Type 2 diabetes is the most common form of diabetes in humans and results from a combination of genetic and acquired factors that impair β-cell function and tissue insulin sensitivity (1,2). However, there is growing evidence that β-cell dysfunction is crucial for the development and progression of this form of diabetes (3,4). Reduced β-cell functional mass in diabetes, and other categories of glucose intolerance, has been described by several authors, and decreased islet and/or β-cell volume in the pancreas of type 2 diabetic patients has been consistently reported. In addition, studies conducted in patients, and isolated islets, have shown both quantitative and qualitative defects of glucose-stimulated insulin secretion. Predictably, therefore, much interest is focused on the possibility of preserving the β-cell to prevent the onset of diabetes, or impede the progressive deterioration of glycemic control, observed after diagnosis and developing over the years. In this brief overview, several major features of β-cell dysfunction in different conditions (from normal glucose tolerance [NGT] to overt diabetes) will be described; thereafter, the possibility/feasibility of maintaining or restoring β-cell functional mass in type 2 diabetes, to prevent deterioration of glucose control, will be discussed.
β-CELL DYSFUNCTION AND TYPE 2 DIABETES: IN VIVO STUDIES
Several cross-sectional and prospective studies showed that β-cell dysfunction plays a major role in determining the onset and progression of type 2 diabetes. When insulin response to an oral glucose tolerance test and insulin sensitivity during euglycemic insulin clamp were measured in 388 individuals (138 with NGT; 49 with impaired glucose tolerance [IGT], and 201 with type 2 diabetes), a progressive decline of β-cell function (with insulin release corrected for glycemic stimulus and degree of insulin resistance) was observed; this decline commenced in normal glucose tolerant subjects (5). Furthermore, in 188 subjects, spanning the range from NGT to IGT or overt diabetes, it was found that the sensitivity of β-cells to glucose decreased already within the range of NGT in association with rising 2-h glucose levels during an oral glucose tolerance test (6). Moreover, dynamic parameters of β-cell function were independent determinants of prevailing plasma glucose concentrations throughout the glucose tolerance interval (6).
More stringent results have derived from longitudinal studies. Insulin action and insulin secretion were measured in 17 Pima Indians, in whom glucose tolerance deteriorated from NGT to IGT, or diabetes, over 5.1 ± 1.4 years (7). Transition from NGT to IGT was associated with a decline in insulin-stimulated glucose disposal and a more marked decrease of the acute insulin secretory response to intravenous glucose. Progression from IGT to diabetes was accompanied by further reductions in insulin sensitivity and acute insulin response. Nevertheless, 31 subjects who retained NGT over a similar period also showed reduced insulin-stimulated glucose disposal, but their acute insulin response increased sufficiently to maintain normoglycemia (7). Changes in β-cell function and insulin sensitivity were evaluated in Caucasian and African American individuals with NGT, IGT, or type 2 diabetes, over 5.2 years of follow-up (8). At baseline, decreasing levels of both β-cell function (acute insulin response) and insulin sensitivity (obtained from a frequently sampled intravenous glucose tolerance test) mirrored deteriorating glucose tolerance condition at baseline and at follow-up. Over time, insulin sensitivity declined in each glucose tolerance category. However, subjects who maintained NGT exhibited a compensatory increase in insulin secretion, whereas failure to augment insulin release led to IGT, or overt diabetes (8).
Secondary failure of plasma glucose control after initial successful response to diet therapy in 432 newly diagnosed type 2 diabetic patients was evaluated in the Belfast Diet Study (9). Secondary failure to diet therapy occurred in 41 patients in years 2–4, in 67 patients in years 5–7, and in 51 patients in years 8–10; 173 patients remained on diet alone until death or the end of the study (10 years). Loss of efficacy of diet alone was associated with greater β-cell failure, and the ongoing decline in β-cell function (assessed by homeostasis model assessment [HOMA]-β) closely mirrored the steady rise in fasting plasma glucose. It is of interest that there was no change in mean insulin sensitivity in any of the groups (9).
First-degree relatives of patients with type 2 diabetes are at increased risk of developing hyperglycemia. When 531 first-degree relatives with no known history of diabetes were studied (10), it was found that in all ethnic groups (Caucasian, African American, Asian-American, and Hispanic-American), impaired β-cell function was more important than insulin resistance in determining alterations of glucose metabolism. Accordingly, in a group of 33 nondiabetic first-degree relatives followed-up for 7 years, decline in glucose tolerance over time was strongly associated with loss of β-cell function (11). All this is in agreement with the data from the U.K. Prospective Diabetes Study, showing that at the time of diagnosis of diabetes, there is an ∼50% loss of β-cell function (as calculated by HOMA-β), which is followed by a further progressive decline over time, whatever the treatment (12).
Whereas all this work provides valuable information, it has to be kept in mind that each test used in the aforementioned studies for the assessment of β-cell function in vivo has of course merits but also caveats (rev. in 13,14). The HOMA-β index is useful when studying large populations, but it provides an estimate of how the β-cell is performing under fasting conditions only. The oral glucose tolerance test is relatively easy to administer, but it provides limited information on early phase insulin release, and the intravenous glucose tolerance test does not allow the assessment of the incretin effect. On the other hand, measurement of β-cell mass in vivo in humans remains elusive (13,14). Work done with human donors who underwent hemipancreatectomy (15) and recipients of islet auto- or allograft (16,17) has shown that acute insulin response to glucose or arginine and glucose potentiation of arginine-induced insulin secretion appear to best correlate with β-cell mass.
β-CELL DYSFUNCTION AND TYPE 2 DIABETES: HISTOLOGICAL AND EX VIVO STUDIES
The role of reduced β-cell mass in human type 2 diabetes, the primary importance of β-cell apoptosis, and the insufficiency of replication/neogenesis have been studied by several authors using histological pancreatic samples, or isolated islets. The number of islets in the pancreas of diabetic subjects has been generally found to be reduced (up to 40%) compared with nondiabetic individuals (18–21). Moreover, β-cell mass and/or volume have been consistently found to be ∼20–60% lower in type 2 diabetic pancreata (21–24), with reduction already occurring in the presence of impaired fasting glycemia (IFG) (23). In this latter study, the authors found that obesity in nondiabetic individuals was accompanied by a 50% increase in relative β-cell volume compared with lean nondiabetic individuals. However, obese subjects with IFG and type 2 diabetes had a 40 and 63% deficit, respectively, in relative β-cell volume, and lean subjects with type 2 diabetes had a 41% deficit in relative β-cell volume than lean nondiabetic subjects. These differences were due to a reduced number of β-cells, rather than a smaller volume of individual cells. Neogenesis, while increased in obesity, was comparable in all groups. Furthermore, β-cell replication was found to be not significantly decreased in patients with diabetes or IFG. However, a significantly increased frequency of apoptotic events was detected in type 2 diabetic subjects versus nondiabetic subjects. In a detailed study published more recently (25), the authors analyzed autopsy samples from 57 type 2 diabetic and 52 nondiabetic European subjects and confirmed that β-cell mass was lower in the former. However, there was marked inter-subject variability and overlap between the two groups. β-Cell mass reduction was more pronounced with longer duration of diabetes (25). Using electron microscopy, it was observed that diabetic β-cells have a decreased number of insulin granules (24). Interestingly, the percentage of pancreas volume occupied by β-cells (as assessed in autopsy pancreas samples from obese humans with NGT, IFG, or diabetes) has been found to be significantly correlated with fasting plasma glucose (26) (Fig. 1). Furthermore, research with isolated human islets has consistently shown that β-cells from type 2 diabetic subjects display several defects that include reduced insulin content, diminished insulin mRNA expression, and decreased, or absent, first-phase insulin secretion in response to glucose (3,27–29).
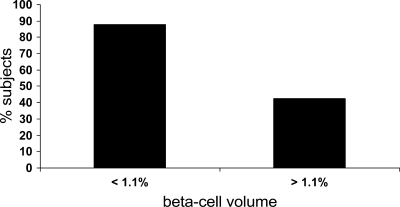
Figure 1.
Percentage of subjects with fasting plasma glucose higher than 100 mg/dl in relation to β-cell volume (lower or greater than 1.1%). Case subjects were obese and nondiabetic either with IFG or with diabetes. A higher percentage of individuals with increased glucose values had reduced β-cell volume. Adapted from Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC: Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 2006;29:717–718.
Obviously, these studies are cross-sectional. Therefore, prospective information on β-cell mass changes to be correlated with diabetes progression is not available. In addition, at the present time, it is not possible to exclude that subjects who develop diabetes start with a lower β-cell mass due to genetic reasons. As a matter of fact, several genes associated with type 2 diabetes may affect β-cell development (30,31).
β-CELL PRESERVATION BY CURRENT PHARMACOLOGICAL THERAPIES: IN VIVO STUDIES
As aforementioned, research performed in different categories of subjects by cross-sectional and longitudinal studies, together with histological analysis and ex vivo islet investigations, strongly suggest that β-cell failure is crucial for the onset of diabetes and progressive deterioration of glycemic control. Consequently, much interest is being focused on the potential of preserving the β-cell during the different natural history stages of type 2 diabetes.
Prevention of diabetes has been achieved in variable percentages of high-risk individuals by lifestyle changes, or pharmacological intervention, but only in a few cases has β-cell function been assessed. In the U.S. Diabetes Prevention Program study, subjects with IGT were assigned to either placebo, a lifestyle modification program (with a goal of 150 min of physical activity per week and 7% weight loss), or metformin (32). During an average follow-up of 2.8 years, the incidence of diabetes was significantly lower in the metformin-treated individuals (4.8%) than in those receiving placebo (7.8%). When the role of insulin sensitivity and insulin secretion was evaluated (33), it was found that diabetes prevention was associated with a significant improvement of insulin secretion relative to insulin sensitivity. Thiazolidinediones have also been shown to be effective in preventing diabetes. In the Troglitazone Prevention of Diabetes (TRIPOD) study, a significant reduction in the incidence of type 2 diabetes (from 45 to 20%) was observed in the group of individuals treated with troglitazone, which was associated with preservation of β-cell compensation for insulin resistance (34). In addition, preliminary results of the ACT NOW study showed that pioglitazone was able to significantly reduce the incidence of type 2 diabetes in patients with IGT, which was associated with better insulin secretory function (DeFronzo et al., late-breaking abstract, American Diabetes Association 68th Scientific Sessions, San Francisco, 6–10 June 2008).
After the diagnosis of type 2 diabetes, the use of intensive treatment with insulin, metformin, glibenclamide, or chlorpropamide was effective in improving glycemic control compared with conventional therapy in the U.K. Prospective Diabetes Study (35). However, the various drug treatments were similarly unable to prevent progressive deterioration of glucose control and reduction of β-cell function, as assessed by measuring the HOMA-β index. Successive studies have shown that treatment with some medications is associated with improved β-cell function. When 53 type 2 diabetic patients were randomized to receive 4 months of treatment with placebo, 45 mg/day pioglitazone, or 8 mg/day rosiglitazone, improved glycemic control was achieved in the thiazolidinedione groups (36). In this study, glucose values were significantly associated with β-cell function (36). Postprandial β-cell performance was assessed by a mathematical model in a group of subjects with type 2 diabetes treated with metformin or a sulfonylurea, who were administered exenatide as add-on for 4 weeks (37). The authors showed that the glucagon-like peptide 1 (GLP-1) mimetic improved β-cell function significantly at the end of the treatment period. In a recent study (38), 69 metformin-treated patients with type 2 diabetes were randomized to exenatide or insulin glargine, and β-cell function was measured during an arginine-stimulated hyperglycemic clamp at week 0, at week 52, and after a 4-week off-drug period. Both drugs reduced A1C levels significantly during treatment, which was associated with a significant improvement of β-cell function parameters in the exenatide arm (Table 1). However, glycemic control and β-cell function measures returned to pretreatment values in both groups after 4 weeks off-drug (Table 1).
Table 1.
Measures of β-cell function during hypeglycemic clamp and ratio to pretreatment in the groups of type 2 diabetic patients treated with exenatide or insulin glargine
| Pretreatment | End of treatment | Off-drug | End of treatment ratio to pretreatment (geometric mean) | End of treatment ratio to pretreatment (between group difference) | End of treatment ratio to pretreatment (P) | Off-drug ratio to pretreatment (geometric mean) | Off-drug ratio to pretreatment (between group difference) | Off-drug ratio to pretreatment (P) | |
|---|---|---|---|---|---|---|---|---|---|
| First-phase C-peptide response to glucose | |||||||||
| Glargine | 5.4 ± 0.6 | 6.1 ± 0.5 | 6.1 ± 0.6 | 1.17 ± 0.06 | 1.13 ± 0.05 | ||||
| Exenatide | 5.4 ± 0.6 | 9.4 ± 1.0 | 5.0 ± 0.6 | 1.78 ± 0.11 | 1.53 ± 0.11 | <0.0001 | 1.00 ± 0.05 | 0.90 ± 0.06 | 0.11 |
| Second-phase C-peptide response to glucose | |||||||||
| Glargine | 77.4 ± 8.8 | 80.7 ± 6.9 | 86.2 ± 9.1 | 1.08 ± 0.05 | 1.10 ± 0.05 | ||||
| Exenatide | 78.5 ± 8.3 | 235.6 ± 23.0 | 79.5 ± 9.1 | 3.05 ± 0.22 | 2.85 ± 0.22 | <0.0001 | 1.01 ± 0.04 | 0.92 ± 0.06 | 0.19 |
| C-peptide response to arginine at 15 mM glucose | |||||||||
| Glargine | 20.0 ± 2.5 | 24.8 ± 2.2 | 21.4 ± 2.5 | 1.31 ± 0.07 | 1.03 ± 0.08 | ||||
| Exenatide | 19.7 ± 2.1 | 62.2 ± 7.0 | 22.0 ± 2.6 | 3.19 ± 0.24 | 2.46 ± 0.20 | <0.0001 | 1.12 ± 0.06 | 1.08 ± 0.10 | 0.40 |
Data are means ± SD. Table adapted from Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ: One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009;32:762–768.
Therefore, a few studies have shown that certain pharmacological treatments can prevent the onset of diabetes and slow its progression in humans and that these effects are associated with ameliorated β-cell function. However, from such in vivo studies, it is impossible to understand whether the effects on the insulin-secreting cells are mediated by an improvement of the metabolic milieu or are due to an action of the drug(s) directly on β-cells.
β-CELL PRESERVATION BY CURRENT PHARMACOLOGICAL THERAPIES: EX VIVO STUDIES
The possibility that pancreatic β-cell damage can be prevented, or even reverted, has been tested in isolated human nondiabetic islets exposed to different metabolic perturbations and, more importantly, with islets from type 2 diabetic donors. In early work, it was assessed whether metformin could affect the phenomenon of glucotoxicity (39). Human islets were incubated for 24 h in culture medium containing either 5.5 or 22.2 mmol/l glucose, with or without a therapeutic concentration of metformin. After incubation in the absence of metformin, the islets pre-exposed to 22.2 mmol/l glucose showed no significant increase in insulin release when challenged acutely with 16.7 mmol/l glucose (confirming that hyperglycemia desensitizes pancreatic β-cells). In the presence of metformin, the islets maintained the ability to significantly increase their insulin release in response to 16.7 mmol/l glucose, even when previously cultured with high glucose. Metformin could also protect human islets from lipotoxicity. When islets were incubated for 48 h in the presence of 2.0 mmol/l free fatty acid (oleate to palmitate, 2 to 1), acute insulin secretion in response to 16.7 mmol/l glucose was significantly reduced (40). Impairment of insulin secretion after exposure to free fatty acids was mainly accounted for by defective early-phase release. Addition of metformin to high–free fatty acid media prevented the impairment of glucose-mediated insulin release and the decline of first-phase insulin secretion (40). Other drugs used to reduce insulin resistance in peripheral tissues (i.e., peroxisome proliferator–activated receptor [PPAR]-γ) seem to protect islet cells from metabolic insults. Exposure of isolated islets to free fatty acids decreased glucose-stimulated insulin release and islet insulin content, and these alterations were prevented by the PPAR-γ agonist rosiglitazone (41). Interestingly, it has been reported that β-cell rest induced by an ATP-dependent potassium channel (KATP) opener protected human islets from the functional damage induced by prolonged pre-exposure to relatively (11 mmol/l) high glucose concentrations (42).
Of greater importance, however, is to assess whether the functional and molecular alterations of human type 2 diabetic β-cells are reversible. In a recent article, it was found that when type 2 diabetic islets were incubated with metformin for 24 h, insulin content and insulin granule amount increased significantly (Fig. 2), which was accompanied by partial restoration of glucose responsiveness (24). Metformin also improved β-cell survival (24). Recently, the role of incretins (GLP-1, glucose-dependent insulinotropic polypeptide [GIP], and some of their analogs) in the therapy of diabetes has received much attention (43). In vitro and laboratory animal models have shown that these molecules can protect the β-cell from apoptosis and promote β-cell differentiation and proliferation. In a study from our laboratory (44), pancreatic islets were prepared from the pancreas of nondiabetic and type 2 diabetic donors and then incubated in the presence of 5.5 mmol/l glucose, with or without the addition of exendin-4 (a long-acting GLP-1 mimetic). Insulin secretion from the type 2 diabetic islets improved after incubation with exendin-4, which also induced a significantly higher expression of insulin, GLUT2, glucokinase, and some β-cell regeneration and differentiation factors, including pancreas duodenum homeobox-1 (Pdx-1).
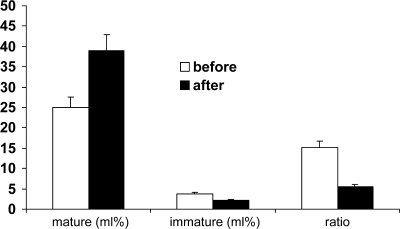
Figure 2.
Volume density of mature and immature insulin granules and its ratio in β-cells from type 2 diabetic subjects before and after ex vivo metformin exposure. Treatment caused significant increase of mature granules, decrease of immature granules, and higher ratio. Adapted from Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S: Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab 2004;89:5535–5541.
Amelioration of insulin release per se may have direct beneficial actions on β-cells (45). Insulin receptor phosphorylation leads to activation of phosphoinositide 3 (PI-3) and mitogen-activated (MAP) kinases, protecting the β-cell from apoptosis (46). In addition, several data have demonstrated that pro-insulin–derived C-peptide can affect the function and survival of a number of cell types, including β-cells (47). When isolated human islets were cultured in the presence of 50 ng/ml C-peptide, islet cell apoptotic rate decreased significantly, which was accompanied by increased mRNA and protein expression of the anti-apoptotic molecule B-cell CLL/lymphoma 2 (Bcl-2), without changes in the expression of the pro-apoptotic protein Bax (48).
CONCLUSIONS
Pancreatic β-cell dysfunction is key to the development and progression of type 2 diabetes. Both altered β-cell function and decreased β-cell mass are likely to contribute to the defects in insulin release typical of diabetes. These defects cause a progressive increase of glucose levels, with deterioration of glycemic control over the years. Interestingly, however, some evidence is emerging to show that the onset of diabetes may be delayed and the progression of glucose control deterioration slowed by certain therapies. These beneficial effects are associated, at least in part, to better maintained β-cell function. For these approaches to be more effective, strategies should be developed to deliver potentially useful drugs to the β-cell at the desired concentrations and for the necessary duration. In addition, efforts should be made to better understand which alterations at the level of β-cell microenvironment are present and may impede the complete success of therapeutic interventions.
Acknowledgments
This work was supported in part by the Italian Ministry of University and Research (PRIN 2007–2008).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31 ( Suppl. 1): S55– S60 [DOI] [PubMed] [Google Scholar]
- 2. Stumvoll M, Goldstein BJ, van Haeften TW: Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333– 1346 [DOI] [PubMed] [Google Scholar]
- 3. Marchetti P, Dotta F, Lauro D, Purrello F: An overview of pancreatic beta-cell defects in human type 2 diabetes: implications for treatment. Regul Pept 2008; 146: 4– 11 [DOI] [PubMed] [Google Scholar]
- 4. Kahn SE: The relative contribution of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3– 19 [DOI] [PubMed] [Google Scholar]
- 5. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: San Antonio Metabolism Study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio Metabolism (SAM) study. Diabetologia 2004; 47: 31– 39 [DOI] [PubMed] [Google Scholar]
- 6. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005; 90: 493– 500 [DOI] [PubMed] [Google Scholar]
- 7. Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM: The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006; 55: 1114– 1120 [DOI] [PubMed] [Google Scholar]
- 9. Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR: Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med 1998; 15: 290– 296 [DOI] [PubMed] [Google Scholar]
- 10. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE: American Diabetes Association GENNID Study Group. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002; 51: 2170– 2178 [DOI] [PubMed] [Google Scholar]
- 11. Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE: Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007; 30: 677– 682 [DOI] [PubMed] [Google Scholar]
- 12. U.K. Prospective Diabetes Study 16 Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995; 44: 1249– 1258 [PubMed] [Google Scholar]
- 13. Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM: An examination of beta-cell function measures and their potential use for estimating beta-cell mass. Diabetes Obes Metab 2008; 10 ( Suppl. 4): 63– 76 [DOI] [PubMed] [Google Scholar]
- 14. Robertson RP: Estimation of beta-cell mass by metabolic tests: necessary but how sufficient? Diabetes 2007; 56: 2420– 2424 [DOI] [PubMed] [Google Scholar]
- 15. Seaquist ER, Roberson RP: Effects of hemipancreatectomy on pancreatic alpha and beta-cell function in healthy human donors. J Clin Invest 1992; 89: 1761– 1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland SE, Roberson RP: Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998; 47: 324– 330 [DOI] [PubMed] [Google Scholar]
- 17. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AM: Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 2002; 51: 2148– 2157 [DOI] [PubMed] [Google Scholar]
- 18. Westermark P, Wilander E: The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia 1978; 15: 417– 421 [DOI] [PubMed] [Google Scholar]
- 19. Saito K, Iwama N, Takahashi T: Morphometrical analysis on topographical difference in size distribution, number and volume of islets in the human pancreas. Tohoku J Exp Med 1978; 124: 177– 186 [DOI] [PubMed] [Google Scholar]
- 20. Gepts W, Lecompte PM: The pancreatic islets in diabetes. Am J Med 1981; 70: 105– 115 [DOI] [PubMed] [Google Scholar]
- 21. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S: Reduced beta cell mass and expression of oxidative stress related DNA damage in the islets of Japanese type 2 diabetic patients. Diabetologia 2002; 45: 85– 96 [DOI] [PubMed] [Google Scholar]
- 22. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S: Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes in Korea. J Clin Endocrinol Metab 2003; 88: 2300– 2308 [DOI] [PubMed] [Google Scholar]
- 23. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 24. Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S: Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab 2004; 89: 5535– 5541 [DOI] [PubMed] [Google Scholar]
- 25. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC: Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10 ( Suppl. 4): 32– 42 [DOI] [PubMed] [Google Scholar]
- 26. Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC: Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care 2006; 29: 717– 718 [DOI] [PubMed] [Google Scholar]
- 27. Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P: Functional and molecular alterations of pancreatic islets in human type 2 diabetes. Diabetes 2005; 54: 727– 735 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Alvarez J, Conget I, Rasschaert J, Sener A, Gomis R, Malaisse WJ: Enzymatic, metabolic and secretory patterns in human islets of type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1994; 37: 177– 181 [DOI] [PubMed] [Google Scholar]
- 29. Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF: Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 2004; 53: 624– 632 [DOI] [PubMed] [Google Scholar]
- 30. Jafar-Mohammadi B, McCarthy MI: Genetics of type 2 diabetes mellitus and obesity: a review. Ann Med 2008; 40: 2– 10 [DOI] [PubMed] [Google Scholar]
- 31. Groop L, Lyssenko V: Genes and type 2 diabetes mellitus. Curr Diab Rep 2008; 8: 192– 197 [DOI] [PubMed] [Google Scholar]
- 32. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program. Diabetes 2005; 54: 2404– 2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroq uin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2796– 2803 [DOI] [PubMed] [Google Scholar]
- 35. U.K. Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 36. Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ: One-year treatment with exenatide improves β-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762– 768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A: Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38: 838– 844 [DOI] [PubMed] [Google Scholar]
- 38. Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ: One-year treatment with exenatide improves beta-cell function, compared to insulin glargine, in metformin treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762– 768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lupi R, Del Guerra S, Tellini C, Giannarelli R, Coppelli A, Lorenzetti M, Carmellini M, Mosca F, Navalesi R, Marchetti P: The biguanide compound metformin prevents desensitization of human pancreatic islets induced by high glucose. Eur J Pharmacol 1999; 364: 205– 209 [DOI] [PubMed] [Google Scholar]
- 40. Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patane G, Boggi U, Mosca F, Piro S, Del Prato S, Marchetti P: Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes 2002; 51 ( Suppl. 1): S134– S137 [DOI] [PubMed] [Google Scholar]
- 41. Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S: Rosiglitazone prevents the impairment of human islet function induced by fatty-acids: evidence for a role PPAR-γ2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab 2004; 286: E560– E567 [DOI] [PubMed] [Google Scholar]
- 42. Ritzel RA, Hansen JB, Veldhuis JD, Butler PC: Induction of beta-cell rest by a Kir6.2/SUR1-selective K(ATP)-channel opener preserves beta-cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab 2004; 89: 795– 805 [DOI] [PubMed] [Google Scholar]
- 43. Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide 1 receptor agonists and dipeptidyl peptidase 4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696– 1705 [DOI] [PubMed] [Google Scholar]
- 44. Lupi R, Mancarella R, Del Guerra S, Bugliani M, Del Prato S, Boggi U, Filipponi F, Torri S, D'Aleo V, Marchetti P: Effects of exendin-4 on human type 2 diabetes islets. Diabetes Obes Metab 2008; 10: 515– 519 [DOI] [PubMed] [Google Scholar]
- 45. Persaud SJ, Muller D, Jones PM: Insulin signalling in islets. Biochem Soc Trans 2008; 36: 290– 293 [DOI] [PubMed] [Google Scholar]
- 46. Federici M, Hribal ML, Ranalli M, Marselli L, Porzio O, Lauro D, Borboni P, Lauro R, Marchetti P, Melino G, Sesti G: The common Arg972 polymorphism in insulin receptor substrate-1 causes apoptosis of human pancreatic islets. FASEB J 2001; 15: 22– 24 [DOI] [PubMed] [Google Scholar]
- 47. Wahren J, Ekberg K, Jörnvall H: C-peptide is a bioactive peptide. Diabetologia 2007; 50: 503– 509 [DOI] [PubMed] [Google Scholar]
- 48. Bugliani M, Torri S, Lupi R, Del Guerra S, Grupillo M, Del Chiaro M, Mosca F, Boggi U, Del Prato S, Marchetti P: Effects of C-peptide on isolated human pancreatic islet cells. Diabetes Metab Res Rev 2007; 23: 215– 219 [DOI] [PubMed] [Google Scholar]