antibodies to rat-EGF (cat no AF3214), human HB-EGF (AF259-NA), or mouse Amphiregulin (AF989) were also from R&D Systems. Note the cross-reactivity of antibody #06-102 with HB-EGF in lane 2. (b), Western blot of REK lift cultures harvested at different times after acute puncture injury, and probed with anti-human HB-EGF (R&D Systems, AF259-NA). Purified recombinant human HB-EGF was used as a protein standard (downward arrows). GAPDH served as a loading control.
Requirement of HB-EGF for the HA-induction response to wounding
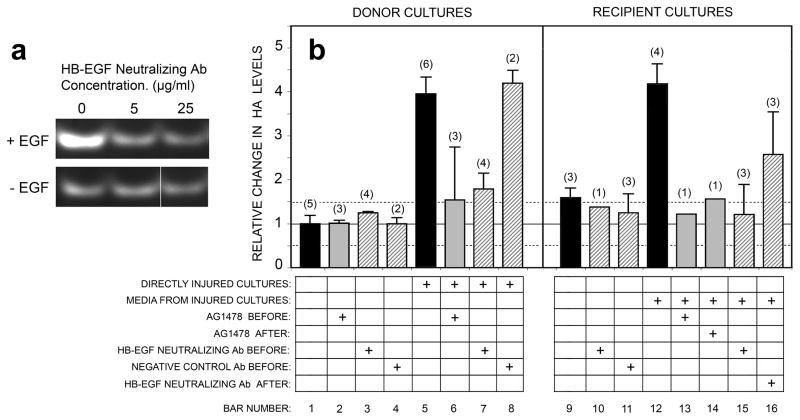
Since EGF is not expressed in keratinocytes (see above), yet the anti neutralizing antibody cross-reacted with HB-EGF and was reportedly capable of functionally blocking EGFR-mediated pathways1, we used it in an attempt to block injury-induced HA accumulation. Pilot titration studies were first performed to determine the antibody concentration needed to completely block HA stimulation from exogenously-added EGF; an effective neutralizing dose was determined to be 5 μg/ml (Fig. 10a). Using that dose, a complete puncture experiment in which conditioned media from cultures injured in the absence or presence of the antibody were transferred to recipient cultures, was performed (Fig. 10b, hatched bars). (For comparison, quantitative data from experiments using AG1478 are also included in these graphs.) In either the donor or recipient cultures, when compared to HA induction after puncture alone (bars 5, 12), addition of the neutralizing antibody prior to puncture (bar 7, 15) blocked the wounding-induced increase in HA nearly completely. However, when the neutralizing antibody was added at the time of media transfer (bar 16), blockade by this antibody was only partial, i.e., a reduction to ~50% of the HA maximum after wounding. This was true even though AG1478 (inhibition of EGFR kinase) was able to fully block HA induction (bars 13, 14) in the recipient cultures. These results could be due to the fact that the neutralizing antibody only cross-reacts with a ~19 kDa band of HB-EGF, and not the ~14 kDa form which might be differentially expressed in donor vs. recipient cultures. Alternatively, additional ErbB/HER growth factors may be required. In summary, we conclude that HB-EGF is a very important factor but perhaps not the only factor required to mediate injury-induced HA synthesis.
Figure 10. Injury-induced HA production can be blocked by an EGFR function-neutralizing antibody, demonstrating that release of soluble EGFR-family ligands (including HB-EGF) is required for new synthesis of HA.
(a), Titration of neutralizing antibody (see Methods) for its ability to prevent EGF-induced stimulation of HA synthesis. EGF was added at 20 ng/ml for 6 h; the antibody was added at 5 or 25 μg/ml. (b), Donor cultures were treated as illustrated in Figure 3, either in the absence of inhibitors (black bars), or in the presence of the neutralizing antibody (5 μg/ml; hatched bars), an IgG isotype matched control antibody (5 μg/ml; hatched bars), or AG1478 at a maximally effective dose (1 μM; gray bars). The inhibitors were added either 1 hr before needle injury (BEFORE), or 6 h after injury at the time of media transfer (AFTER), as indicated in the figure. HA was assayed by FACE or by immunofluorescent analysis of bHABP binding with image-processing, and the relative increases in HA relative to uninjured donor controls were determined from pooled experiments. Bars, mean ± range (if n=2), or mean ± SD (if n >2); n = number of experiments.
DISCUSSION
Hyaluronanic acid (HA) in the epidermis is of current interest because recent studies indicate that HA is important for regulating keratinocyte proliferation and differentiation in vivo, both under homeostatic conditions (Passi et al., 2004; Tammi et al., 2000) and in response to tissue trauma (Tammi et al., 2005) and this paper). In this study, we used organotypic 3-D cultures comprising rat keratinocytes (REKs) to study mechanisms of HA accumulation that occur in the epidermal compartment after wounding (Passi et al., 2004). Full-thickness needle (puncture) injury of these 3-D cultures led to elevated HA production as measured by two independent methods, namely, histological detection with a biotinylated HA-binding protein and biochemical detection using enzymatic digestion and fluorophore-labeling of the HA disaccharides. The time course of HA induction was quite rapid, reaching 50% of maximum by 15 min after wounding, then peaking at 24 h and subsiding at 48 – 72 h. In contrast, induction of HA in murine epidermis in vivo (observed both here and in a previously published study of tape-strip injury (Tammi et al., 2005)) was measurable at 24 h, reached a maximum at 3 days, and persisted for at least one week. The reason that HA levels remain elevated longer in the native epidermis than in the organotypic model is unknown, but because the REK 3-D model contains no fibroblasts, it is possible that paracrine factors from the dermis are necessary to sustain HA synthesis by keratinocytes in vivo.
Increased HA expression after skin wounding in vivo can be observed far from the site of injury. This phenomenon was also seen in our culture system. Histological analysis of REK 3-D cultures, injured in a small localized area, displayed HA induction throughout the culture and confirmed that HA induction is not confined to the immediate vicinity of the injury. To explain this action at a distance, we hypothesized that a soluble HA-inducing factor is released. Previous reports had shown that certain growth factors and cytokines can induce the expression of HA-synthetic genes (Has2 and Has3) in keratinocytes, and most of these are also released after tissue injury, including the following: EGF (Pasonen-Seppanen et al., 2003; Pienimaki et al., 2001), KGF (Karvinen et al., 2003), IL-1β (Yamada et al., 2004) and interferon-γ (Sayo et al., 2002). Ligands of the EGFR/ErbB1/HER-1 family of receptors were particularly strong candidates as soluble inducers of HA, given published evidence that EGF (Pasonen-Seppanen et al., 2003; Pienimaki et al., 2001), HB-EGF (Pasonen-Seppanen et al., 2006) and TGF-alpha (Bachem et al., 1989) were each capable of stimulating HA synthesis. Particularly intriguing to us was a report demonstrating that blockade of the EGFR signaling pathway can prevent the migration of cultured epithelial cells into an open wound in vitro (Block et al., 2004), a process known to be influenced by HA in the matrix (Rilla et al., 2002). Using AG1478 (an inhibitor of EGFR tyrosine kinase), we showed that injury-induced HA production was completely blocked by pre-treatment of the REK 3-D cultures with this inhibitor (Figs. 7 and 10). HA synthesis was prevented regardless of whether AG1478 was added to the donor cultures before injury, or to the conditioned media after injury. These data suggested, albeit indirectly, that EGFR signaling is required to trigger the increase in HA production. As a more direct means of identifying ligand(s) responsible for HA induction, we found an antibody (Millipore/Upstate #06-102) that is capable of binding to the larger of two proteolytic forms of rat HB-EGF (Fig. 9). Because EGF (as opposed to HB-EGF) is not produced in keratinocytes, even after wounding (data not shown), we reasoned that any effect that the neutralizing antibody might exert in functional experiments should be due to its neutralizing effect upon HB-EGF. Indeed, addition of the antibody to wounded cultures blocked HA induction by ~ 50% (Fig. 10). This shows that HB-EGF is an important ligand for the EGFR-mediated induction of HA synthesis. It may or may not be the only factor involved. Amongst the six ligands known to bind EGFR, namely EGF, HB-EGF, amphiregulin, epiregulin, betacellulin, and TGFα (Harris et al., 2003), human keratinocytes have been reported to express AR, HB-EGF, and epiregulin under nonstimulated conditions (Pastore et al., 2008). Here in rat keratinocytes, we showed that HB-EGF is rapidly cleaved and expressed within 5 min after injury. AR is constitutively expressed but not altered after injury. Epiregulin, betacellulin, and TGFα remain to be tested. However, based upon the frequency of reports in which HB-EGF is produced (Mathay et al., 2008; Pasonen-Seppanen et al., 2008; Rittie et al., 2006; Shirakata et al., 2005) and released after epithelial injury (Tokumaru et al., 2000; Xu et al., 2006), our proposition that HB-EGF is a major ligand required for HA upregulation after wounding appears solid.
Because several growth factors had already been shown to regulate one or more Has genes ((Ijuin et al., 2001; Jacobson et al., 2000; Karvinen et al., 2003; Pasonen-Seppanen et al., 2003; Saavalainen et al., 2005) ), we suspected that HA accumulation after injury is due to increased synthesis (versus decreased catabolism) of HA. Expression of genes for the major Has enzymes (Has1-3) and HA-degrading enzymes (Hyal1-3) was measured in injured REK 3-D cultures, and Has2 and Has3 were upregulated whereas expression of the hyaluronidases remained unchanged after injury (Fig. 6). Interestingly, the fact that Has3 is induced more than Has2 in our system is consistent with reports that Has3 is preferentially induced over Has2 in human keratinocytes exposed to cytokines (Sayo et al., 2002) or to serum in order to stimulate the “wound repair transcriptome” (Qi et al., 2008). From these data we conclude that the soluble factor (HB-EGF) induces overall HA production by increasing Has gene expression. One caveat is that HA induction during the initial phase (15 min) after injury is too rapid to result from transcriptional upregulation of Has genes, since the appearance of new mRNA is typically expected to take a few hours. We plan to test the hypothesis that a more rapid mechanism, involving stimulation of pre-existing Has2 and Has3, accounts for the initial surge in HA synthesis.
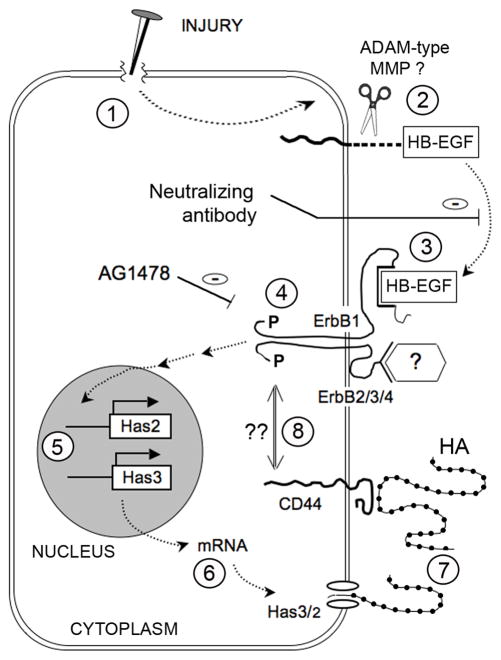
Based upon current data, our working hypothesis is summarized in Fig. 11. We have shown that physical injury to epidermal keratinocytes (Fig. 11, step 1) causes the release of processed forms of HB-EGF. Because the antibody we used can only recognize the cleaved (and not the pro-) forms of HB-EGF, the processed HB-EGF must have arisen from proteolytic cleavage of pro-HB-EGF through the action of a metalloprotease (probably a “sheddase” of the ADAM family; reviewed in (Blobel, 2005; Sahin et al., 2004)) as previously demonstrated in keratinocytes (Tokumaru et al., 2000). Our experimental data showed that soluble HB-EGF (step 3) was need for binding to the EGFR/ErbB1 receptor, and activation of the EGFR signaling pathway (i.e., receptor autophosphorylation) is required for HA synthesis (step 4). Elevated transcription of Has3/Has2 (step 5), as reflected by RT-PCR measurements of the mRNAs (step 6), play an important role. Ultimately, the increased accumulation of HA (step 7) is observed. An important question for the future is whether activation of the HA receptor, CD44, might exert autoregulatory feedback effects by interacting with EGFR (step 8). Mounting evidence from the cancer literature has revealed functional interactions between ErbB receptors (mainly ErbB2, but also the EGF receptor) and CD44 (Ellis et al., 2007; Misra et al., 2006; Turley et al., 2002), at least in cancer cell lines. This suggests the tantalizing hypothesis that HA may be involved in autoregulating its own synthesis via feedback interactions between CD44 and EGFR.
Figure 11. Hypothetical model of injury-induced HB-EGF production and upregulation of HA production.
(See text for details).
In conclusion, we have shown that physical injury in an organotypic model of epidermal keratinocytes causes the release of HB-EGF and activation of EGFR, and that these are prerequisites for a significant accumulation of HA. Given that HA is involved in the regulation of keratinocyte migration and of the epidermal hyperplastic response after injury (Maytin et al., 2004), future studies to elucidate details of this pathway should provide new opportunities for modifying the wound healing response and for ameliorating clinical conditions that involve excessive thickening of the epidermis.
MATERIALS AND METHODS
Source of reagents
All general reagents were purchased from Fisher Scientific (Hanover Park, IL), and all tissue culture reagents were purchased from Invitrogen (Carlsbad, CA), unless stated otherwise. The AG1478 inhibitor of EGFR tyrosine kinase was from EMD Biosciences (Calbiochem), La Jolla, CA, cat. no. 658548. The EGF-neutralizing antibody was a rabbit polyclonal from Millipore/Upstate, Lake Placid, NY, Millipore Cat. no. 06-102. Other antibodies are listed under “Western blot analyses,” below.
Wounding of murine skin in vivo
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at our institution. C57BL/6J adult mice, 8–10 weeks old, were anesthetized with a 0.04-ml injection of a general anesthetic cocktail (ketamine 100 mg ml; xylazine 20 mg ml; acepromazine 10 mg/ml), and the upper dorsal back shaved and disinfected with 70% alcohol. Each animal received a 1.5-cm full thickness incisional wound that was closed with interrupted 6.0 nylon suture. All wounds were midline and made in the same location. Wounded animals were housed in individual cages for the duration of the study. At days 0, 3, 7, and 14 post-wounding, the wound plus surrounding tissue was harvested and fixed overnight in 4% paraformaldehyde in phosphate buffered saline (PBS) in preparation for HA analysis.
Preparation of collagen gels
Rat tail collagen type 1 (BD Biosciences Clontech, Palo Alto, CA) was mixed with Hanks’ Salt Solution containing phenol red, and buffered with 20 mM HEPES. The pH was adjusted by adding small (25 μl) aliquots of 1 N sodium hydroxide until a homogeneous, pale orange solution was formed (at ~pH 7.0). Care was taken to avoid bubble formation during mixing. The solution was added to plastic Transwell inserts (1 ml per insert; diameter 2.4 cm; pore size 3.0 μm; Corning Incorporated, Corning, NY) housed in 6-well plates. Polymerized collagen fibrils were formed after incubation at 37°C for 2 h in a 5% CO2 incubator. Once formed, the collagen gels were stored immersed in PBS with 50 μg/ml gentamycin sulphate at 4°C until required. Before use, the gels were soaked twice in DMEM for 20 min and finally equilibrated at 37°C in DMEM in the CO2 incubator.
Preparation of basement membranes
Maden Darby canine kidney (MDCK) cells (a gift from Donald MacCallum, University of Michigan) were seeded onto the collagen gels at a density of 2 × 105 cells per transwell in MDCK medium (DMEM supplemented with 4.5 g/L glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B and 10% FBS). The cells were grown for 21 days at 37°C in a 5% CO2 incubator and the media was changed every 2 days. The cells were removed by detergent lysis, as follows. First, cells were immersed in hypotonic lysis buffer (10 mM Tris-HCl, pH 7.5, with 0.1% bovine serum albumin and 0.1 mM CaCl2) for 10 min in a humidified atmosphere at 37°C. Then, they were treated with 0.2% deoxycholate in hypotonic lysis buffer twice for 5 min. The complete removal of adherent MDCK cells was confirmed by light microscopy. Collagen gels with the basement membranes on their surfaces were stored immersed in PBS supplemented with 50 μg/ml gentamycin sulphate at 4°C until required. Prior to use, the gels were soaked 3 times in PBS for 10 min, then twice in MDCK medium for 20 min before finally being equilibrated in MDCK medium overnight at 37°C in a 5% CO2 incubator.
REK cell line
The REK cell line was originally developed by Donald MacCallum from neonatal rat epidermal keratinocytes (MacCallum & Lillie 1990). These keratinocytes possess the unique ability to stratify and terminally differentiate in organotypic cultures without the aid of feeder fibroblasts. The cells were grown in REK medium (DMEM supplemented with 1 g/L glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B, 50 μg/ml gentamycin sulphate and 10% FBS) at 37°C in a 5% CO2 humidified incubator. Confluent REK monolayer cultures were passaged using trypsin/EDTA solution (0.05% trypsin, 0.02% EDTA in Ca2+/Mg2+-free Earles’ balanced salt solution) at a ratio of 1:3.
Establishment of organotypic cultures
REKs were seeded onto inserts containing collagen and a basement membrane at a density of 2 × 105 cells per transwell. They were grown immersed in REK medium for 48 h. The cells were then ‘lifted’ by removing the medium from the upper chamber to expose the cells to air. Medium in the lower chamber was changed daily (3.5 ml) for the duration of the experiment. Lifted cultures were grown for up to 5 days at 37°C in a 5% CO2 incubator.
Puncture wounding and harvesting for biochemical and histological analyses
Fully stratified lift cultures (lifted for 5 days) were injured using a 28 gauge, 0.5 inch needle. The needle was pushed through the entire lift culture, collagen layer and porous nylon membrane and was designated as 1 ‘puncture’. To randomly cover a circular Transwell of 25 mm diameter, 100 punctures were “randomly” administered ~2 mm apart, delivering five rows (12, 11, 10, 9, and 8 punctures per row) on each side of the midline. To administer a more localized pattern of injury, 100 punctures were made ~1 mm apart in four parallel rows of 25 punctures grouped near the midline. After injury, cultures were incubated for designated times (typically, 6 h) at 37°C in a 5% CO2 incubator. Lift cultures were then harvested, as follows. For analysis of carbohydrate (HA), the epithelial and collagen components were separated and processed using the FACE technique; see below. For molecular analysis of RNA or protein, cultures were cut in half and snap-frozen for later lysis in the appropriate buffer. For histologic analysis, the lift cultures were fixed in Histochoice fixative (Amresco, Solon, OH) prior to rolling and embedding in paraffin using the “cigarette roll” technique described previously (Passi et al., 2004).
Experiments involving the transfer of conditioned medium
In transfer experiments, the culture medium from the lower chamber of a REK lift culture at 6 h after injury was transferred from the donor (injured) culture to the corresponding well of a recipient (unwounded) culture, as shown in the schematic timeline in Fig. 3. The recipient REK lift cultures were incubated for a further 24 h at 37°C in a 5% CO2 incubator, then harvested in the desired manner for the experiment intended. In experiments where inhibitors of the EGFR pathway were tested, an EGFR kinase inhibitor (AG1478), a neutralizing antibody to EGFR ligands (5 μg/ml, rabbit polyclonal IgG; Millipore/Upstate 06-102), or an irrelevant, IgG isotype-matched control antibody (5 μg/ml, anti-keratin 10, Covance, Inc., #PRB-159P), was added 1 h prior to the time of puncture injury or at the point of medium transfer.
Histological detection of HA
HA visualization in histological sections was performed using immunofluorescence microscopy, after labelling the HA in situ with biotinylated hyaluronan binding protein (bHABP; Seikagaku Ltd, Tokyo, Japan) and Cy3-conjugated streptavidin (Jackson Immunoresearch; West Grove, PA), as described (Passi et al., 2004). Digitally-captured images were analyzed using IPLab Spectrum (Signal Analytics, Vienna, VA), a software program that allows one to trace out regions of interest within the tissue (done either manually, or by setting a baseline threshold), followed by background subtraction and summation to provide an integrated intensity value. To establish that the observed signal was specific for HA, histological sections were treated overnight with hyaluronidase from Streptomyces hyaluronidase (2.5 units in saline, overnight at 37 °C; Calbiochem, Inc.) or with hyaluronidase from Streptococcus dysgalactiae (5 milliunits in 0.1 M ammonium acetate, overnight at 37 °C; Seikagaku Corp.); see Fig. 1.
Fluorophore-Assisted-Carbohydrate Electrophoresis (FACE) of HA
The technique of Calabro et al. (Calabro et al., 2000) was used with several modifications. REK lift cultures were excised from the Transwell insert (using a scalpel around the perimeter), and placed epidermis-side down onto a 3 × 3 cm square of blotting paper. The porous nylon membrane and collagen gel were removed and discarded. The paper with adherent cell layers was cut in two, to provide duplicate samples (one for FACE analysis, the other for storage at −20°C for future use). For analysis, samples were sliced into small pieces and digested in 1 ml of proteinase K solution (100 μg/ml proteinase K (Invitrogen), 0.5% SDS, 0.1 M ammonium acetate) at 60°C overnight. Following a brief vortex, the blotting paper was removed and samples were concentrated in a Speed-Vac to a volume of 250 μl. Ice-cold 100% ethanol (1 ml) was added and the samples were incubated overnight at −20°C. Samples were centrifuged at 13,000 rpm for 20 min at 4°C and the supernatants were discarded. Ice-cold 75% ethanol (1 ml) was added and the samples were centrifuged for a further 20 min as described above. The supernatants were again removed and samples were air-dried at room temperature for 20 min. Ammonium acetate 0.1 M (35 μl) was added to the dried pellets, which were then resuspended by vortexing, heated to 100°C for 3 min, and placed on ice. Next, 1.4 μl of a digestion solution (0.6 μl 1% glacial acetic acid, 0.4 μl SD hyaluronidase (2.5 mU/μl; Seikagaku Corporation, Tokyo, Japan), 0.4 μl chondroitinase ABC (25 mU/μl; Seikagaku) was added to each sample, then mixed and incubated at 37°C for 3 h. A further 160 μl of 100% ice-cold ethanol was added to each sample and mixed before being incubated overnight at −20°C.
The samples were centrifuged for 20 min (as above) and the supernatants were collected and transferred to separate tubes. More 75% ice-cold ethanol (160 μl) was added to the pellets which were then centrifuged for 20 min (as above). The supernatants from these samples were then pooled with those previously collected, and dried to completion in a Speed-Vac. The dried samples were resuspended in 20 μl 0.1 M ammonium acetate, transferred to 200 μl tubes, then dried to completion for a second time. Next, 8 μl of AMAC dye solution (7.5% glacial acetic acid, 6.25 mM 2-aminoacridone (Sigma, St. Louis, MO), 625 μM sodium cyanoborohydride (Sigma)) was added and each sample was incubated overnight at 37°C in the dark. Finally, 3 μl of 80% glycerol was added to prepare each sample for gel analysis.
A Hoefer SE 600 vertical gel apparatus (Amersham Biosciences, Buckinghamshire, UK) was used for sample separations. Each sample was loaded and run on a 20% acrylamide gel (20 ml acrylamide (40% 37.5:1; Bio-Rad), 4 ml Tris-acetate (400 mM; pH 7), 1 ml glycerol, 15 ml H2O) in 1 × TBE buffer for 2 h at 4°C). The fluorescent disaccharide bands were visualized by illumination with 365 nm UV light (Ultra Lum Transillumninator), and the fluorescence emission was collected after passage through an ethidium bromide orange barrier filter onto a Quantix cooled CCD camera, as described in detail elsewhere (Calabro et al., 2000). Images were analyzed using the Gel-Pro Analyzer 3.0 program (Media Cybernetics, Bethesda, MD).
RT-PCR analysis of Has and Hyal gene expression
REK lift cultures were excised from the transwell inserts and placed onto blotting paper, as described above. Half of this sample was sliced up and submerged in 1 ml TRIZOL reagent (Invitrogen), vortexed, and stored overnight at −80°C. The next day, samples were thawed on ice and the blotting paper was removed. Chloroform (200 μl) was added to each sample, mixed by inversion and incubated on ice for 5 min. Samples were centrifuged at 13000 rpm for 20 min at 4°C with the brake off. The upper, aqueous phase of each sample (500 μl) was transferred to a second tube containing 500 μl of isopropanol. Samples were mixed and precipitated overnight at −20°C, then centrifuged at 13,000 rpm for 15 min at 4°C. The supernatants were removed, and 1 ml 100% ice-cold ethanol was added. Samples were centrifuged as described above and the supernatants were again discarded. 1 ml 70% ice-cold ethanol was added, and the samples were centrifuged for a third time. After removal of the supernatant, samples were air-dried for 1 h at room temperature. Purified RNA samples were resuspended in 20 μl H2O and stored at −80°C.
Semi-quantitative PCR was performed using the random hexamer method. Sample RNA (1 μg) was added to 250 ng random primers (Invitrogen) and 1 μl 10 mM dNTP mix (Invitrogen). The mixture was heated for 5 min at 65°C then placed on ice. To this mixture, 4 μl of 5× First Strand buffer, 1 μl of 0.1 M dithiothreitol, 1 μl of RNaseOUT (40 U/μl) and 1 μl of Superscript III (200 U/μl) reverse transcriptase (all from Invitrogen) were added and the mixture heated for 5 min at 25°C, 60 min at 50°C and 15 min at 75°C. Then, 1 μl of the generated cDNA was used per 25 μl PCR reaction as follows: 1 μl cDNA was mixed with 5 μl GoTaq 5× colorless buffer (with 7.5 mM MgCl2; Promega, Madison, WI), 0.1 μl GoTaq polymerase (5 U/μl l; Promega), 0.5 μl dNTPs (10 mM), 2.5 μl sense strand primer (10 μM) and 2.5 μl antisense strand primer (10 μM) and 13.4 μl H2O. The primer sequences, predicted product sizes, TMs and cycle numbers are given in Supplemental Table 1. PCR was performed using a Mastercycler Personal thermal cycler (Eppendorf, Westbury, NY). Amplification for each primer set consisted of a preliminary denaturation at 94°C for 5 min, followed by a cycle of 94°C for 30 s, a TM of 60/65°C for 30 s and an elongation at 72°C for 1 min. A final elongation step at 72°C for 15 min concluded the amplification. Each reaction product (5 μl) was analyzed by agarose gel electrophoresis (2.5% agarose gel; 1× TAE buffer containing 0.5 mg/ml ethidium bromide; 1.5 h at 90 V). Gels were visualized and digitally photographed under UV light, and densitometry performed using IPLab Spectrum software.
Western blot analyses of pEGFR and EGF-family ligands
The REK lift cultures were harvested for protein as follows. The cell layer was separated from the collagen gel and placed directly into 100 μl RIPA buffer (PBS, pH 7.4 with 1% Nonidet P-40 (Sigma), 0.5% sodium deoxycholate (Sigma), 0.1% SDS, 100 μg/ml PMSF (USB Scientific), 100 μg/ml aprotinin (USB Scientific) and 10 μg/ml sodium orthovanadate (Sigma). Samples were vortexed briefly and incubated on ice for 20 min. After a brief (4 s) sonication, samples were centrifuged for 10 min at 13000 rpm at 4°C. The supernatants were transferred to new tubes and stored at −80°C until required.
Protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad) as per manufacturers’ protocol. 20 μg protein from each sample was then resolved by SDS-PAGE under reducing conditions. For the detection of pEGFR, samples were resolved using 10% Bis-Tris gels in NuPage® MOPS SDS running buffer (Invitrogen). For the detection of all other proteins, 4–12% pre-cast NuPage® Novex® Bis-Tris gels (Invitrogen) and NuPage® MES SDS running buffer (Invitrogen) were used. Samples were then transferred onto Immobilon P® membranes (Millipore) using NuPage® transfer buffer (Invitrogen) at 100 V for 1.5 h. Membranes were incubated in blocking buffer (TBST containing 5% non-fat dried milk) for 30 min at RT then incubated with the desired primary antibody in blocking buffer overnight at 4°C, using the following antibody dilutions: anti-pEGFR [pY1068 44-788G] 1:300 (Invitrogen); anti-GAPDH [FL-335] 1:200 (Santa Cruz); anti-mouse EGF [neutralizing 06-102] 1:100 (Millipore); anti-rat EGF [AF3214] 1:200 (R&D Systems); anti-mouse AR [AF989] 1:200 (R&D Systems); anti-human HB-EGF [AF259NA] 1:500 (R&D Systems), or anti-mouse K14 [PRB-155P] 1:5,000 (Covance). After washing the membranes 3 times for 5 min per wash in Tris-saline buffer (100 mM Tris, 0.9% NaCl, 0.1% Triton-X100), the blots were incubated with the desired horseradish peroxidase-secondary antibody at 1:20,000 dilution in blocking buffer for 1 h at RT, using the following antibodies: goat-anti rabbit IgG [111-035-003]; donkey anti-sheep IgG [713-35-003], donkey anti-goat IgG [705-035-147] (all Jackson). After three, 5 min washes with Tris-saline buffer, proteins were visualized using the ECL-Plus detection reagent (GE Healthcare) following manufacturers’ instructions, and HyBlot CL autoradiography film (Denville, Inc).
Statistics
Two-sample t-tests were used to compare the difference between data points of experimental conditions and untreated controls. A p value of 0.05 or less was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Samantha Keevey for expert technical assistance, and Dr. Vincent C. Hascall for advice and encouragement on all things HA-related. This work was supported by grant R01 AR049249 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS/NIH).
Abbreviations
- HA
hyaluronic acid
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FACE
fluorophore assisted carbohydrate electrophoresis
- Has
hyaluronan synthase
- Hyal
hyaluronidase
- HB-EGF
heparin binding EGF-like growth factor
- pEGFR
phosphorylated EGFR
- REK
rat epidermal keratinocyte
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
CONFLICT OF INTEREST
The authors state no conflict of interest.
Information about the neutralizing antibody (Millipore/Upstate), and its ability to inhibit 3H-thymidine incorporation in primary cultured hepatocytes treated with 10 ng/ml EGF, was obtained from the certificate of analysis from the company.
References
- Agren UM, Tammi M, Ryynanen M, Tammi R. Developmentally programmed expression of hyaluronan in human skin and its appendages. J Invest Dermatol. 1997;109:219–224. doi: 10.1111/1523-1747.ep12319412. [DOI] [PubMed] [Google Scholar]
- Ajani G, Sato N, Mack JA, Maytin EV. Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp Cell Res. 2007 doi: 10.1016/j.yexcr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem MG, Riess U, Melchior R, Sell KM, Gressner AM. Transforming growth factors (TGF alpha and TGF beta 1) stimulate chondroitin sulfate and hyaluronate synthesis in cultured rat liver fat storing cells. FEBS Lett. 1989;257:134–137. doi: 10.1016/0014-5793(89)81804-6. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- Calabro A, Benavides M, Tammi M, Hascall VC, Midura RJ. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE) Glycobiology. 2000;10:273–281. doi: 10.1093/glycob/10.3.273. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RAF, editor. The molecular and cellular biology of wound repair. 2. New York: Kluwer Academic/Plenum Publishers; pp. 1–611. [Google Scholar]
- Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- Ellis IR, Schor AM, Schor SL. EGF AND TGF-alpha motogenic activities are mediated by the EGF receptor via distinct matrix-dependent mechanisms. Exp Cell Res. 2007;313:732–741. doi: 10.1016/j.yexcr.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Hascall VC, Laurent TC. Hyaluronan: structure and physical properties. In: Hascall VC, Yanagishita M, editors. Glycoforum/Science of Hyaluronan review series. 1997. http://wwwglycoforumgrjp. [Google Scholar]
- Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, et al. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Ijuin C, Ohno S, Tanimoto K, Honda K, Tanne K. Regulation of hyaluronan synthase gene expression in human periodontal ligament cells by tumour necrosis factor-alpha, interleukin-1beta and interferon-gamma. Arch Oral Biol. 2001;46:767–772. doi: 10.1016/s0003-9969(01)00032-2. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan synthases in response to external stimuli. Biochem J 348 Pt. 2000;1:29–35. [PMC free article] [PubMed] [Google Scholar]
- Karvinen S, Pasonen-Seppanen S, Hyttinen JM, Pienimaki JP, Torronen K, Jokela TA, et al. Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem. 2003;278:49495–49504. doi: 10.1074/jbc.M310445200. [DOI] [PubMed] [Google Scholar]
- Mack JA, Abramson SR, Ben Y, Coffin JC, Rothrock JK, Maytin EV, et al. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. Faseb J. 2003;17:1352–1354. doi: 10.1096/fj.02-0959fje. [DOI] [PubMed] [Google Scholar]
- Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y. Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. J Invest Dermatol. 2008;128:717–727. doi: 10.1038/sj.jid.5701069. [DOI] [PubMed] [Google Scholar]
- Maytin EV, Chung HH, Seetharaman VM. Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am J Pathol. 2004;165:1331–1341. doi: 10.1016/S0002-9440(10)63391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen S, Karvinen S, Torronen K, Hyttinen JM, Jokela T, Lammi MJ, et al. EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol. 2003;120:1038–1044. doi: 10.1046/j.1523-1747.2003.12249.x. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen S, Maytin EV, Torronen K, Hyttinen JM, Kultti A, Tammi MI, et al. All-trans retinoic acid induced hyaluronan production and hyperplasia are mediated by EGFR-ERK signaling in epidermal keratinocytes. J Invest Dermatol. 2006;126:87. doi: 10.1038/sj.jid.5701098. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen S, Suhonen TM, Kirjavainen M, Miettinen M, Urtti A, Tammi M, et al. Formation of permeability barrier in epidermal organotypic culture for studies on drug transport. J Invest Dermatol. 2001;117:1322–1324. doi: 10.1046/j.0022-202x.2001.01529.x. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen SM, Maytin EV, Torronen KJ, Hyttinen JM, Hascall VC, MacCallum DK, et al. All-trans retinoic acid-induced hyaluronan production and hyperplasia are partly mediated by EGFR signaling in epidermal keratinocytes. J Invest Dermatol. 2008;128:797–807. doi: 10.1038/sj.jid.5701098. [DOI] [PubMed] [Google Scholar]
- Passi A, Sadeghi P, Kawamura H, Anand S, Sato N, White LE, et al. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp Cell Res. 2004;296:123–134. doi: 10.1016/j.yexcr.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- Pienimaki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S, et al. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem. 2001;276:20428–20435. doi: 10.1074/jbc.M007601200. [DOI] [PubMed] [Google Scholar]
- Qi L, Higgins SP, Lu Q, Samarakoon R, Wilkins-Port CE, Ye Q, et al. SERPINE1 (PAI-1) is a prominent member of the early G0 --> G1 transition “wound repair” transcriptome in p53 mutant human keratinocytes. J Invest Dermatol. 2008;128:749–753. doi: 10.1038/sj.jid.5701068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilla K, Lammi MJ, Sironen R, Torronen K, Luukkonen M, Hascall VC, et al. Changed lamellipodial extension, adhesion plaques and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J Cell Sci. 2002;115:3633–3643. doi: 10.1242/jcs.00042. [DOI] [PubMed] [Google Scholar]
- Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732–739. doi: 10.1038/sj.jid.5700202. [DOI] [PubMed] [Google Scholar]
- Saavalainen K, Pasonen-Seppanen S, Dunlop TW, Tammi R, Tammi MI, Carlberg C. The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J Biol Chem. 2005;280:14636–14644. doi: 10.1074/jbc.M500206200. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayo T, Sugiyama Y, Takahashi Y, Ozawa N, Sakai S, Ishikawa O, et al. Hyaluronan synthase 3 regulates hyaluronan synthesis in cultured human keratinocytes. J Invest Dermatol. 2002;118:43–48. doi: 10.1046/j.0022-202x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, Nanney LB, King LE., Jr Quantitative determination of EGF-R during epidermal wound healing. J Invest Dermatol. 1992;99:645–649. doi: 10.1111/1523-1747.ep12668143. [DOI] [PubMed] [Google Scholar]
- Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, et al. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- Tammi RH, Tammi MI, Hascall VC, Hogg M, Pasonen S, MacCallum DK. A preformed basal lamina alters the metabolism and distribution of hyaluronan in epidermal keratinocyte “organotypic” cultures grown on collagen matrices. Histochem Cell Biol. 2000;113:265–277. doi: 10.1007/s004180000128. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol. 1997;29:201–210. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KP, Yin J, Yu FS. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:2832–2839. doi: 10.1167/iovs.05-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol. 2004;122:631–639. doi: 10.1111/j.0022-202X.2004.22332.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.