Abstract
Amino alcohols, diamines, benzenesulfonyl chlorides and bromoketones were used to prepare polymer-supported 2-(2-(2-(amino/hydroxyl)ethylamino)ethyl)-3-benzoyl-2H-indazoles. Acid-mediated release yielded 2-((amino/hydroxyl)ethyl)-1-aryl-3,4-dihydropyrazino[1,2-b]indazole-2-iums. In neutral pH, iminiums spontaneously cyclized to complex fused heterocycles 3,6,9,10-tetraazatetracyclo[7.7.0.02,6.011,16]hexadeca-11,13,15-trienes, 3-oxa-6,9,10-triazatetracyclo[7.7.0.02,6.011,16]hexadeca-11,13,15-trienes, and 3,7,10,11-tetraazatetracyclo[8.7.0.02,7.012,17]heptadeca-12,14,16-trienes. Transformations were carried out under mild conditions and tolerated diverse substitution patterns.
Introduction
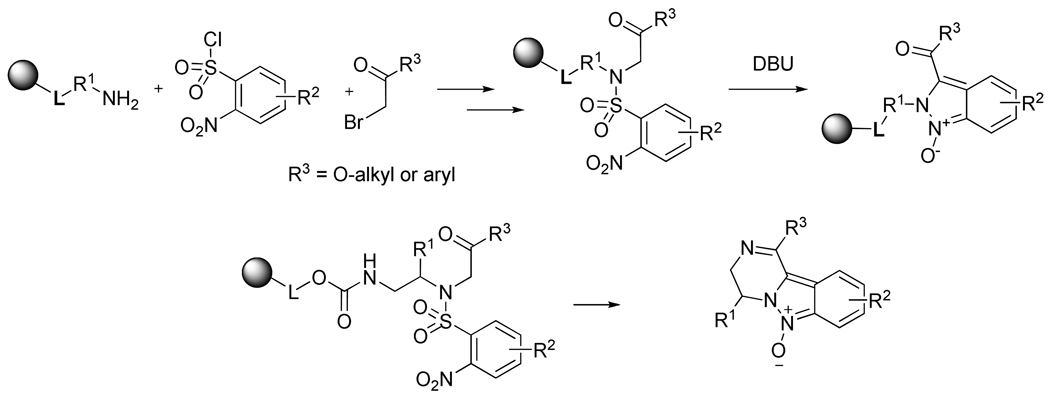
As part of our ongoing research on solid-phase synthesis of heterocyclic compounds and the generation of combinatorial libraries for drug discovery, we recently developed a novel synthetic route to indazoles.1 N-alkylation of polymer-supported 2-nitrobenzenesulfonyl (2-Nos)-activated/protected amines by bromoketones (a variant of the Fukuyama method2) provided α-sulfonylamino ketones. Treatment of these 2-Nos derivatives with DBU led to tandem carbon-carbon followed by nitrogen-nitrogen bond formation to produce indazole oxides of excellent purity (Scheme 1). The target indazoles contained a carbonyl functional group attached to the side-chain of the substituent in position 2 of the indazole ring. The carbonyl group enabled subsequent transformations of indazoles and formation of a fused pyrazine ring via an intramolecular nucleophile located on the R1 side-chain, providing a route for pyrazino[1,2-b]indazole synthesis.3
Scheme 1.
Synthesis of indazoles and pyrazino[1,2-b]indazoles
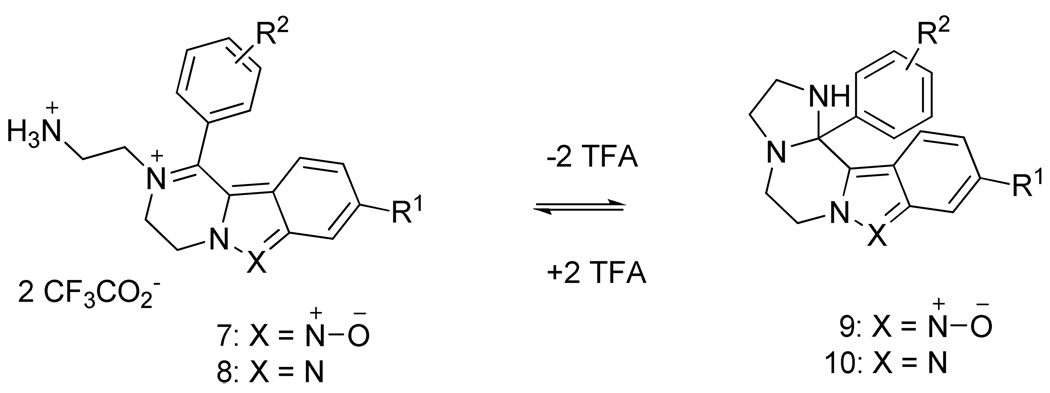
Herein, we extend our chemistry to indazole derivatives with a secondary, rather than primary, amino group in the β-position with respect to the sulfonamide. After TFA-mediated cleavage from the resin, these resin-bound intermediates yielded iminium trifluoroacetes. The presence of a nucleophile on the side-chain of the amino group enabled ring closure in neutral pH and formation of complex fused heterocycles.
Iminium ions are well-established powerful intermediates used in many chemical transformations.4–7 The wide applicability of the iminium intermediate is documented through their use in the construction of complex heterocycles8–10 including polycyclic alkaloids.11–14 Among the various methods for iminium ion synthesis, the condensation of secondary amines with aldehydes (ketones) is regarded as one of the most versatile procedures.
Iminium salts were applied in combinatorial solid-phase syntheses. Polymer-supported syntheses of bi-, tri and tetracyclic derivatives of 1-acyl-3-oxopiperazines employed tandem N-acyliminium ion cyclization-nucleophilic addition as the ring-forming process.15 Polymer-supported synthesis with the aromatic aza Diels-Alder reaction via iminium-initiated cyclization provided an efficient approach to produce (pyrido-fused) tetrahydroquinoline.16,17 A novel strategy of the Pictet-Spengler reaction provided for the synthesis of triazabenzoazulenes.18 Various ring systems with a pyrrolidino-lactam core were prepared by intramolecular [3+2] azomethine ylide cycloaddition via iminium intermediates. These syntheses were carried out in solid phase as well as in solution.19
Results and discussion
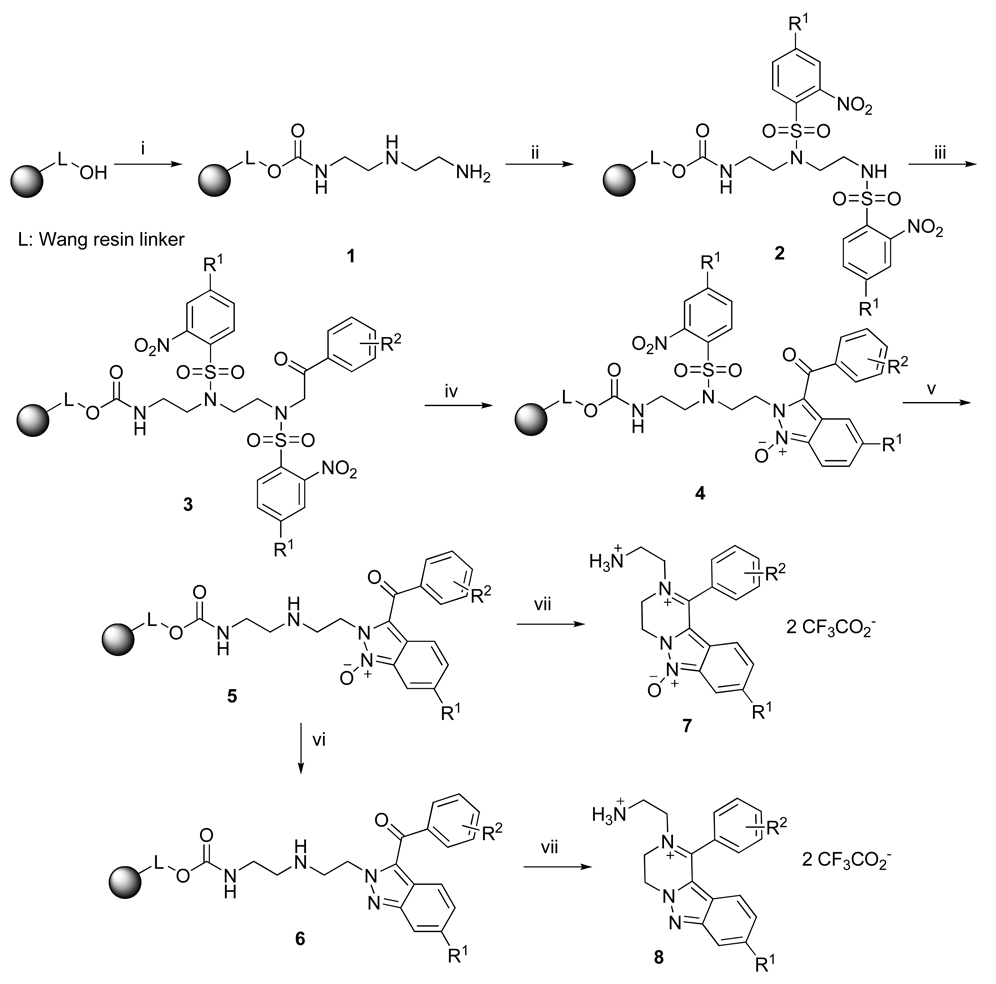
To investigate the outcome of the iminium-forming reaction, we synthesized the resin-bound precursor using diethylenetriamine, 2-nitrobenzenesulfonyl chlorides, and bromoketones. The commercially available diethylenetriamine was immobilized to Wang resin via a carbamate linkage using carbonyldiimidazole (CDI) activation to provide resin 1 (Scheme 2). The reaction occurred exclusively through the primary amino group; there was no need to distinguish between primary amino groups due to the symmetrical nature of diethylenetriamine. A large excess of diethylenetriamine essentially eliminated the potential cross-linking reaction (reaction of the amino group of already immobilized diethylenetriamine). Resin 1 was used for the synthesis of the model indazole derivatives.
Scheme 2.
Synthesis of 1,2-substituted-3,4-dihydropyrazino[1,2-b]indazole-2-ium 6-oxides using 2-Nos protecting groups
Reagents and conditions: (i) CDI, pyridine, DCM, rt, 3 h, then diethylenetriamine, DCM, rt, 16 h; (ii) 2-nitrobenzenesulfonyl chlorides, lutidine, DCM, rt, 16 h; (iii) bromoketones, DIEA, DMF, rt, 16 h; (iv) DBU, DMF, rt, 30 min; (v) 2-mercaptoethanol, DBU, DMF, rt, 5 min; (vi) methanesulfonyl chloride, TEA, DCM, 16 h; (vii) 50% TFA, DCM, rt, 1 h.
Synthesis using dual 2-Nos protecting groups
To enable the selective alkylation of the primary group of resin 1, we protected/activated both amino groups with a 2-nitrobenzenesulfonyl (2-Nos) group (LC purity of crude 2 was >90%). The secondary amino group was protected against subsequent reaction with bromoketones and the 2-Nos-activated primary amino group facilitated N-monoalkylation (Scheme 2). Reaction with four 2-nitrobenzenesylfonyl chlorides containing both electron-donating and electron-withdrawing groups (H, CF3, NO2, and OMe, Table 1) yielded the sulfonamides 2. The reaction outcome was monitored by LCMS analysis of a sample released from the resin using 50% TFA in dichloromethane (DCM) cleavage cocktail.
Table 1.
Selection of 2-nitrobenzenesulfonyl chlorides and bromoketones
| BB # | R1 | R2 |
|---|---|---|
| 1 | H | 4-Me |
| 2 | CF3 | 4-OMe |
| 3 | NO2 | 4-Cl |
| 4 | OMe | 4-CN |
| 5 | - | 3,5-diCl-4-NH2 |
| 6 | - | 4-CF3 |
Alkylation of resin 2 was carried out with four bromoketones (4-Me, 4-OMe, 4-Cl, and 4-CF3, Table 1) to assess the effect of substitution on the aromatic ring. The reaction was carried out in DMF in the presence of a tertiary base.1 All four 2-nosyl derivatives yielded the expected products 3. However, LC analysis of the 2,4-diNO2 derivatives 3(3,1), 3(3,2) and 3(3,6) showed a complex mixture. The next step, DBU-mediated cyclization of alkylated sulfonamides 3 to indazole-6-oxides 4, was carried out in DMF. Only the 4(1,2), 4(1,3) and 4(1,6) derivatives were obtained in high purity (~95%) and the purity of the crude derivatives (4(2,1), 4(2,2) and 4(2,3)) was still acceptable (80%). The cyclization of the 4(4,1), 4(4,3), and 4(4,6) derivatives was characterized by very low conversion and the crude product was accompanied by several impurities. The cleavage of the 2-nitrobenzenesulfonyl groups was carried out with 2-mercaptoethanol and DBU in DMF.2 The 2-NO2 and 2-NO2-4-CF3 derivatives provided products 7 with acceptable purity (80%). Cleavage of the 2-NO2-4-OCH3-benzenesulfonyl group was not successful, probably due to the presence of the electron-donating methoxy group. Compounds that provided acceptable purity were deoxygenated using methanesulfonyl chloride in the presence of triethylamine (TEA)20 to provide resin-bound parent heterocycles 6.
Although synthesis of resins 5 using the 2-Nos protection/activation strategy was not generally applicable for preparation of compounds with diverse side-chains, it provided straightforward access to compounds with electron-neutral and electron-withdrawing substituents and allowed for full characterization and structure determination of model compounds.
Equilibrium iminium – imidazolidine derivatives
All compounds were cleaved from the resin 6 with a TFA-containing cleavage cocktail, and the crude products were isolated as trifluoroacetates. The crude compounds were purified by semi-preparative reversed-phase HPLC using a gradient elution with two different aqueous buffers, acidic 0.1% TFA and neutral ammonium acetate. There were remarkable differences in the 1H and 13C spectra of compounds purified using the two different buffers.
The structures of the compounds were determined by 1D 1H and 13C NMR with 2D homo-(COSY) and heteronuclear 1H-13C (HETCOR, gHMBC) experiments. Signals of all carbons with directly attached protons were assigned using the HETCOR spectra. gHMBC spectra were used to analyze connectivities. The compounds purified in 0.1% aqueous TFA corresponded to iminium ions 7 and 8 (Scheme 3), whereas compounds purified in aqueous ammonium acetate cyclized to complex fused heterocycles 9 and 10. All spectra and interpretations are included in the supporting material.
Scheme 3.
Equilibrium iminium - imidazolidine
An analogous pH-dependent equilibrium between iminium ions and the corresponding cyclic products has previously been reported, e.g., between 3,4-dihydroisoquinolinium21 and 1-(2-hydroxyethyl)-2,3,4,5-tetrahydropyridinium cations.22
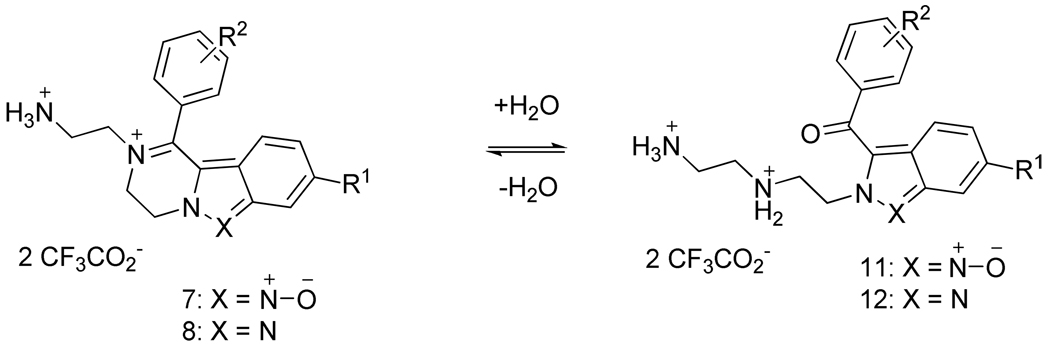
Hydrolysis of iminiums
An LCMS analysis of the products highlighted the tendency of iminiums to hydrolyze in aqueous solutions (Scheme 4). The LC traces revealed the presence of two components, the iminiums 7 (or 8) and the keto-amines 11 (or 12). The relative ratio of the keto-amine was dependent on the substitution pattern on both aromatic rings (Table 2). The ratio was significantly influenced by the R2 substitution with a clear preference for the open form with increasing electron-donating character of the substituent. The electron-donating character of the 4-OMe and 3,5-diCl-4-NH2 groups increased the electron density on the carbonyl group, and caused the hydrated form to be less prone to cyclization. The effect of substitution on the indazole ring was less profound. In a few cases, the difference of retention times of both components was too small to reliably integrate the individual peaks, and therefore, the ratio was not determined. The NMR spectra (collected in anhydrous d6 DMSO) did not indicate the presence of any hydrolyzed components. The hydrolysis of structurally unrelated iminium intermediates was reviewed23 and also exploited for preparative purposes.24,25
Scheme 4.
Equilibrium iminium – amino ketone
Table 2.
Relative contents of amino-ketones as a function of substitution
| R1/R2 | CN | Me | Cl | OMe | diCl-NH2 |
|---|---|---|---|---|---|
| H | 5% | 5% | 9% | ND | 30% |
| CF3 | 3% | 8% | ND | 14% | 21% |
| NO2 | 3% | 5% | 6% | 9% | 16% |
ND - not determined
Synthesis using Pht and Boc protecting groups
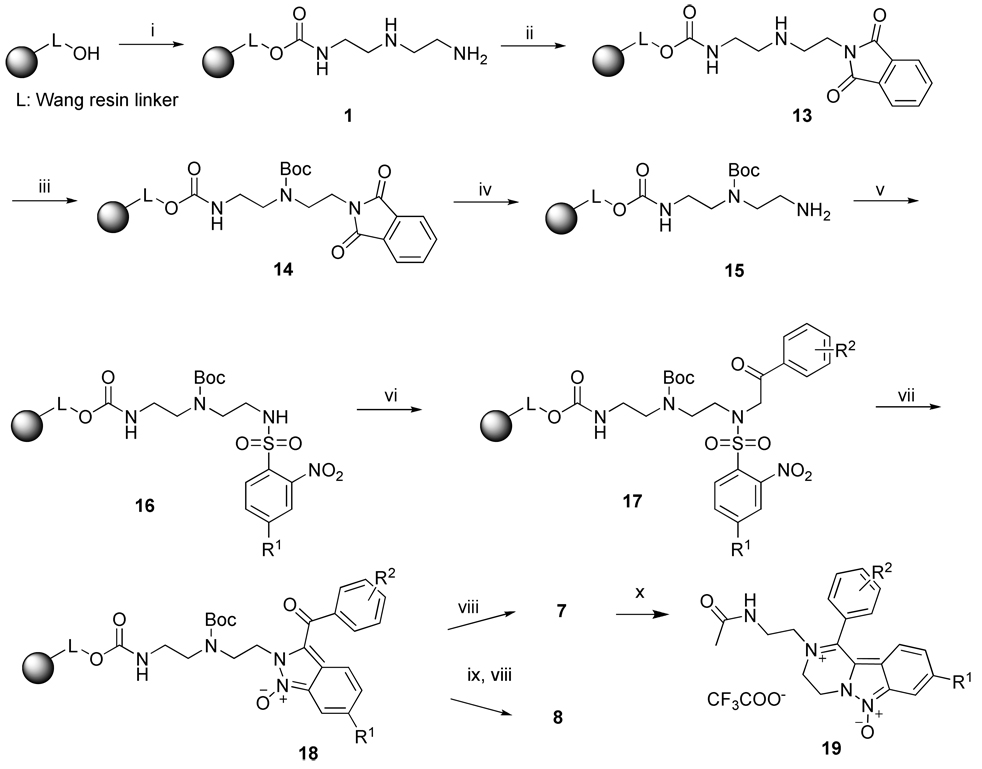
Although synthesis of resins 5 using a dual 2-Nos protection/activation strategy was straightforward, it was not generally applicable and did not allow for the synthesis of target compounds with a diverse side-chain group profile. Encouraged by the smooth transition of iminium intermediates to complex fused heterocycles, we altered the protection strategy and prepared the resin-bound triamine 1 using orthogonal protection, which enabled the use of building blocks with diverse substituents.
To differentiate between the primary and secondary amino groups on resin 1, we protected the primary amino group with a phthaloyl (Pht) group and the secondary amino group by a Boc-protecting group (Scheme 5). We have shown that a Pht group can be introduced under very mild conditions using N-hydroxyphthalimide in DMF.26 The reaction of resin 1 containing both the primary and secondary amino groups with N-hydroxyphthalimide yielded the protected amine resin 13. Quantification of this on-resin protection reaction was confirmed by reaction of resin 13 with Fmoc-OSu and subsequent analysis of a cleaved sample. No double Fmoc-ylated compound was detected in the LC traces. The reaction of resin 13 with (Boc)2O was accomplished in 2 h in the presence of pyridine as a base in DCM to produce resin 14. The completion of reactions was again verified by treatment with Fmoc-OSu. The absence of the Fmoc-derivative in a cleaved sample confirmed the complete reaction with (Boc)2O. The Pht group was cleaved by a hydrazine monohydrate solution with a 50% methanol/THF mixture. The best results were obtained with 2M hydrazine hydrate and a reaction time of 4 h.
Scheme 5.
Synthesis using Pht and Boc protecting groups
Reagents and conditions: (i) CDI, pyridine, DCM, rt, 3 h, then diethylenetriamine, DCM, rt, 16 h; (ii) N-hydroxyphthalimide, DMF, rt, 16 h; (iii) Boc2O, pyridine, DCM, rt, 2 h; (iv) hydrazine monohydrate, MeOH/THF (1:1), rt, 4 h; (v) 2-Nitrobenzenesulfonyl chloride, lutidine, DCM, rt, 16 h; (vi) bromoketone, DIEA, DMF, rt, 16 h; (vii) DBU, DMF, rt, 30 min; (viii) 50% TFA / DCM, rt, 1 h., (ix) MsCl, TEA, DCM, 30 min at 0°C, then rt, 16 h; (x) Ac2O, acetonitrile, 2 h.
The resin-bound primary amine 15 was then reacted with 2-nitrobenzenesylfonyl chlorides and the sulfonamides 16 were alkylated with bromoketones to yield the corresponding sulfonamide ketones 17. The intermediates 17 were cyclized to indazole 6-oxide 18, the resin was split, and one part was deoxygenated using methanesulfonyl chloride and TEA in DCM.20 Removal of the Boc protecting group with concurrent cleavage from the resin in 50% TFA / DCM yielded target compounds 7 and 8.
This protection strategy was compatible with the diverse substitution pattern on both the 2-nitrobenzenesulfonyl chlorides and bromoketones and enabled the efficient synthesis of a small combinatorial array of target compounds (Table 1). We used three 2-nitrobenzenesulfonyl chlorides (2-NO2, 2,4-diNO2, 2-NO2-4-CF3) and five bromoketones; the set of bromoketones used for optimization (4-OMe, 4-CF3, 4-Cl) was expanded by 4-CN and 3,5-diCl-4-NH2 derivatives. We eliminated compounds having the same substituent on both aromatic rings (e.g., compound 7(2,6) was not made). At the polymer-supported indazoles 6-oxide 18 stage, the resins were split and one half was deoxygenated using methanesulfonyl chloride in the presence of TEA1 to provide parent heterocycles 8 after cleavage from the resin. The rate of deoxygenation was substitution-dependent; target compounds without substitution on the indazole (R1 = H) were quantitatively deoxygenated after overnight treatment, with the exception of the R2 = CN derivative. Deoxygenation of compounds with R1 = CF3 and R1 = NO2 was repeated to complete the reaction. The compounds with the most electron-withdrawing groups on both rings (R1 = NO2 and R2 = CN) required a substantially extended reaction time of 4 days. Synthetic data are listed in Table 3.
Table 3.
Analytical data on synthesized compounds
| Compound | R1 | R2 | MS ESI+ | Puritya(%) | Yieldb(%) |
|---|---|---|---|---|---|
| 7(1,1) | H | 4-Me | 321 | 89 | 23 |
| 7(1,2) | H | 4-OMe | 337 | 86 | 81 |
| 7(1,3) | H | 4-Cl | 341 | 91 | 59 |
| 7(1,4) | H | 4-CN | 332 | 65 | 28 |
| 7(1,5) | H | 3,5-diCl-4-NH2 | 390 | 89 | 99 |
| 7(2,1) | CF3 | 4-Me | 389 | 95 | 82 |
| 7(2,2) | CF3 | 4-OMe | 405 | 95 | 55 |
| 7(2,3) | CF3 | 4-Cl | 409 | 84 | 59 |
| 7(2,4) | CF3 | 4-CN | 400 | 88 | 63 |
| 7(2,5) | CF3 | 3,5-diCl-4-NH2 | 458 | 95 | 99 |
| 7(3,1) | NO2 | 4-Me | 366 | 93 | 33 |
| 7(3,2) | NO2 | 4-OMe | 382 | 96 | 64 |
| 7(3,3) | NO2 | 4-Cl | 386 | 90 | 59 |
| 7(3,4) | NO2 | 4-CN | 377 | 89 | 55 |
| 7(3,5) | NO2 | 3,5-diCl-4-NH2 | 435 | 92 | 78 |
| 8(1,1) | H | 4-Me | 305 | 89 | 56 |
| 8(1,2) | H | 4-OMe | 321 | 96 | 76 |
| 8(2,2) | CF3 | 4-OMe | 389 | 94 | 97 |
| 8(2,3) | CF3 | 4-Cl | 393 | 81 | 56 |
| 8(2,4) | CF3 | 4-CN | 384 | 84 | 49 |
| 8(3,3) | NO2 | 4-Cl | 370 | 81 | 31 |
| 8(3,4) | NO2 | 4-CN | 361 | 67 | 41 |
| 8(3,5) | NO2 | 3,5-diCl-4-NH2 | 419 | 84 | 43 |
| 10(1,2) | H | 4-OMe | 321 | 96 | 69 |
| 10(1,3) | H | 4-Cl | 325 | 90 | 35 |
| 10(1,4) | H | 4-CN | 316 | 76 | 11 |
| 10(1,5) | H | 3,5-diCl-NH2 | 374 | 86 | 45 |
| 10(2,5) | CF3 | 3,5-diCl-4-NH2 | 442 | 92 | 68 |
| 10(3,2) | NO2 | 4-OMe | 366 | 87 | 73 |
| 19(1,1) | H | 4-Me | 344 | 94 | 53 |
| 29(1,1) | H | 4-Me | 322 | 91 | 64 |
| 29(1,3) | H | 4-Cl | 342 | 85 | 67 |
| 29(1,4) | H | 4-CN | 333 | 78 | 47 |
| 30(1,4) | H | 4-CN | 317 | 85 | 63 |
| 34(1,1) | H | 4-Me | 335 | 81 | 57 |
| 34(1,3) | H | 4-Cl | 355 | 87 | 63 |
| 40(1,2) | H | 4-OMe | 427 | 98 | 68 |
| 40(1,3) | H | 4-Cl | 430 | 97 | 83 |
| 40(1,4) | H | 4-CN | 422 | 54 | 19 |
Purity of crude product;
Yield after purification
The target compound 7(1,1) was further derivatized in solution by acylation of the amino group by acetanhydride (Scheme 5). The product 19 was isolated, purified in methanol with an 0.1% aqueous TFA gradient and fully characterized. The presence of the amide proton in the 1H NMR spectrum provided further evidence for the iminium structure.
Combinatorial synthesis of triamines
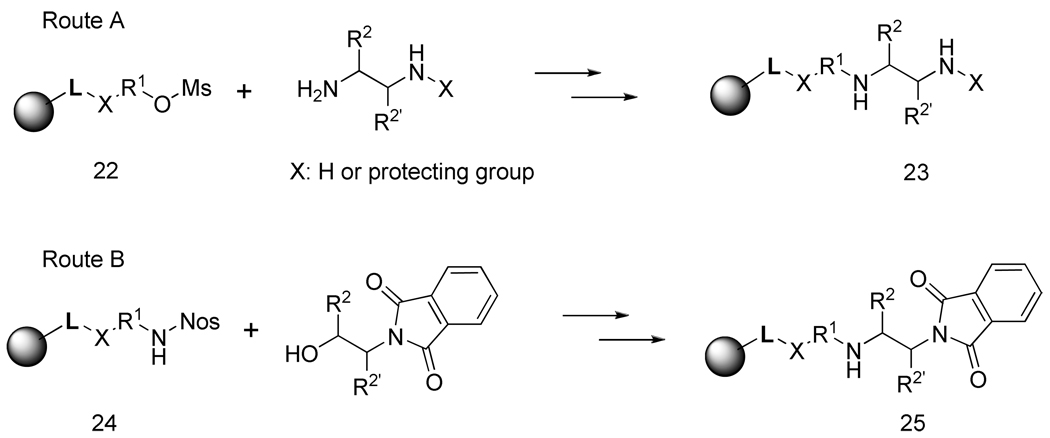
The orthogonal Pht/Boc protection strategy proved to be the preferred route for the synthesis of target compounds with diverse side-chains on both aromatic rings. However, the use of diethylenetriamine prevented the introduction of side-chain functional groups on the triamine and limited the substitution pattern of the products. In addition, it did not allow for the modification of the substituent attached to the secondary amine. Thus, as a next step in the development of these syntheses, we prepared amine resins from two building blocks while maintaining the same protection strategy. Two different scenarios were tested and successfully applied for the synthesis.
Route A (Scheme 6) involved the immobilization of an amino alcohol using CDI-activated Wang resin. The alcohol resin was converted to mesylate 22 using methanesulfonyl chloride and reacted with ethylenediamine to yield amine resin 23. An alternative route B involved the immobilization of an amine, reaction with 2-Nos-Cl (resin 24) and Mitsunobu reaction with the β-amino alcohol protected by the Pht group (resin 25).
Scheme 6.
Combinatorial assembly of amine resins
These routes were used to prepare compounds that enabled iminium-mediated cyclization with different ring sizes (hexahydro-pyrimidine and oxazolidine in addition to imidazolidine) as well as compounds that did not contain an internal nucleophile amenable to reaction with the iminium (4-carbamoyl-benzyl).
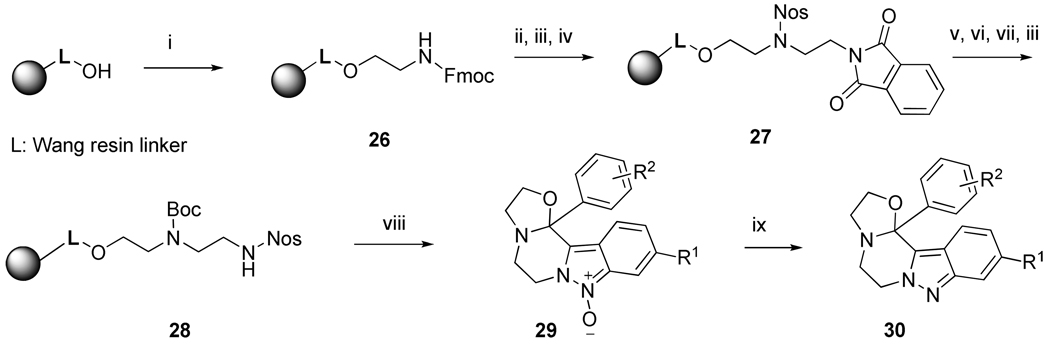
Synthesis of oxazolidine derivatives 30
Wang resin was activated via trichloroacetmimidate27 (Scheme 7) and reacted with Fmoc-ethanolamine (resin 26). The Fmoc group was cleaved, and the amine resin reacted with 2-Nos-Cl. The Nos resin underwent a Mitsunobu reaction with N-(2-hydroxyethyl)phthalimide (resin 27). The Nos group was cleaved, and the amine resin was protected with Boc2O. The Pht group was cleaved by hydrazine hydrate, and the amine resin was reacted with 2-Nos-Cl to yield resin 28. Target compounds were obtained after finishing the reaction sequence described for the preparation of compounds 7 and 8.
Scheme 7.
Synthesis of oxazolidine derivatives
Reagents and conditions: (i) trichloroacetonitrile, DBU, DCM, 0 °C to rt, DCM wash, then Fmoc-ethanolamine, BF3•Et2O, anhydrous THF, rt, 30 min; (ii) piperidine, DMF, rt, 20 min; (iii) 2-nitrobenzenesulfonyl chloride, lutidine, DCM, rt, 16 h; (iv) N-(2-hydroxyethyl)phthalimide, DIAD, anhydrous THF, 5 h; (v) mercaptoethanol, DBU, DMF, rt, 30 min; (vi) Boc2O, pyridine, DCM, rt, 2 h; (vii) hydrazine monohydrate, MeOH/THF (1:1), rt, 4 h; (viii) cf. Scheme 5, reactions vi to viii; (ix) MsCl, TEA, DCM, 30 min at 0°C, then rt, 16 h
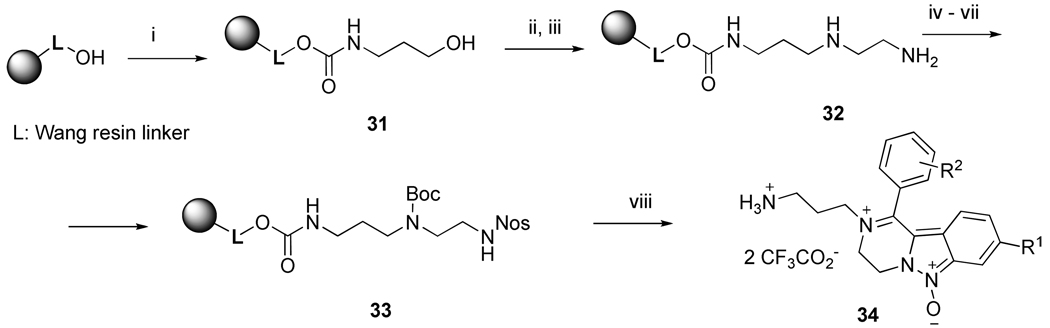
Synthesis of 2-(3-aminopropyl)-1-aryl-3,4-dihydropyrazino[1,2-b]indazole-2-ium 6-oxides 34
The expanded ring analogs of compounds 7 and 8 were prepared by reacting CDI-activated Wang resin with 1-aminopropanol (Scheme 8). The alcohol resin 31 was converted to a mesyl derivative by reaction with methanesulfonyl chloride. The resin-bound unsymmetrical triamine 32 was obtained after reaction with ethylenediamine. The reaction sequence was finished using the previously described protocol and products 34 were obtained after TFA-mediated cleavage from the resin.
Scheme 8.
Synthesis of hexahydro-pyrimidine derivatives
Reagents and conditions: (i) CDI, pyridine, DCM, rt, 3 h, then 1-aminopropanol, DCM, rt, 16 h; (ii) mesyl chloride, pyridine, 1h; (iii) ethylenediamine, DMSO, 2 days; (iv) N-hydroxyphthalimide, DMF, rt, 16 h; (v) Boc2O, pyridine, DCM, rt, 2 h; (vi) hydrazine monohydrate, MeOH/THF (1:1), rt, 4 h; (vii) 2-nitrobenzenesulfonyl chloride, lutidine, DCM, rt, 16 h, (viii) cf. Scheme 5, reactions vi to viii
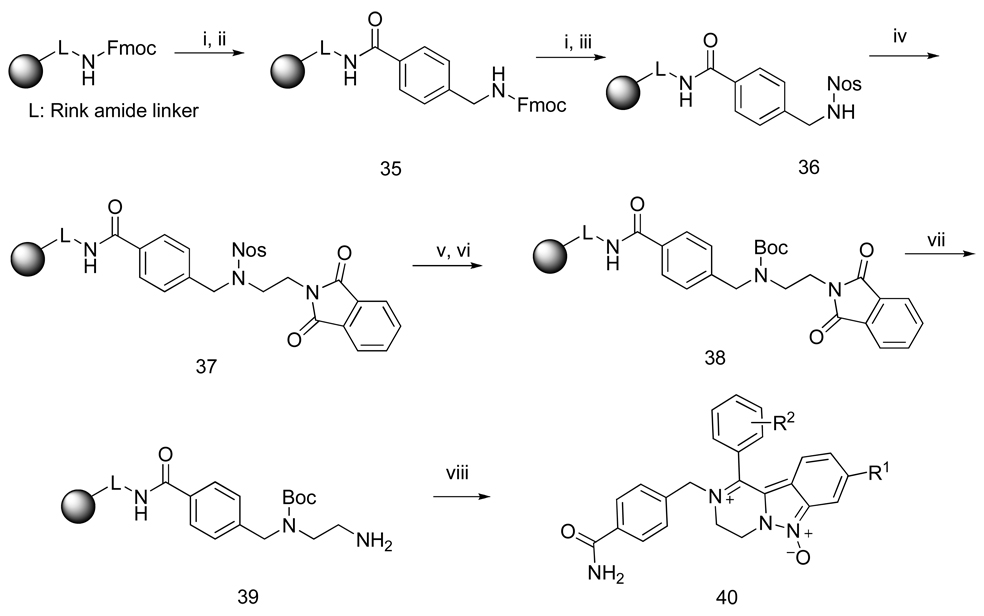
Synthesis of 4-carbamoyl-benzyl derivatives 40
All compounds described so far contained a nucleophile (amino or hydroxyl group) that facilitated ring closure of iminiums to produce fused five or six-membered rings. We also prepared 4-carbamoyl derivatives 40 (Scheme 9) that eliminated the nucleophile from the side-chain. Briefly, Rink amide resin was acylated with 4-(Fmoc-aminomethyl)benzoic acid (resin 35). The Fmoc group was cleaved and the amine resin was reacted with 2-Nos-Cl to generate sulfonamide resin 36. N-alkylation with N-(2-hydroxyethyl)phthalimide under Mitsunobu conditions yielded resin 37. At this stage, the 2-Nos group was cleaved and the secondary amine was reacted with Boc2O in the presence of pyridine to afford the protected amine 38. After cleavage of the phthalimide group, the resin 39 was reacted with 2-Nos-Cl. Alkylation with three bromoketones (4-MeO, 4-Cl, and 4-CN) proceeded smoothly. After cyclization to indazoles and cleavage from resins, the LC-MS analyses of final products indicated iminium/amino ketone equilibriums of 86%/11%, 71%/25%, and 66%/31% for the 4-OMe, 4-Cl, and 4-CN derivatives, respectively.
Scheme 9.
Synthesis of 4-carbamoyl-benzyl derivatives 40
Reagents and conditions: (i) 50% piperidine, DMF, 15 min; (ii) 4-(Fmoc-aminomethyl)-benzoic acid, HOBt, DIC, DCM / DMF (1:1), 16; (iii) 2-nitrobenzenesulfonyl chloride, lutidine, DCM, 16 h; (iv) N-(2-hydroxyethyl)phthalimide, PPh3, DIAD, 0°C, 30 min then rt, 16 h; (v) 2-mercaptoethanol, DBU, DMF, 5 min; (vi) Boc2O, pyridine, DCM, 2 h; (vii) hydrazine monohydrate, MeOH/THF (1:1), rt, 4 h; (viii) cf. Scheme 5, reactions vi to viii
All library compounds were submitted for evaluation of biological activities to High Throughput Screening in the Molecular Libraries Probe Production Centers Network. The results are available in PubChem (http://pubchem.ncbi.nlm.nih.gov/).
Conclusion
Efficient solid-phase synthesis of complex fused heterocyclic systems was developed using amino alcohols, diamines, benzenesulfonyl chlorides and bromoketones. Syntheses tolerated a wide range of substitution patterns on building blocks, and all reactions were carried out under mild conditions. Acid-mediated cleavage of resin-bound indazole precursors yielded 1,2-substituted 3,4-dihydropyrazino[1,2-b]indazole-2-ium trifluoroacetates that spontaneously cyclized to complex fused heterocycles including 3,6,9,10-tetraazatetracyclo[7.7.0.02,6.011,16]hexadeca-11,13,15-trienes, 3-oxa-6,9,10-triazatetracyclo[7.7.0.02,6.011,16]hexadeca-11,13,15-trienes, and 3,7,10,11-tetraazatetracyclo[8.7.0.02,7.012,17]heptadeca-12,14,16-trienes.
Experimental Section
Solid-phase syntheses were carried out using a manually operated Domino Block synthesizer28 (www.torviq.com) in disposable polypropylene reaction vessels. Commercially available solvents, resins, and reagents were used. The Rink resin (100–200 mesh, 1% DVB, 0.75 mmol/g), aminomethyl resin (100–200 mesh, 1% DVB, 0.9 mmol/g) and Wang resin (100–200 mesh, 1% DVB, 1.0 mmol/g) were obtained from Advanced ChemTech (Louisville, KY, www.peptide.com). The swelling of resins in DCM was measured before syntheses, and those with swollen volumes greater than 7 mL/g of dry resin were used.29 All reactions were carried out at ambient temperature (21 °C) unless otherwise stated.
Experimental procedures
Reaction with CDI and diethylenetriamine (resin 1)
Wang resin, 1 g, was swollen in DCM and a solution of CDI (5 mmol, 810 mg) and pyridine (5 mmol, 400 µL) in 10 mL of DCM was added and the slurry was shaken for 3 h. Resin was washed 3 × with DCM and a solution of diethylenetriamine (5 mmol, 540 µL) in 10 mL DCM was added. Resin slurry was shaken for 16 h and washed 3 × with DCM.
Reaction with N-hydroxyphthalimide (resins 13)
Resin 1, 1 g, was washed 3 × with DCM, 3 × DMF and a solution of N-hydroxyphthalimide (10 mmol, 1.63 g) in 10 mL DMF was added to the resin and reaction slurry was shaken for 16 h. The resin was washed with 5 × DMF and 3 × DCM.
Reaction with Boc2O (resins 14)
Resin 13, 1 g, was washed 3 × with DCM and a solution of Boc2O (5 mmol, 1.1 g) and pyridine (5 mmol, 400 µL) in 10 mL DCM was added to the resin and reaction slurry was shaken for 2 h. The resin was washed 3 × with DCM.
Reaction with Hydrazine monohydrate (resins 15)
Resin 14, 1 g, was washed 3 × with DCM, 3 × THF and a solution of hydrazine monohydrate (41.2 mmol, 2 mL) in 10 mL MeOH / THF (1:1) was added to the resin and reaction slurry was shaken for 6 h. The resin was washed with MeOH (5 ×), 3 × DCM.
Reaction with 2-Nos-Cl (resin 16)
Resin 15, 1 g, was washed 3 × with DCM, a solution of 2-Nos-Cl (3 mmol, 663 mg) and lutidine (3.3 mmol, 382 µL) in 10 mL DCM was added to the resin and reaction slurry was shaken for 16 h. The resin was washed with 5 × DCM.
Reaction with bromoketone (resins 17)
Resin 16, 1 g, was washed 3 × with DCM and 3 × DMF. A solution of 0.5 M bromoketone (5 mmol) and 1 M DIEA (10 mmol, 1.73 mL) in 10 mL DMF was added. The resin slurry was shaken for 16 h. The resin was washed 3 × with DMF and 5 × DCM.
Cyclization to indazole oxides (resins 18)
Resin 17, 250 mg, was washed 3 × with DCM, 3 × DMF and a solution of 0.2 M DBU (1 mmol, 150 µL) in 5 mL DMF was added and resin slurry was shaken for 30 min. Resin was washed 3 × with DMF, 3 × MeOH, and 5 × DCM.
Deoxygenation of indazole oxides
Syringe with indazole oxide resin 18, 250 mg, was washed 3 × with DCM and 0.7 M TEA (245 µL) in 2.5 mL DCM was added. The syringe was connected with the second syringe containing 0.5 M mesyl chloride (96 µL) in 2.5 mL DCM. Connected syringes were left in a freezer for 30 min and then mesyl chloride solution was drawn into the syringe with the resin. The resin slurry was shaken for 16 h. Resin was washed with 5 × DCM.
Acylation of Rink resin with 4-(Fmoc-aminomethyl) benzoic acid (resin 35)
Rink resin, 1 g, was swollen in DCM, washed 3 × with DMF and treated with 50 % piperidine in DMF for 15 min. After washing 3 × with DMF and 3 × DCM a solution of 4-(Fmoc-aminomethyl) benzoic acid (3 mmol, 1.12 g), HOBt (3 mmol, 405 mg), and DIC (3 mmol, 463 µL) in 10 mL of DCM / DMF (1:1) was added to syringe and the slurry was shaken for 16 h. The resin was washed with 5 × DMF and 5 × DCM.
Mitsunobu reaction with N-(2-hydroxyethyl)phthalimide (resin 37´)
Resin 36, 1 g, was washed 3 × with DCM, anhydrous 3 × THF. Solution of N-(2-hydroxyethyl)phthalimide (2.6 mmol, 480 mg) and PPh3 (2.5 mmol, 655 mg) in 10 mL of anhydrous THF was added to the syringe, and cooled in freezer over 30 min. Then DIAD (2.4 mmol, 480 µL) was added into syringe and shaken for 16 h. The resin was washed 5 × with THF and 5 × DCM.
Cleavage and purification of products
Resins with target compounds, typically ~250 mg, were treated with 50 % TFA / DCM for 1 h. TFA solution was collected, the resin was washed with 50 % TFA / 3 × DCM, combined extracts were evaporated by a stream of nitrogen and purified by semi-preparative HPLC.
Reaction with acetic anhydride (21)
The compound 7(1,1) (0.07 mmol, 24.6 mg) was dissolved in acetonitrile (2 mL) and acetic anhydride (2.3 mmol, 217 µL) was added and the reaction mixture was stirred for 3 h, evaporated under reduced pressure and crude product purified by semi-preparative HPLC (MeOH - 0.1% aqueous TFA).
Supplementary Material
Acknowledgement
The work was supported by the Department of Chemistry and Biochemistry, University of Notre Dame and the NIH (GM079576). We gratefully appreciate the use of the NMR facility at the University of Notre Dame.
Footnotes
Supporting Information Available. Details regarding experimental procedures, spectroscopic data and NMR spectra for new compounds are available free of charge via the Internet at http://pubs.acs.org
Reference List
- 1.Bouillon I, Zajicek J, Pudelova N, Krchnak V. J. Org. Chem. 2008;73:9027–9032. doi: 10.1021/jo8018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuyama T, Jow CK, Cheung M. Tetrahedron Lett. 1995;36:6377–6374. [Google Scholar]
- 3.Pudelova N, Krchnak V. J. Comb. Chem. 2009;11:370–374. doi: 10.1021/cc800181y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royer J, Bonin M, Micouin L. Chem. Rev. 2004;104(5):2311–2352. doi: 10.1021/cr020083x. [DOI] [PubMed] [Google Scholar]
- 5.Remuson R, Gelas-Mialhe Y. Mini-Rev. Org. Chem. 2008;5(3):193–208. [Google Scholar]
- 6.Speckamp WN, Moolenaar MJ. Tetrahedron. 2000;56(24):3817–3856. [Google Scholar]
- 7.Overman LE. Acc. Chem. Res. 1992;25:352–359. [Google Scholar]
- 8.Sumner JS, Matthews RG. J. Am. Chem. Soc. 1992;114(18):6949–6956. [Google Scholar]
- 9.Sharma S, Saha B, Sawant D, Kundu B. J. Comb. Chem. 2007;9(5):783–792. doi: 10.1021/cc0700445. [DOI] [PubMed] [Google Scholar]
- 10.Petersen JS, Toeteberg-Kaulen S, Rapoport H. J. Org. Chem. 1984;49(16):2948–2953. [Google Scholar]
- 11.Larouche-Gauthier R, Be¦ülanger G. Org. Lett. 2008;10(20):4501–4504. doi: 10.1021/ol801705s. [DOI] [PubMed] [Google Scholar]
- 12.Belanger G, Larouche-Gauthier R, Menard F, Nantel M, Barabe F. J. Org. Chem. 2006;71(2):704–712. doi: 10.1021/jo052141v. [DOI] [PubMed] [Google Scholar]
- 13.Bates HA, Rapoport H. J. Am. Chem. Soc. 1979;101(5):1259–1265. [Google Scholar]
- 14.Hiemstra HC, Bieräugel H, Wijnberg M, Pandit UK. Tetrahedron. 1983;39:3981–3986. [Google Scholar]
- 15.Vojkovsky T, Weichsel A, Patek M. J. Org. Chem. 1998;63:3162–3163. [Google Scholar]
- 16.Spaller MR, Thielemann WT, Brennan PE, Bartlett PA. J. Comb. Chem. 2002;4(5):516–522. doi: 10.1021/cc020027+. [DOI] [PubMed] [Google Scholar]
- 17.Carranco I, Diaz JL, Jimenez O, Vendrell M, Albericio F, Royo M, Lavilla R. J.Comb. Chem. 2005;7(1):33–41. doi: 10.1021/cc049877a. [DOI] [PubMed] [Google Scholar]
- 18.Kundu B, Sawant D, Partani P, Kesarwani AP. J. Org. Chem. 2005;70(12):4889–4892. doi: 10.1021/jo050384h. [DOI] [PubMed] [Google Scholar]
- 19.Marx MA, Grillot A, Louer CT, Beaver KA, Bartlett PA. J. Am. Chem. Soc. 1997;119:6153–6167. [Google Scholar]
- 20.Morimoto Y, Kurihara H, Yokoe C, Kinoshita T. Chem. Lett. 1998;(8):829–830. [Google Scholar]
- 21.Degutyte R, Sackus A, Berg U. Journal of Chemical Research, Synopses. 2001:540–542. [Google Scholar]
- 22.Moehrle H, Berkenkemper T. Z. Naturforsch. B. 2007;62:1514–1524. [Google Scholar]
- 23.Jencks WP. Chem. Rev. 1972;72(6):705–718. [Google Scholar]
- 24.Gouesnard JP. Bull. Soc. Chim. Fr. 1988:132–138. [Google Scholar]
- 25.D'hooghe M, Boelens M, Piqueur J, De Kimpe N. Chem. Commun. 2007;(1927):1929. doi: 10.1039/b703147e. [DOI] [PubMed] [Google Scholar]
- 26.Krchnak V, Zajicek J. J. Comb. Chem. 2005;7:523–525. doi: 10.1021/cc050026s. [DOI] [PubMed] [Google Scholar]
- 27.Hanessian S, Xie F. Tetrahedron Lett. 1998;39:733–736. [Google Scholar]
- 28.Krchnak V, Padera V. Bioorg. Med. Chem. Lett. 1998;22:3261–3264. doi: 10.1016/s0960-894x(98)00594-0. [DOI] [PubMed] [Google Scholar]
- 29.Bouillon I, Soural M, Miller MJ, Krchnak V. J. Comb. Chem. 2009;11:213–215. doi: 10.1021/cc800143e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.