Adipose tissue is a heterogeneous organ with respect to embryonic origin, body distribution, and function. In addition to playing a major role in the regulation of nutrient and energy homeostasis, it is involved in the modulation of the immune response, reproductive function, hemostasis, mechanical support, bone mass growth, and thermogenesis.
To postulate that insulin resistance starts in adipose tissue, there should be evidence of 1) potential mechanisms for such a causal relationship, 2) the manifestation of such mechanisms in insulin-resistant individuals, and 3) their early occurrence in the development of insulin resistance.
PLAUSIBILITY: ADIPOSE TISSUE CAN CAUSE INSULIN RESISTANCE
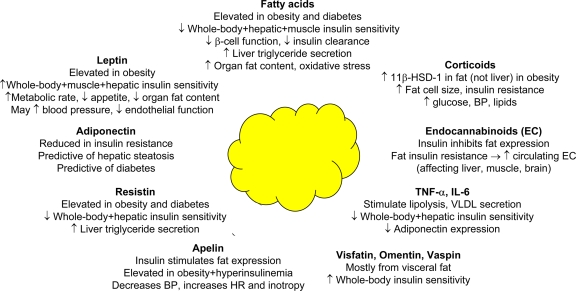
Adipose tissue releases a variety of factors known to modulate insulin sensitivity, and their effects are summarized in Fig. 1.
Figure 1.
Adipose tissue is the largest endocrine organ in the body. The diagram summarizes the main roles and effects of representative fat-derived products that have been related to insulin resistance and metabolic risk. BP, blood pressure; HR, heart rate.
Fatty acids
The concept that fatty acids (FAs) provoke cardiac, skeletal muscle, and hepatic insulin resistance and impair β-cell function has been extensively confirmed in humans, and mechanisms are reviewed in detail elsewhere (1). A sustained pharmacologic inhibition of lipolysis, with reduction in the plasma FA concentration, reverses these defects (2–4). Elevated FA levels also promote the synthesis and release of VLDL by the liver by 1) increasing substrate availability, 2) inhibiting insulin-mediated apoB degradation (5), and 3) reducing hepatic insulin clearance, contributing to hyperinsulinemia. In the brain, excessive FA uptake and its response to weight loss have been documented in subjects with the metabolic syndrome (6), and FAs are implicated in the central regulation of glucose production (7).
FA overflow from adipocytes to skeletal muscle and other tissues may result in free radical formation during oxidative phosphorylation, the intramyocellular accumulation of triglyceride, and the production of toxic lipid metabolites (fatty-acyl CoAs, diacylglycerol, and ceramides) and metabolic intermediates, which reflect oxidative damage (4), both of which can interfere with the insulin signaling cascade. Consistent with this hypothesis, lipid oxidation is increased systemically and regionally, i.e., in the myocardium and skeletal muscle, and in the liver in states of insulin resistance and/or in steatosis (8–10), and recent evidence supports a role of β-cell oxidative stress in mediating FA-induced β-cell dysfunction (11). Local production of reactive oxygen species within adipose tissue likely initiates lipotoxicity and insulin resistance at the immediate site of FA release (12). Oxidative damage is amplified by peroxidation of lipid stores and could, in turn, impair mitochondrial function and insulin sensitivity and produce inflammation in different target organs.
Adipokines
Adipose tissue is the largest endocrine organ in the body and generates multiple signals that regulate metabolism in other tissues. Leptin acts centrally to enhance the resting metabolic rate and decrease appetite, thus reducing tissue triglyceride accumulation. In lipodystrophic humans with severe insulin resistance, the administration of leptin restores insulin sensitivity and reduces organ steatosis and hyperglycemia (13). However, leptin deficiency and resistance are rare causes of disease in humans. Interestingly, leptin receptors have been identified in the vessel wall, and leptin infusion increases arterial blood pressure (14). Adiponectin is produced by adipose tissue in inverse amounts to the fat mass and is one relevant mediator of the action of peroxisome proliferator–activated receptor-γ (PPAR-γ) agonists. Administration of adiponectin reverses the insulin resistance associated with obesity or lipodystrophy by reducing FA and triglyceride levels (15). Plasma adiponectin concentrations typically are reduced in obese normal glucose tolerant, insulin-resistant and lean, and obese type 2 diabetic subjects, and decreased plasma adiponectin levels are predictive of hepatic steatosis and insulin resistance. However, the observation that weight loss has a dramatic effect on insulin sensitivity without change in the plasma adiponectin concentration mitigates against a causal role of this hormone in the pathogenesis of insulin resistance (16). The role of resistin, which is elevated in animal models of obesity and diabetes, and of visfatin and omentin (which are produced by visceral fat) in the development of insulin resistance remains controversial.
Adipocytokines
Tumor necrosis factor (TNF)-α stimulates adipose tissue lipolysis, promotes VLDL production (17), interferes with insulin signaling and expression of adiponectin, and increases the expression of interleukins. In humans, tissue expression, rather than circulating levels of TNF-α, correlates with obesity and insulin resistance (18). Interleukin (IL)-6 also is associated with insulin resistance, increased fat mass, and elevated circulating FA levels, consistent with the lipolytic action of this cytokine. IL-6 interferes with the insulin signaling pathway in hepatocytes, skeletal muscle, and adipose tissue (19) and inhibits the production of adiponectin.
Glucocorticoids and endocannabinoids
Sex steroids and corticosteroids have a major impact on fat distribution and enzymes involved in steroid synthesis and metabolism, such as 11-β hydroxysteroid dehydrogenase (11-β-HSD1) (20) and aromatase, and are present in adipose tissue. Activation of 11-β-HSD1 results in tissue-specific glucocorticoid excess, causing central obesity with increased fat cell size, insulin resistance, hyperglycemia, hyperlipidemia, and hypertension. In obese Zucker rats, adipose, but not hepatic, tissue expression of 11-β-HSD1 is increased. In humans, subcutaneous adipose tissue expression and activity of 11-β-HSD1 correlate with indexes of obesity.
In adipose tissue, fat-specific overproduction of endocannabinoids reduces adiponectin expression, decreases adipose tissue energy expenditure and fat oxidation, and enhances lipogenesis. Recent evidence demonstrates that insulin inhibits the synthesis of endocannabinoids and that fat-specific insulin resistance may be responsible for the augmented plasma endocannabinoid levels found in insulin-resistant states. The increased plasma endocannabinoid levels could influence liver, muscle, and brain regulation of glucose homeostasis (21).
EVIDENCE FOR ADIPOSE TISSUE DYSFUNCTION AND ALTERED TOPOGRAPHY IN INSULIN RESISTANCE
By buffering postprandial FA influx, adipose tissue plasticity plays an important role in controlling nutrient supply to other organs, and the ability of adipocytes to dispose of ingested fat load is impaired in both obesity and lipodystrophy (22). Adipose tissue is composed of adipocytes, pre-adipocytes, nerves, macrophages, fibroblasts, and vascular cells, and this constitutes a network that can lead to a variety of dysfunctional manifestations.
Adipocyte size and buffering ability
Adipocytes can maximally store ∼0.8 μg lipid per cell. Larger adipocytes become insulin resistant, leading to reduced triglyceride and glucose clearance. Lean offspring of type 2 diabetic subjects, who do not manifest overt insulin resistance and who have no apparent metabolic or hemodynamic abnormalities (23), have a significant enlargement in adipocyte size, enhanced regional subcutaneous glycerol release in response to insulin, and normal FA levels. In Pima Indians, large fat cell volume and circulating FA levels are an independent predictor of the future development of diabetes (24). Interventions that reduce adipocyte size, either by increasing the total amount of fat via recruitment of new adipocytes (e.g., thiazolidinediones) or by depleting triglyceride stores in existing fat cells (e.g., exercise, diet), reverse most of the features associated with fat dysfunction. Smaller adipocytes have more buffering capacity, and this may explain the link between the decrease in adipocyte size and reversal of insulin resistance. Indeed, ob/ob PPAR-γ2 knockout mice are lean and have a reduced number of small adipocytes. They develop an early defect in GLUT4 expression in adipose tissue, severe insulin resistance, β-cell failure, and dyslipidemia (25).
Adipose tissue metabolism, perfusion, and inflammation
Adipose tissue accounts for 10–20% of whole-body glucose utilization. Insulin-mediated glucose disposal is impaired in obese nondiabetic subjects and patients with type 2 diabetes. It has been suggested that expansion of fat mass partly compensates for the defect in insulin action, supporting a role for adipose tissue in attenuating insulin resistance (26). Notably, fat-specific GLUT4 deletion causes whole-body insulin resistance, whereas muscle GLUT4 ablation stimulates adipose tissue glucose disposal (27). Recent data in humans document higher fasting FA uptake in adipose tissue in obese subjects (28). Circulating FA levels are typically increased in insulin-resistant states and generally correlate with the severity of insulin resistance; normal FA levels may be observed, if a compensatory increase in FA clearance occurs. An acute increase in FFA concentrations in normal subjects leads to acute cellular inflammation, as revealed by the increase in nuclear factor κB and macrophage migration inhibitory factor. Both of these factors have been implicated as potential causes of insulin resistance, thus suggesting an additional indirect mechanism of FA-induced insulin resistance (29).
Adipose tissue blood flow is reduced in obese nondiabetic and type 2 diabetic subjects (22), and this could lead to a defect in triglyceride clearance by adipocytes and FA spillover from adipose tissue. The perfusion defect may be due, in part, to adipocyte enlargement, which cannot be compensated by adequate angiogenesis (30). The diameter (140–180 μm) achieved by enlarged adipocytes is greater than the diffusion distance of oxygen, thus limiting the exchange between blood and adipocyte cytoplasm. Hypoxia may inhibit adipocyte differentiation and adiponectin expression, promote formation of free radicals and inflammation (31), and lead to cell death. Consistent with this, macrophages in adipose tissue are localized primarily around dead adipocytes (32). Increased numbers of macrophages in fat are observed in obese individuals, and newly recruited macrophages have a greater increase in proinflammatory properties than resident ones (33). Inflammation and macrophage infiltration intensify with increasing obesity and can be reversed by weight loss (34).
Adiposity and fat topography
In humans, white adipose tissue is found in the subcutaneous, intra-abdominal, epicardial, extramyocellular, perivascular, lymphnodal, retroorbital, and facial regions. It also is present in bone marrow and mammary gland. Brown fat occurs during fetal development, with some remnants in the mediastinal, paravertebral, and neck areas in adults (35). Each depot has specific gene expression and different responsiveness to nutrients, hormones, and temperature, thereby reflecting specific tasks. Metabolically active fat in the intra-abdominal, intermuscular, perivascular, and epicardial areas may serve for the immediate provision of energy to respective vital organs (i.e., liver, heart, vessels, and skeletal muscle). By directly providing FAs to the liver, visceral fat also represents a source of ketones for the brain. Omental fat acts as a sensor that regulates the disposal of ingested nutrients and directs their delivery from the liver to the rest of the body, with feedback to the brainstem through autonomic neurons (36). Deep subcutaneous adipose tissue in the trunk is restrained by the fascia superficialis and can nourish the underlying extensive muscle apparatus. These adipose depots share similarities in adipocytokine and FA release patterns and display resistance to insulin (37–39). Compared with visceral adipocytes, superficial subcutaneous adipose tissue primarily serves as a storage organ, consistent with its anatomical location, response to the anabolic action of insulin, and its more efficient proliferation and differentiation in vitro (40). Thus, relative insulin resistance in intra-abdominal fat and intrathoracic and intermuscular depots optimizes their ability to release energy to proximal organs, while limiting the expansion upon surrounding structures.
Epidemiological evidence demonstrates that obesity, high energy intake, and sedentary lifestyle are significant contributors to the epidemic of chronic disease, i.e., type 2 diabetes and atherosclerosis, related to insulin resistance. However, despite similar lifestyles, 1) some subjects are more susceptible to weight gain than others; 2) insulin sensitivity varies over a wide range in obese and lean individuals; 3) ∼20% of obese individuals do not develop metabolic abnormalities, whereas 18% of lean subjects do (41); and 4) elderly individuals, offspring of diabetic parents, and lipodystrophic subjects are typically insulin resistant despite a lean phenotype.
These apparent incongruities can be reconciled by postulating that a defect in subcutaneous adipose tissue expandability, independent of body weight or adiposity, may be the primary cause of insulin resistance. In fact, hyperplastic obesity—in which newly differentiated adipocytes maintain the ability to store triglycerides—is typically more benign than fat hypertrophy. Longitudinal studies in Pima Indians (42) have documented that insulin-sensitive subjects, in whom the anabolic effect of insulin is enhanced, gain more weight and have a fourfold more rapid decline in insulin sensitivity than less insulin-sensitive individuals. Thus, both fat mass and insulin resistance should be regarded as relative and dynamic features, and this could explain the relatively weak correlation between the two variables.
The primacy of defective subcutaneous fat storage versus visceral fat enlargement in the development of metabolic complications is supported by evidence in patients with total lipodystrophy, who experience severe insulin resistance despite the lack of visceral fat. In addition, treatments that selectively augment the ability of subcutaneous tissue to take up and store fat (including glitazones in humans and subcutaneous fat re-implantation in animals [43]) have a major impact to reverse insulin resistance and normalize metabolic risk factors without modifying (or even increasing) the total mass of ectopic fat depots. Conversely, liposuction fails to correct the metabolic disturbances, i.e., insulin resistance and glucose intolerance, in obese humans (44); this may be partly accounted for by the fact that lifestyle is not modified, and macronutrient intake is a demonstrated source of oxidative stress and inflammation, potentially interfering with insulin signaling (45–47). Subcutaneous tissue is at least 10-fold larger than visceral fat mass, and its production of FAs and adipokines outweighs the contribution of those from nonsubcutaneous fat depots. The immediate consequence of a defect in subcutaneous storage capability is the uncontrolled outflow of substrates (FAs) and compensatory expansion of alternative fat depots or deposition of triglycerides in nonadipose tissues. Thus, removal of part, or all, of a fat depot leads to expansion of alternative fat tissue (48).
A high visceral-to-subcutaneous fat mass ratio correlates closely with the metabolic syndrome phenotype in obese and lean individuals (41) and is a typical feature of aging and lean insulin-resistant offspring of type 2 diabetic individuals. Targeted expansion of the visceral fat mass by steroids and the selective surgical removal of visceral fat in animals (49) and omental fat in humans (50) support the detrimental role of this depot. Numerous large cohort studies have demonstrated that excessive visceral, intermuscular, and trunkal fat mass correlates with the severity of metabolic dysregulation, cardiovascular risk, and presence of type 2 diabetes (51–53). Smaller adipocyte size was shown to be associated with increased leg fat mass, whereas larger adipocyte size was related to more trunkal fat mass (53).
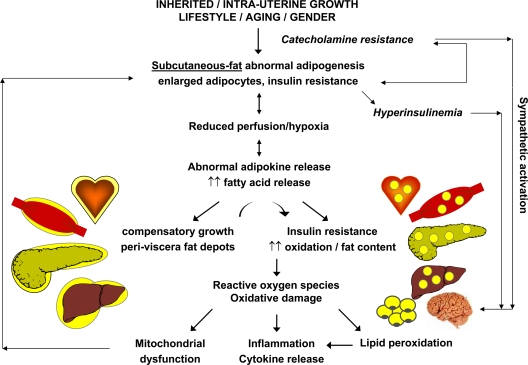
In summary, the inability of adipose tissue to store FA and glucose, independent of total body adiposity, appears to be a common feature in all disorders characterized by insulin resistance. The fat insulin resistance is related to excessive FA accumulation and cell enlargement. Oxidative stress in fat cells is exacerbated by reduced perfusion and relative hypoxia, which promote chemo-attraction and inflammation. These abnormalities adversely influence metabolic pathways throughout the body in the form of lipotoxicity, ectopic fat storage, systemic low-grade inflammation, and insulin resistance. Epicardial, intermuscular, and visceral abdominal fat depots are physiologically more metabolically active and less insulin sensitive. Their expansion exacerbates the flow of substrates and cytokines both locally and to vital organs, provoking triglyceride accumulation and oxidative damage. In the liver, FA overflow reduces insulin clearance, thereby promoting the exposure of peripheral tissues, brain, and vessels to hyperinsulinemia, which exacerbates insulin resistance and causes sympathetic activation. The cascade of events hypothesized above is summarized in Fig. 2.
Figure 2.
Proposed cascade of events via which abnormal adipose tissue development leads to insulin resistance in nonadipose organs. Ectopic fat deposition, oxidative damage, and inflammation play a central role in the development of insulin resistance in muscle, liver, and other organs and establish a negative reverberating cycle.
PRIMACY: INSULIN RESISTANCE STARTS IN ADIPOSE TISSUE
Studies in twins, who have been raised apart and together (54), have documented that body fat distribution and metabolic abnormalities are a clustering genetic trait. Heritability in the Framingham study, including genetic and early environment contributions, was 57% for subcutaneous and 36% for visceral fat mass (51). As reviewed by Fernandez-Twinn and Ozanne (55), because of intrauterine nutritional and hormonal factors, low–birth weight newborns have more fat and are at higher risk of metabolic and cardiovascular diseases in adulthood. It is noteworthy that fat accretion occurs during the last trimester and maternal under-nutrition in animals during late pregnancy leads to glucose intolerance in adult offspring, in association with reduced adipose, but not muscle GLUT4, content and increased adipose tissue mass. The embryonic origin of adipose tissue, i.e., the paraxial mesoderm, gives rise to truncal fat, whereas the lateral plate mesoderm gives rise to fat in the limbs (35), which also reflects the relationship between fat distribution and adult metabolic risk.
Recent human studies from our lab (16) have documented that whereas systemic inflammation, whole-body/skeletal muscle/hepatic insulin resistance, hypertension, dyslipidemia, and hyperglycemia all are reversed by weight loss, adipose tissue insulin resistance and hypoperfusion are not. This is consistent with the notion that GLUT4 translocation defects are inducible and reversible in myocytes but not in adipocytes (27). Like fat-specific insulin resistance in obesity-prone individuals, catecholamine resistance of adipose tissue does not regress with weight loss (56), partly explaining a tendency to regain the lost weight.
In conclusion, fat-specific insulin resistance appears to be an early and irreversible defect that can explain the causal relationship between adipocyte dysfunction, extra-adipose tissue (i.e., muscle and liver), and insulin resistance. The origin of this association can be traced to genetic and embryonic programming, long before the development of metabolic disease in adulthood.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. Randle PJ: Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998; 14: 263– 283 [DOI] [PubMed] [Google Scholar]
- 2. Rigazio S, Lehto HR, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P: The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab 2008; 295: E413– E419 [DOI] [PubMed] [Google Scholar]
- 3. Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E: Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E1775– E1781 [DOI] [PubMed] [Google Scholar]
- 4. Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA: Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005; 54: 3148– 3153 [DOI] [PubMed] [Google Scholar]
- 5. Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G: Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 1995; 95: 158– 166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nuutila P, Viljanen A, Hirvonen J, Virtanen K, Karmi A, Oikonen V, Kemppainen J, Viljanen T, Haaparanta M, Nagren K, Solin O, Iozzo P: Brain unsaturated fatty acid (FA) uptake is increased in obesity and reversed by weight reduction. Diabetologia 2007; 50 ( Suppl. 1): S13 [Google Scholar]
- 7. Lam TK, Schwartz GJ, Rossetti L: Hypothalamic sensing of fatty acids. Nat Neurosci 2005; 8: 579– 584 [DOI] [PubMed] [Google Scholar]
- 8. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ: Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004; 109: 2191– 2196 [DOI] [PubMed] [Google Scholar]
- 9. Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ: Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007; 56: 2085– 2092 [DOI] [PubMed] [Google Scholar]
- 10. Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M: Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005; 48: 634– 642 [DOI] [PubMed] [Google Scholar]
- 11. Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A: Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 2007; 56: 2927– 2937 [DOI] [PubMed] [Google Scholar]
- 12. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I: Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752– 1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI: Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 2002; 109: 1345– 1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shek EW, Brands MW, Hall JE: Chronic leptin infusion increases arterial pressure. Hypertension 1998; 31: 409– 414 [DOI] [PubMed] [Google Scholar]
- 15. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T: The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941– 946 [DOI] [PubMed] [Google Scholar]
- 16. Viljanen AP, Lautamäki R, Järvisalo M, Parkkola R, Huupponen R, Lehtimäki T, Rönnemaa T, Raitakari OT, Iozzo P, Nuutila P: Effects of weight loss on visceral and abdominal subcutaneous adipose tissue blood-flow and insulin-mediated glucose uptake in healthy obese subjects. Ann Med 2009; 41: 152– 160 [DOI] [PubMed] [Google Scholar]
- 17. Grunfeld C, Feingold KR: Tumor necrosis factor, interleukin, and interferon induced changes in lipid metabolism as part of host defense. Proc Soc Exp Biol Med 1992; 200: 224– 227 [DOI] [PubMed] [Google Scholar]
- 18. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409– 2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H: Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes 2004; 53: 2232– 2241 [DOI] [PubMed] [Google Scholar]
- 20. Tomlinson JW, Stewart PM: Modulation of glucocorticoid action and the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 2007; 21: 607– 619 [DOI] [PubMed] [Google Scholar]
- 21. D'Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR: The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes 2008; 57: 1262– 1268 [DOI] [PubMed] [Google Scholar]
- 22. Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW: Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 2003; 27: 875– 888 [DOI] [PubMed] [Google Scholar]
- 23. Eriksson JW, Smith U, Waagstein F, Wysocki M, Jansson PA: Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients: is cellular insulin resistance a secondary phenomenon? Diabetes 1999; 48: 1572– 1578 [DOI] [PubMed] [Google Scholar]
- 24. Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E: A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 1995; 38: 1213– 1217 [DOI] [PubMed] [Google Scholar]
- 25. Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppänen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A: PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet 2007; 3: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Virtanen KA, Iozzo P, Hällsten K, Huupponen R, Parkkola R, Janatuinen T, Lönnqvist F, Viljanen T, Rönnemaa T, Lönnroth P, Knuuti J, Ferrannini E, Nuutila P: Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron-emitting tomography study. Diabetes 2005; 54: 2720– 2726 [DOI] [PubMed] [Google Scholar]
- 27. Cahová M, Vavrínková H, Kazdová L: Glucose-fatty acid interaction in skeletal muscle and adipose tissue in insulin resistance. Physiol Res 2007; 56: 1– 15 [DOI] [PubMed] [Google Scholar]
- 28. Koutsari C, Ali AH, Jensen MD: Direct FFA storage into subcutaneous adipose tissue depots in lean and obese humans. Diabetes 2008; 57 ( Suppl. 1): 96– OR [Google Scholar]
- 29. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P: Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003; 52: 2882– 2887 [DOI] [PubMed] [Google Scholar]
- 30. Trayhurn P, Wang B, Wood IS: Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 2008; 100: 227– 235 [DOI] [PubMed] [Google Scholar]
- 31. Ye J, Gao Z, Yin J, He Q: Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007; 293: E1118– E1128 [DOI] [PubMed] [Google Scholar]
- 32. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS: Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347– 2355 [DOI] [PubMed] [Google Scholar]
- 33. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR: Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007; 56: 16– 23 [DOI] [PubMed] [Google Scholar]
- 34. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54: 2277– 2286 [DOI] [PubMed] [Google Scholar]
- 35. Gesta S, Tseng YH, Kahn CR: Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242– 256 [DOI] [PubMed] [Google Scholar]
- 36. Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM: Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 2006; 147: 1140– 1147 [DOI] [PubMed] [Google Scholar]
- 37. van Harmelen V, Dicker A, Rydén M, Hauner H, Lönnqvist F, Näslund E, Arner P: Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes 2002; 51: 2029– 2036 [DOI] [PubMed] [Google Scholar]
- 38. Iacobellis G, Corradi D, Sharma AM: Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005; 2: 536– 543 [DOI] [PubMed] [Google Scholar]
- 39. Monzon JR, Basile R, Heneghan S, Udupi V, Green A: Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes Res 2002; 10: 266– 269 [DOI] [PubMed] [Google Scholar]
- 40. Van Harmelen V, Röhrig K, Hauner H: Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 2004; 53: 632– 637 [DOI] [PubMed] [Google Scholar]
- 41. Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET: Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569– 2575 [DOI] [PubMed] [Google Scholar]
- 42. Swinburn BA, Nyomba BL, Saad MF, Zurlo F, Raz I, Knowler WC, Lillioja S, Bogardus C, Ravussin E: Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest 1991; 88: 168– 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML: Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 2000; 105: 271– 278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS: Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004; 350: 2549– 2557 [DOI] [PubMed] [Google Scholar]
- 45. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P: Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000; 85: 2970– 2973 [DOI] [PubMed] [Google Scholar]
- 46. Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P: Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006; 55: 1177– 1785 [DOI] [PubMed] [Google Scholar]
- 47. Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P: Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr 2004; 80: 51– 57 [DOI] [PubMed] [Google Scholar]
- 48. Mauer MM, Harris RB, Bartness TJ: The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev 2001; 25: 15– 28 [DOI] [PubMed] [Google Scholar]
- 49. Gabriely I, Barzilai N: Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep 2003; 3: 201– 206 [DOI] [PubMed] [Google Scholar]
- 50. Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P: A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord 2002; 26: 193– 199 [DOI] [PubMed] [Google Scholar]
- 51. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ: Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007; 116: 39– 48 [DOI] [PubMed] [Google Scholar]
- 52. Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM: Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006; 91: 4459– 4466 [DOI] [PubMed] [Google Scholar]
- 53. Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE: the Look AHEAD Adipose Research Group. Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2007; 293: E435– E442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nelson TL, Vogler GP, Pedersen NL, Hong Y, Miles TP: Genetic and environmental influences on body fat distribution, fasting insulin levels and CVD: are the influences shared? Twin Res 2000; 3: 43– 50 [DOI] [PubMed] [Google Scholar]
- 55. Fernandez-Twinn DS, Ozanne SE: Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 2006; 88: 234– 243 [DOI] [PubMed] [Google Scholar]
- 56. Knittle JL, Ginsberg-Fellner F, Brown RE: Adipose tissue development in man. Am J Clin Nutr 1977; 30: 762– 766 [DOI] [PubMed] [Google Scholar]