Abstract
Several phosphoinositide-3-kinase (PI3K) catalytic subunit inhibitors are currently in clinical trial. We therefore sought to examine relationships between pharmacological inhibition and somatic mutations in PI3K catalytic subunits in ER+ breast cancer, where these mutations are particularly common. RNA interference (RNAi) was used to determine the effect of selective inhibition of PI3K catalytic subunits, p110α and p110β, in ER+ breast cancer cells harboring either mutation (PIK3CA) or gene amplification (PIK3CB). p110α RNAi inhibited growth and promoted apoptosis in all tested ER+ breast cancer cells under estrogen deprived-conditions, whereas p110β RNAi only affected cells harboring PIK3CB amplification. Moreover, dual p110α/p110β inhibition potentiated these effects. In addition, treatment with the clinical grade PI3K catalytic subunit inhibitor BEZ235 also promoted apoptosis in ER+ breast cancer cells. Importantly, estradiol suppressed apoptosis induced by both gene knockdowns and by BEZ235 treatment. Our results suggest that PI3K inhibitors should target both p110α and p110β catalytic subunits, whether wild-type or mutant, and be combined with endocrine therapy for maximal efficacy when treating ER+ breast cancer.
Keywords: breast cancer, estrogen receptor, PI3 kinase, endocrine therapy, synthetic lethality
INTRODUCTION
Despite the use of adjuvant endocrine treatment prognosis remains poor for a significant population of patients with estrogen receptor positive (ER+) breast cancer (1). The cellular basis for the efficacy of endocrine therapy treatment is principally through inhibitory effects on the tumor cell cycle (2) because, unlike cytotoxic chemotherapy, it has never been clearly demonstrated that endocrine therapy promotes cell death through apoptosis (3). A logical approach to improving ER+ breast cancer treatment is, therefore, to inhibit gene activities that promote survival in the presence of ER targeting agents. To address this hypothesis we focused on combining endocrine agents with inhibitors of phosphoinositide 3-kinase (PI3K) because this pathway promotes cell survival in a number of tumor types (4).
Aberrant activation of the PI3K pathway through mutation and epigenetic silencing of genes within the PI3K signaling cascade frequently occurs in breast cancer. Gain of function mutations in the PI3K alpha catalytic subunit (PIK3CA) occur in ~30% of ER+ breast cancer and, much less commonly, activating AKT1 mutations. Loss of function mutations affect the PI3K negative regulator PTEN and gene amplification in S6 protein kinase-1 (RPS6KB1) and AKT2 have also been reported (5–8). Whereas the precise consequences of these aberrations on the clinical outcome of ER+ disease remain to be fully defined, RPS6KB1 amplification and PTEN loss are both associated with poor prognosis and PTEN loss may correlate with endocrine therapy resistance in ER+ tumors (9–15). In contrast, PIK3CA presents a more complex picture and mutations may differentially impact prognosis depending upon the affected PIK3CA functional domain (15). Finally, a role for PIK3CB, the gene encoding the PI3K beta catalytic subunit, has also recently been postulated in breast cancer, although mutations in this gene have not been detected (16, 17).
Several PI3K catalytic subunit inhibitors are advancing towards Phase II clinical testing (18). The targets for these agents are the products of the Class 1A PI3K catalytic subunit genes (PIK3CA, PIK3CB and PIK3CD). PIK3CA and PIK3CB are believed to be broadly expressed in breast cancer, whereas PIK3CD gene expression is more limited (19). We sought to address a number of issues related to the clinical development of these compounds. First, it is not clear if PIK3CA mutation status restricts the efficacy of PI3K inhibitors. Second, catalytic subunit targeting strategies for achieving maximum therapeutic effect have not been developed. Finally, a rationale for the combination of a PI3K inhibitor and endocrine therapy in ER+ breast cancer has not been established.
MATERIALS AND METHODS
Human Tumor Samples
Fresh-frozen and formalin-fixed paraffin embedded (FFPE) human breast tumor biopsies for paired aCGH and PIK3CB fluorescence in situ hybridization (FISH) were obtained from ER+ breast cancer patients undergoing preoperative letrozole treatment (POL) (20). RNA for transcriptional profiling and cDNA synthesis (samples ≥ 50% tumor) and DNA for aCGH (samples ≥ 70% tumor cellularity) were prepared from sectioned fresh-frozen samples using RNeasy Mini and QIAamp DNA Micro kits (Qiagen, Valencia, CA) for RNA and DNA extractions. Tumor enrichment was performed using macrodissection or an Arcturus Veritas laser capture microdissection instrument (Arcturus Bioscience, Mountain View, CA). A human breast tissue microarray (TMA) obtained at the Siteman Cancer Center Tissue Core Facility and used for PIK3CB FISH was described previously (21).
aCGH
Details are provided in Supplementary Materials and Methods.
Transcriptional Profiling
Details are provided in Supplementary Materials and Methods.
Gene Re-sequencing
Details for PIK3CA and PIK3CB re-sequencing are provided in Supplementary Materials and Methods.
Cell Culture
The HCC712 cell line (22) was provided by Dr. Adi Gazdar. Other cell lines were obtained from ATCC (Manassas, VA). Cell lines were propagated in RPM1 1640 containing 10% FBS with antibiotics and supplements (50 µg/mL gentamycin, pyruvate, 10 mM Hepes and glucose to 4.5 g/L) in a humidified 37°C incubator containing 5% CO2. To test the effects of estradiol (E2, Sigma-Aldrich) treatment and withdrawal, cells were maintained in phenol red-free RPM1 1640 containing 5% charcoal-stripped serum (CSS, Invitrogen, Carlsbad, CA) (CSS medium) for at least 7 days prior to siRNA transfection or drug treatments.
Protein Extracts
Details are provided in Supplementary Materials and Methods.
RNAi Transfection
Nuclease-resistant Stealth duplex siRNAs (Invitrogen) were used for RNAi experiments. The following siRNAs were used: Universal Low GC Negative Control; PIK3CA siRNAs (target sequence 5'–GGUGGUGCGAAAUUCUCACACUAUU–3' for primary siRNA duplex and 5’–CCCAAGAAUCCUAGUAGAAUGUUUA–3’ for alternative siRNA duplex); PIK3CB siRNAs (target sequence 5'–GCUGUCAAUCAAGUGGAAUAAACUU–3' for primary siRNA duplex and 5’–GCGCUUGAUGGAUUUACUCUGGAAA –3’ for alternative siRNA duplex). PIK3CA and PIK3CB siRNA knockdown efficiencies were determined by reverse transfection of siRNAs into cells and immunoblotting cell lysates prepared three days after transfection. Maximal knockdown efficiency (>70%) was achieved with 10 nM PIK3CA or PIK3CB siRNA. Transfection efficiency assessed by the BLOCK-IT fluorescent oligo (Invitrogen) was >90% in all cell lines.
Immunoblotting
Details are provided in Supplementary Materials and Methods.
Cell Growth Assay
Details are provided in Supplementary Materials and Methods.
Cell Death Assay
Details are provided in Supplementary Materials and Methods.
PIK3CB FISH
Details are provided in Supplementary Materials and Methods.
Statistical Analysis
Unless indicated otherwise, quantitative data are presented as mean ± SEM. The effect of siRNA knockdowns and pharmacologic treatments on cell growth and apoptosis was analyzed using ANOVA. If within group comparisons reached statistical significance (p< 0.05), comparisons between specific treatments were made with Student’s t test. Interactions between PAM50 subtypes or PIK3CA mutation status and PIK3CA, PIK3CB and PIK3CD expression were analyzed by t-tests using the SAS version 9.1 Statistical Package (SAS Institute Inc., Cary, NC).
RESULTS
p110α and p110β Expression in Breast Cancer Cells
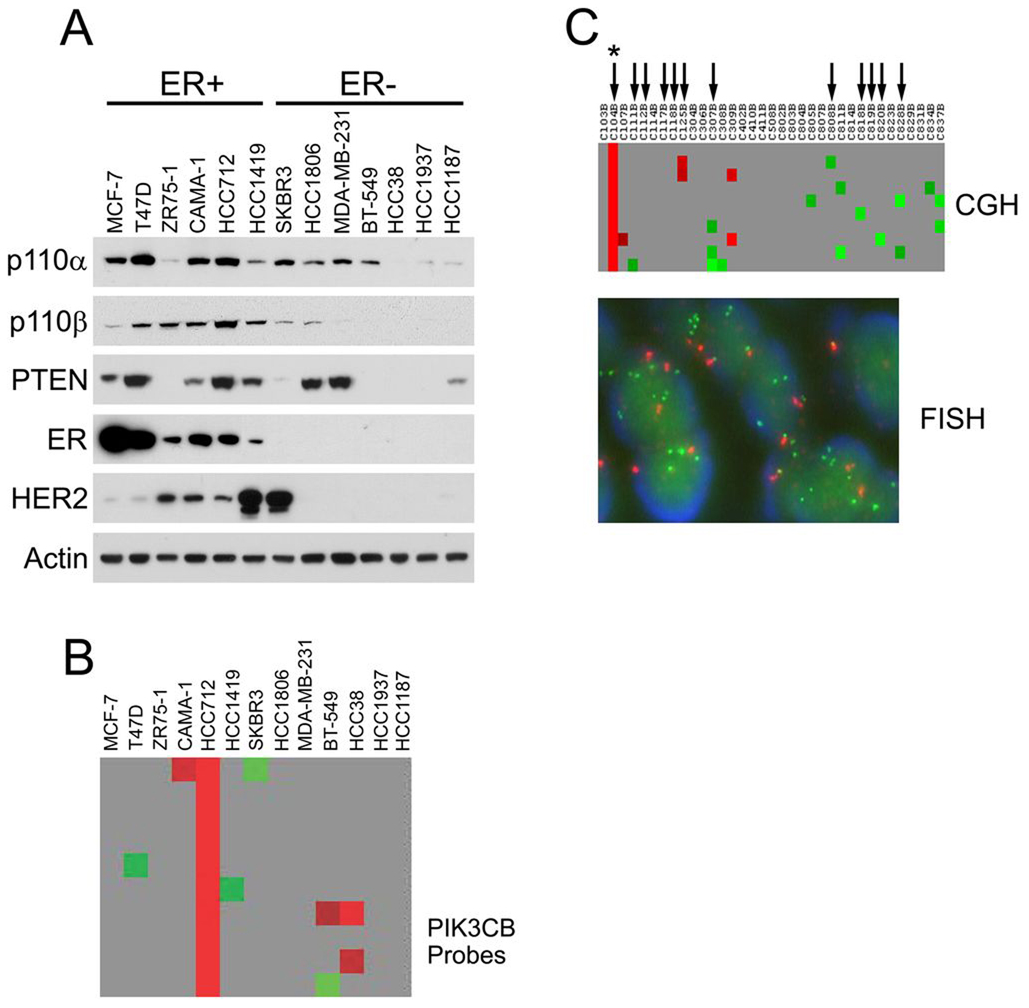
The expression of p110α and p110β was examined in breast cancer cell lines by western blot analysis (Figure 1A). The panel included ER+ breast cancer cells with activating PIK3CA mutations (MCF-7 and T47D) or wild-type PIK3CA (HCC712) (11). Both PI3K catalytic subunit isoforms were ubiquitously present, however p110α and p110β expression varied widely. Notably, p110β protein expression was higher in ER+ cells with the HCC712 cell line (22) expressing the most. To determine if increased expression was associated with gene copy gain, high resolution array comparative genomic hybridization (aCGH) was performed. This analysis revealed PIK3CB copy gain at 3q22.3 in the HCC712 cell line, but not in other cell lines (Figure 1B). PIK3CB gene copy number in HCC712 cells was confirmed by fluorescent in situ hybridization (FISH) (gene to centromere ratio ~2.5).
Figure 1. PIK3CB is amplified in breast cancer.
A, p110α and p110β expression in breast cancer cell lines. Equal amounts (25µg) of protein from each cell line were immunoblotted for the indicated proteins. Longer exposures revealed that both p110α and p110β proteins were expressed in all cell lines. B, PIK3CB aCGH analysis in breast cancer cell lines. Shown is a section of probes on chromosome 3 corresponding to the PIK3CB locus. Individual array probes indicate probable copy number gain (red), loss (green), or no change (gray) relative to female diploid DNA. C, aCGH and PIK3CB FISH in ER+ breast tumors. Top panel, aCGH probes corresponding to the PIK3CB locus. Arrows indicate tumor samples subjected to PIK3CB FISH. One of the FISH-tested tumor samples (*) contained PIK3CB amplification. Bottom panel, FISH results from the PIK3CB-amplified breast tumor above. The CEP3 probe is red; the PIK3CB-specific probe is green.
PIK3CB Amplification in Primary Breast Cancer
Array CGH analysis on ER+ primary breast tumors revealed PIK3CB copy gain in at least one tumor examined (1/35) which was confirmed by FISH (amplification ration 2.3, Figure 1C). In another series of primary breast cancer samples from a breast tissue microarray, low-level PIK3CB amplification or copy number gain was observed by FISH in different breast cancer subtypes (1 ER−, 1 ER+, 2 ER+/HER2+), suggesting that PIK3CB copy number gain occurs with an incidence of ~5% (Supplementary Table1). To determine if PIK3CB was mutant, the PIK3CB helical and kinase domains in 22 primary ER+ breast tumors were sequenced, including the PIK3CB amplified breast tumor illustrated in Figure 1C and three breast cancer cell lines (HCC712, MCF-7 and T47D). No sequence anomalies were detected.
PIK3CA and PIK3CB Are Expressed at Higher Levels in Luminal B Breast Cancer in Comparison to Luminal A Breast Cancer
The variation in PIK3CA and PIK3CB expression observed in breast cancer cell lines led to an expression analysis in a series of ER+ primary breast cancers. First, microarray studies were examined using the PAM50 model (23) in order to subtype cases into Luminal A (good prognosis - 31 cases) or Luminal B (poor prognosis - 44 cases (Table 1). There was strong evidence for higher expression of both PIK3CA and PIK3CB in poor prognosis Luminal B tumors when compared to Luminal A tumors. Higher expression of PIK3CD was also observed in Luminal B tumors but the result was less striking. The presence of a PIK3CA mutation was associated with higher levels of PIK3CA mRNA but not PIK3CB or PIK3CD.
Table 1. PIK3CA, PIK3CB and PIK3CD Expression in Relation to ER+ Breast Cancer Subtype and PIK3CA Mutation Status.
Gene expression in primary breast tumors was measured by whole genome expression arrays and ER+ tumors were subtyped by PAM50 subclassification to Luminal A (LumA) and Luminal B (LumB) and PIK3CA mutation status was determined. Means and standard deviations (in parenthesis) were calculated for subtypes and mutation status. The 95% confidence intervals (CI) were calculated for the mean difference of LumB to LumA and PIK3CA mutant to wild-type. Two-sample t-tests were used to determine differences in the expression of PIK3CA, PIK3CB and PIK3CD based on LumB versus LumA subtypes and PIK3CA mutant (MUT) versus PIK3CA wild-type (WT) tumors.
| Expression Mean (SD) |
95% CI for mean difference |
P-Value | |
|---|---|---|---|
| PIK3CA Expression | |||
| LumB | 0.41 (0.42) | 0.11 ~ 0.54 | 0.003 |
| LumA | 0.08 (0.50) | ||
| PIK3CA MUT | 0.45 (0.47) | 0.04 ~ 0.49 | 0.02 |
| PIK3CA WT | 0.18 (0.47) | ||
| PIK3CB Expression | |||
| LumB | −0.31 (0.46) | 0.14 ~ 0.66 | 0.005 |
| LumA | −0.72 (0.66) | ||
| PIK3CA MUT | −0.41 (0.59) | −0.16 ~ 0.41 | 0.37 |
| PIK3CA WT | −0.53 (0.58) | ||
| PIK3CD Expression | |||
| LumB | −0.1 (0.16) | −0.16 ~ −0.01 | 0.03 |
| LumA | −0.01 (0.16) | ||
| PIK3CA MUT | −0.09 (0.16) | −0.13 ~ 0.03 | 0.25 |
| PIK3CA WT | −0.05 (0.17) |
p110α is the Predominant Mediator of PI3K signaling in Breast Cancer Cells but p110β Contributes in a Cell Line Restricted Manner
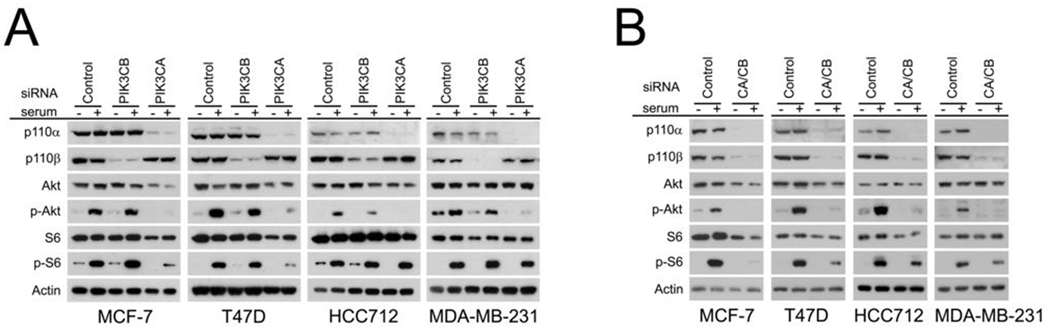
To determine the individual effects of PIK3CA and PIK3CB on PI3K signaling, small interfering (si)RNAs were used to selectively knock down p110α and p110β expression (Figure 2A). An analysis of signal transduction demonstrated that PIK3CB RNAi had no effect on serum-stimulated AKT phosphorylation in MCF-7, T47D and MDA-MB-231 cells, but partially inhibited Akt phosphorylation in HCC712 cells (Figure 2A). Knockdown of p110β had no clear effect on serum-stimulated phosphorylation of S6 protein in any of the cell lines tested. In contrast, PIK3CA RNAi suppressed serum-stimulated Akt phosphorylation in all cell lines tested. S6 phosphorylation was also significantly inhibited in MCF-7 and T47D cells but not in the HCC712 and MDA-MB-231 cell lines. Consistent with previous studies (24), we observed reductions in Akt and S6 protein levels in some experiments, particularly for MCF-7 cells. To test whether the lack of inhibition of serum-stimulated S6 phosphorylation by p110α knockdown in MDA-MB-231 and HCC712 cells was due to compensatory signaling through p110β, dual p110α/p110β knockdowns were performed (Figure 2B). Combined p110α/p110β knockdown had no clear effect on S6 phosphorylation in MDA-MB-231 cells. However the combination partially inhibited S6 phosphorylation in HCC712 cells indicating that both PIK3CA and PIK3CB must be inhibited to impact S6 kinase activation in this cell line. Overall, this analysis indicated that p110α is the major catalytic subunit that transduces PI3K pathway signals in ER+ breast cancer cells, but p110β significantly contributes to pathway activation, particularly in cells containing higher levels of p110β expression.
Figure 2. p110α is the predominant mediator of PI3K signaling in breast cancer cells.
A, effect of p110α and p110β knockdown on PI3K signaling. Cells were transfected with control siRNAs (Control) or siRNAs against PIK3CB (PIK3CB) or PIK3CA (PIK3CA). Three days after transfection, serum-deprived cells were stimulated with 20% FBS (final concentration) and lysates were analyzed for effects on PI3K pathway signaling through phospho-Akt (p-Akt) and phospho-S6 (p-S6) immunoblotting. Shown are representative immunoblots obtained from at least three experiments per cell line. B, effect of p110α/p110β dual knockdown on PI3K signaling. Cells were transfected with control siRNAs or a mixture of PIK3CA and PIK3CB siRNAs (CA/CB), treated as above and subjected to immunoblot analysis. Representative results obtained in at least two experiments per cell line are shown.
PIK3CA and PIK3CB RNAi Inhibit ER+ Breast Cancer Cell Growth and Survival
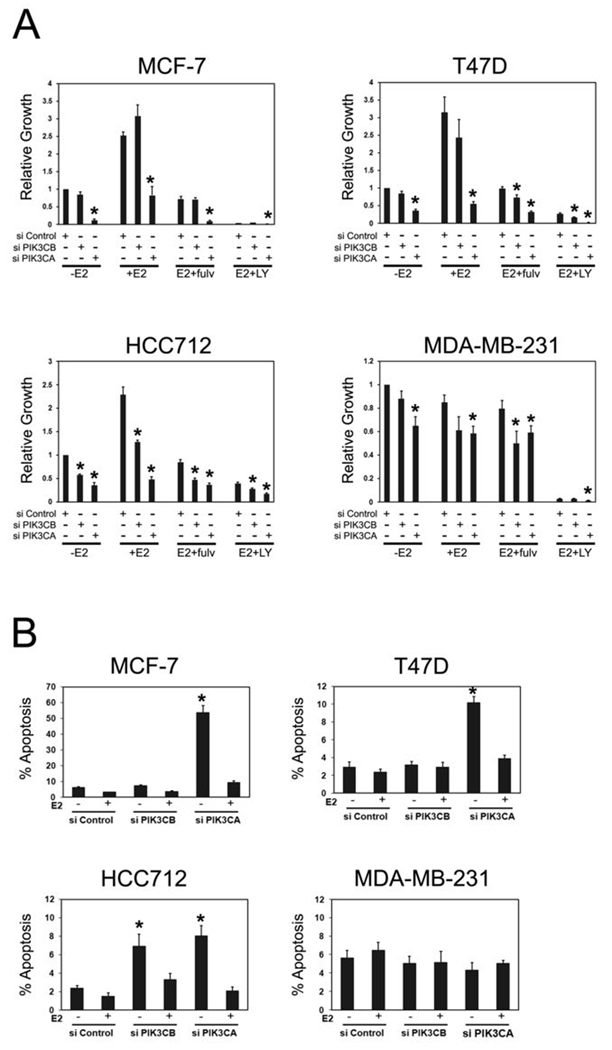
To more precisely determine the cellular response of ER+ cells, the effects of RNAi mediated p110α and p110β inhibition were examined under estrogen-dependent growth conditions (Figure 3A). PIK3CA RNAi inhibited growth in all cell lines, ranging from a modest reduction in growth in the MDA-MB-231 cell line to a greater than 90% reduction in MCF-7 cells. In contrast, PIK3CB RNAi inhibited growth only in HCC712 cells. To determine if PIK3CA and PIK3CB RNAi promoted cell death, apoptosis was quantified in the presence and absence of estradiol (Figure 3B). Estrogen deprivation alone resulted in no significant increase in cell death in HCC712 and T47D cells but a modest (significant) increase in cell death in MCF-7 cells. However, PIK3CA RNAi resulted in significant activation of apoptosis in estrogen-deprived MCF-7, T47D and HCC712 cells. In particular, p110α knockdown dramatically induced cell death in estrogen-deprived MCF-7 cells, with approximately 50% of cells dying via apoptosis seven days after transfection (Figure 3B). Consistent with data on cell growth, PIK3CB RNAi promoted apoptosis in HCC712 cells but not in the other cell lines examined. In contrast to the effects on ER+ cells, neither PIK3CA nor PIK3CB RNAi affected the survival of ER- MDA-MB-231 cells. Importantly, estradiol treatment suppressed the induction of apoptosis by PIK3CB RNAi in HCC712 cells and PIK3CA RNAi in all three ER+ cell lines, indicating that the combination of estrogen deprivation with specific PI3K inhibition caused synthetic lethality.
Figure 3. PIK3CA and PIK3CB RNAi cause synthetic lethality in estrogen-deprived ER+ breast cancer cells.
A, PIK3CA and PIK3CB RNAi inhibit growth of ER+ breast cancer cells. Cells in CSS medium were transfected with 10 nM Control (si Control), PIK3CB (si PIK3CB) or PIK3CA (si PIK3CA) siRNAs. Cells were treated without (−E2) or with 10 nM estradiol (+E2) in the absence or presence of 300 nM Fulvestrant (Fulv) or 20 µM LY294002 (LY). Growth was assessed after 10 d of treatment and is expressed relative to untreated (−E2), Control siRNA transfected cells. Results from five experiments per cell line are shown. Significant differences (p< 0.05, *) between treatments in PIK3CB or PIK3CA siRNA transfected cells and identical treatments in Control siRNA transfected cells are indicated. B, PIK3CA and PIK3CB RNAi promote apoptosis in estrogen-deprived ER+ cells. Cells growing in CSS medium were transfected with siRNAs as in A, above and treated without or with 10 nM estradiol for 7d. Apoptosis was assessed by counting TUNEL-positive or pyknotic Hoechst-stained nuclei. Results from four experiments per cell line are shown. Significant differences (p< 0.05. *) between estrogen-deprived Control siRNA and estrogen-deprived PIK3CB or PIK3CA siRNA transfected cells are indicated. Estrogen suppression of PIK3CB RNAi-induced apoptosis in HCC712 cells was not statistically significant (p= 0.06).
Combined PIK3CA/PIK3CB RNAi Enhances Apoptosis in Estrogen-deprived ER+ Breast Cancer Cells Compared to Either Single Gene Knockdown
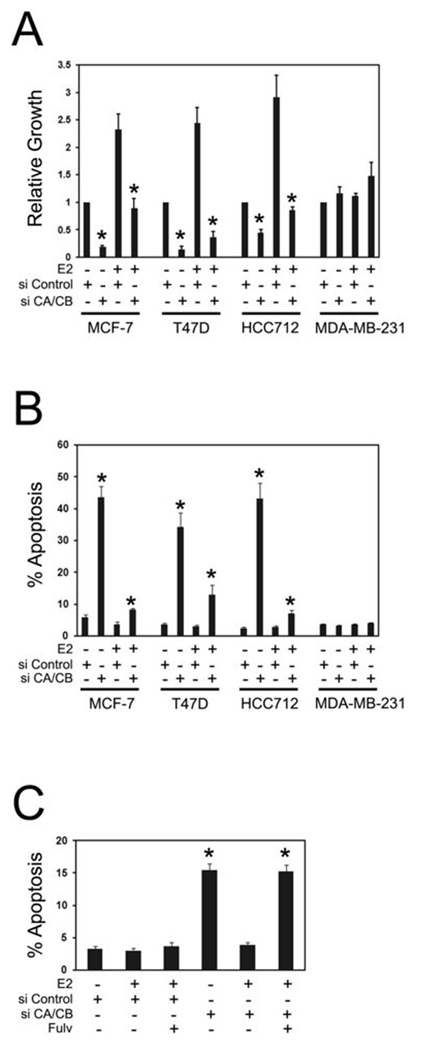
Next, we examined the effects of simultaneous inhibition of PIK3CA and PIK3CB on cell growth and survival using RNAi (Figure 4A, B). Dual knockdown of p110α and p110β reduced cell growth by ~90% in estrogen-deprived MCF-7 cells, similar to the inhibition of cell growth caused by p110α knockdown alone (Figure 4A). In contrast, dual p110α/p110β knockdowns produced a greater reduction in cell growth in estrogen-deprived T47D cells (90% inhibition of cell growth) in comparison to the single subunit knockdowns (65% growth inhibition for PIK3CA RNAi, no significant growth inhibition for PIK3CB RNAi). Combined RNAi was also effective in inhibiting HCC712 cell growth; however the growth of ER- MDA-MB-231 cells was unaffected. While dual p110α/p110β knockdown did not enhance cell death in estrogen-deprived MCF-7 cells compared to the marked effect already achieved by p110α knockdown alone, combined 110α/p110β knockdown resulted in approximately 4-fold higher levels of apoptosis in T47D and HCC712, similar to that achieved in MCF7 cells with single PIK3CA knockdown. In contrast, the survival of MDA-MB-231 cells was unaffected by dual p110α/p110β knockdown (Figure 4B). Importantly, estradiol treatment significantly rescued all three ER+ cell lines from cell death caused by dual p110α/p110β knockdown. It remained possible that the induction of apoptosis observed with RNAi knockdown was be caused by off-target siRNA effects and that rescue of apoptosis with estradiol is not ER-dependent. However, dual p110α/p110β knockdowns in T47D cells with different siRNA than those used in Figure 3A, 3B, Figure 4A and 4B also induced apoptosis in estrogen-deprived cells. In addition, treatment with the ER-specific inhibitor fulvestrant abrogated rescue by estradiol indicating that estradiol rescue was mediated by the ER (Figure 4C).
Figure 4. Dual PIK3CA/PIK3CB RNAi enhances apoptosis in estrogen-deprived ER+ cells.
A, dual PIK3CA/PIK3CB RNAi inhibits growth of ER+ cells. Cells in CSS medium were transfected with 20 nM Control (si Control) or 10 nM each (20 nM final) PIK3CA and PIK3CB siRNAs (si CA/CB). Cells were left untreated or treated with 10 nM estradiol (E2) and growth was assessed after 10d. Growth is expressed relative to untreated, Control siRNA transfected cells. Results are from 4–6 experiments per cell line. Significant differences (p< 0.05, *) between Control siRNA and PIK3CA/PIK3CB siRNA transfected cells in the presence or absence of estradiol are indicated. B, dual p110α/p110β knockdown enhances apoptosis in ER+ cells. Cells were transfected with 20 nM Control or 10 nM each PIK3CA and PIK3CB siRNAs. Cells were left untreated or treated with 10 nM estradiol for 7d and apoptosis was assessed by counting Hoechst-stained nuclei. Results from four experiments per cell line are shown. Significant differences (p< 0.05, *) between Control siRNA and dual PIK3CA/PIK3CA siRNA transfected cells in either the presence or absence of estradiol are indicated. Estradiol significantly suppressed PIK3CA/PIK3CB RNAi-induced apoptosis MCF-7, T47D and HCC712 cells. C, dual p110α/p110β knockdown was performed in T47D cells with alternative PIK3CA and PIK3CB siRNAs. Cells were left untreated, treated with 10 nM estradiol or treated with estradiol + 300 nM Fulvestrant (Fulv) for 7d. Apoptosis was assessed by counting Hoechst-stained nuclei. Results from four experiments are shown. Significant differences (p< 0.05, *) between Control and PIK3CA/PIK3CA siRNA transfected cells in either the presence or absence of estradiol are indicated.
BEZ235 Induces Apoptosis in Estrogen-Deprived ER+ Breast Cancer Cells
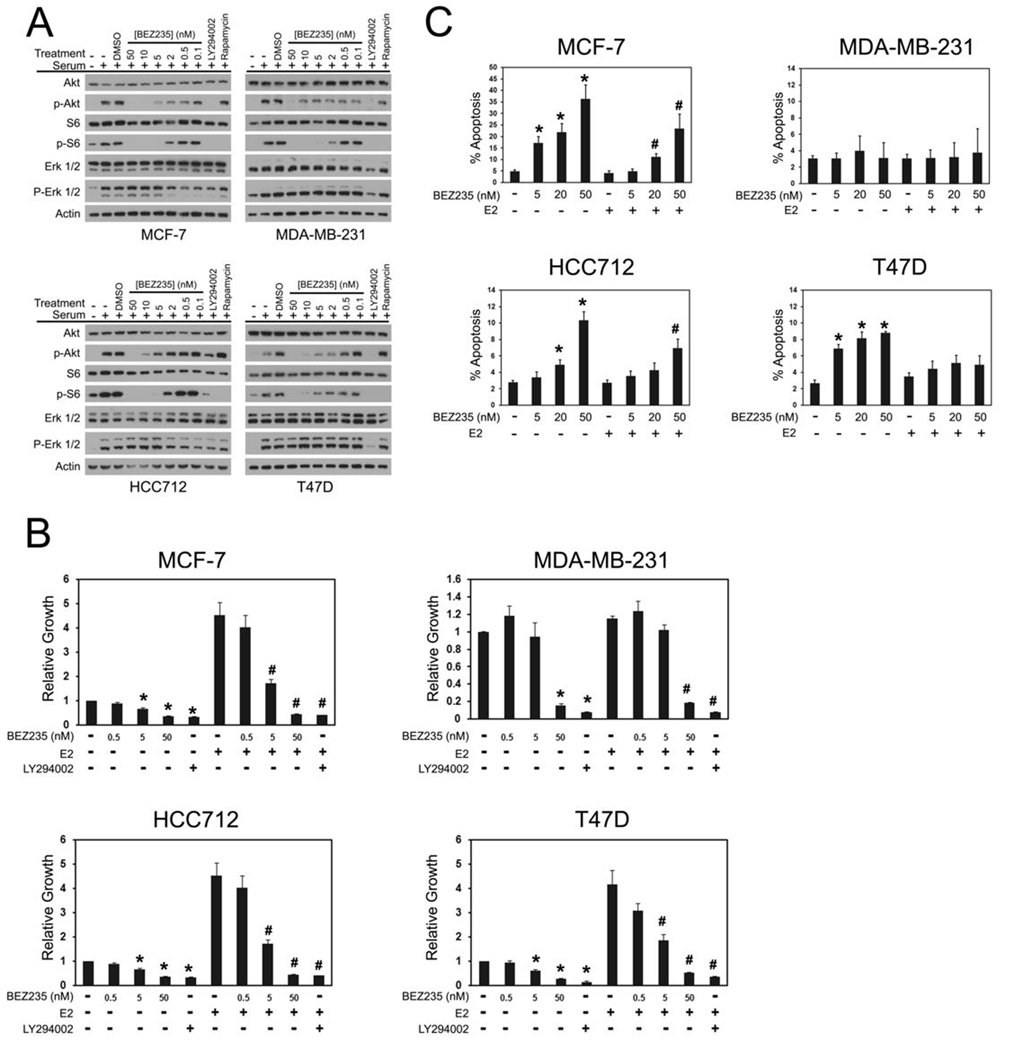
The PIK3CA and PIK3CB RNAi experiments in the cell line panel provide a defined system for examining the potential of pharmacological PI3K inhibitors in breast cancer cells. The effects of BEZ235 (a dual PI3K Class 1 catalytic subunit/mTOR inhibitor) was therefore investigated. BEZ235 has been shown to potently inhibit wild-type and mutant p110α at low nanomolar concentrations (IC50 ~ 5nM) and p110β at significantly higher concentrations (IC50 ~ 75 nM) (25). Signaling effects in the cell line panel are consistent with a selective p110α inhibitor (summarized in Figure 5A). Low concentrations (5nM) of BEZ235 significantly inhibited the growth of all three ER+ breast cancer cell lines both in the presence and absence of estrogen (Figure 5B). In contrast, only high concentrations (50 nM) of BEZ235 inhibited MDA-MB-231 cell growth. When the effect of BEZ235 on cell survival in the presence and absence of estrogen was examined, marked differences between the three ER+ cell lines emerged (Figure 5C). Treatment of estrogen-deprived MCF-7 and T47D cells with concentrations of BEZ235 as low as 5 nM promoted cell death. However, effect of BEZ235 on survival was maximal using 5 nM BEZ235 in T47D cells. In contrast, the level of apoptosis in MCF-7 cells increased with BEZ235 concentration and approached the levels observed with PIK3CA RNAi at 50nM. In estradiol rescue experiments, induction of cell death by 5 nM BEZ235 in MCF7 cells was completely blocked by estradiol and in the presence of estradiol four-fold higher doses ≥ 20 nM were required to induce cell death. Remarkably, estradiol abrogated the BEZ235-induced cell death in T47D cells at all doses tested. HCC712 cells were the least sensitive to BEZ235 treatment and required higher concentrations (≥ 20 nM) to promote cell death under estrogen-deprived conditions. However, estrogen did suppress BEZ235-induced apoptosis in HCC712 cells such that cell death only occurred in estradiol treated cells at the highest concentration tested (50nM). Consistent with the PIK3CA and PIK3CB single and combination knockdown results, BEZ235 treatment did not induce apoptosis in MDA-MB-231 cells.
Figure 5. BEZ235 causes synthetic lethality in estrogen-deprived ER+ breast cancer cells.
A, BEZ235 treatment inhibits PI3K pathway signaling in breast cancer cells. Serum-starved cells were treated with vehicle (DMSO), the indicated concentrations of BEZ235, LY294002 (20µM), or rapamycin (100 nM) then stimulated with 20% FBS. Cell lysates were immunoblotted to determine effects on PI3K signaling by phospho-Akt (p-Akt) and phospho-S6 (p-S6) antibodies and on MAPK signaling via phospho-ERK (p-ERK 1/2) antibodies. Shown are representative results from at least two experiments per cell line. B, BEZ235 inhibits growth of breast cancer cells. Cells in CSS medium were treated without or with BEZ235 in the absence or presence of 10 nM estradiol (E2). Cell growth was measured after 10 d and is calculated relative to growth in untreated (−E2) cells. Shown are results from five experiments per cell line. Significant differences in growth between estrogen-deprived and estrogen-deprived, drug-treated cells are indicated (p<0.05, *). Significant differences in growth between estrogen-stimulated and estrogen-stimulated cells, drug- treated cells are indicated (p<0.05, #). C, BEZ235 promotes apoptosis in ER+ cells. Cells growing in CSS medium were treated with the indicated concentrations of BEZ235 without or with 10 nM estradiol for 7d. Apoptosis was assessed by counting Hoechst-stained nuclei. Results from 4–6 experiments per cell line are shown. Significant induction of cell death in estrogen-deprived, BEZ235 treated cells (p< 0.05, *) and cells treated with BEZ235 in the presence of estradiol (p< 0.05, #) is indicated.
DISCUSSION
The role of estrogen in the proliferation of ER+ breast tumors is well established. However, the role of estrogen as a survival factor is less clear. Preclinical studies with the MCF-7 cell line demonstrated that treatment with antiestrogens or estrogen deprivation increases apoptosis, as we can confirm (26–28). However, MCF-7 cells are unusual in this regard since we did not observe estrogen-deprivation induced apoptosis in the HCC712 or T47D cell lines and in the neoadjuvant endocrine therapy setting, treatment did not increase apoptosis (3, 29). Our data indicates that signaling through the PI3K pathway may explain these observations since estradiol promotes survival only when PI3K is inhibited. The presence of two apparently independent cell survival mechanisms, one PI3K dependent and one estradiol dependent, creates an opportunity for synthetic lethality. At the current time it is not known whether estrogen promotes survival through ER-dependent transcription or by rapid, non-genomic activation of signal transduction pathways (30, 31). Nevertheless, our data strongly suggest that the effectiveness of PI3K catalytic subunit inhibitors in treating ER+ breast cancer will be greatest when combined with endocrine therapy.
In vitro studies have demonstrated that activated forms of p110α or p110β transform mammary epithelium (32, 33). Knock-in and knock-out transgenic mouse studies confirmed these findings and p110β appears to be particularly important for ERBB2-driven breast cancer (16, 34) and in the promotion of proliferation, survival and invasiveness in a variety of cancer types (35–37). The data presented in this study is the first to demonstrate PIK3CB amplification in primary breast cancer and suggests that this amplification event may promote oncogenesis. Our initial screen indicates PIK3CB copy number gain occurs at a low frequency (~5%) in tumors of breast cancer patients, however this may be clinically significant, since breast cancer is common (38). We also find that PIK3CB is preferentially expressed in Luminal B breast cancer regardless of gene copy number, indicating that this isoform is a potentially important therapeutic target, perhaps as a conduit for the effects of other somatic mutations that activate the PI3K such as PTEN loss (17). Interestingly, our in vitro data indicates that PIK3CB supports cell survival in HCC712 cells under estrogen deprived conditions, implying that targeted p110β inhibition could be effective in treating PIK3CB-amplified breast tumors. However our data also suggests that both PIK3CA and PIK3CB may have to be inhibited under these circumstances since high level apoptosis only occurred when both catalytic subunits were targeted. Since PIK3CA is wild-type in HCC712 cells we also conclude that PIK3CA gain of function mutations are not a prerequisite for the synthetic lethal effect when combining estrogen deprivation and PI3K inhibition. Additionally, inhibition of p110β also appears relevant in the presence of a PIK3CA mutation. T47D cells express modest levels of wild-type p110β as well as a mutant PIK3CA, raising the question of whether PIK3CB provides an escape from mutant p110α inhibition. A comparison between single and combined knockdowns suggests that this is the case, since both isoforms must be inhibited for maximal synthetic lethality. MCF-7 cells appear unusual in their extreme sensitivity to p110α inhibition alone, however this may possibly reflect the low levels of PIK3CB expression in this cell line.
BEZ235 is an example of a new generation of PI3K inhibitors to enter clinical investigation in breast cancer (25). A comparison between the effects of BEZ235 and RNAi against PIK3CA and PIK3CB supports the conclusion that BEZ235 functions as a selective p110α inhibitor at low nanomolar concentrations (25). However the apoptotic effect remained very modest in T47D cells, even at higher doses, consistent with lack of PIK3CB inhibition which, based on the RNAi experiments, is necessary for the full synthetic lethal effect. Estradiol suppresses BEZ235-induced apoptosis in the three ER+ cell lines, but estrogen rescue was not as dramatic as that observed in the PIK3CA and PIK3CB RNAi experiments. The reduced sensitivity to estradiol rescue likely reflects inhibition of other kinases by BEZ235.
We conclude that there is a strong rationale for the combination of endocrine therapy and PI3K inhibitors. In terms of the population of patients suitable for a clinical trial of a PI3K inhibitor combined with endocrine treatment, the data suggests that eligibility should not restricted by PIK3CA mutation status. Furthermore, the association between PIK3CA and PIK3CB expression and Luminal B status indicates the addition of a PI3K inhibitor may be particularly important in more aggressive forms of ER+ disease. Finally PIK3CB is emerging an important therapeutic target, whose inhibition is important not only to increase the efficacy of PIK3CA inhibition, but as a target in its own right, perhaps particularly in the setting of gene amplification. A combined inhibitor with low nM inhibitory properties for both PIK3CA and PIK3CB may be necessary for maximal clinical efficacy in combination with an endocrine agent in the treatment of ER+ breast cancer. However this clinical treatment strategy may be problematic since systemic inhibition of both catalytic subunits will cause derangements in insulin signaling and glucose homeostasis (16, 17, 39, 40), and metabolic toxicity could be further enhanced by estrogen deprivation. Nonetheless it may be possible to pursue this strategy clinically because endocrine therapy in combination with a PI3 kinase inhibitor is cytotoxic. Therefore short-course high-toxicity combinations of PI3 kinase inhibitors with endocrine therapy, analogous to conventional chemotherapy, rather than prolonged exposure, may be sufficient to increase the cure rate for ER+ breast cancer.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by awards from the NIH (R01 CA095614; U01 CA114722); the St. Louis Affiliate of the Susan G Komen Breast Cancer Foundation, Inc; Breast Cancer Research Foundation; and the Barnes-Jewish Hospital Research Foundation (to M.J.E). This work was also supported by awards to J.A.O. (3P50 CA68438-07S2). The Siteman Comprehensive Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Conflicts of Interest: No pharmaceutical company funding was received for this research project. Dr Ellis has received honoraria, grants and has served as a consultant for AstraZenica, Novartis and Pfizer.
REFERENCES
- 1.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Ellis MJ, Tao Y, Luo J, et al. Outcome Prediction for Estrogen Receptor-Positive Breast Cancer Based on Postneoadjuvant Endocrine Therapy Tumor Characteristics. JNCI. 2008;100 doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowsett M, Smith IE, Ebbs SR, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res. 2006;12:1024s–1030s. doi: 10.1158/1078-0432.CCR-05-2127. [DOI] [PubMed] [Google Scholar]
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 6.Barlund M, Monni O, Kononen J, et al. Multiple genes at 17q23 undergo amplification and overexpression in breast cancer. Cancer Res. 2000;60:5340–5344. [PubMed] [Google Scholar]
- 7.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 8.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 9.Shoman N, Klassen S, McFadden A, Bickis MG, Torlakovic E, Chibbar R. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod Pathol. 2005;18:250–259. doi: 10.1038/modpathol.3800296. [DOI] [PubMed] [Google Scholar]
- 10.van der Hage JA, van den Broek LJ, Legrand C, et al. Overexpression of P70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004;90:1543–1550. doi: 10.1038/sj.bjc.6601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 12.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007;13:408–414. doi: 10.1158/1078-0432.CCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 15.Barbareschi M, Buttitta F, Felicioni L, et al. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007;13:6064–6069. doi: 10.1158/1078-0432.CCR-07-0266. [DOI] [PubMed] [Google Scholar]
- 16.Ciraolo E, Iezzi M, Marone R, et al. Phosphoinositide 3-Kinase p110{beta} Activity: Key Role in Metabolism and Mammary Gland Cancer but Not Development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia S, Liu Z, Zhang S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer C, Sturge J, Bennett DC, et al. Regulation of breast cancer cell chemotaxis by the phosphoinositide 3-kinase p110delta. Cancer Res. 2003;63:1667–1675. [PubMed] [Google Scholar]
- 20.Olson JA, Budd GTLA, Carey LA, et al. Improved Surgical Outcomes for Breast Cancer Patients Receiving Neoadjuvant Aromatase Inhibitor Therapy: Results from a Multicenter II Trial. J Am Coll Surg. 2009 doi: 10.1016/j.jamcollsurg.2009.01.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown LA, Hoog J, Chin SF, et al. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:806–807. doi: 10.1038/ng0708-806. author reply 10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazdar AF, Kurvari V, Virmani A, et al. Characterization of paired tumor and non-tumor cell lines established from patients with breast cancer. Int J Cancer. 1998;78:766–774. doi: 10.1002/(sici)1097-0215(19981209)78:6<766::aid-ijc15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Parker JS, Mullins M, Cheang MC, et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Ahmad N. RNA interference-mediated depletion of phosphoinositide 3-kinase activates forkhead box class O transcription factors and induces cell cycle arrest and apoptosis in breast carcinoma cells. Cancer Res. 2006;66:1062–1069. doi: 10.1158/0008-5472.CAN-05-1018. [DOI] [PubMed] [Google Scholar]
- 25.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 26.Kyprianou N, English HF, Davidson NE, Isaacs JT. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991;51:162–166. [PubMed] [Google Scholar]
- 27.Warri AM, Huovinen RL, Laine AM, Martikainen PM, Harkonen PL. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J Natl Cancer Inst. 1993;85:1412–1418. doi: 10.1093/jnci/85.17.1412. [DOI] [PubMed] [Google Scholar]
- 28.Detre S, Salter J, Barnes DM, et al. Time-related effects of estrogen withdrawal on proliferation- and cell death-related events in MCF-7 xenografts. Int J Cancer. 1999;81:309–313. doi: 10.1002/(sici)1097-0215(19990412)81:2<309::aid-ijc23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 30.Migliaccio A, Di Domenico M, Castoria G, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. Embo J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 31.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci U S A. 2005;102:18443–18448. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 34.Zhao JJ, Cheng H, Jia S, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czauderna F, Fechtner M, Aygun H, et al. Functional studies of the PI(3)-kinase signalling pathway employing synthetic and expressed siRNA. Nucleic Acids Res. 2003;31:670–682. doi: 10.1093/nar/gkg141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu P, Kang C, Zhang Z, Liu X, Jiang H. Downregulation of PIK3CB by siRNA suppresses malignant glioma cell growth in vitro and in vivo. Technol Cancer Res Treat. 2006;5:271–280. doi: 10.1177/153303460600500308. [DOI] [PubMed] [Google Scholar]
- 37.An HJ, Cho NH, Yang HS, et al. Targeted RNA interference of phosphatidylinositol 3-kinase p110-beta induces apoptosis and proliferation arrest in endometrial carcinoma cells. J Pathol. 2007;212:161–169. doi: 10.1002/path.2158. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Jeong EG, Yoo NJ, Lee SH. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. Br J Cancer. 2008;98:1533–1535. doi: 10.1038/sj.bjc.6604212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foukas LC, Claret M, Pearce W, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.