Summation
This update summarizes the growing application of “click” chemistry in diverse areas such as bioconjugation, drug discovery, materials science, and radiochemistry. This update also discusses click chemistry reactions that proceed rapidly with high selectivity, specificity, and yield. Two important characteristics make click chemistry so attractive for assembling compounds, reagents, and biomolecules for preclinical and clinical applications. First, click reactions are bio-orthogonal; neither the reactants nor their product's functional groups interact with functionalized biomolecules. Second, the reactions proceed with ease under mild nontoxic conditions, such as at room temperature and, usually, in water. The copper-catalyzed Huisgen cycloaddition, azide-alkyne [3 + 2] dipolar cycloaddition, Staudinger ligation, and azide-phosphine ligation each possess these unique qualities. These reactions can be used to modify one cellular component while leaving others unharmed or untouched. Click chemistry has found increasing applications in all aspects of drug discovery in medicinal chemistry, such as for generating lead compounds through combinatorial methods. Bioconjugation via click chemistry is rigorously employed in proteomics and nucleic research. In radiochemistry, selective radiolabeling of biomolecules in cells and living organisms for imaging and therapy has been realized by this technology. Bifunctional chelating agents for several radionuclides useful for positron emission tomography and single-photon emission computed tomography imaging have also been prepared by using click chemistry. This review concludes that click chemistry is not the perfect conjugation and assembly technology for all applications, but provides a powerful, attractive alternative to conventional chemistry. This chemistry has proven itself to be superior in satisfying many criteria (e.g., biocompatibility, selectivity, yield, stereospecificity, and so forth); thus, one can expect it will consequently become a more routine strategy in the near future for a wide range of applications.
Key words: click chemistry, bioconjugation, bioorthogonal, radiochemistry, imaging, bifunctional
Introduction
Generally, the preparation of bifunctional chelating agents involves multistep syntheses, and their subsequent incorporation into biomolecules is often hampered by cross-reactivity or nonspecific interactions with other functional groups present. The quest for novel, efficient strategies for the synthesis of bifunctional chelating agents, and their incorporation into biomolecules, has led to a burgeoning interest in “click” chemistry. One should not consider click chemistry as a replacement of existing methods for drug discovery, but rather as an extension and a complementary technology. This chemistry provides mimics of traditional pharmacophores, drugs, and natural products through the choice of appropriate building blocks.1,2 Click chemistry also has the ability not only to generate novel structures that might not resemble known pharmacophores, but also to simplify the covalent assembly of two very chemically dissimilar molecules, such as complex carbohydrates with peptides, or chemoreporters, such as fluorescent dyes with biopolymers. This highly selective, specific chemistry has emerged as an attractive technique. There are growing numbers of applications of click chemistry in research areas, such as organic chemistry,3 bioconjugation,4 drug discovery,5 polymers,6,7 and radiochemistry.8 In this Update, we provide a brief summary of two of the click reactions, namely Huisgen cycloaddition and Staudinger ligation; however, the focus will primarily be on the former chemistry, as this route appears to be the most popular.
Click Chemistry
Click chemistry, a term first coined by Kolb et al., described the goal to develop a set of powerful, selective, and modular building blocks, such as azide and alkyne, that work for both small and large scales.2 It is worth noting that the unactivated azide-alkyne cycloaddition was first discovered by Huisgen in 1963, but was ignored for decades primarily due to the requirements of high temperatures and pressures.9 Kolb et al. revitalized this reaction in 2001 by employing Cu(I) as a catalyst. They went on to define the criteria for successful click chemistry by stating that “the reaction must be modular, wide in scope, give very high yields, generate only inoffensive byproducts that can be removed by non-chromatographic methods, and be stereo-specific (but not necessarily enantioselective).” For a reaction to meet these criteria, starting materials and reagents must be readily available, and the reaction should proceed under friendly reaction conditions, such as room temperature and a benign solvent (i.e., water).
Click Reaction
A close examination of nature's favorite molecules, such as nucleic acids and proteins, unsurprisingly reveals a preference for making carbon-heteroatom bonds over carbon-carbon bonds; carbon dioxide is a common component with water as the reaction media.2 For example, proteins are formed from amino-acid building blocks joined by reversible amide heteroatom links (carbonyl). Inspired by nature, click chemistry focuses on carbon-heteroatom bond formation, but unlike nature, these reactions are irreversible. Click chemistry reactions rely on highly energetic reagents or reactants that are often described as being “spring-loaded.”10 Examples of click chemistry reactions, include: cycloaddition reactions, such as the 1,3-dipolar family, and hetero Diels-Alder reactions11; nucleophilic ring-opening reactions (e.g., epoxides, aziridines, cyclic sulfates, and so forth)2; carbonyl chemistry, such as the formation of oxime ethers, hydrazones, and aromatic heterocycles; in addition to carbon-carbon multiple bonds, such as epoxidation12 and dihydroxylation13 and azide-phosphine coupling (Staudinger ligation).14,15
The Huisgen 1,3-dipolar cycloaddition reaction, also termed the [3 + 2] azide-alkyne cycloaddition, has become the most popular click reaction, which fuses together two unsaturated reactants (azides and alkynes; Fig. 1),16 and provides access to a variety of five-membered heterocycles. This click reaction is unique in that the azide moiety is absent in almost all naturally existing compounds, lacks reactivity with natural biomolecules, and, consequently, only undergoes ligation with a limited set of partners. The popularity of this reaction is attributable to the fulfillment of the aforementioned criteria, such as mild reaction conditions, high yield, simple work-up, selectivity, and specificity. The small and stable azide functional group readily and selectively reacts with phosphines or activated alkynes. Functionalized azides have been incorporated into glycans, lipids, and proteins in living cells with little physiologic side-effects.17 This click reaction utilizes functional groups that are mostly compatible with enzymes under physiologic conditions and can be readily incorporated into diverse organic building blocks. However, the original Huisgen 1,3-cycloaddition reaction (i.e., the reaction of unactivated azides and alkynes) proceeds slowly in the absence of a catalyst, usually require high temperatures or pressures, and yields a mixture of 1,4-and 1,5-triazoles, rendering this reaction generally unsuitable for most applications involving biomedicals or biomaterials. Rostovtsev et al. and Tornoe et al. independently modified this reaction to proceed rapidly at room temperature by employing copper as a catalyst, which also promotes the regiospecific formation of 1,4-disubstituted 1,2,3-triazole products (Fig. 1).3,18 The Cu(I) catalyst used in click reactions is known to lower the activation barrier by 11 kcal/mol, which is sufficient to rapidly drive the reaction forward with high selectivity.19,20
FIG. 1.
Cu-catalyzed Huisgen 1,3-cycloaddition reaction.
A number of Cu(I) sources, such as CuI, or CuOTfC6H6 can be used in this type of Cu(I)-catalyzed reaction, but the formation of byproducts was often observed.18,21 (A detailed mechanism of the Cu(I) catalysis has been reported by Chassaing et al.22 and Bock et al.19) This is also true in cases wherein the Cu(I) can potentially be sequestered by ligating groups present in the reaction and other transition metals present can directly be replaced by Cu(I).23 To overcome these problems, Cabrera et al. have developed a method in which an alkyne-derivatized bis-pyridin-2-ylmethylamine ligand (Fig. 2) was first protected with Cu(II) or Cr(III) prior to performing a Cu(I)-catalyzed click reaction. The metal (Cu(II) or Cr(III)) was removed after the reaction by washing the product with a saturated solution of tetrasodium ethylenediamine tetraacetate (Na4EDTA).23 Knor et al. simply permitted the chelate moiety of their octreotate DOTA chelate conjugate to become filled with copper during the course of the reaction and, subsequently, expelled that copper by treatment with H2S.24 These strategies enabled the chelating agents to coordinate another metal ion afterward. Rostovtsev et al. pointed out that in order to obtain the 1,4-triazole product with high yields and purity, the Cu(I) catalyst is better prepared in situ by reducing copper(II) salts, such as CuSO45H2O, with a reducing agent, such as sodium ascorbate.3 This method suppress the formation of undesired byproducts associated with the use of Cu(I) and has the advantage of not requiring inert conditions to prevent the oxidation of Cu(I) to Cu(II) by atmospheric oxygen.21 The Cu(II) catalyst (CuSO4) can then be removed by simply washing the product with water25 or by using metal resin scavengers, such as Chelex resin.25,26
FIG. 2.
Structures of bis-pyridin-2-ylmethylamine derivatives.
Although copper catalysis has been widely employed to activate terminal alkynes in click chemistry for [3 + 2] cycloaddition, this may be incompatible with living systems due to toxicity of the metal. Codelli et al. reported an alternative method to lower the activation barrier for [3 + 2] cycloaddition by employing intrinsically highly strained cyclic alkynes that readily and selectively react with azides (Fig. 3).27,28 These click chemistry products form at ambient temperature and pressure with no apparent cytotoxicity. They concluded that the rate of this copper-free reaction of azide with this strained cyclic alkyne is comparable to that of the Cu(I)-catalyzed reaction of azide with terminal alkynes, validating the potential of this strategy.28,29 The potential drawback of this method is the production of a mixture of regioisomeric products that may deleteriously impact the application, unlike the Cu(I)-catalyzed reaction, which produces one regioisomer with high yield.
FIG. 3.
Uncatalyzed Huisgen 1,3-cycloaddition reaction.
Another widely used click chemistry reaction in building macromolecules is Staudinger ligation, which forms phosphazo compounds by reacting tertiary phosphines with organic azides (Fig. 4).15 The first synthesis of phosphazo compounds was reported by Staudinger and Meyer in 1919.14 They reported the imination of tertiary phosphines by reacting trialkyl, triaryl, mixed, unsaturated phosphines with various aliphatic or aromatic azides, cyanogen azide, sulfur-, phosphorus-, and metal-containing organic azides. Following this pioneering work, a significantly large body of research from diverse groups defined this strategy as a synthetic tool for making compounds containing a P = N bond. Note that it is possible to make use of a phosphine group derivatized with an alkyne for Staudinger ligation reactions, and that the reaction is selective toward the phosphine, yielding a P = N bond instead of a cyclic triazole product as with a Huisgen cycloaddition reaction.
FIG. 4.
Staudinger ligation reaction.
Click Chemistry in Drug Discovery
Drug discovery based on natural products can be hampered by slow, complex synthesis. Click chemistry, on the other hand, simplifies and optimizes syntheses, providing faster, efficient reactions. Click chemistry was employed by Zhang et al. to produce the peroxisome proliferator-activated receptor γ (PPAR-γ) agonists for the treatment of type II diabetes (Fig. 5).30 Copper-catalyzed coupling of acetylene derivatives of carbohydrates and azides was used in the preparation of a series of multivalent triazole-linked neoglycoconjugates.31,32 This approach mimics nature's preparation of multivalent carbohydrates to increase the interaction between carbohydrates and receptors or enzymes.
FIG. 5.
Structures of one of the tested compounds for peroxisome proliferator-activated receptor-γ
Lewis et al. extended the Huisgen 1,3-dipolar cycloaddition to prepare an enzyme-bound inhibitor.33 They performed the click reaction in the presence of the enzyme, acetylcholinesterase (AChE), so that the association of the triazole click product with AChE would produce an inhibitor. The enzyme, AChE, is known to be involved, to a high degree, in the hydrolysis of neurotransmitter in the central and peripheral nervous systems.34,35 The results showed that AChE catalyzed the 1,3-dipolar cycloaddition reaction of one of the azide-alkyne combinations tested to form a triazole product. When the active site of the enzyme was blocked, the triazole product was not detected, indicating that AChE was acting as a reaction template.
HIV-1 protease (HIV-1-PR) is well known to be responsible for virus maturation, and that the appearance of mutant proteases demand effective inhibitors.36,37 Brik et al. prepared libraries based on two different azide-bearing hydroxyethylamine and two different acetylene derivatives.38 Both azide-alkyne libraries were tested against wild-type HIV-1-PR and three mutant proteases (G48V, V82F, and V82A). Their data showed that one of the tested azide-alkyne combinations gave >50% inhibition against all four enzymes, with the inhibition of HIV-1-PR at 1 nanometer (nM) concentrations. Recently, Whitey et al. applied in situ click chemistry to identify HIV-1-PR inhibitors.39 Click reactions were performed in the presence and absence of the enzyme (SF-2-Pr) to probe for an enzyme-mediated inhibitor. The results showed that the enzyme accelerates product formation with one specific regioisomer up to 10-fold, when compared to the reaction without the enzyme.
The 1,2,3-triazole click reaction product displays biologic activities, such as anti-HIV activity40 and antimicrobial activity against Gram-positive bacteria.41 Alvarez et al. prepared a series of 1,2,3-triazole derivatives and evaluated their inhibitory activity against HIV-1 and HIV-2 in MT-4 and CEM cell cultures. No inhibition was observed against HIV-2, but one of the compounds, the unsubstituted triazole, displayed an EC50 (effective concentration at 50%) for HIV-1 in both MT-4 and CEM cells at 3.7 and 3.4 μM, respectively. They also observed that substitutions at the triazole ring promoted 5–10-fold more inhibition to HIV-1 than the unsubstituted triazole. Genin et al. synthesized a series of 1,2,3-triazole derivatives and found that most compounds tested had antibacterial activity several times more potent against Gram-positive and negative bacterial isolates than currently used antibiotics, such as linezolid, eperezolid, and vancomycin.41 Natarajan et al. reported the synthesis of a divalent single-chain fragment (di-scFv) of a monoclonal antibody (mAb) by using Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition chemistry.42 Immunosorbant assay (ELISA) and immunohistochemistry (IHC) were used to compare the binding of this di-scFv and single scFv to its antigen and cancer cells, with the di-scFv demonstrating a higher affinity toward antigen and cancer cells than the single scFv.
Click Chemistry in Bioconjugation
Click chemistry has become a burgeoning strategy of bioconjugation in the development of bifunctional molecules. Bioconjugation involves the attachment of synthetic labels to biomolecular building blocks, such as fusing two or more proteins together or linking a carbohydrate with a peptide, and covers a wide range of science between molecular biology and chemistry. Although bioconjugation is applicable to the in vivo labeling of biomolecules, only a handful of reactions are actually useful.2,10 The possibility of applying click chemistry in bioconjugation was first demonstrated by Tornoe et al. for the preparation of peptidotriazoles via solid-state synthesis.18 Their goal was to develop new, more efficient synthetic methods to prepare various [1,2,3]-triazole pharmacophores for potential biologic targets. This initial report makes possible the introduction of various novel functional and reporter groups into biomolecules, such as peptides and proteins,29 for DNA labeling and modification,43–45 and for cell-surface labeling.46
Most bioconjugation reactions, such as isothiocyanateamine, thiol-maleimide, and amine-carboxylic acid couplings,47–50 cannot be used for labeling in vivo because of competing nucleophiles on proteins, nucleic acids, and other biopolymers. Labeling of biomolecules in living systems, using condensation reactions between ketones or aldehydes, and hydrazides or aminoxy derivatives, is not feasible. At the optimum pH of 5–6, such linkages are reversible, and ketones or aldehyde functionalities are present inside of cells.47 Click chemistry overcomes these obstacles by being bioorthogonal and by proceeding irreversibly in water at neutral pH and biocompatible temperatures (25–37°C) without any cytotoxic reagents or byproducts.
Click chemistry continues to attract attention for the labeling of proteins and live organisms. Wang et al. successfully labeled Cowpea mosaic virus (CPMV) particles with fluorescein with >95% yield.4 The labeling was performed by modifying the surface of viral protein (either lysine or cysteine residues) with azides or alkynes, followed by reaction with fluorescein-bearing complementary groups. Similarly, Link and Tirrell were able to modify Eschericia coli with an azide-bearing outer membrane protein C (OmpC). The modified cell was then biotinylated by reacting with a biotinalkyne derivative under copper-catalyzed click chemistry conditions.46 Deiters et al. developed a method to genetically encode proteins of Saccharomyces cerevisiae with azide- or acetylene-based synthetic amino acids (Fig. 6).51 The genetic modification was done by reacting an alkyne- or an azide-bearing protein with the counterpart unnatural amino acid. In the same study, the possibility of inserting organic molecules to proteins by an azide-alkyne [3 + 2] cycloaddition reaction was demonstrated by reacting an azide- or alkyne-bearing proteins with azide- or alkyne-bearing dyes.
FIG. 6.
Structures of synthetic amino acids (top) and dyes (bottom).
Click in Oligonucleotide and Carbohydrate Chemistry
Owing to its biocompatibility, click chemistry has become an efficient strategy for synthesizing oligonucleotides (ONs) for applications such as labeled carbohydrate ONs,43,52 fluorescent ONs,53 and multimodified ONs.45 Covalent attachment of a lipid moiety to an ON was published by Godeaes et al.54 They chose the 17-mer 2′-O-methylribonucleotide antisense (ON17mer) of the hepatitis C virus RNA and reported that the lipid-modified ON enhanced antisense activity, as compared to the nonconjugated parental ON. The conjugates displayed no cytotoxicity in human hepatic Huh7 cells. It is notable that the 1,3-dipolar cycloaddition reaction has become an efficient method to covalently assemble different components of multivalent and supramolecular compounds and has found an application in the preparation of higher order molecular conjugates, such as glycoproteins,55 neoglycoconjugates32 (e.g., glycopolymers, glycoclusters, oligomers, polyamino acids, and glycol dendrimers), proteinoligonucleotides,56 and DNA-peptide conjugates.57
Wang et al. prepared fluorescent-labeled ssDNA by using Staudinger ligation without gel electrophoresis or high-performance liquid chromatography (HPLC) for purification.58 Their goal was to develop a fluorescent-based oligonucleotide to be used as a primer in a Sanger dideoxy chain termination reaction.59 The results showed that the fluorescent-labeled oligonucleotide was an efficient primer with high specificity in a Sanger dideoxy DNA-sequencing reaction to produce DNA-sequencing fragments. Baccaro et al. developed a straightforward syntheses of two azide-modified thymidine analogs and incorporated them into a DNA by using primer extension reactions and polymerase chain reaction (PCR).60 The resulting DNA assembly was ultimately conjugated to biotin by using Staudinger ligation. They reported that a less sterically hindered DNA-azide assembly was superior in conjugation to biotin through Staudinger ligation. This advancement facilitates the investigation of complex biologic systems.
Saxon and Bertozzi demonstrated cell-surface engineering by using a modified Staudinger ligation strategy.61 They modified Jurkat cell surfaces by first metabolically incorporating an azide derivative (azidoacetylmannosamine) into cells. Thereafter, the modified cells were allowed to react with a phosphine derivative (a biotinylated phosphine). A side-by-side comparison with a conventional method, cell-surface engineering with a ketone derivative,62 demonstrated that the azidosugar metabolism route produced a 2-fold higher desired product. This is due to the abiotic nature of the azide, whereas ketones are known to have cross-reactions with biomoelcules. Prescher et al. have remodeled cell surfaces in mice by using a Staudinger ligation reaction.63 Mice were injected with an azide carbohydrate derivative (Man-NAz) and then with a phosphine-derived peptide conjugate while the reaction progress was monitored by flow cytometry.
The triazole moiety is stable to metabolic degradation and capable of hydrogen bonding, which may be helpful for target binding or solubility. In a quest for a better contrast agent for magnetic resonance imaging (MRI), Bryson et al. successfully synthesized a novel β-cyclodextrin coated with seven paramagnetic Gd-DTTA chelates, with each gadolinium ion potentially having two water-exchangeable sites, using click chemistry.64 The ligand was synthesized by reacting an alkyne-functionalized diethylenetriaminetetraacetic acetic acid (DTTA) with an azide-bearing β-cyclodextrin. This provided a macromolecular agent with a molar relaxivity nearly twice that of the clinically approved contrast agent, Magnevist® (6.2mM−1s−1 vs. 3.2mM−1s−1; Bayer, Waye, NJ). This easy-to-make β-cyclodextrin assembly can be further functionalized with a receptor-binding tag for biomolecular imaging. Its high stability along with enhanced relaxivity might improve the diagnosis, understanding, and treatment of diseases. Laughlin et al. were able to image glycans in developing zebrafish by the use of click chemistry.65 In this study, embryonic zebrafish were incubated with an azide-peracetylated N-azidoacetylgalactosamine derivative (Ac4-GalNAz), which was then reacted with a difluorinated cyclooctyne attached to a dye (Fig. 7). The images obtained by flow cytometry showed neither background interference nor cytotoxicity.
FIG. 7.
Schematic depiction of metabolic labeling.
Click in Peptide Chemistry
Recently, Gauthier and Klok published an article compiling the existing strategies, including Huisgen cycloaddition and Staudinger ligation, for the preparation of peptide/protein-polymer conjugates.47 The click reaction has proven to be very useful for modifying functional biomolecules because of its high chemoselectivity. Biologic oligomers and polymers, such as peptides, nucleic acids, and carbohydrates, have been modified by using the copper-catalyzed azide-alkyne cycloaddition click reaction. Functionalization of an oligopeptide was demonstrated by Tornoe et al. in early 2002.18 They performed solid-phase syntheses of peptide-peptide conjugates by reacting a peptide-containing alkyne group with its counterpart peptide-containing azide group to yield peptidotriazoles (>95%). Similarly, Gogoi et al. developed a versatile method where peptide-linked terminal alkynes were allowed to react with nucleic acids containing an azide functionality and vice versa in both solid and solution (i.e., water) phases to obtain peptide-oligonucleotide conjugates.57 This method proved to be far more efficient than the normally used (4 + 2) Diels-Alder cycloaddition, minimizing cross-reactions between the dienophile and other nucleophilic centers on the peptides.66,67
The copper-catalyzed click reaction has been used to modify proteins with high selectivity under physiologic conditions. Deiters et al. demonstrated this novel approach by adding amino acids to the genetic code of S. cerevisiae.68 The method involved incorporation of either azide- or alkyne-containing amino acids genetically inserted into proteins in response to the amber nonsense codon (TAG). The cycloaddition reaction was then performed with their counterpart alkyne or azide to study and manipulate cellular processes in eukaryotic cells.
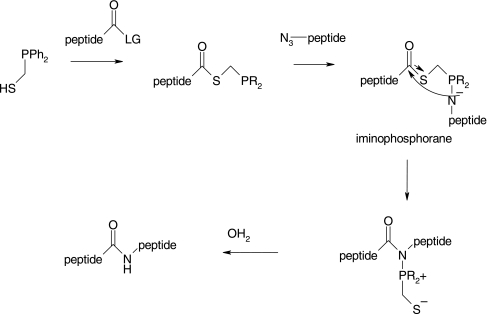
Staudinger ligation is also useful for the synthesis of large biomolecules. Most peptide syntheses require a terminal Cys residue at the active site and are hampered by low yields and inconvenient work-ups.69 Nilsson et al. traversed those obatacles by developing a high yield Staudinger ligation method that does not require a Cys residue.70–72 As shown in Figure 8, an amide bond was formed from a peptide-linked phosphinothiol at the C-terminus and a peptide-linked azide at the N-terminus. The end-product, an amidophosphonium salt, formed after the intramolecular rearrangement of the intermediate iminophosphorane with > 90% yield.
FIG. 8.
Staudinger ligation reaction for peptide synthesis.
Click in Radiochemistry
The use of click chemistry is receiving more interest in the field of radiopharmacy, and there have been several reports recently based on the click-to-chelate approach for radiopharmaceutical applications.73–76 Since noninvasive nuclear-imaging techniques, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), with high sensitivity have become available, radiolabeling of biologically active molecules has become an important tool to assess novel drug candidates. Nonmetallic positron-emitting isotopes, such as 18F and 11C, possessing short half-lives (t1/2 = 109.8 minutes t1/2 = 20.5 minutes), have to be produced on-site or within range of a production facility. Another conflict for labeling biomolecules of higher molecular weight with high specificity is the requirement of harsh reaction conditions. To overcome these, a bifunctional approach is usually applied, wherein the 18F or 11C is linked to, or incorporated into, a small molecule that is subsequently capable of being attached to biomolecules, such as proteins and peptides, under mild conditions.
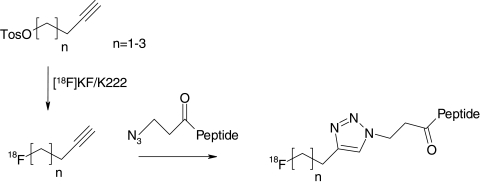
The increase in availability of methods to prepare targeting peptides labeled with 18F currently makes PET more widely accessible for imaging various physiologic and pathologic processes in vivo. The synthesis of 18F-labeled peptides through click chemistry, in which 18F-fluoroalkynes were conjugated to various azide-functionalized peptides, was first reported by Marik and Sutcliffe, with good radiochemical yields being achieved within 10 minutes.77 Their synthetic method to prepare functionalized peptides attached to 18F-fluoroalkynes (e.g., butyne, pentyne, and hexyne) achieved high radiochemical purities (81%–99%).77 The 18F-fluoroalkynes were prepared from the reaction of the corresponding tosylalkynes with [18F]KF/K222 complex and purified by codistillation with acetonitrile in 10 minutes, and the labeling was achieved by reacting peptides bearing N-(3-azidopropionyl)-groups with ω-[18F]fluoroalkynes (Fig. 9). Although the reaction yields were not optimal, the short reaction times and mild reaction conditions were more than sufficient to compensate for loss in radiochemical yield. Glaser and Arstad also published a similar study using an 18F-azide agent instead of an 18F-alkyne.78 Their reported yields were higher, but HPLC was required for purification and their reaction times were longer. One can assume the two methods are equally applicable to prepare 18F-labeled peptides for PET imaging. Both groups still need to prove the applicability of their methods, in vitro and in vivo, for PET imaging.
FIG. 9.
Method to label peptides by using 1,3-dipolar cycloaddition.
Hausner et al. demonstrated the applicability of click chemistry for PET imaging in vivo in mice.79 They modified a peptide, A20FMDV2, that selectively binds to integrin αvβ6 by attaching a 3-azidopropionyl group to the N-terminus. The peptide was then reacted with three 18F-derivatives with similar overall radiochemical yields. Differences in tumor uptake, rates of clearance, and biodistribution data for each prosthetic group highlight the influence of chemical structures on their metabolic fate, which, in turn, suggests the necessity for close, careful study of each individual compound. The study highlights the exciting possibilities and challenges, and obstacles, that need to be resolved in future studies.
A series of 18F-labeled peptides for PET imaging of integrin αvβ3 in vivo was also developed and provided high sensitivity with good spatial/temporal resolution.80 Ross et al. demonstrated new efficient synthetic strategies, based on click chemistry, to prepare PET imaging probes.81 They prepared an azido folate derivative as a precursor to develop a folate PET (18F) tracer. The in vitro binding studies demonstrated that the synthetic probe binds to the folate receptor that is overexpressed on most epithelial cancer cells with nanomolar affinity. The 18F-labeled click folate product was successfully used for in vivo PET imaging of nude mice bearing tumor xenografts. Von Maltzahn et al., through click chemistry, successfully linked the cyclic peptide, LyP-1, bearing an alkyne group to polymer-coated magneto-fluorescent nanoparticles bearing azide groups to target an overexpressed mitochondrial protein, p32, localized at the surface of tumor cells.82 The click nanoparticles were highly stable and also able to penetrate the tumor interstitium to specifically bind to target receptors. This provides motivation for the future application of click chemistry in nanotechnology aspects of nuclear medicine imaging and, ultimately, therapy and drug-delivery applications.
Click chemistry has not been as widely applied to the synthesis of 11C-labeled compounds for PET imaging, no doubt, due to the very short half-life (20 minutes) of 11C. The short half-life demands rapid, efficient methods for the introduction of 11C into biomolecules, because the specific activity (in MBq/μmol) is a function of time. The feasibility to apply click chemistry for the preparation of 11C-labeled compounds was explored by Schirrmacher et al.83 They reported a method to prepare a 11C-labeled compound within 5–10 minutes under nontoxic aqueous conditions with the radiochemical yield of 60% at room temperature.
Knor et al. prepared a full octadentate bifunctional DOTA ligand functionalized with an alkyne group.24 The ligand was then used in a Cu(I)-catalyzed click reaction to form an octreotate peptide conjugate without the need for protecting any functional groups in either reactant. This resulted in the formation of, what some have considered, a liability to this conjugation chemistry wherein the chelation chemistry becomes compromised by the conjugation chemistry (i.e., filling the DOTA with nonradioactive copper). However, these researchers were able to expel the copper from the DOTA with H2S, thereby demonstrating that those concerns could, in fact, be successfully obviated.
Generally, there are two reasons why SPECT can be preferable to PET imaging, especially with respect to the use of 18F and 11C agents. The primary reason is the greater accessibility and availability of radionuclides for in vivo SPECT (e.g., 99mTc, 111In) and, in general, their longer half-lives, ranging from several hours to several days, making the handling and processing far more convenient. Second, small-animal SPECT scanners with submillimeter spatial resolution, that can outperform other PET devices, are now available. Preparation of the metal chelators for radiolabeling of biomolecules with 99mTc (t1/2 = 6 hours, 140 keV γ-radiation) have been performed via multistep syntheses that involved extensive purification and isolation protocols. Incorporation of those metal chelates into biomolecules generally lacks efficiency and is often complicated by the reactivity of the metal chelators with other functional groups.82,83 Protective groups are often required to prevent unintended side reactions. There is an abundance of literature reporting strategies for developing bifunctional chelating agents, most of which are cumbersome and result in low product yields. However, with click chemistry, this type of metal complex can be prepared via a “one-pot” prelabeling synthetic procedure that avoids the isolation of the chelator prior to the incorporation of the metal. This method is applicable because none of the starting reagents alone (azide or alkyne) form a stable complex with the metal ion. Compared to the conventional methods from the literature,84–86 a one-pot click method is superior for the preparation of 99mTc chelate conjugates in terms of product yield, stereospecificity, and reaction time, as demonstrated by Mindt et al. and Struthers et al.73,76
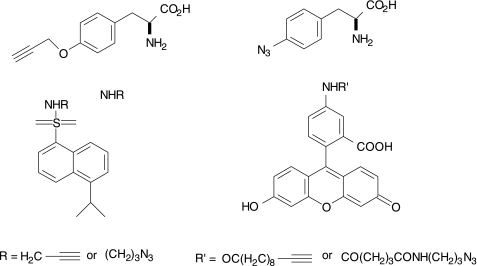
The classic Cu(I)-catalyzed click chemistry has found an application in the design of bifunctional chelating agents.19 As noted previously, the reaction is thermodynamically favored (∼11 kcal mol−1) and regiospecifically resulted in the formation of 1,4-bifunctionalyzed 1,2,3-triazole products with quantitative yield under mild conditions and in aqueous media.19,87 The 1,2,3-triazole ring itself is an efficient ligand for various transition metals, coordinating the metal via the N3 nitrogen of the 1,4-triazole ring (Figs. 10 and 11), and forms highly stable compounds in vivo.73 Mindt et al. have synthesized tridentate bifunctional chelating agents, products of click reactions, that are potent metal chelators and form stable complexes in vitro and in vivo for SPECT (Fig. 10).88 Their in vitro study with folate receptor-positive KB tumor cells showed that there was 50% cell uptake of the two radiotracers tested, in which one third was internalized. Biodistribution experiments revealed that both complexes displayed a fast clearance from the blood and there was specific tumor and kidney uptake.
FIG. 10.
The two bifunctional chelates containing folic-acid derivatives and 1,2,3-triazole.
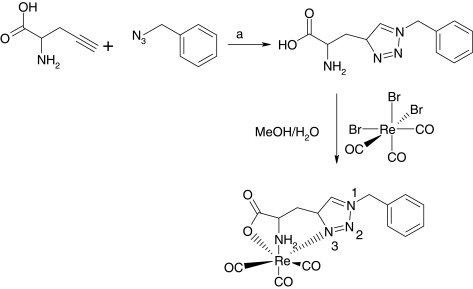
FIG. 11.
Synthetic scheme of a Re(CO)3 complex. a) 0.1 equiv. Cu(OAc)2, 0.1 equiv. sodium ascorbate, tBuOH/H2O, 12 h, room temperature.
Struthers et al. also reported applications of functionalized bifunctional chelating agents from a combination of azide and alkyne building blocks with different substituents (Fig. 11).76 They successfully synthesized and radiolabeled a series of transition metal complexes of thymidine derivatives, which differed in size, overall charge, and hydrophobicity, employing the click chemistry approach. The activity of each individual complex toward human cytosolic thymidine kinase (hTH1) was assayed, reaching a conclusion that overall charge was the key contributing factor to their activity.
Disadvantages of Click Chemistry
The Huisgen 1,3-dipolar cycloaddition of azides and acetylenes is not without limitations and a few of them will be discussed here. One obvious disadvantage is alkyne homocoupling. Alkynes can react with another alkyne instead of the azide.18 For the click reaction to occur efficiently, both the alkyne and the azide should be at the terminal position of an alkyl chain. Tornoe et al. found that a sterically hindered azide failed to react even at elevated temperatures and with extended reaction times.18 Some click chemistry reactions require metal, such as copper, as a catalyst, which can be incompatible in vivo. Excess copper is known to cause physiologic side-effects, such as hepatitis and neurologic and renal diseases. Another concern is the stability of some azide derivatives. Some heavy metal azides or methyl azide are known to be explosive, but this property certainly should not be a major issue at the small-scale pharmaceutical research level and scope. One of the drawbacks for the Staudinger ligation reaction is air oxidation of phosphine reagents that can be problematic and affect reaction kinetics.28 Even though click chemistry is easy to perform with high yield in most cases and widely accepted in diverse research areas, attaining commercial availability of the general reagents and building units required remains to be achieved.
Conclusions
Regardless of negative aspects, click chemistry obviously will be more influential in the design of future drugs owing to its simplicity. One of the unique, important properties of click chemistry is its being bio-orthogonal. Covalent and rapid linkage of two dissimilar components together under friendly, nontoxic conditions are properties that are completely complementary to the creation of novel agents for the development of both imaging and therapeutic products. Of course, there are areas in click chemistry that require optimization, such as real time usage in live animals, imaging of live cells, and, ultimately, the substitution of the azide group with other less potentially hazardous species. Although these may present as significant challenges, considering the pace and applications that click chemistry facilitates for the creation of novel biomolecules for preclinical evaluation, one can be confident that these requirements will be met in the foreseeable future.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors would also like to thank Kwamena Baidoo for his assistance in the preparation and revision of the manuscript for this article.
Disclosure Statement
No competing financial conflicts exist.
About the Authors

Kido Nwe graduated at the University of Buffalo, New York, in 2008 under the supervision of Professor Janet R. Morrow. His Ph.D research was dedicated to the preparations of lanthanide catalysts for RNA cleavage and lanthanide complexes evaluated as PARACEST agents. Currently, he is a post doctoral fellow at the National Cancer Institute in the Radioimmune & Inorganic Chemistry Section under the supervision of Dr. Martin W. Brechbiel. Dr. Nwe's present research interests include preparation of macromolecular contrast agents for magnetic resonance imaging and novel bifunctional chelating agents for radio-nuclides that are useful for imaging or therapy.

Martin W. Brechbiel received his B.A. in 1979 from Gettysburg College, Gettysburg, PA, and an M.S. in 1982 from the University of Delaware, Newark, DE, under the guidance of Professor Harold Kwart. After working for FMC Corporation, he joined the National Cancer Institute (NCI) in 1984. Thereafter, he worked to develop novel bifunctional chelating agents for sequestering radionuclides and their conjugation to immunoproteins under the direction of Dr. Otto A. Gansow, while simultaneously obtaining a Ph.D from American University, Washington, DC, in 1988 with Professor Thomas Cantrell. He remained with the NCI, and, in 1997, was appointed acting section chief of the Radioimmune & Inorganic Chemistry Section and was tenured at the Section Chief in 2001. His research group's activities include continuing development of novel chelating agents for radionuclides, the development of contrast media for magnetic resonance imaging, electron paramagnetic resonance, and computed tomography imaging, and the medicinal chemistry of novel metal complexes.
References
- 1.Bemis GW. Murcko MA. The properties of known drugs. Molecular frameworks. J Med Chem. 1996;39:2887. doi: 10.1021/jm9602928. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HC. Finn MG. Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtsev VV. Green LG. Fokin VV, et al. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q. Chan TR. Hilgraf R, et al. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 5.Manetsch R. Krasinski A. Radic Z, et al. In situ click chemistry: Enzyme inhibitors made to their own specifications. J Am Chem Soc. 2004;126:12809. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- 6.Helms B. Mynar JL. Hawker CJ, et al. Dendronized linear polymers via “Click chemistry.”. J Am Chem Soc. 2004;126:15020. doi: 10.1021/ja044744e. [DOI] [PubMed] [Google Scholar]
- 7.Karim MA. Cho Y-R. Park JS, et al. Comparison of three different click reaction methods for the synthesis of fluorene-based polymers and performance in quasi-solid-state DSSCs. Macromol Chem Phys. 2008;209:1967. [Google Scholar]
- 8.Schirrmacher R. Wangler C. Schirrmacher E. Recent developments and trends in 18F-radiochemistry: Syntheses and applications. Mini-Rev Org Chem. 2007;4:317. [Google Scholar]
- 9.Rolf H. 1,3-dipolar cycloadditions. Past and Future. Angew Chem Int Ed. 1963;2:565. [Google Scholar]
- 10.Kolb HC. Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 11.Karl Anker J. Catalytic asymmetric hetero-diels-alder reactions of carbonyl compounds and imines. Angew Chem. 2000;39:3558. doi: 10.1002/1521-3773(20001016)39:20<3558::aid-anie3558>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Adolfsson H. Converso A. Sharpless KB. Comparison of amine additives most effective in the new methyltrioxorhenium-catalyzed epoxidation process. Tetrahedron Lett. 1999;40:3991. [Google Scholar]
- 13.Kolb HC. Van Nieuwenhze MS. Sharpless KB. Catalytic asymmetric dihydroxylation. Chem Rev. 1994;94:2483. [Google Scholar]
- 14.Staudinger H. Meyer J. New organic compounds of phosphorus: III. Phosphinemethylene derivatives and phosphinimines [in German] Helv Chim Acta. 1919;2:635. [Google Scholar]
- 15.Gololobov YG. Zhmurova IN. Kasukhin LF. Sixty years of Staudinger reaction. Tetrahedron. 1981;37:437. [Google Scholar]
- 16.Huisgen R. Kinetic and reaction mechanisms: Selected examples from the experience of 40 years. Pure Appl Chem. 1989;61:618. [Google Scholar]
- 17.Prescher JA. Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 18.Tornoe CW. Christensen C. Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 19.Bock VB. Hiemstra H. van Maarseveen JH. Cu-catalyzed alkyne-azide click cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem. 2006;2006:51. [Google Scholar]
- 20.Hein C. Liu X-M. Wang D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res. 2008;25:2216. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegfried S. Reactivity of copper(I) complexes towards dioxygen. Eur J Inorg Chem. 2000;2000:2311. [Google Scholar]
- 22.Chassaing S. Sido ASS. Alix A, et al. “Click Chemistry” in zeolites: Copper(I) zeolites as new heterogeneous and ligand-free catalysts for the Huisgen [3 + 2] cycloaddition. Chem Eur J. 2008;14:6713. doi: 10.1002/chem.200800479. [DOI] [PubMed] [Google Scholar]
- 23.Cabrera DG. Koivisto BD. Leigh DA. A metal-complex-tolerant CuAAC ‘click’ protocol exemplified through the preparation of homo- and mixed-metal-coordinated [2]rotaxanes. Chem Commun. 2007 doi: 10.1039/b713501g. [DOI] [PubMed] [Google Scholar]
- 24.Knor S. Modlinger A. Poethko T, et al. Synthesis of novel 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) derivatives for chemoselective attachment to unprotected polyfunctionalized compounds. Chem Eur J. 2007;13:6082. doi: 10.1002/chem.200700231. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet D. Ilien B. Galzi JL, et al. A rapid and versatile method to label receptor ligands using “click” chemistry: Validation with the muscarinic M1 antagonist pirenzepine. Bioconjugate Chem. 2006;17:1618. doi: 10.1021/bc060140j. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg RR. Kingston HM. Trace element analysis of natural water samples by neutron activation analysis with chelating resin. Analy Chem. 1983;55:1160. [Google Scholar]
- 27.Codelli JA. Baskin JM. Agard NJ, et al. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J Am Chem Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baskin JM. Prescher JA. Laughlin ST, et al. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskin JM. Bertozzi CR. Bioorthogonal click chemistry: Covalent labeling inliving systems. QSAR Comb Sci. 2007;26:1211. [Google Scholar]
- 30.Zhang H. Ryono DE. Devasthale P, et al. Design, synthesis, and structure-activity relationships of azole acids as novel, potent dual PPAR [alpha]/[gamma] agonists. Bioorg Med Chem Lett. 2009;19:1451. doi: 10.1016/j.bmcl.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Calvo-Flores FG. Isac-Garcia J. Hernandez-Mateo F, et al. 1,3-dipolar cycloadditions as a tool for the preparation of multivalent structures. Org Lett. 2000;2:2499. doi: 10.1021/ol006175v. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Balderas F. Ortega-Munoz M. Morales-Sanfrutos J, et al. Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org Lett. 2003;5:1951. doi: 10.1021/ol034534r. [DOI] [PubMed] [Google Scholar]
- 33.Lewis WG. Green LG. Grynszpan R, et al. Click chemistry in situ: Acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew Chem Int Ed. 2002;41:1053. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Taylor P. Radic Z. The cholinesterases: From genes to proteins. Ann Rev Pharmacol Toxicol. 1994;34:281. doi: 10.1146/annurev.pa.34.040194.001433. [DOI] [PubMed] [Google Scholar]
- 35.Quinn DM. Acetylcholinesterase: Enzyme structure, reaction dynamics, and virtual transition states. Chem Rev. 1987;87:955. [Google Scholar]
- 36.Kohl NE. Emini EA. Schleif WA, et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988;85:4686. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Condra JH. Schleif WA. Blahy OM, et al. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995;374:569. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 38.Brik A. Muldoon J. Lin Y-C, et al. Rapid diversity-oriented synthesis in microtiter plates for in situ screening of HIV protease inhibitors. ChemBioChem. 2003;4:1246. doi: 10.1002/cbic.200300724. [DOI] [PubMed] [Google Scholar]
- 39.Whiting M. Muldoon J. Lin Y-C, et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew Chem Int Ed. 2006;45:1435. doi: 10.1002/anie.200502161. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez R. Velazquez S. San-Felix A, et al. A, Balzarini J, Camarasa MJ. 1,2,3-triazole-[2,5-bis-O-(tert-butyldimethylsilyl)-.beta.-D-ribofuranosyl]-3′-spiro-5″-(4″-amino-1″,2″-oxathiole 2″, 2″-dioxide) (TSAO) analogs: synthesis and anti-HIV-1 activity. J Med Chem. 1994;37:4185. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 41.Genin MJ. Allwine DA. Anderson DJ, et al. Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms haemophilus influenzae and moraxella catarrhalis. J Med Chem. 2000;43:953. doi: 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan A. Du W. Xiong C-Y, et al. Construction of di-scFv through a trivalent alkyne-azide 1,3-dipolar cycloaddition. Chem Commun. 2007:695. doi: 10.1039/b611636a. [DOI] [PubMed] [Google Scholar]
- 43.Chevolot Y. Bouillon C. Vidal S, et al. DNA-based carbohydrate biochips: A platform for surface glyco-engineering13. Angew Chem Int Ed. 2007;46:2398. doi: 10.1002/anie.200604955. [DOI] [PubMed] [Google Scholar]
- 44.Weisbrod SH. Marx A. Novel strategies for the site-specific covalent labelling of nucleic acids. Chem Commun. 2008:5675. doi: 10.1039/b809528k. [DOI] [PubMed] [Google Scholar]
- 45.Gramlich PME. Warncke S. Gierlich J, et al. Click-click-click: Single to triple modification of DNA. Angew Chem Int Ed. 2008;47:3442. doi: 10.1002/anie.200705664. [DOI] [PubMed] [Google Scholar]
- 46.Link AJ. Tirrell DA. Cell surface labeling of escherichia coli via copper(I)-catalyzed [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:11164. doi: 10.1021/ja036765z. [DOI] [PubMed] [Google Scholar]
- 47.Gauthier MA. Klok H-A. Peptide/protein-polymer conjugates: Synthetic strategies and design concepts. Chem Commun. 2008:2591. doi: 10.1039/b719689j. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T. Kamiya N. Nagamune T. N-terminal glycine-specific protein conjugation catalyzed by microbial transglutaminase. FEBS Lett. 2005;579:2092. doi: 10.1016/j.febslet.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 49.Joshi NS. Whitaker LR. Francis MB. A three-component Mannich-type reaction for selective tyrosine bioconjugation. J Am Chem Soc. 2004;126:15942. doi: 10.1021/ja0439017. [DOI] [PubMed] [Google Scholar]
- 50.Antos JM. Francis MB. Transition metal catalyzed methods for site-selective protein modification. Curr Opin Chem Biol. 2006;10:253. doi: 10.1016/j.cbpa.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Deiters A. Cropp TA. Mukherji M, et al. Adding amino acids with novel reactivity to the genetic code of Saccharomyces cerevisiae. J Am Chem Soc. 2003;125:11782. doi: 10.1021/ja0370037. [DOI] [PubMed] [Google Scholar]
- 52.Bouillon C. Meyer A. Vidal S, et al. Microwave-assisted “click” chemistry for the cynthesis of multiple labeledcarbohydrate oligonucleotides on solid support. J Org Chem. 2006;71:4700. doi: 10.1021/jo060572n. [DOI] [PubMed] [Google Scholar]
- 53.Seo TS. Li Z. Ruparel H, et al. Click chemistry to construct fluorescent oligonucleotides for DNA sequencing. J Org Chem. 2003;68:609. doi: 10.1021/jo026615r. [DOI] [PubMed] [Google Scholar]
- 54.Godeau G. Staedel C. Barthe le my P. Lipid-conjugated oligonucleotides via “click chemistry” efficiently inhibit hepatitis C virus translation. J Med Chem. 2008;51:4374. doi: 10.1021/jm800518u. [DOI] [PubMed] [Google Scholar]
- 55.Hang HC. Bertozzi CR. Chemoselective approaches to glycoprotein assembly. Acc Chem Res. 2001;34:727. doi: 10.1021/ar9901570. [DOI] [PubMed] [Google Scholar]
- 56.Humenik M. Huang Y. Wang Y, et al. C-terminal incorporation of bio-orthogonal azide groups into a protein and preparation of protein-oligodeoxynucleotide conjugates by Cu(I)-catalyzed cycloaddition. ChemBioChem. 2007;8:1103. doi: 10.1002/cbic.200700070. [DOI] [PubMed] [Google Scholar]
- 57.Gogoi K. Mane MV. Kunte SS, et al. A versatile method for the preparation of conjugates of peptides with DNA/PNA/analog by employing chemoselective click reaction in water. Nucl Acids Res. 2007;35:e139. doi: 10.1093/nar/gkm935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang CCY. Seo TS. Li Z, et al. Site-specific fluorescent labeling of DNA using Staudinger ligation. Bioconj Chem. 2003;14:697. doi: 10.1021/bc0256392. [DOI] [PubMed] [Google Scholar]
- 59.Sanger F. Nicklen S. Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baccaro A. Weisbrod SH. Marx A. DNA conjugation by Staudinger ligation: New thymidine analogues. Synthesis. 2007;2007:1949. doi: 10.1093/nass/nrn195. [DOI] [PubMed] [Google Scholar]
- 61.Saxon E. Bertozzi CR. Cell-surface engineering by a modified Staudinger reaction. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 62.Mahal LK. Yarema KJ. Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 63.Prescher JA. Dube DH. Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 64.Bryson JM. Chu W-J. Lee J-H, et al. A β-cyclodextrin “click cluster” decorated with seven paramagnetic chelates containing two water-exchange sites. Bioconj Chem. 2008;19 doi: 10.1021/bc800200q. [DOI] [PubMed] [Google Scholar]
- 65.Laughlin ST. Baskin JM. Amacher SL, et al. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchan V. Ortega S. Pulido D, et al. Diels-Alder cycloadditions in water for the straightforward preparation of peptide-oligonucleotide conjugates. Nucl Acids Res. 2006;34:e24. doi: 10.1093/nar/gnj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkatesan N. Kim BH. Peptide conjugates of oligonucleotides: Synthesis and applications. Chem Rev. 2006;106:3712. doi: 10.1021/cr0502448. [DOI] [PubMed] [Google Scholar]
- 68.Deiters A. Cropp TA. Mukherji M, et al. Adding amino acids with novel reactivity to the genetic code of Saccharomyces cerevisiae. J Am Chem Soc. 2003;125:11782. doi: 10.1021/ja0370037. [DOI] [PubMed] [Google Scholar]
- 69.Dawson PE. Muir TW. Clark-Lewis I, et al. Synthesis of proteins by native chemical ligation. Science. 1994;266:776. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson BL. Kiessling LL. Raines RT. High-yielding Staudinger ligation of a phosphinothioester and azide to form a peptide. Org Lett. 2001;3:9. doi: 10.1021/ol006739v. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson BL. Kiessling LL. Raines RT. Staudinger ligation: A peptide from a thioester and azide. Org Lett. 2000;2:1939. doi: 10.1021/ol0060174. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson BL. Hondal RJ. Soellner MB, et al. Protein assembly by orthogonal chemical ligation methods. J Am Chem Soc. 2003;125:5268. doi: 10.1021/ja029752e. [DOI] [PubMed] [Google Scholar]
- 73.Mindt TL. Struthers H. Brans L, et al. “Click to chelate”: Synthesis and installation of metal chelates into biomolecules in a single step. J Am Chem Soc. 2006;128:15096. doi: 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- 74.Mindt TL. Schibli R. Cu(I)-catalyzed intramolecular cyclization of alkynoic acids in aqueous media: A “click side reaction.”. J Org Chem. 2007;72:10247. doi: 10.1021/jo702030e. [DOI] [PubMed] [Google Scholar]
- 75.Mindt TL. Ross T. Schibli R. Session 6: Click labelling methods. J Label Compd Radiopharm. 2007;50:S34. [Google Scholar]
- 76.Struthers H. Spingler B. Mindt TL, et al. ‘click-to-chelate’: Design and incorporation of triazole-containing metal-chelating systems into biomolecules of diagnostic and therapeutic interest. Chem Eur J. 2008;14:6173. doi: 10.1002/chem.200702024. [DOI] [PubMed] [Google Scholar]
- 77.Marik J. Sutcliffe JL. Click for PET: Rapid preparation of [18F]fluoropeptides using CuI-catalyzed 1,3-dipolar cyclo-addition. Tetrahedron Lett. 2006;47:6681. [Google Scholar]
- 78.Glaser M. Arstad E. “Click Labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjugate Chem. 2007;18:989. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 79.Hausner SH. Marik J. Gagnon MKJ, et al. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-“click” chemistry: Evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem. 2008;51:5901. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Z-B. Wu Z. Chen K, et al. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin expression. Bioconj Chem. 2007;18:1987. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ross TL. Honer M. Lam PYH, et al. Fluorine-18 click radiosynthesis and preclinical evaluation of a new 18F-labeled folic acid derivative. Bioconj Chem. 2008;19:2462. doi: 10.1021/bc800356r. [DOI] [PubMed] [Google Scholar]
- 82.von Maltzahn G. Ren Y. Park J-H, et al. In vivo tumor cell targeting with “click” nanoparticles. Bioconj Chem. 2008;19:1570. doi: 10.1021/bc800077y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schirrmacher R. Lakhrissi Y. Jolly D, et al. Rapid in situ synthesis of [11C]methyl azide and its application in 11C click-chemistry. Tetrahedron Lett. 2008;49:4824. [Google Scholar]
- 84.Levadala MK. Banerjee SR. Maresca KP, et al. Direct reductive alkylation of amino acids: Synthesis of bifunctional chelates for nuclear imaging. Synthesis. 2004;2004:1759. [Google Scholar]
- 85.van Staveren DR. Benny PD. Waibel R, et al. S-functionalized cysteine: Powerful ligands for the labelling of bioactive molecules with triaquatricarbonyltechnetium-99m(1+) ([99mTc(OH2)3(CO)3]+) Helv Chim Acta. 2005;88:447. [Google Scholar]
- 86.Stephenson KA. Zubieta J. Banerjee SR, et al. A new strategy for the preparation of peptide-targeted radiopharmaceuticals based on an FMOC-lysine-derived single amino acid chelate (SAAC). Automated solid-phase synthesis, NMR characterization, and in vitro screening of fMLF(SAAC)G and fMLF[(SAAC-Re(CO)3)+]G. Bioconj Chem. 2004;15:128. doi: 10.1021/bc034128s. [DOI] [PubMed] [Google Scholar]
- 87.Bock VD. Perciaccante R. Jansen TP, et al. Click chemistry as a coute to cyclic tetrapeptide analogues: Synthesis of cyclo-[Pro-Val-(triazole)-Pro-Tyr] Org Lett. 2006;8:919. doi: 10.1021/ol053095o. [DOI] [PubMed] [Google Scholar]
- 88.Mindt TL. Muller C. Melis M, et al. “Click-to-chelate”: In vitro and in vivo comparison of a 99mTc(CO)3-labeled N(τ)-histidine folate derivative with its isostructural, clicked 1,2,3-triazole analogue. Bioconj Chem. 2008;19:1689. doi: 10.1021/bc800183r. [DOI] [PubMed] [Google Scholar]