Abstract
Targeted inactivation of p27kip1 was sufficient for intestinal tumor formation in mice, but this was strictly a function of diet: tumors formed in p27+/− or p27−/− mice fed control AIN-76A diet and were increased by a western-style diet but did not develop in mice fed standard chow diet. When crossed with the Apc1638N+/− mouse, Apc+/−,p27+/− or Apc+/−,p27−/− mice not only formed twice as many tumors than the sum of the tumors from mutation at either locus alone, but on AIN76A diet also developed intestinal intussusception, a tumor-associated pathology in patients leading to intestinal blockage that has not been reported for intestinal cancer in mouse models. Moreover, the frequency of intussusception was increased when the compound mutant mice were maintained on the western diet, leading to early death. Despite this more aggressive tumor phenotype generated by inactivation of p27 than by inactivation of another cyclin-dependent kinase inhibitor, p21WAF1/cip1, the nonsteroidal anti-inflammatory drug sulindac was still effective in inhibiting intestinal tumor formation in Apc+/−,p27+/− or Apc+/−,p27−/− mice, which contrasts with the abrogation of the effects of sulindac in Apc+/−,p21+/− or Apc+/−,p21−/− mice, indicating that p27 is not necessary for tumor inhibition by sulindac. Furthermore, tumor inhibition by sulindac was linked to the induction of p21 expression by the drug, regardless of p27 status, leading to suppression of cell proliferation and promotion of cell differentiation and apoptosis in the intestinal mucosa.
Introduction
p27kip1 and p21WAF1/cip1 are both cyclin-dependent kinase (cdk) inhibitors (1, 2), but they do not have identical functions in regulating multifactor complexes that regulate cell maturation. For example, p27 has been shown to have a significant role in colonic cell differentiation outside of its role in regulating cell proliferation, with forced increases and decreases in expression linked to stimulation and inhibition, respectively, of differentiation along the absorptive cell lineage (3). In addition, there is much better evidence in the literature for level of expression of p27 as a marker of prognosis in human colon cancer than there is for p21 (3–9). Therefore, it was important that we found that, unlike targeted inactivation of p21, targeting of p27 was sufficient for tumor formation, not only in the pituitary, as had been reported (10–12), but also for intestinal adenomas and adenocarcinoma (13). This intestinal tumorigenesis by inactivation of p27 was linked to disruption of intestinal cell maturation caused by elevated expression of c-myc and cyclin D1, as well as the c-myc target gene, cdk4 (13).
Although it was also reported that the targeted inactivation of p27 enhanced adenomatosis polyposis coli (Apc)–initiated tumor formation in compound mutant mice, that report did not address tumor formation by inactivation of p27 in the absence of an Apc mutation (14). Here, we show that this is likely because p27-initiated tumor formation is strictly dependent on the diet fed the animals. Moreover, we show that compound Apc+/−,p27+/− or Apc+/−,p27−/− mutant mice develop intussusception, an unusual, aggressive tumor-associated pathology seen in patients that has not been previously reported in genetically initiated intestinal cancer in mice. Not only are the frequency and size of the tumors substantially increased in the Apc,p27 compound model by feeding of a western-style, high-risk diet but also the incidence of intussusception, leading to a severe shortening of life span.
Finally, the nonsteroidal anti-inflammatory drug sulindac has a substantial effect in inhibiting Apc-initiated tumors in the intestine of either the human (15, 16) or the mouse (17, 18). We show that the inactivation of p27 does not interfere with this tumor inhibition by sulindac, which contrasts markedly with the abrogation of the effects of sulindac on tumor formation in Apc+/− mice that have a targeted inactivation of the gene encoding another cdk inhibitor, p21WAF1/cip1 (17, 19). This inhibition of tumorigenesis in the Apc,p27 compound mice was associated with the ability of sulindac to induce p21, which led to reduced proliferation and apoptosis in both the intestinal mucosa and tumors of Apc+/− mice in which one or both p27 alleles had been inactivated. All of these responses were lost in the Apc+/−,p21+/− and Apc+/−,p21−/− mice.
Materials and Methods
The Apc1638N+/− and p27−/− mouse models and methods for genotyping have been reported (13, 20). Apc1638N+/− mice (C57Bl/6 background) were mated with p27−/− mice (mixture of 129S1/sv × C57Bl/6) to generate Apc+/−,p27+/− offspring (F1). F1 mice were mated to produce desired genotypes: Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/−. At weaning (~3–4 weeks), littermates of different genotypes were randomized to different dietary groups and fed, ad libitum, either AIN-76A control–defined diet, AIN-76A diet supplemented with 0.02% sulindac, or a western-style diet based on AIN-76A that is formulated on the principle of nutrient density to mimic the intake of major risk factors for colon cancer in the diet of populations in developed countries: high in fat and phosphate and low in calcium and vitamin D (21, 22). Diets were from Research Diets (New Brunswick, NJ). The mice with targeted inactivation of p27 without an Apc mutation were also maintained on standard chow diet (LabDiet, Somerville, NJ), AIN-76A diet, or western-style diet.
Mice were weighed weekly and maintained on diet for 16 or 36 weeks, or until they exhibited significant weight loss or other signs of extensive tumor formation. Mice were killed by CO2 overdose and rapidly dissected for evaluation of tumors and fixation of tissues, as described previously (13, 17, 23).
Total RNA and protein were isolated from the frozen tissues using TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA), as previously described (13, 19). The quantity of RNA and protein were measured spectrophotometrically, as we described previously (13, 19). cDNA was synthesized from DNase-treated total RNA using Taqman Multiscribe Reverse Transcriptase (Applied Biosystems, Inc., Foster City, CA). Quantitative PCR analysis was done using the ABI Prism 7900-HT Sequence Detection System (96 wells). The primers for p21, p27, and β-actin; the amplification conditions for the quantitative real-time reverse transcription-PCR; and methods of data analysis have been reported (13, 19).
Western blot analysis of specific proteins was done by standard methods (13, 19) using the following primary antibodies for detection: anti-p21, anti-p27 (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin (Sigma, St. Louis, MO). Signal was detected by the enhanced chemiluminescence technique (Amersham Life Science, Piscataway, NJ).
Results
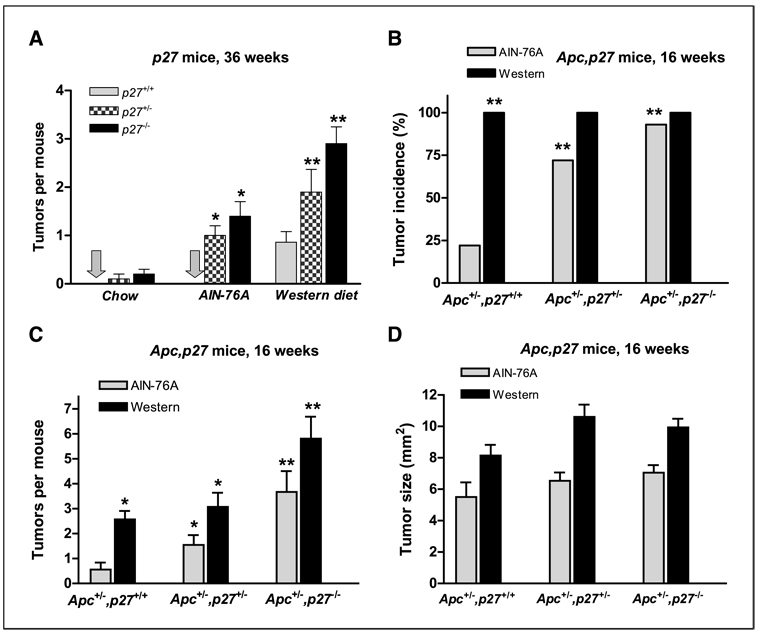
Figure 1A shows that in mice that inherit a heterozygous or homozygous inactivation of the p27 gene (p27+/− and p27−/−, respectively), intestinal tumors formed when the animals were fed a control-defined AIN-76A diet for 36 weeks and increased substantially when they were fed a western-style diet for 36 weeks that is formulated based on nutrient density to mimic major risk factors (high fat and phosphate/low calcium and vitamin D) for intestinal cancer in the United States and other developed countries. These tumors were both adenomas and adenocarcinomas. Importantly, the incidence of tumors was very low in these mice when they were fed a standard chow diet. Thus, in the p27+/− and p27−/− mice, intestinal tumor formation is strictly a function of diet.
Figure 1.
A, the number of intestinal tumors that developed in p27+/+, p27+/−, or p27−/− mice fed on standard chow diet, AIN-76A, or Western-style diet for 36 weeks. Incidence (B), frequency (C), and size (D) of intestinal tumors in the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice fed AIN-76A diet or western-style diet for 16 weeks. *, P < 0.05; **, P < 0.01.
Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice were generated by mating Apc1638N+/− mice with p27−/− mice. When maintained on the defined AIN-76A diet, by 16 weeks, 22% of the Apc+/−,p27+/+ mice developed intestinal tumors (Fig. 1B), at a frequency of 0.6 tumors per mouse (Fig. 1C). However, elimination of one or both wild-type p27 alleles in the Apc+/−,p27+/− and Apc+/−,p27−/− mice increased tumor incidence dramatically to 72% and 93%, respectively (Fig. 1B; P = 0.01, compared with Apc+/−,p27+/+ mice). Inactivation of p27 in these compound mice also substantially increased tumor frequency by 3- and 6-fold (Fig. 1C; P < 0.02 and P < 0.002, compared with Apc+/−,p27+/+ mice; Fig. 2A) and tumor size by 20% to 30% (Fig. 1D) in the mice heterozygote and homozygote for p27, respectively.
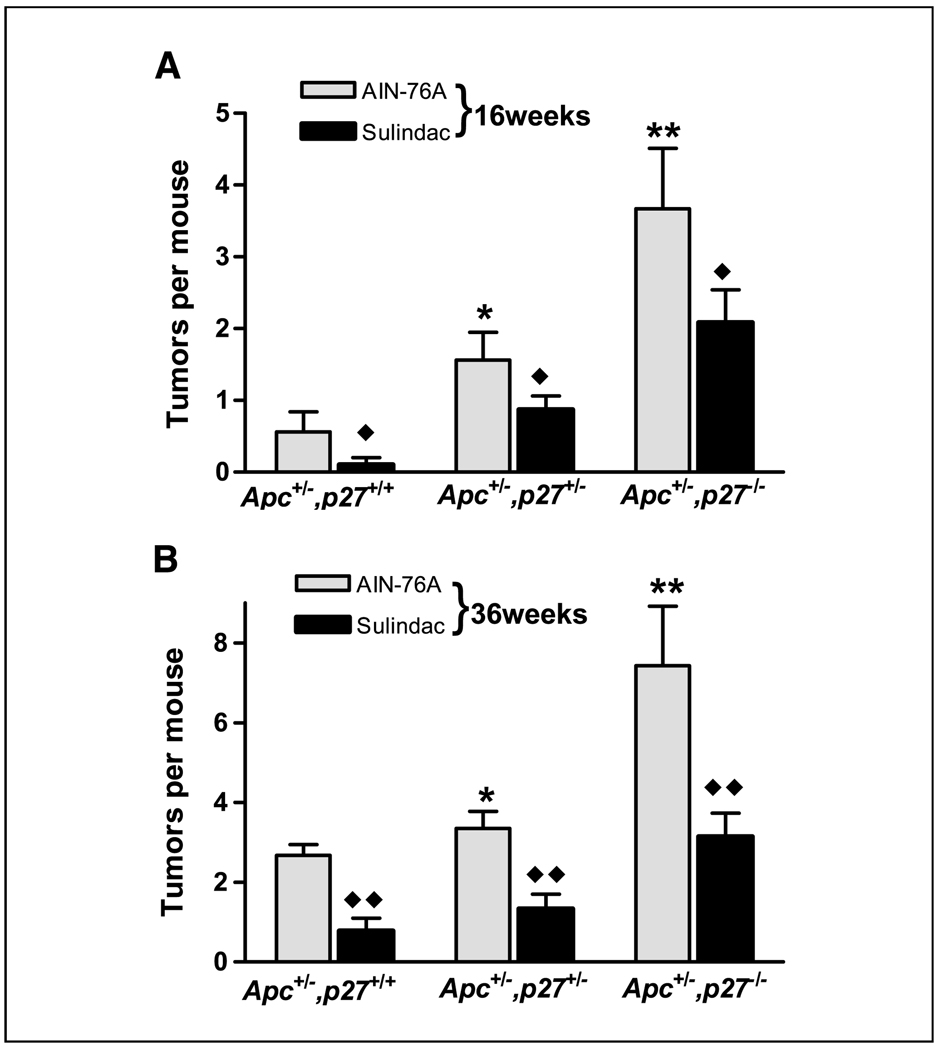
Figure 2.
The number of intestinal tumors in the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice fed AIN-76A diet or sulindac supplemented diet (sulindac), for 16 weeks (A) and 36 weeks (B). *, P < 0.05; **, P < 0.01, compared with Apc+/−,p27+/+ mice. ♦, P < 0.05; ♦♦, P < 0.01, compared with the mice fed AIN-76A diet.
At an older age of 36 weeks, all the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice fed the AIN-76A diet had at least one intestinal tumor (data not shown). In littermates that were Apc+/−,p27+/+, the mean frequency was 2.67 tumors per mouse (Fig. 2B). This was similar to that of Apc1638N+/− mice on a homogeneous Black 6 background (20), thus reducing the probability that unlinked loci from the p27 mice had a significant effect on the Apc-initiated intestinal tumor formation. However, littermates that were Apc+/− and either p27+/− or p27−/− exhibited significantly higher tumor frequencies at 36 weeks of 3.35 and 7.43 tumors per mouse (Fig. 2B; P < 0.05 and P < 0.01, respectively). The 7.43 tumors in the compound Apc+/−,p27−/− mice fed the AIN-76A diet was nearly twice the sum (i.e., 4.1) of the ~2.67 tumors that developed in the Apc+/− mice (Fig. 2B; Apc+/−,p27+/+ mice on AIN-76A diet for 36 weeks) plus the 1.5 intestinal tumors that developed in the p27−/− mice on the same diet at 36 weeks (Fig. 1A; ref. 13). This contrasts with our data on inactivation of another cdk inhibitor, p21WAF1/cip1, which was only additive with Apc inactivation regarding intestinal tumor formation (23).
Inactivation of p27 also caused more aggressive intestinal tumor development. That is, only 10% of the intestinal tumors in Apc+/− mice were invasive adenocarcinomas, but the incidence was significantly increased to 17% in Apc+/−,p27+/− mice and 19% in Apc+/−,p27−/− mice (P < 0.05), respectively (Table 1).
Table 1.
Histopathologic features of the intestinal tumors in Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice
| AIN-76A diet, n (%) |
Western diet, n (%) |
Sulindac, n (%) |
|
|---|---|---|---|
| Apc+/−,p27+/+ | |||
| TA | 19*(63) | 53 (75) | 6 (86) |
| VA | 3 (10) | 5 (7) | 1 (16) |
| TVA | 5 (17) | 4 (5) | 0 (0) |
| CA | 3 (10) | 9 (13) | 0 (0) |
| Total | 30 (100) | 71 (100) | 7 (100) |
| Apc+/−,p27+/− | |||
| TA | 54 (72) | 72 (67) | 21 (95) |
| VA | 2 (3) | 4 (4) | 0 (0) |
| TVA | 6 (8) | 12 (11) | 1 (5) |
| CA | 13 (17) | 19 (18) | 0 (0)† |
| Total | 75 (100) | 107 (100) | 22 (100) |
| Apc+/−,p27−/− | |||
| TA | 63 (71) | 48 (69) | 28 (80) |
| VA | 1 (1) | 1 (1) | 3 (8) |
| TVA | 8 (9) | 7 (10) | 2 (6) |
| CA | 17 (19)‡ | 14 (20) | 2 (6) † |
| Total | 89 (100) | 70 (100) | 35 (100) |
Abbreviations: TA, tubular adenoma; VA, villous adenoma; TVA, tubulovillous adenoma; CA, adenocarcinoma.
The number of tumors in each group.
P < 0.05, compared with the mice on AIN-76A diet.
P < 0.05, compared with Apc+/−,p27+/+ mice on AIN-76A diet.
The western-style diet, which increased tumor formation in the p27+/− and p27−/− mice (Fig. 1A), has been shown to be highly effective in augmenting intestinal tumorigenesis in rodent models (13, 21–24). This was also seen in this study, in that the same western-style diet significantly increased tumor incidence, tumor frequency per mouse, and tumor size, regardless of Apc and/or p27 genotype (Fig. 1A–D). Moreover, the western diet increased the incidence of invasive adenocarcinoma in the p27 wild type, Apc+/− mice (Table 1). Because the Apc+/− mice with heterozygote or homozygote inactivation of p27 led to early morbidity and death, it was necessary to sacrifice these mice earlier than 36 weeks. Therefore, the increase of adenocarcinoma incidence was likely minimized in the observations on Apc+/−,p27+/− or Apc+/−,p27−/− mice (Table 1). Thus, the western-style, high-risk diet was additive to intestinal tumor formation in the compound Apc,p27 model and in either the Apc or the p27 models alone.
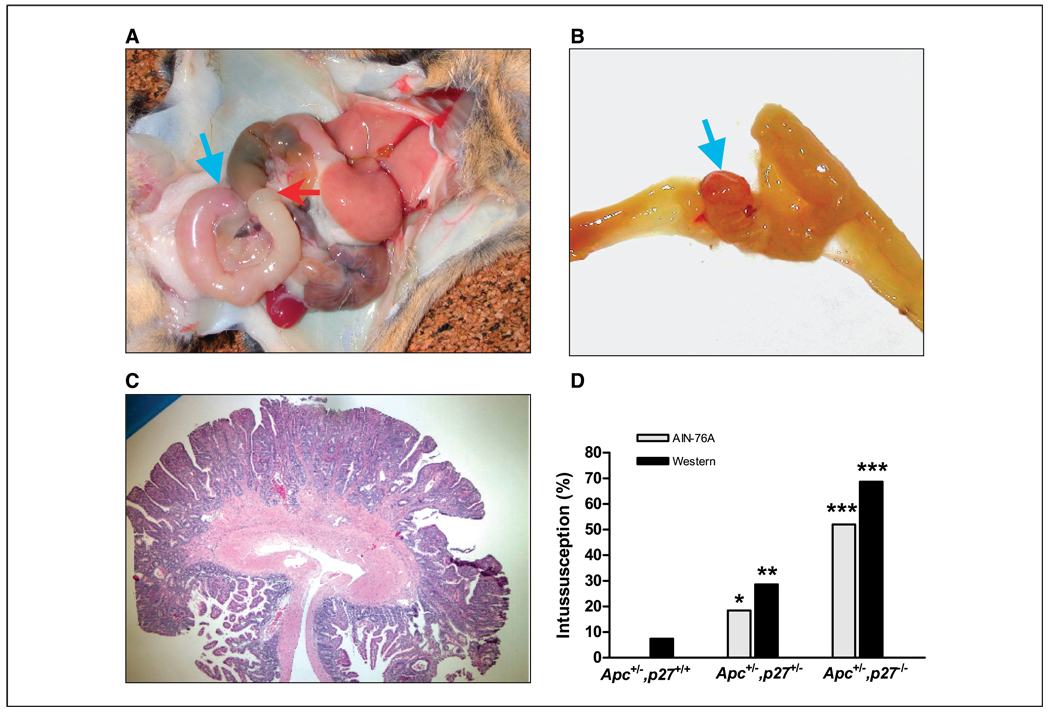
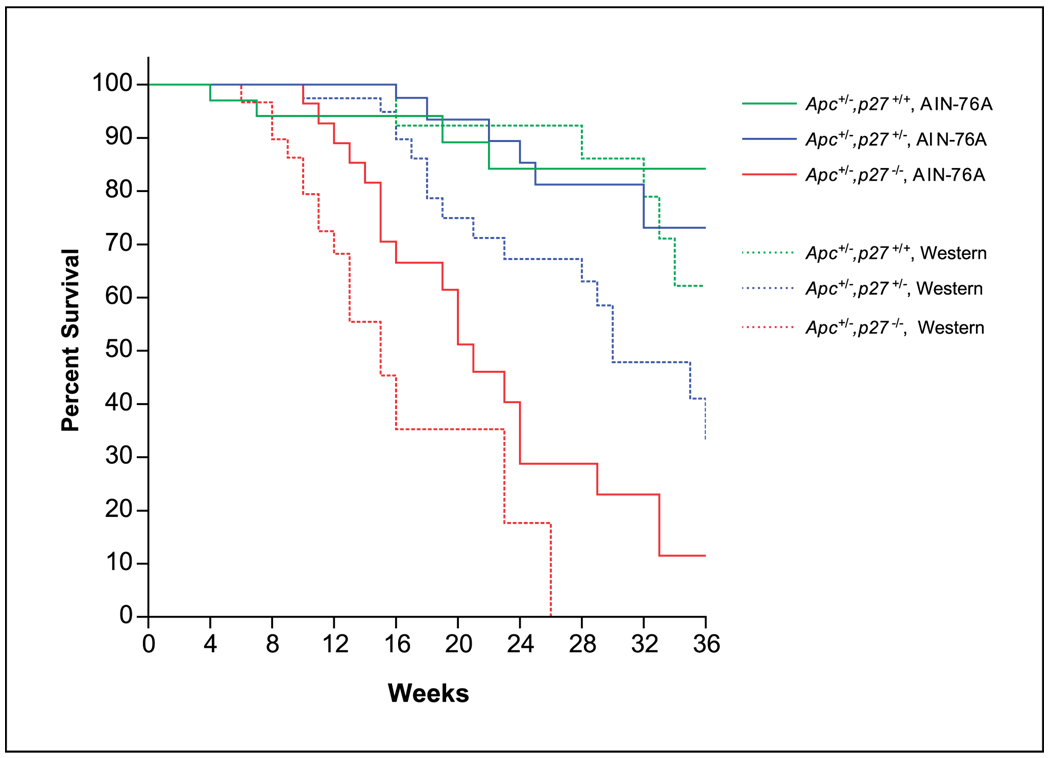
A more detailed examination of the gross pathology of the compound Apc+/−,p27+/− or Apc+/−,p27−/− mice revealed the presence of intussusception of the intestine (Fig. 3). Intussusception is usually caused in humans by the presence of an intestinal tumor (25). Peristaltic seizure and propulsion of the intestine causes the more proximal segment of the intestine, where the tumor is located, to invaginate into the next lower segment. This produces intestinal obstruction. Intussusception was found in 18% (7 of 38) of the Apc+/−,p27+/− mice fed the AIN-76A diet and was significantly increased to 52% (12 of 23) in the Apc+/−,p27−/− mice (Fig. 3D; P < 0.01, compared with Apc+/−,p27+/− mice). No intussusception was found in Apc+/−,p27 wild-type mice fed AIN-76A control diet and was rarely detected in this genotype fed the western-style diet (P < 0.01 and P < 0.001, compared with Apc+/−,p27+/− or Apc+/−,p27−/− mice fed AIN-76A diet, respectively). However, the incidence of intussusception in the Apc+/−,p27+/− or Apc+/−,p27−/− mice fed the western-style diet significantly increased to 29% (8 of 28) and 68% (11 of 16; Fig. 3D; P < 0.05 and P < 0.001, compared with Apc+/−,p27+/+ mice fed the western diet, respectively). Most of the intussusception was formed in the jejunum or ileum, and the tumors at sites of intussusception were either adenoma or adenocarcinoma (Fig. 3). In some mice, there were two sets of intussusception. Because of the intussusception, which caused intestinal obstruction, the survival time of the mice dramatically decreased; for example, 50% of the Apc+/−,p27−/− mice fed the western-style diet died at an age of 16 weeks, and none of these mice survived to 30 weeks of age (Fig. 4; P < 0.001).
Figure 3.
Intussusception in the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice. A, a representative photograph of intussusception at the jejunum (red arrow, start site; green arrow, tumor), which caused intestinal obstruction. B, the jejunum in (A) was longitudinally opened, and a tumor was found in the middle of the obstruction (blue arrow). C, an adenocarcinoma was taken from the intussusception site; the tumor had invaded the basal membrane and penetrated into the muscle layer. D, the incidence of intussusception in Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice. *, P < 0.01; **, P < 0.05, compared with Apc+/−,p27+/+ mice fed AIN-76A or western diet, respectively. ***, P < 0.001, compared with Apc+/−,p27+/+ mice fed AIN-76A or western diet, respectively.
Figure 4.
Survival of Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice fed the AIN-76A diet or the Western style diet.
We have previously studied the role of p21, another cdk inhibitor that is intimately associated with epithelial cell maturation in the intestinal tract, in tumors initiated in Apc+/− mice, and in the response to sulindac, a nonsteroidal anti-inflammatory drug (17, 23). This was prompted by findings from a number of laboratories, including our own, that p21 was a target of sulindac both in vitro and in vivo, with p21 mRNA and protein induced significantly as concomitants of the cell cycle arrest and apoptosis stimulated by the drug (17, 19, 26, 27). In comparison with the effects of p21 on intestinal tumor formation, the inactivation of p27 was far more pronounced and produced a much more aggressive phenotype (see above). We therefore extended our studies on sulindac to the Apc,p27 compound mouse model. As shown in Fig. 2, sulindac caused an equivalent decrease in intestinal tumors in the compound Apc,p27 mouse model, regardless of p27 genotype, at both 16 weeks (Fig. 2A) and 36 weeks (Fig. 2B) of age. This is in marked contrast to the Apc,p21 model, where inactivation of even one p21 allele was sufficient to eliminate the tumor inhibitory effect of sulindac (17). Moreover, sulindac significantly inhibited tumor progression to malignancy: no adenocarcinomas were seen in Apc+/−,p27+/+ or Apc+/−,p27+/− mice fed the sulindac diet (P < 0.05, compared with the mice fed AIN-76A diet), and adenocarcinoma formation was decreased in Apc+/−,p27−/− mice from 19% (17 of 89) to only 6% (2 of 35; P < 0.05) in mice fed the sulindac diet (Table 1). In addition, no intussusception was observed in the mice fed the sulindac-supplemented diet (not shown). Consequently, Apc+/−,p27−/− mice fed AIN-76A diet supplemented with the drug had a life span equivalent to that of Apc+/−,p27 wild-type mice fed just the AIN-76A (data not shown).
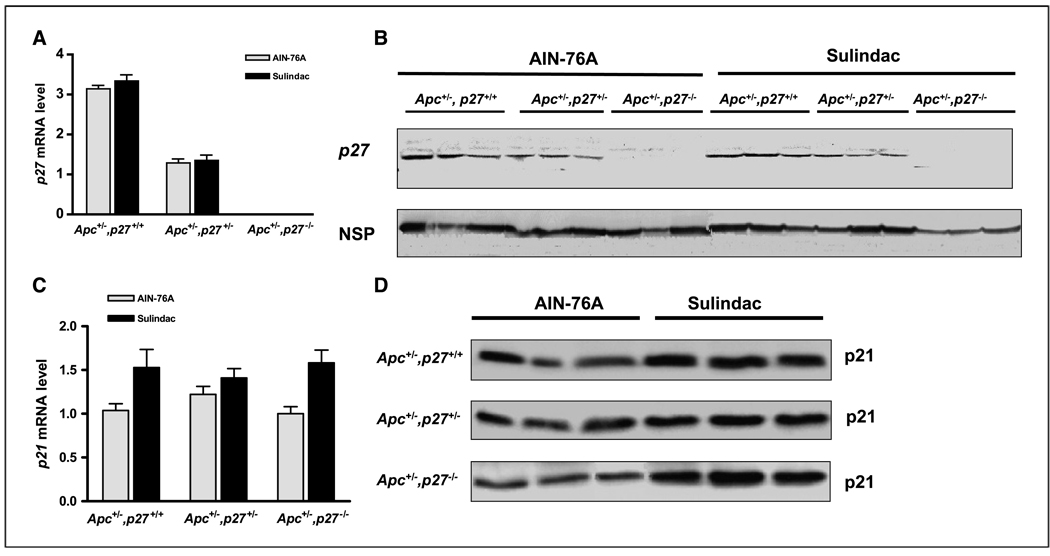
In the Apc,p21 model, we have recently shown that there is no induction of p21 even in the Apc+/−,p21+/− mouse (17), apparently due to the methylation and functional inactivation of the wild-type p21 allele (19). This strongly suggests that induction of p21 is a critical event in the inhibition of tumors by sulindac. Because tumors were inhibited by sulindac in the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice, p21 and p27 mRNA and protein were measured in these animals by quantitative real-time RT-PCR and by Western blot, respectively. First, as expected, p27 mRNA and protein were significantly expressed in the Apc+/−,p27+/+ mice, were decreased by ~50% in the Apc+/−,p27+/− mice, and were not detected in the Apc+/−,p27−/− mice (Fig. 5A–B). These levels of p27 were not changed by sulindac (Fig. 5A–B), suggesting that p27 is not a target of this drug. Second, p21 was expressed in each genotype, and at a similar level regardless of p27 genotype, when the mice were fed the control AIN-76A diet (Fig. 5C–D). Thus, there was no compensatory overexpression of p21 in the heterozygous or homozygous p27 mice. However, unlike p27, both mRNA and protein expression of p21 were significantly induced by sulindac regardless of the status of p27 (Fig. 5C–D). Therefore, inhibition of tumorigenesis by sulindac in the Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice was linked to the induction of p21. This again provides evidence that a wild-type, functional p21 gene is required for intestinal cells to respond to sulindac to inhibit tumor formation (17, 19), but that they do not require p27 for this tumor inhibition.
Figure 5.
The expression of p27 (A–B) and p21 (C–D) in the colon of Apc+/−,p27+/+, Apc+/−,p27+/−, or Apc+/−,p27−/− mice. A and C, mRNA level, assayed by quantitative RT-PCR. Columns, means of five different mice per group; bars, ±SE. B and D, protein level assayed by Western blot. A nonspecific band (NSP) was used as a loading control.
Discussion
In this report, we have established that the ability of p27 inactivation to cause tumor formation in the intestine is strictly linked to the diet that the mice are fed. Almost no tumors form in p27+/− or p27−/− mice maintained on standard chow diet, but those fed a defined AIN-76A diet show a significant tumor incidence. Both the control AIN-76A diet and the chow diet have been widely used for maintaining rodent growth, but the components differ significantly. The fat in the AIN-76A diet is from corn oil, but the fat in standard chow diet is from soybean oil. The minerals and vitamins in the diets also differ. Both type of dietary fat, as well as other phytochemicals in the chow diet, but not in AIN-76A, have been shown to have profound effects on tumor formation in human populations and in a number of model systems (28–33). Our findings show that dietary factors were critical and additive with inactivation of p27 in initiating intestinal tumor formation. Moreover, we only detected intestinal intussusception in the Apc+/−,p27 compound mouse model when the animals were fed the AIN76A control diet, not the chow diet, and the incidence of this pathology was substantially increased by the western-style diet. Intestinal intussusception is seen in intestinal cancer patients but has not been reported for any other mouse genetic model of intestinal cancer.
The western-style diet is formulated on the principle of nutrient density to mimic the intake of major, established risk factors for intestinal cancer in the human (increased fat and phosphate/decreased calcium and vitamin D; refs. 21, 22). It is important that both the type of control diet and the western-style diet had such a pronounced effect on tumor formation and pathology initiated by genetic factors, because there is overwhelming evidence in human populations that diet can significantly affect risk for tumor development in different genetic populations (34–36). Therefore, not only is it imperative that diet be considered in the explanation of the large variations in risk for tumor formation at different organ sites in developed and undeveloped countries, but as a practical matter, our data show that use of diets that reflect the human diet of different populations is fundamental to the successful development of mouse genetic models that efficiently and accurately reflect tumorigenesis in the human.
The at-least-additive effects of Apc mutation, p27 mutation, and a western diet on tumor formation in the mouse intestine argues that each affects different steps and/or stages in tumor development. There is increasing evidence that the Apc-initiated transformation is due to the inability of mutant or absent APC protein to successfully target β-catenin for degradation thus resulting in elevated β-catenin-Tcf transcriptional activity (37). Moreover, this pathway seems fundamental not only in controlling epithelial cell growth but also in regulating lineage-specific cell differentiation in the intestinal mucosa (38–43). Consistent with this, inactivation of Muc2, the gene encoding the major gastrointestinal mucin synthesized and secreted by goblet cells, causes tumor formation (44). It is therefore important that the inactivation of either p21 or p27 in the Apc+/− mice caused a decrease in mucin-secreting goblet cells (13, 23) and that introducing inactivated p21 into the Muc2−/− mice exacerbated affects on this lineage, causing decreased expression of ITF, another marker of this cell type (45). Thus, although the mechanisms of interaction between different genetic factors and nutritional factors in increasing risk for tumor formation and progression are not yet entirely clear, we predict that these will converge on effects on intestinal maturation and specific aspects of lineage specific differentiation.
Finally, because we had shown that targeted inactivation of p21 abrogated the ability of sulindac to inhibit Apc-initiated tumor formation (17), we tested this in the Apc+/−,p27 mouse model. It was surprising that although inactivation of p27 produced a much more aggressive tumor phenotype than did inactivation of p21, sulindac was still able to inhibit tumor formation and development of intussusception in the Apc+/−,p27+/− or Apc+/−,p27−/− mice as it did in the Apc+/− mice wild type for p27. Thus, p27 apparently plays no role in intestinal cell response to this powerful tumor inhibitor. However, consistent with the critical role of p21 in the response to sulindac (17, 19), p21 mRNA and protein were both induced in the Apc,p27 compound mouse model regardless of p27 genotype. These data have important implications for predicting clinical response to this drug.
Acknowledgments
Grant support: National Cancer Institute grants CA112081, U54 CA100926, CA96605, CA100926 and P01 13330.
We thank Drs. J. Pollard (Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY) and A. Koff (Cell Cycle Regulation Laboratory, Memorial Sloan-Kettering Cancer Center, New York, NY) for providing the p27−/− mice.
References
- 1.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Deschenes C, Vezina A, Beaulieu JF, Rivard N. Role of p27(Kip1) in human intestinal cell differentiation. Gastroenterology. 2001;120:423–438. doi: 10.1053/gast.2001.21199. [DOI] [PubMed] [Google Scholar]
- 4.Cariou S, Catzavelos C, Slingerland JM. Prognostic implications of expression of the cell cycle inhibitor protein p27Kip1. Breast Cancer Res Treat. 1998;52:29–41. doi: 10.1023/a:1006154900130. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd RV, Erickson LA, Jin L, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas GV, Szigeti K, Murphy M, Draetta G, Pagano M, Loda M. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol. 1998;153:681–687. doi: 10.1016/S0002-9440(10)65610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 8.Yao J, Eu KW, Seow-Choen F, Cheah PY. Down-regulation of p27 is a significant predictor of poor overall survival and may facilitate metastasis in colorectal carcinomas. Int J Cancer. 2000;89:213–216. [PubMed] [Google Scholar]
- 9.Zhang H, Sun XF. Loss of p27 expression predicts poor prognosis in patients with Dukes’ B stage or proximal colorectal cancer. Int J Oncol. 2001;19:49–52. [PubMed] [Google Scholar]
- 10.Kagawa Y, Yoshida K, Hirai T, Toge T. Significance of the expression of p27Kip1 in esophageal squamous cell carcinomas. Dis Esophagus. 2000;13:179–184. doi: 10.1046/j.1442-2050.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiyokawa H, Kineman RD, Manova-Todorova KO, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 13.Yang WC, Bancroft L, Nicholas C, Lozonschi I, Augenlicht LH. Targeted inactivation of p27kip1 is sufficient for large and small intestinal tumorigenesis in the mouse, which can be augmented by a Western-style high-risk diet. Cancer Res. 2003;63:4990–4996. [PubMed] [Google Scholar]
- 14.Philipp-Staheli J, Kim KH, Payne SR, et al. Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell. 2002;1:355–368. doi: 10.1016/s1535-6108(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 15.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 16.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 17.Yang WC, Velcich A, Mariadason J, et al. p21(WAF1/cip1) is an important determinant of intestinal cell response to sulindac in vitro and in vivo. Cancer Res. 2001;61:6297–6302. [PubMed] [Google Scholar]
- 18.Boolbol SK, Dannenberg AJ, Chadburn A, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 19.Yang W, Bancroft L, Augenlicht LH. Methylation in the p21(WAF1/cip1) promoter of Apc(+/−), p21(+/−) mice and lack of response to sulindac. Oncogene. 2005;24:2104–2109. doi: 10.1038/sj.onc.1208444. [DOI] [PubMed] [Google Scholar]
- 20.Fodde R, Edelmann W, Yang K, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newmark HL, Lipkin M, Maheshwari N. Colonic hyperplasia and hyperproliferation induced by a nutritional stress diet with four components of Western-style diet. J Natl Cancer Inst. 1990;82:491–496. doi: 10.1093/jnci/82.6.491. [DOI] [PubMed] [Google Scholar]
- 22.Newmark HL, Lipkin M, Maheshwari N. Colonic hyperproliferation induced in rats and mice by nutritional-stress diets containing four components of a human Western-style diet (series 2) Am J Clin Nutr. 1991;54:209S–214S. doi: 10.1093/ajcn/54.1.209S. [DOI] [PubMed] [Google Scholar]
- 23.Yang WC, Mathew J, Velcich A, et al. Targeted inactivation of the p21(WAF1/cip1) gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosal. Cancer Res. 2001;61:565–569. [PubMed] [Google Scholar]
- 24.Yang K, Edelmann W, Fan K, et al. Dietary modulation of carcinoma development in a mouse model for human familial adenomatous polyposis. Cancer Res. 1998;58:5713–5717. [PubMed] [Google Scholar]
- 25.Sleisenger MH, Fordtran JS. Gastrointestinal disease. 5th ed. Philadelphia: W.B. Saunders Company; 1993. [Google Scholar]
- 26.Goldberg Y, Nassif II, Pittas A, et al. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996;12:893–901. [PubMed] [Google Scholar]
- 27.Augenlicht LH, Velcich A, Klampfer L, et al. Application of gene expression profiling to colon cell maturation, transformation and chemoprevention. J Nutr. 2003;133:2410S–2416S. doi: 10.1093/jn/133.7.2410S. [DOI] [PubMed] [Google Scholar]
- 28.Wasan HS, Novelli M, Bee J, Bodmer WF. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc Natl Acad Sci U S A. 1997;94:3308–3313. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang WL, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 30.Reddy BS. Omega-3 fatty acids in colorectal cancer prevention. Int J Cancer. 2004;112:1–7. doi: 10.1002/ijc.20320. [DOI] [PubMed] [Google Scholar]
- 31.Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001;61:1927–1933. [PubMed] [Google Scholar]
- 32.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–586. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 33.Hong MY, Chapkin RS, Davidson LA, et al. Fish oil enhances targeted apoptosis during colon tumor initiation in part by downregulating Bcl-2. Nutr Cancer. 2003;46:44–51. doi: 10.1207/S15327914NC4601_06. [DOI] [PubMed] [Google Scholar]
- 34.Little J, Sharp L, Duthie S, Narayanan S. Colon cancer and genetic variation in folate metabolism: the clinical bottom line. J Nutr. 2003;133:3758S–3766S. doi: 10.1093/jn/133.11.3758S. [DOI] [PubMed] [Google Scholar]
- 35.Diergaarde B, van Geloof WL, van Muijen GN, Kok FJ, Kampman E. Dietary factors and the occurrence of truncating APC mutations in sporadic colon carcinomas: a Dutch population-based study. Carcinogenesis. 2003;24:283–290. doi: 10.1093/carcin/24.2.283. [DOI] [PubMed] [Google Scholar]
- 36.Slattery ML, Curtin K, Schaffer D, Anderson K, Samowitz W. Associations between family history of colorectal cancer and genetic alterations in tumors. Int J Cancer. 2002;97:823–827. doi: 10.1002/ijc.10148. [DOI] [PubMed] [Google Scholar]
- 37.Fodde R, Smits R. Cancer biology. A matter of dosage. Science. 2002;298:761–763. doi: 10.1126/science.1077707. [DOI] [PubMed] [Google Scholar]
- 38.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–770. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batlle E, Henderson JT, Beghtel H, et al. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 41.van de Wetering M, Sancho E, Verweij C, et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 42.Mariadason JM, Bordonaro M, Aslam F, et al. Down-regulation of β-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 43.Kielman MF, Rindapaa M, Gaspar C, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nat Genet. 2002;32:594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 44.Velcich A, Yang W, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Velcich A, Lozonschi I, et al. Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2−/− mice. Am J Pathol. 2005;166:1239–1246. doi: 10.1016/S0002-9440(10)62342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]