Abstract
Autoimmune diabetes (T1D) is characterized by CD4+ T cell reactivity to a variety of islet-associated antigens. At-risk individuals, genetically predisposed to T1D, often have similar T cell reactivity, but nevertheless fail to progress to clinically overt disease. In order to study the immune tolerance and regulatory environment permissive for such autoreactive T cells, we expressed TcR transgenes derived from two autoreactive human T cells, 4.13 and 164, in HLA-DR4 transgenic mice on a C57Bl/6-derived “diabetes-resistant” background. Both TcR are responsive to an immunodominant epitope of glutamic acid decarboxylase 65 (555–567), which is identical in sequence between humans and mice, is restricted by HLA-DR4, and is a naturally processed self antigen associated with T1D. Although both TcR use the identical Vα and Vβ genes, differing only in CDR3, we found stark differences in the mechanisms utilized in vivo in the maintenance of immune tolerance. A combination of thymic deletion (negative selection), TcR down-regulation, and peripheral activation-induced cell death dominated the phenotype of 164 T cells, which nevertheless still maintain their antigen responsiveness in the periphery. In contrast, 4.13 T cells are much less influenced by central and deletional tolerance mechanisms, and instead display a peripheral immune deviation including differentiation into IL-10 secreting Tr1 cells. These findings indicate a distinct set of regulatory alternatives for autoreactive T cells, even within a single highly restricted HLA-peptide-TcR recognition profile.
Keywords: Tolerance, T cell, self-antigen, HLA
Introduction
Central and peripheral mechanisms maintaining T cell tolerance to self antigens are variable in degree of completeness, and autoreactive T cells populate the peripheral immune system. Central tolerance in the thymus is largely governed through the interaction of the T cell receptor with self-peptide-MHC complexes, in which high avidity T cells are eliminated through apoptosis(1–3) or potentially differentiated into CD4+/CD25+/Foxp3 expressing regulatory T cells (Treg)(4,5). Strategies by which autoreactive T cells may escape central tolerance to self antigens include down modulation of receptor or costimulatory molecules (6) and skewing of CD4/CD8 coreceptor expression (7,8). These mechanisms are incomplete, however, such that self reactivity by some peripheral T cells is an intrinsic property of normal immunity, perhaps required to enable the immune repertoire to respond to the diverse nature of foreign antigens (9).
Once in the periphery, several additional mechanisms operate as checkpoints to limit T cell activation to self-antigens, including functional inactivation or anergy of the T cell(10,11), activation-induced T cell deletion(12–14), generation of suppressive cytokine secreting T cells (Tr1 and Th3) (15,16) and differentiation of uncommitted T cells into Foxp3 expressing regulatory T cells(17,18).
While several TcR transgenic mice have been developed to study tolerance to self antigens, the vast majority of studies use either alloreactive T cells or a foreign antigen reactive T cell expressed as a TcR transgene along with the foreign antigen as a second transgene(4,19,20). In human type 1 diabetes (T1D), HLA-DR4 subjects commonly carry peripheral T cells reactive to a variety of islet associated self antigens, including the immunodominant GAD65 555–567 peptide, a naturally-processed epitope of glutamic acid decarboxylase(21–24). Interestingly, recognition of this epitope displays a biased TcR repertoire, with prevalent use of Vβ5.1/Vα12.1, although CDR3 regions are variable (22). In order to study tolerance mechanisms associated with this dominant autoreactive specificity, we introduced transgenic TcR from two human CD4+ T cells specific for GAD65 555–567, that differ only in their CDR3 regions, intercrossed into HLA-DR4 transgenic mice. In spite of the close structural features of these two autoreactive TcR, stark differences in both central and peripheral tolerance mechanisms were elicited.
Materials and Methods
Mice
DR0401-IE mice (DR4) were obtained from Taconic Laboratories (Germantown, NY). These C57BL/6 I-Abo/o mice express a human-mouse chimeric class II molecule in which the TcR interacting and peptide binding domains of mouse I-E (domains α1 and β1, exon 2 in both genes) have been replaced with the α1 and β1 domains from DRA1*0101 and DRB1*0401 respectively. Retention of the murine α2 and β2 domains allows for the cognate murine CD4-murine MHC interaction(25).
TcR sequences for generation of the two T cell transgenic mice were obtained from human CD4+ T cell clones 164(26)and 4.13(22). Both human T cells are responsive to the same self antigen GAD65 (555–567) and both use human Vα12.1/Vβ5.1 T cell receptors. The 164 T cell was cloned from peripheral blood from a HLA DRA1*0101/B1*0401 diabetes at-risk individual as previously described(26). Clone 4.13 was cloned from the peripheral blood of a HLA DRA1*0101/B1*0401 diabetic individual(22). Human-mouse chimeric TcR transgenes were constructed by subcloning PCR amplified regions encoding rearranged VαJα and VβDβJβ domains from the human clones into pTαcass and pTβcass TcR transgenic vectors respectively (27). TcR transgenic vectors pTαcass and pTβcass contain the natural mouse TcR α and β promoter/enhancer elements and mouse Cα and Cβ constant regions respectively. DNA injection into C57BL/6/I-Abo/o (164 TcR) or F1-B6/C3H (4.13 TcR) mouse embryos was performed at the University of Washington (Seattle, WA) in the Comparative Medicine animal facility. Founder mice containing the human TcR transgenes were then crossed onto DR0401-IE mice to generate DR4/164 and DR4/4.13 mice. Additional crosses were made onto Rag2o/o mice. A founder mouse was also identified that contained only the 164 TcR beta transgene. This was also crossed onto DR0401-IE mice. 4.13 TcR transgenic mice generated in F1-B6/C3H were crossed for 9 generations into DR0401-IE mice. All animal work was approved by the Benaroya Research Institute (BRI) Animal Care and Use Committee (ACUC) and animals were housed in the BRI AAALAC-accredited animal facility.
Tissue processing and Flow cytometry
Thymus, spleen, and lymph node tissues were processed into single cell suspensions by gently pressing through 0.40 µm cell strainers (BD-Falcon REF 352340, Bedford MD) using the rubber end of a 1 ml tuberculin syringe in DMEM-10 media (DMEM cat 11965-092 (Gibco, Rockville MD.) supplemented with 10% FBS (Hyclone, Logan Utah), 100µg/ml Penicillin, 100U/ml Streptomycin, 50µM βme, 2mM glutamine and 1mM sodium pyruvate (Gibco, Rockville MD)). Cell suspensions were centrifuged at 200g for 10 minutes, aspirated, and either: a.) resuspended in DMEM-10 media (LN and thymus) or b.) red blood cells were lysed (for spleens) using 1 ml ACK lysis buffer(28) for 5’ at 37°C at which time 30 ml of media was added and cells spun down (200g), aspirated, and resuspended in DMEM-10 media. The following chromophore-labeled antibodies were used in flow cytometric analysis: anti-mouse CD4 (clone RM4-5), CD8 (clone 53-6.7), CD25 (clone PC61), CD62L(Mel-14), anti-human active Caspase-3( polyclonal cat# 557091), CD44(IM7), Fc-Block (2.4G2), and PE-labeled Annexin-V (all from BD-Pharmingen, San Jose, CA), anti-human Vβ5.1-PE (clone IMMU 157 Immunotech-Coulter, Miami, FL) and Vα12.1-FITC (clone 6D6 Endogen Woburn, MA). FACS samples in media were prestained with Fc-Block for 10’ on ice and then stained with specific antibodies on ice for 45 minutes, washed once, and resuspended in FACS stain buffer (PBS containing 1% FBS, 0.1% Na-azide) before being run on a FACS Caliber or LSR II flow cytometer (Becton Dickinson). Intracellular staining of cells for FoxP3 was performed using eBioscience kit (FJK.16a Ab, San Diego, CA) according to manufacturer’s instructions. Intracellular staining for active Caspase 3, mouse anti-IFN-γ (XMG1.2, eBioscience), and anti-IL-10 (clone JES5-16E3, eBioscience) was performed using eBioscience intracellular staining kit (cat 88-8823-88, eBioscience San Diego, CA).
Proliferation assays
In lymph node or purified CD4+ T cell proliferation assays 1×105 lymph node cells were cultured with 2×105 3000 Rads Cs-γ irradiated splenocytes (final volume 150 µl). Supernatants for cytokine analysis were taken (50 µl) at 48 hrs. and 1 µCi/well of 3H-thymidine was added at 72 hours. Thymidine incorporation was assayed at 96 hours using liquid scintillation counting analyzed on a Microbeta TriLux 1450 scintillation counter (Wallac-Perkin/Elmer Life Sciences, Boston MD.). Splenocyte responses were measured in the same manner using 5×105 splenocytes per well. CD4 and CD8 single positive cells were obtained using Mitenyi beads with purity of 90 percent or greater or by antibody labeling with CD4 and CD8 and sorting by flow cytometry.
Cytokine analysis
Cytokines IL-2, IL-4, IL-5, TNF-α, and IFN-γ were assayed using a Mouse Th1/Th2 Cytokine CBA kit (BD Bioscience, San Diego, CA 92121, cat # 551287). IL-10 was assayed using a BD OptEIA mouse IL-10 Elisa Set and mouse TGF beta1 was measured using Human/Mouse TGF-beta1 ELISA Ready-Set-Go kit (BD Bioscience, San Diego, CA 92121 cat # 555252, #88-7344 respectively). Supernatants from triplicate proliferation wells (50 µl/well) were combined for cytokine analysis with 50 µl used for CBA analysis and 50 µl for IL-10 Elisa.
Results
Thymic selection of autoreactive T cells
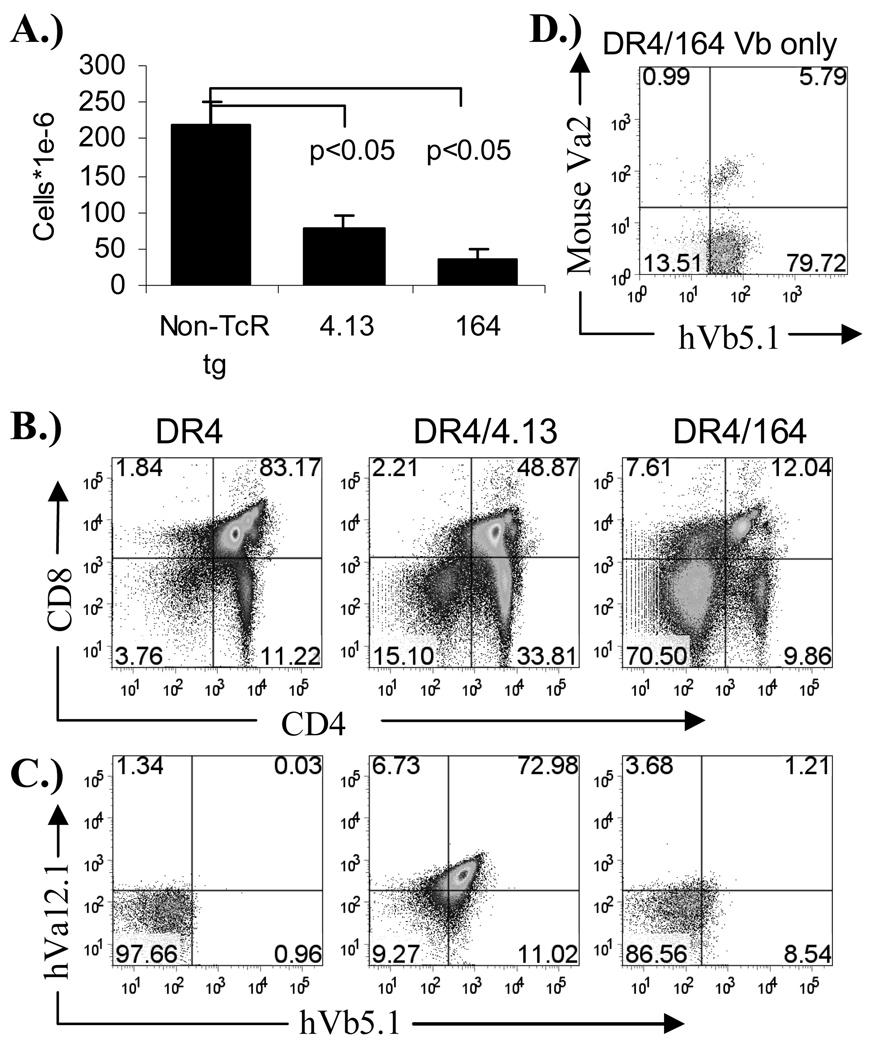
Utilizing TcR from two structurally related DRB1*0401 (DR4) restricted human CD4+ T cell clones reactive to the autoantigen glutamic acid decarboxylase 65 (GAD65), human TcR transgenic mice were generated to investigate differential modes of T cell tolerance to the naturally processed GAD65 (555–567) autoantigen. The human CD4+ T cell clones 164 and 4.13 (obtained from two different subjects) are structurally related in that they both use TcR with human Vα12.1 (hVα12.1) and Vβ5.1 (hVβ5.1) gene sequences which differ only in their CDR3 regions (Table 1). Both of the human T cells recognize GAD65 555–567(22,26), a region within the naturally processed and presented GAD65 552–572 epitope(21,23). The sequence of the DR4-binding minimal stimulating epitope GAD65 555–567 is identical for GAD65 and GAD67 in both human and mouse and thus serves as a naturally processed self antigen T cell epitope in both species(29). Both 4.13/Rag2+/+ and 164/Rag2+/+ mice display reduced thymus cellularity (Figure 1A.) with 164 mice exhibiting a profound reduction in CD4+/CD8+ double positive cells (Figure 1B.). The reduction in cellularity and a decrease in double positive cells is indicative of negative selection(20,30,31). While positively selected 4.13 T cells are heavily skewed toward single positive CD4+/CD8− phenotype reflecting their class II restriction, single positive thymic T cells in 164 mice are matured into both CD4−/CD8+ and CD4+/CD8− phenotype, a profile similar to that observed in other self-antigen responsive TcR transgenic mice under conditions of strong negative selection(8). In addition to the stronger negative selection observed in 164 mice is the down-modulated expression of the T cell receptor on CD4+CD8− thymocytes where only about 1% of mature CD4+/CD8− T cells express both Vα and Vβ transgenes (Figure 1C). This is in stark contrast to the greater than 70% expression of hVα12.1 and hVβ5.1 on CD4+/CD8− thymocytes from 4.13 mice. As the amino acid sequence in the CDR3 region of 164 TcR is different from 4.13 TcR it was possible that the low level of hVβ5.1 and hVα12.1 staining on 164 mice could be the result of differential binding of the antibody itself, however, the hVβ5.1 antibody does stain the 164 TcR from 164 beta chain only TcR transgenic mice (lacking the human TcR Vα12.1 transgene) suggesting that the low level of 164 TcR expression on matured CD4+/CD8− thymocytes is the result of down modulation of the TcR under thymic selection pressures (Figure 1D.). Based on thymic cellularity , CD4 vs CD8 profiles, and TcR expression levels we conclude that 164 TcR thymocytes, likely due to a higher avidity for peptide-MHC of the 164 TcR relative to the 4.13 TcR, undergo stronger central tolerance and maintain a down modulated TcR.
Table I.
Comparison of 164 and 4.13 TcR. Gray highlighted areas denote differences between 164 and 4.13 TcR CD3 sequences, bold fonts denote non-polar to non-polar amino acid changes, underlined residues denote polar-to-polar changes, and parentheses denote charge changes.
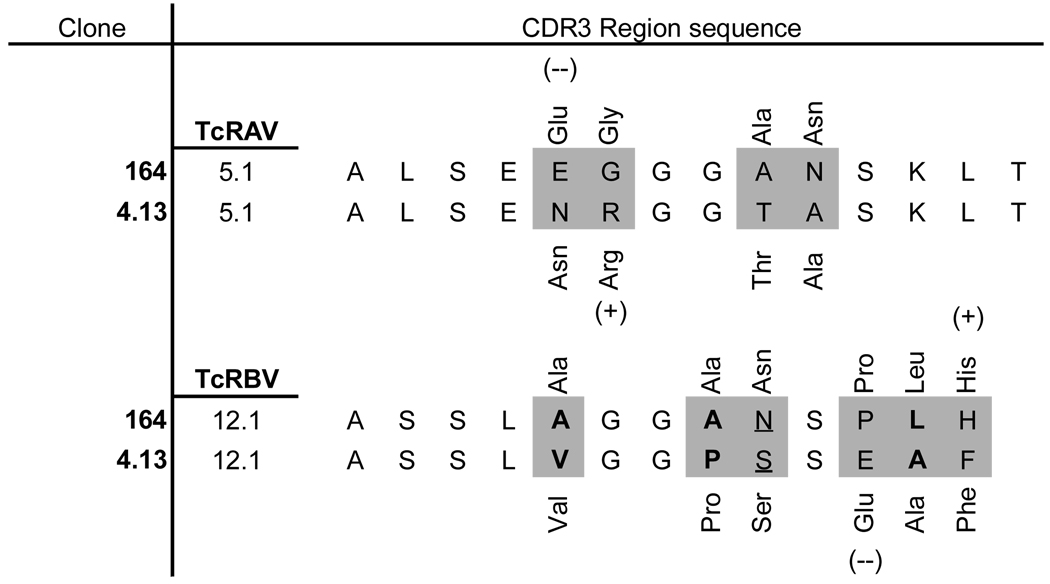 |
Figure 1.
Thymic lymphocyte profiles in 164 and 4.13 human TcR transgenic HLA DR4 mice. Thymus cellularity of 8–10 week old mice (n=3) (A), CD4/CD8 profiles (B), and TcR hVb5.1 vs hVa12.1 expression on CD4+/CD8− gated cells (C). Human Vb5.1 expression on CD4+/CD8− thymocytes from 164 Vb only TcR mice (D).
Peripheral skewing of autoreactive T cells
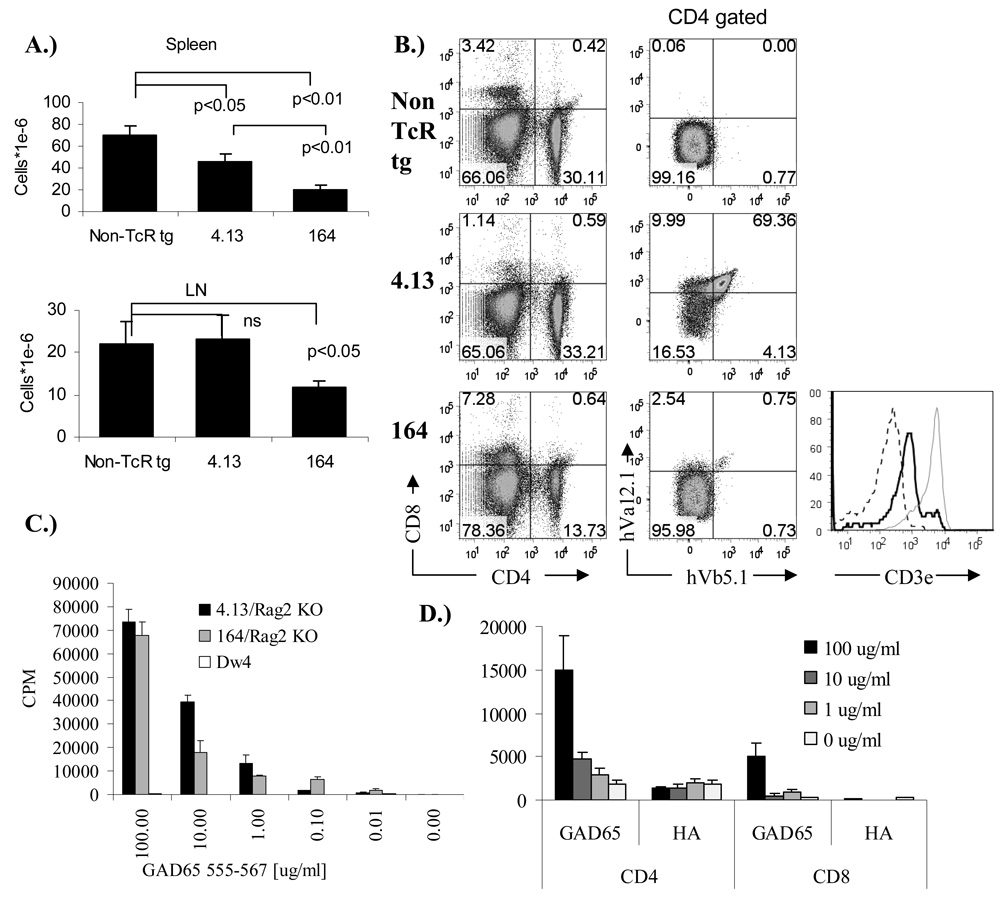
4.13/Rag2+/+ and 164/Rag2+/+ mice both show reduced cellularity in the spleen (Figure 2A), however only 164 mice show a reduction in peripheral lymph nodes. 4.13 T cells in the spleen as in the thymus are heavily skewed toward CD4+/CD8− lineage reflecting their class II restriction (Figure 2B). 164/Rag2+/+ mice have fewer cells in the spleen and less than one percent of CD4+/CD8− T cells are hVα12.1 and hVβ5.1 positive (Figure 2B). Coinciding with the weak TcR expression in 164/Rag2+/+ mice is also a low expression of CD3e on CD4+/CD8− gated cells (Figure 2B histogram). In contrast to the near absence of CD4−/CD8+ cells in 4.13/Rag2+/+ mice, 164/Rag2+/+ mice have nearly one-third of their T cells as CD8+/CD4− cells which is also greater than that seen in non-TcR transgenic mice (Figure 2B). The percentages of CD4 cells among all T cells (CD4/(CD4+CD8)) (average of 3 mice) is 98% ±1% in 4.13 mice, 73% ± 2% in164 mice compared to 90% ± 1% in non TcR transgenic mice, indicating that 4.13 T cells are strongly selected toward their MHC class II restriction, while T cell selection in 164/Rag2+/+ mice is skewed toward the CD8 compartment, similar to what is observed in the thymus. The stronger central tolerance in 164/Rag2+/+ mice is also reflected in the periphery by the greater expression of endogenous mouse mVα and mVβ T cell receptors (supplemental Figure S1.). In assaying for antigen specificity we used splenocytes from Rag2o/o TcR transgenic mice to ensure that all alpha/beta T cells only express the hVα12.1 and hVβ5.1 transgenes. Splenocytes from both 4.13/Rag2o/o and 164/Rag2o/o mice respond to GAD65 555–567 in an antigen specific manner confirming their specificity for the GAD65 epitope (Figure 2C). Because of the of the skewing of 164 T cells from 164/Rag2+/+ mice (also seen in 164/Rag2o/o mice) into a CD8+/CD4− pathway we sorted 164/Rag2o/o T cells into CD4+/CD8− and CD4−/CD8+ fractions and stimulated these fractions with irradiated splenocytes and peptide. We find that both populations are antigen specific with the CD8 164 cells having a lower proliferative response (lower functional avidity) (Figure 2D).
Figure 2.
Peripheral lymphocyte profiles in 8–10 week old 164 and 4.13 TcR transgenic DR4 mice. Peripheral spleen and LN (inguinal and para-aortic combined) cellularity (n=3)(A). CD4 vs CD8 profile and TcR human Va12.1 and Vb5.1 expression on CD4+/CD8− gated cells from spleen (B). Histogram in (B.) shows CD3e expression on CD4+/CD8− gated cells from 164 mice (black line), 4.13 mice (gray line), and isotype control (dotted line). Splenocyte antigen dose response in 164 (gray) and 4.13 (black) TcR transgenic DR4 mice on a Rag2o/o background (C). For CD4+/CD8− and CD4−/CD8+ T cell stimulation (D) cells were sorted by flow and tested for proliferation in response to GAD65 555–567, or control HA antigen. All experiments were repeated at least 3 times with similar results.
Peripheral tolerance mediated by apoptosis
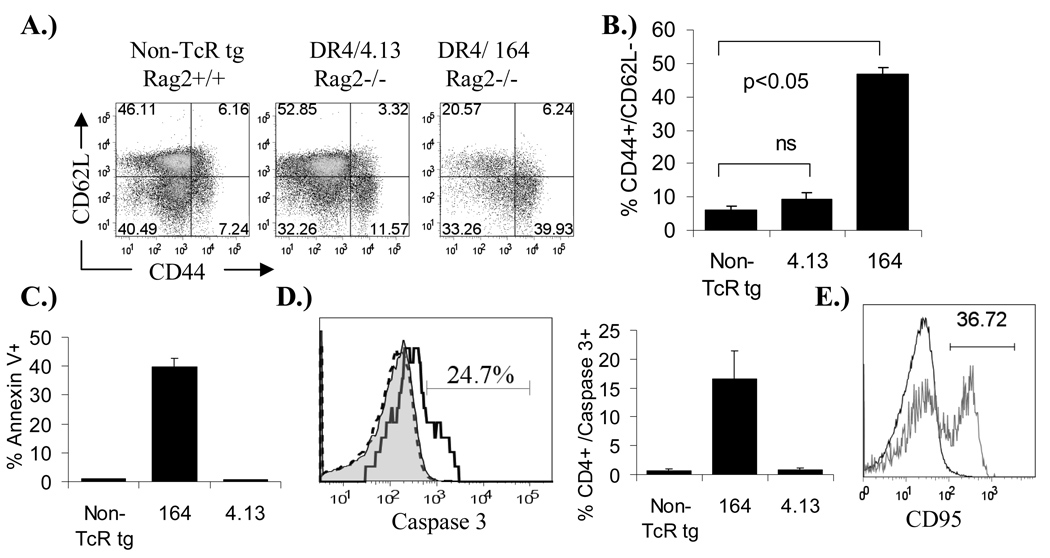
As with the low expression of the transgenic TcR on 164/Rag2+/+ thymocytes in the thymus (Figure 2B) the TcR expression on 164/Rag2+/+ T cells in the periphery is also nearly absent (also true in 164/Rag2o/o mice). This suggested that perhaps the ligand inducing negative selection in the thymus is also activating these cells in the periphery and thus the extremely low level of TcR expression in the periphery is in part the result of constant activation of 164 cells in the periphery. By surface phenotyping we found that the majority of peripheral 4.13/Rag2o/o CD4+ T cells, like CD4+ cells from non-TcR /Rag2+/+ transgenic mice, are of a naïve nature expressing high levels of CD62L and intermediate levels of CD44 (CD62LHI/CD44Med)(Figure 3A). In contrast about 40% of peripheral spleen CD4+ cells from 164/Rag2o/o mice are CD62Llow/CD44Hi compared to about 10% in 4.13 and non-TcR transgenic mice indicating an activated phenotype (Figure 3A and B). A similar activation profile of 164/Rag2o/o CD4+ T cells was observed in other lymph nodes (pancreatic and inguinal, data not shown) and also in Rag2+/+ mice (supplemental Figure S3). Therefore, we tested whether the low numbers of T cells in the peripheral tissues of 164/Rag2o/o mice could be the result of constant peripheral activation and subsequent activation induced cell death (AICD). As shown in Figure 3C peripheral CD4+ 164/Rag2o/o T cells compared to 4.13/Rag2o/o and non-TcR transgenic cells stain with the apoptotic marker Annexin V and additional staining indicated that the CD4+ 164/ Rag2o/o T cells are also activated caspase-3 positive (Figure 3D). Peripheral CD4+ 4.13/Rag2o/o cells were negative for both Annexin V and activated Caspase-3 staining. Surface staining on CD4+ 164/ Rag2o/o T cells indicated that a significant portion of these cells are also CD95 positive suggesting that apoptotic signaling may occur through CD95 (Figure 3E).
Figure 3.
Activation and apoptosis in 164 and 4.13 TcR transgenic mice. Spleen cells from 8–12 week old DR4 mice on a Rag2o/o background were stained with activation markers CD44 and CD62L, gated on CD4+/CD8− cells for analysis (A.& B.). Percentage of gated CD4+/CD8− cells that were Annexin V positive (C.) and active Caspase 3 positive (D.) are shown. Example Caspase 3 histograms in (D.) are non-TcR transgenic (gray-filled), DR4/4.13/Rag2o/o (black-dashed line), and DR4/164/Rago/o (black-heavy line). Percentages are from 3 mice in each group. CD4+ spleen T cells from 164 (gray line) mice are also CD95 positive compared to non-TcR CD4+ T cells (black line) (E.)
Both 164 and 4.13 mice show an enhanced selection of peripheral FOXP3+ cells
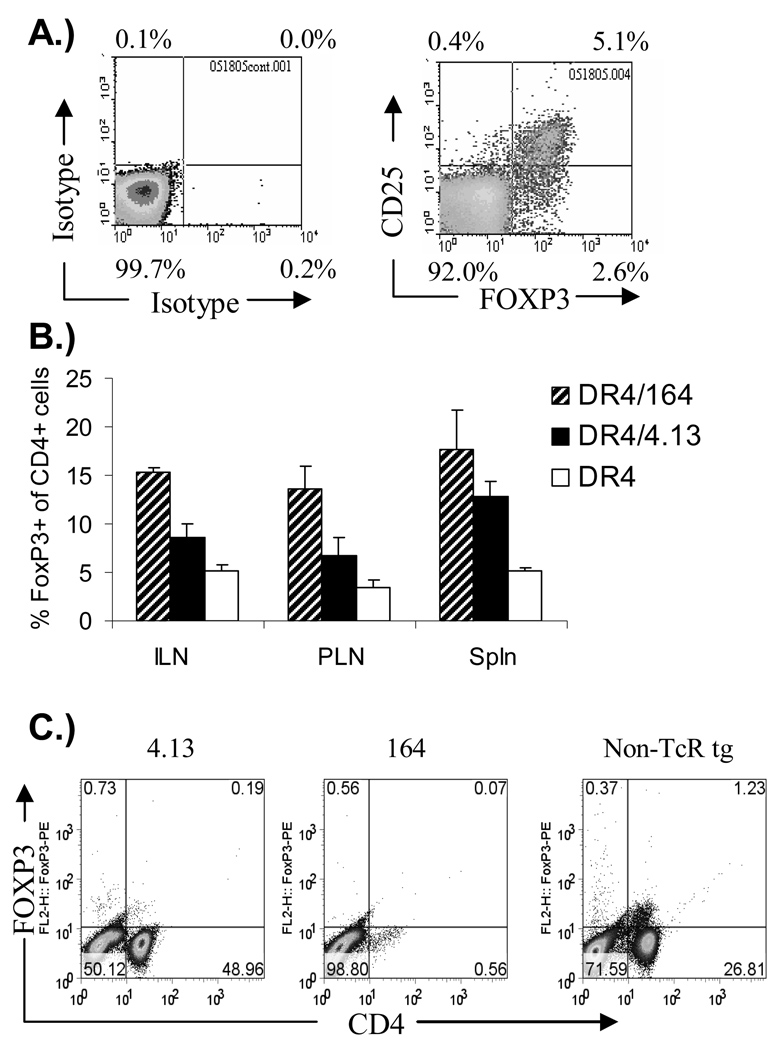
CD4+/CD25+ cells which express FOXP3 participate in immune regulation and the selection of these Treg cells can be mediated in foreign antigen specific TcR transgenic mice by expression of the stimulatory antigen as a neo self peptide driven by tissue specific promoters(4,32). It has also been shown that increasing avidity of the TcR for the peptide-MHC correlates with a propensity to develop along the thymic-derived FOXP3 Treg pathway(4). In our setting involving endogenous self-antigen recognition, we find that peripheral CD4+ T cells from both autoreactive 4.13/ Rag2+/+ and 164/ Rag2+/+ TcR transgenic mice express increased numbers of FOXP3 cells, and that the percent of CD4+ cells which express FOXP3 is highest in 164 mice compared with 4.13 mice, and both are greater than that seen in non-TcR transgenic mice (Figure 4B). However, upon crossing TcR transgenic mice onto a Rag2 deficient background, peripheral FOXP3 positive cells were near undetectable levels in either 164 or 4.13 mice (Figure 4C) consistent with the induction of Treg populations in the non-transgenic fraction of endogenous T cells.
Figure 4.
FOXP3 expression on spleen CD4+/CD8− T cells from non-TcR transgenic, 164 and 4.13 GAD TcR transgenic mice. 8–12 week old mouse spleen cells from TcR and non-TcR transgenic mice were surface stained with CD4, CD25 and then intracellularly for FOXP3. Example of staining is shown in (A.) on non-TcR transgenic DR4 splenocytes. FOXP3 expression in Rag2+/+ mice as a percentage of CD4+ cells is shown in (B.) (Average of 3 mice). FOXP3 expression on CD4+ T cells from 164 and 4.13 TcR transgenic mice on a Rag2o/o background is shown in (C.).
Peripheral 4.13 CD4+ T cells exhibit Th1 and Tr1 profile
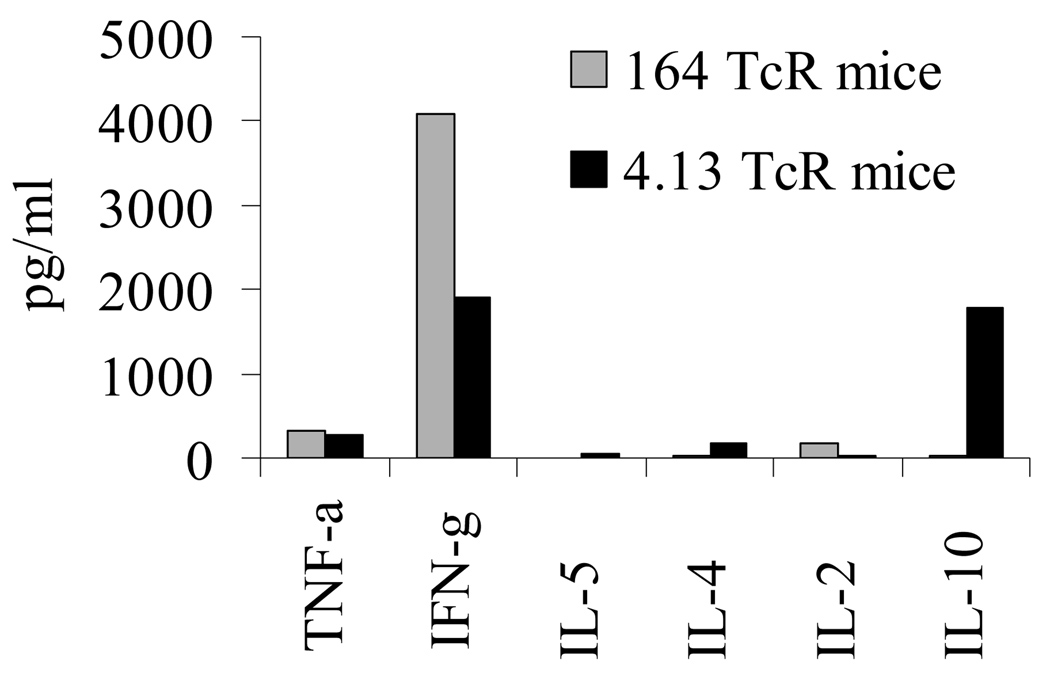
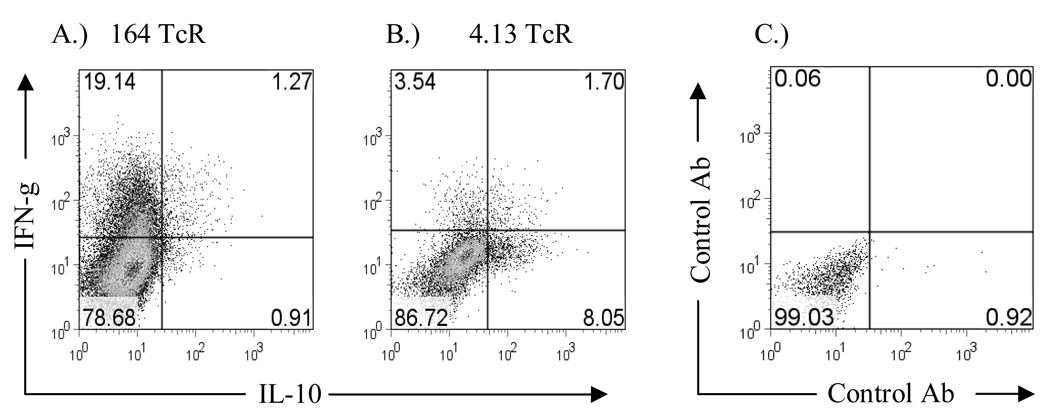
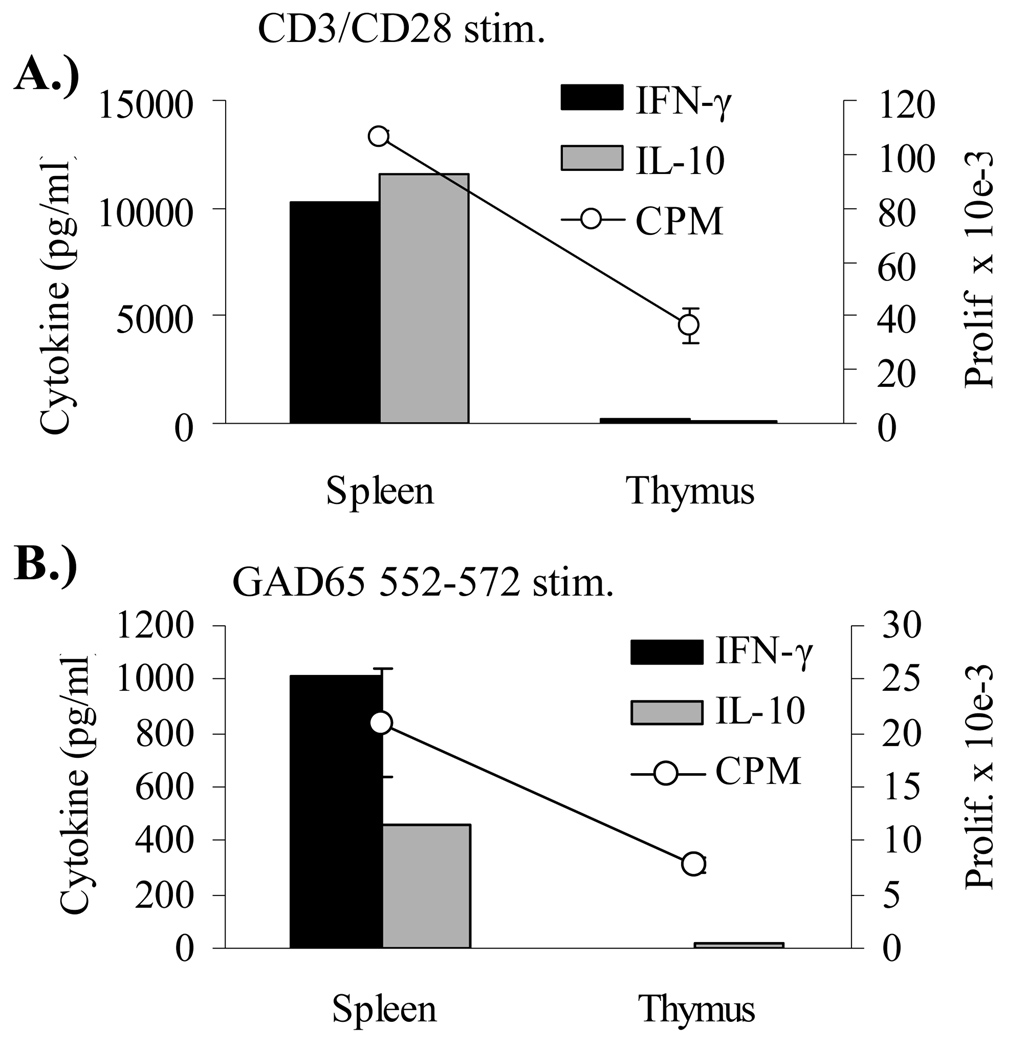
Cytokine analysis on in vitro stimulated cells from both Rag2o/o TcR transgenic mice responding to GAD65 555–567 stimulation is shown in Figure 5. Peripheral 164 T cells are of a Th1 phenotype expressing IFN-γ and little or no IL-4, IL-5, IL-10, or TNF-α while CD4+ 4.13 T cells secrete IFN-γ and IL-10 and little or no IL-4, IL-5, or TNF-α. The same pattern was observed in Rag2+/+ mice (data not shown). Because of the unexpected finding of both IFN-γ and IL-10 from GAD65 555–567 stimulation we performed intracellular staining for IFN-γ and IL-10 to determine if both of these cytokines are derived from the same cell. As shown in Figure 6 we find that T cells from 4.13/Rag2o/o mice generate IFN-γ independently of IL-10 and therefore peripheral 4.13 CD4+ T cells are of a mix of Th1 and Tr1 cells types while 164 T cells are of a Th1 phenotype generating only IFN-γ. Additional cytokine measurements revealed that 4.13 T cells do not secrete TGF-beta1 (supplemental Figure S2). Because IL-10 can be immunoregulatory we addressed whether the commitment of 4.13 T cells to a Tr1 phenotype is a central or peripheral tolerizing event. CD4+ T cells from thymus and spleens of DR4/4.13/Rag2+/+ mice were FACS sorted and stimulated with irradiated APC, and then assayed for IL-10 and IFN-γ production. 4.13 CD4+ T cells from spleen generated IL-10 and IFN-γ in response to either CD3/CD28 or GAD65 552–572 stimulation while thymus derived CD4+/CD8− 4.13 T cells secreted neither cytokine (Figure 7).
Figure 5.
Cytokine profile of 164 and 4.13 T cells to GAD65 552–572. Splenocytes from DR4/164/Rag2o/o and DR4/4.13/Rag2o/o mice were stimulated with 100 ug/ml of GAD65 or control peptide for 96 hours. Supernatants were collected at 48 hours and TNF-α, IFN-γ, IL-2, IL-4, IL-5 were measured using BD mouse Th1/Th2 kit and IL-10 was measured by ELISA. Experiment was done 3 times with similar results.
Figure 6.
Internal cytokine staining for IL-10 and IFN-γ Cells from 164 (A.) and 4.13 (B.) mice were intracellular stained with IL-10 and IFN-γ directly conjugated Antibodies. Spleen cells from Rag2o/o 164 and 4.13 mice were stimulated for 4 days with GAD65 552–572 and then cultured with PMA/Ionomycin for 4 hours with Brefeldin A during the last two hours. Experiment was done 3 times with similar results
Figure 7.
IFN-γ and IL-10 production from stimulated sorted CD4+/CD8− cells from DR4/4.13/Rag2+/+ mice. CD4+/CD8− cells from spleen, LN, and thymus were FACS sorted from tissues taken from 8–12 week old mice and stimulated with irradiated APC and either CD3/CD28 Ab (A) or GAD65 552–572 (B).
Discussion
Limiting pathogenic autoreactivity is of the utmost importance for a successful immune system, and several mechanisms provide functional checkpoints for this control. These mechanisms broadly fit into three categories: Those which involve deletion of autoreactive cells, centrally and/or peripherally; those which involve down-modulation of activation molecules or receptors, changing activation thresholds; and those which involve active immune regulation. In this study we evaluated central and peripheral tolerance mechanisms using two TcR transgenic mice containing structurally similar receptors specific for a naturally processed self antigen. These TcR were derived from autoreactive CD4+ T cells present in humans with immunity to GAD65, an important islet antigen associated with autoimmune diabetes. On a C57Bl/6 “diabetes-resistant” background transgenic for HLA-DR4, the human class II restricting element for these TcR, very potent in vivo tolerance mechanisms were observed. The 164 TcR was associated with strong deletional events, both in the thymus and in the periphery, and surviving 164 T cells down-modulated TcR expression and/or switched from CD4 to CD8 phenotype, even as they maintained specific antigen reactivity. In marked contrast, the 4.13 TcR had less sensitivity to negative selection and no CD4-to-CD8 skewing, but instead used a predominant pathway of immunomodulation, skewing towards an IL-10 phenotype.
Both 164 and 4.13 T cells use Vα12.1/Vβ5.1 TcR and differ only in CDR3, a region that conventionally interacts primarily with the peptide in the antigen-binding MHC(33). Based on the higher thymic cellularity in 4.13 mice compared to 164 mice and the absence of differentiation toward the CD4−/CD8+ pathway it appears that the 164 TcR is of a higher avidity to peptide-MHC complexes in the thymus. As T cell CD4 avidity interaction with the beta 2 domain of the MHC Class II has been shown to contribute positively to thymic T cell selection (21,34) the differentiation of immature CD4+/CD8+ double positive 164 thymocytes into CD4−/CD8+ mature cells would presumably lower the TcR overall avidity to the MHC complex and enable escape from negative selection. This skewing towards a CD4−/CD8+ expression pathway and away from a CD4+CD8− pathway occurred in spite of the class II restriction of the original human 164 T cell clone. Consistent with this interpretation is our observation that peripheral CD4−/CD8+ 164/Rag2o/o T cells have less functional avidity to GAD65 555–567 stimulation than CD4+/CD8− 164/Rag2o/o T cells. The skewing of class II restricted self antigen reactive T cells towards a CD8 lineage has been observed in other TcR transgenic models, also in the context of strong negative selection(7,8).
In addition to thymic deletion and CD4-to-CD8 skewing, T cells surviving in the 164 TcR mice showed significant down-regulation of the TcR molecule itself. This also is consistent with a strategy invoked for lowering avidity, and correlated in the mice with evidence of a very strong activation induced cell death pathway. The end result of all these simultaneous high avidity tolerance checkpoints was the presence in the peripheral circulation of a low number of autoreactive T cells, which nevertheless displayed strong antigen-specific proliferative and Th1 characteristics.
Considering that both 164 and 4.13 TcR use Vα12.1 and Vβ5.1 and are responsive to the same antigen, it was remarkable that 4.13 T cells showed a completely different tolerance induction profile. A more modest central tolerance for 4.13 T cells was reflected in less thymic deletion and normal CD4+/CD8− maturation, and similarly no evidence for peripheral activation induced cell death or receptor down-modulation was observed. A likely explanation for the absence of peripheral activation of 4.13 T cells was the peripheral generation of IL-10 producing Tr1 regulatory cells in these mice. IL-10 is a potent regulatory cytokine, and has been shown to be important in regulating colitis and autoimmunity in EAE and CIA models(35–38). The absence of IL-10 from sorted CD4+/CD8− T cells from the thymus upon stimulation with either CD3/CD28 or antigen specific GAD65 552–572 peptide also indicates that generation of these IL-10 secreting T cells was a peripheral differentiation event. It is interesting to speculate that T cell generated IL-10 in 4.13 mice could be preventing the activation of 4.13 T cells in the periphery which contrast the activated phenotype in peripheral 164 mice. This hypothesis is currently being testing by crossing DR4/4.13/Rag2o/o mice onto IL-10 deficient mice. While both 164 and 4.13 peripheral T cells are specific for GAD65 555–567, because many TcRs are degenerate in peptide recognition(39) we cannot exclude the possibility that cross-reactivity with other unknown ligands might contribute to the differences in functional profiles.
In the periphery, both 4.13 and 164 mice show an increase in FoxP3 positive cells, which is consistent with that seen in quasi-self-antigen models (4,40). The larger increase in the percentage of FOXP3+ cells in 164 mice relative to 4.13 mice correlates with the increase in negative selection (higher avidity TcR) in the thymus. However, upon crossing to Rag2 deficient mice we did not detect peripheral CD4+/CD25+ (FOXP3+) positive cells from either 164 or 4.13 mice. This is in contrast to HA-specific and OVA-specific TcR transgenic mice on a Rag deficient background where the antigen is expressed as a neo self-antigen(40–42). In these models up to half of peripheral T cells are CD25+ and have a regulatory function. However, in TcR transgenic mice where the T cell responsive antigen is endogenously expressed, CD4+/CD25+ (FOXP3+ Treg) do not develop on a Rag deficient background. This includes a MBP specific TcR(43), and the BDC2.5 TcR(44). It has been suggested that a high avidity interaction between T cell and APC in the thymus is required for Treg development(45). Considering the strong negative selection in the thymus of both TcR mice suggesting a high functional avidity of the TcR for MHC-antigen we were surprised to not find CD4+/CD25+/FOXP3+ cells in the periphery on Rag2o/o mice. A possible explanation for a lack of FOXP3 positive Tregs in these mice may be that both of these TcR are of high enough avidity that they are beyond the threshold for FOXP3 differentiation (5).
Peripheral tolerance methods of anergy (10,11), deletion(12–14), or the generation of Tr1(15) and Th3(16) cells are a second line of defense against T cell autoimmunity. Once in the periphery 164 cells displayed a strong activation phenotype in both spleen and lymph nodes resulting in continued down-modulation of their T cell receptor and concomitant AICD through an activated Caspase-3 pathway. Consistent with this is the expression of CD95 (FAS) on 164 T cells through which signaling has been shown to mediate deletion induced peripheral tolerance(46,47). 4.13 T cells which populate the periphery to a greater extent do not undergo this type of peripheral tolerance, most likely due to their apparent lower overall pMHC avidity.
Autoreactive cells, such as those used to derive the 164 and 4.13 TcR in this study, occur frequently in humans with autoimmune disease, in people who are genetically at risk of autoimmune disease, and in normal HLA-matched individuals (48– 52). Nevertheless, overt autoimmune disease is relatively rare, reflecting the importance of tolerance checkpoints in normal immune function. Our study, using human autoimmune TcR and human MHC transgenic mice, directly demonstrates multiple mechanisms which, sometimes simultaneously, elicit both central and peripheral tolerance. Indeed, the two structurally similar TcR used, derived from human HLA-DR4 subjects, with specificity for the same antigen and restriction element, differing only in their CDR3 regions, revealed stark differences in deletional, compensatory and immunomodulatory mechanisms. That such distinction occurs even with closely related autoreactive TcR underscores the importance of understanding the contribution of this variation to disease susceptibility, pathogenic pathways, and response to therapy.
Supplementary Material
Acknowledgements
We would like to acknowledge Alice Long for critical review of the manuscript.
Footnotes
This research was supported by NIH grant AI050864 and USAMRAA grant PR064261.
Reference List
- 1.Bevan MJ, Hogquist KA, Jameson SC. Selecting the T cell receptor repertoire. Science. 1994;264:796–797. doi: 10.1126/science.8171333. [DOI] [PubMed] [Google Scholar]
- 2.Lo D, Reilly CR, Burkly LC, DeKoning J, Laufer TM, Glimcher LH. Thymic stromal cell specialization and the T-cell receptor repertoire. Immunol Res. 1997;16:3–14. doi: 10.1007/BF02786320. [DOI] [PubMed] [Google Scholar]
- 3.Jameson SC, Bevan MJ. T-cell selection. Curr Opin Immunol. 1998;10:214–219. doi: 10.1016/s0952-7915(98)80251-3. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 5.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J. Clin. Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnden MJ, Heath WR, Carbone FR. Down-modulation of CD8 beta-chain in response to an altered peptide ligand enables developing thymocytes to escape negative selection. Cell Immunol. 1997;175:111–119. doi: 10.1006/cimm.1996.1054. [DOI] [PubMed] [Google Scholar]
- 7.Badami E, Maiuri L, Quaratino S. High incidence of spontaneous autoimmune thyroiditis in immunocompetent self-reactive human T cell receptor transgenic mice. J. Autoimmun. 2005;24:85–91. doi: 10.1016/j.jaut.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Ranheim EA, Tarbell KV, Krogsgaard M, Mallet-Designe V, Teyton L, McDevitt HO, Weissman IL. Selection of aberrant class II restricted CD8+ T cells in NOD mice expressing a glutamic acid decarboxylase (GAD)65-specific T cell receptor transgene. Autoimmunity. 2004;37:555–567. doi: 10.1080/08916930400020545. [DOI] [PubMed] [Google Scholar]
- 9.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 11.Saibil SD, Deenick EK, Ohashi PS. The sound of silence: modulating anergy in T lymphocytes. Curr. Opin. Immunol. 2007;19:658–664. doi: 10.1016/j.coi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Redmond WL, Wei CH, Kreuwel HT, Sherman LA. The apoptotic pathway contributing to the deletion of naive CD8 T cells during the induction of peripheral tolerance to a cross-presented self-antigen. J. Immunol. 2008;180:5275–5282. doi: 10.4049/jimmunol.180.8.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster I, Lieberam I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur. J. Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- 14.Morgan DJ, Kreuwel HT, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- 15.You S, Chen C, Lee WH, Brusko T, Atkinson M, Liu CP. Presence of diabetes-inhibiting, glutamic acid decarboxylase-specific, IL-10-dependent, regulatory T cells in naive nonobese diabetic mice. J. Immunol. 2004;173:6777–6785. doi: 10.4049/jimmunol.173.11.6777. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 17.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+ Proc. Natl. Acad. Sci. U. S. A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabarrocas J, Cassan C, Magnusson F, Piaggio E, Mars L, Derbinski J, Kyewski B, Gross DA, Salomon BL, Khazaie K, Saoudi A, Liblau RS. Foxp3+ CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8453–8458. doi: 10.1073/pnas.0603086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 21.Patel SD, Cope AP, Congia M, Chen TT, Kim E, Fugger L, Wherrett D, Sonderstrup-McDevitt G. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR(alpha1*0101,beta1*0401) transgenic mice. Proc Natl Acad Sci U. S. A. 1997;94:8082–8087. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reijonen H, Mallone Roberto, Heninger Anne-Kristin, Laughlin ElseM, Kochik SharonA, Falk Ben, Kwok WilliamW, Greenbaum Carla, Nepom GeraldT. GAD65-Specific CD4+ T-Cells with High Antigen Avidity Are Prevalent in Peripheral Blood of Patients with Type 1 Diabetes. Diabetes. 2004;53:1987–1994. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 23.Nepom GT, Lippolis JD, White FM, Masewicz S, Marto JA, Herman A, Luckey CJ, Falk B, Shabanowitz J, Hunt DF, Engelhard VH, Nepom BS. Identification and modulation of a naturally processed T cell epitope from the diabetes-associated autoantigen human glutamic acid decarboxylase 65 (hGAD65) Proc Natl Acad Sci U. S. A. 2001;98:1763–1768. doi: 10.1073/pnas.98.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masewicz SA, Meldrum N, Gersuk V, Gaur L, Hagopian W, Moriarity L, Nepom GT. Complexity of human immune response profiles for CD4+ T cell epitopes from the diabetes autoantigen GAD65. Autoimmunity. 2001;34:231–240. doi: 10.3109/08916930109014692. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, Falcioni F, Vidovic D, Hammer J, Nagy ZA. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu A, Kwok W, Nepom G. Detection of GAD65 specific T-cells by MHC class II multimers in type 1 diabetes patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 27.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J. Immunol. Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 28.Kruisbeek Ada M. Isolation and Fractionation of Mononuclear Cell Populations. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons, Inc; 2000. pp. 3.1.1–3.1.5. [Google Scholar]
- 29.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, Kwok WW, Nepom GT. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur. J. Immunol. 2003;33:1409–1417. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 30.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 31.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J, Yamamoto K. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr Opin Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 34.Riberdy JM, Mostaghel E, Doyle C. Disruption of the CD4-major histocompatibility complex class II interaction blocks the development of CD4(+) T cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4493–4498. doi: 10.1073/pnas.95.8.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 36.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 38.Johansson AC, Hansson AS, Nandakumar KS, Backlund J, Holmdahl R. IL-10-deficient B10.Q mice develop more severe collagen-induced arthritis, but are protected from arthritis induced with anti-type II collagen antibodies. J. Immunol. 2001;167:3505–3512. doi: 10.4049/jimmunol.167.6.3505. [DOI] [PubMed] [Google Scholar]
- 39.Mazza C, Malissen B. What guides MHC-restricted TCR recognition? Semin. Immunol. 2007;19:225–235. doi: 10.1016/j.smim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lerman MA, Larkin J, III, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J. Immunol. 2004;173:236–244. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 42.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 43.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker LS. CD4+ CD25+ Treg: divide and rule? Immunology. 2004;111:129–137. doi: 10.1111/j.0019-2805.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang HG, Su X, Liu D, Liu W, Yang P, Wang Z, Edwards CK, Bluethmann H, Mountz JD, Zhou T. Induction of specific T cell tolerance by Fas ligand-expressing antigen-presenting cells. J. Immunol. 1999;162:1423–1430. [PubMed] [Google Scholar]
- 47.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J. Immunol. 2005;174:4098–4104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 48.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J. Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 49.Oling V, Marttila J, Ilonen J, Kwok WW, Nepom G, Knip M, Simell O, Reijonen H. GAD65-and proinsulin-specific CD4+ T-cells detected by MHC class II tetramers in peripheral blood of type 1 diabetes patients and at-risk subjects. J. Autoimmun. 2005;25:235–243. doi: 10.1016/j.jaut.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Berthelot L, Laplaud DA, Pettre S, Ballet C, Michel L, Hillion S, Braudeau C, Connan F, Lefrere F, Wiertlewski S, Guillet JG, Brouard S, Choppin J, Soulillou JP. Blood CD8+ T cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur. J. Immunol. 2008;38:1889–1899. doi: 10.1002/eji.200838023. [DOI] [PubMed] [Google Scholar]
- 51.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J. Autoimmun. 2005;25:303–311. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Veldman CM, Gebhard KL, Uter W, Wassmuth R, Grotzinger J, Schultz E, Hertl M. T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. J. Immunol. 2004;172:3883–3892. doi: 10.4049/jimmunol.172.6.3883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.