Type 2 diabetes is a progressive disease in which the risks of myocardial infarction, stroke, microvascular events, and mortality are all strongly associated with hyperglycemia (1). The disease course is primarily characterized by a decline in β-cell function and worsening of insulin resistance. The process is manifested clinically by deteriorations in multiple parameters, including A1C, fasting plasma glucose (FPG), and postprandial glucose levels.
In this review, we will evaluate our current understanding of the role played by deteriorating β-cell function and other abnormalities linked with the progression of type 2 diabetes. An improved understanding of these abnormalities may provide the scientific groundwork for novel therapies that may help achieve and maintain good glycemic control.
CHARACTERISTICS OF DISEASE PROGRESSION
Progression from pre-diabetes to overt diabetes
Because glucose is a continuous variable, the use of thresholds to make a diagnosis is somewhat arbitrary. The term “pre-diabetes” has become well established and implies a risk of progression to overt diabetes. However, although such progression is well studied in prevention trials, little is known about the rate of progression and the characteristics of such progression in the population at large. Table 1 summarizes some of the factors associated with such progression. Nichols et al. (2) studied the progression of pre-diabetes to overt disease and observed that 8.1% of subjects whose initial abnormal fasting glucose was 100–109 mg/dl and 24.3% of subjects whose initial abnormal fasting glucose was 110–125 mg/dl developed diabetes over an average of 29.0 months (1.34 and 5.56% per year, respectively). A steeper rate of increasing fasting glucose; higher BMI, blood pressure, and triglycerides; and lower HDL cholesterol predicted diabetes development.
Table 1.
Factors associated with progression of pre-diabetes to diabetes
| Elevated FPG and increase in FPG |
| High BMI |
| Weight gain |
| Younger age |
| High plasma insulin |
| Decreased insulin response to glucose |
| Dyslipidemia |
| Hypertension |
| Poor β-cell function |
| Choice of treatment |
The Baltimore Longitudinal Study of Aging (3) concluded that although phenotypic differences in rates of progression are partly a function of diagnostic thresholds, fasting and postchallenge hyperglycemia may represent phenotypes with distinct natural histories in the evolution of type 2 diabetes.
Does hyperglycemia evolve from normoglycemia gradually over time or as a step increase? Ferrannini et al. (4) measured plasma glucose and insulin levels during oral glucose testing at baseline and after 3 and 7 years of follow-up. In subjects with normal glucose tolerance on all three occasions (nonconverters), FPG increased only slightly over 7 years. In contrast, conversion to both impaired glucose tolerance (IGT) and diabetes among normal glucose tolerance subjects was marked by a large step-up in FPG. Converters had higher baseline BMI and fasting plasma insulin concentrations than nonconverters; however, no consistent change in either parameter had occurred before conversion. In contrast, changes in 2-h post-glucose insulin levels between time of conversion and preceding measurement were inversely related to the changes in FPG. Thus, within a 3-year time frame, the onset of diabetes is often rapid rather than gradual and is in part explained by a fall in glucose-stimulated insulin response.
The acute insulin response
The natural course of β-cell function suggests that the acute insulin response plays a major role in determining glucose tolerance status over time. Among Pima Indians, over a mean of 5.1 years, progressors (from normal glucose tolerance to IGT and then diabetes) differed significantly from nonprogressors in their acute insulin response. Acute insulin response decreased by 27% during the transition from normal to impaired glucose tolerance and by 51% during the transition from impaired glucose tolerance to diabetes, and in nonprogressors, it actually increased by 30% (5).
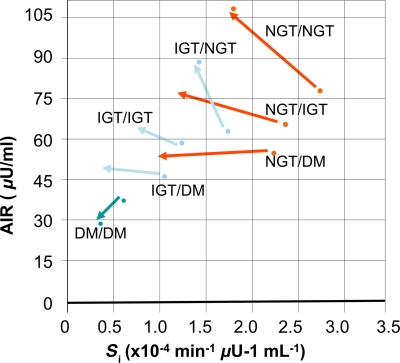
Festa et al. assessed longitudinal changes in β-cell function over 5 years (Fig. 1). Again, the main determinant of glucose tolerance status during follow-up was the change in acute insulin response. Normal glucose tolerance was maintained by a compensatory increase in insulin secretion, whereas failure to increase insulin secretion led to impaired glucose tolerance, and a decrease in insulin secretion led to overt diabetes (6).
Figure 1.
Changes of insulin sensitivity (Si) and acute insulin response to glucose (AIR) from baseline (arrow base) to follow-up (arrow top) in populations stratified by baseline and follow-up glucose tolerance status (6). DM, diabetes; NGT, normal glucose tolerance.
Thus, the progressive decrease in β-cell insulin secretion, particularly the first-phase insulin secretion that occurs acutely after an increase in glycemia, is likely the most critical functional β-cell defect in the development of type 2 diabetes.
Progression to medication
The next step in progression could be defined by the need for medication. Pani et al. (7) examined predictors of diabetes progression (A1C ≥7% or initiation of hypoglycemic agent) over 1 year in 705 patients who had A1C <7% and were not on glucose-lowering medications at baseline. In the 200 patients who progressed, baseline A1C, younger age, and weight gain were independent predictors of progression. Each decade of increasing age reduced the risk of progression by 15%. Each 1-lb increase in weight was associated with a 2% increased odds of progression. Likelihood of medication initiation among progressors decreased by 40% (P = 0.02) with every decade of age and decreased by 2.3% with each 1 mg/dl decrease in LDL level from baseline after adjusting for race, sex, and weight change. Thus, among untreated patients with A1C <7%, younger patients and those with weight gain were more likely to have diabetes progression and should be the focus of aggressive diabetes management. A limitation of this study is that physician bias may have precluded or delayed initiation of treatment in older patients. This study provides indirect evidence that the pathogenesis of type 2 diabetes in subjects who develop diabetes at a younger age is different from that of older subjects and younger patients should be managed more aggressively with earlier initiation of medications. Conversely, there may be an identifiable subset of older patients with stable weight who may be followed without initiating pharmacological therapy.
Loss of glycemic control on medication
Major clinical trials provide evidence of the increasing loss of glycemic control over time in type 2 diabetes. The U.K. Prospective Diabetes Study (UKPDS) showed that therapy with metformin, sulfonylurea, or insulin substantially lowered A1C and FPG compared with conventional therapy, but over 11 years, increased significantly (8,9). A similar pattern with sulfonylureas was observed more recently in A Diabetes Outcome Progression Trial (ADOPT), over a median of 4.0 years (10).
Matthews et al. (11) assessed the predictors of sulfonylurea failure in the UKPDS. By 6 years, 44% had required additional therapy. Of those randomized to glibenclamide, 48% required additional therapy by 6 years, compared with 40% of those allocated to chlorpropamide. Not surprisingly, a higher initial fasting glucose predicted greater need for additional therapy.
In the initial 3 years, nonobese subjects (BMI <30 kg/m2) were more likely to require additional therapy than obese patients (BMI ≥30 kg/m2). Modeled β-cell function showed that those with lower function were more likely to fail (P < 0.0001). Thus, sulfonylureas fail as a therapeutic agent at rates that are dependent both on the phenotype at presentation and perhaps on the agent used initially. Higher failure rates were found in individuals with higher glucose concentrations, those who were younger, those with lower β-cell reserve, and those randomized to glibenclamide compared with chlorpropamide.
β-Cell function decline: the major cause of disease progression
A hallmark of type 2 diabetes is a decline in β-cell function, which begins as early as 12 years before diagnosis and continues throughout the disease process. Using the homeostasis model assessment (HOMA) to quantify β-cell function, the UKPDS demonstrated that, β-cell function continued to deteriorate in association with progressively increasing hyperglycemia despite treatment (12).
As β-cell function continues to decline, monotherapy failure (in ADOPT defined as FPG >180 mg/dl) is almost inevitable. In ADOPT, monotherapy with metformin, rosiglitazone, and glyburide all failed over time, albeit with differences in the rates of decline. At 5 years, the cumulative incidence of monotherapy failure was 15% with rosiglitazone, 21% with metformin, and 34% with glyburide. After initial improvement in glycemia, glyburide had the greatest annual increases in A1C and FPG (0.24 and 5.6 mg/dl, respectively), followed by metformin (0.14 and 2.7 mg/dl, respectively) and rosiglitazone (0.07 and 0.7 mg/dl, respectively) (10).
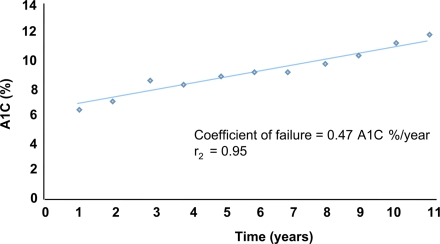
Attempts to quantify β-cell failure include the work of Wallace and Matthews, who plotted A1C against time to derive a coefficient of failure for subjects with diabetes on constant monotherapy. In the UKPDS, the mean coefficient of failure with chlorpropamide and glibenclamide was 0.34 A1C%/year and 0.50 HbA1%/year, respectively (13). The coefficient of failure has several advantages in assessing β-cell failure rates: it uses rates of change, rather than absolute values; it can use other measures of glycemia besides A1C; and it allows comparisons between trials and thus meta-analyses.
Because A1C will increase by ∼1% every 2 years even with most therapies (Fig. 2), patients with diabetes require repeated and vigorous intervention. Failure to implement such interventions, owing to “clinical inertia” or patient noncompliance, results in worsening glucose control and perpetuates a vicious circle of hyperglycemia and glucose toxicity. Importantly, failure of β-cell function in the late stages of the disease is further compounded by the complications of diabetes and by the likelihood of significant comorbidities in elderly patients.
Figure 2.
Illustration of coefficient of β-cell failure over time in relation to A1C (from UKPDS) (13).
Natural history of obesity: progressive weight gain
Weight gain is another common concern as type 2 diabetes progresses. In UKPDS 34, patients treated with insulin experienced the greatest weight gain over 10 years, followed by sulfonylurea treatment; weight gain was lowest and similar in conventional (diet) and metformin treatment groups (8). In the Treat-to-Target trial, weight gain at the end of 6 months was 3.0 and 2.8 kg with bedtime glargine and NPH insulin, respectively (14). In ADOPT, weight gain with rosiglitazone was almost 5 kg over 5 years. In contrast, with glyburide, weight gain of 1.6 kg occurred in the first year, but stabilized thereafter, and weight decreased by ∼3 kg in the metformin group (10). The contribution of weight gain to disease progression is unclear. On the other hand, weight loss is associated with improved β-cell function and a decreased need for treatment.
PATHOPHYSIOLOGY OF β-CELL FAILURE
Pancreatic β-cells normally respond to insulin resistance by increasing their output of insulin to meet the needs of tissues. Development of type 2 diabetes essentially stems from a failure of the β-cell to adequately compensate for insulin resistance. The β-cell dysfunction progresses over time and is well advanced by the time a person's plasma glucose level is in the diabetic range and continues to worsen after diabetes develops (12).
Many obese individuals, who tend to have insulin resistance, progress to diabetes. Yet some do not: their β-cells continue to function adequately and they are able to maintain glucose homeostasis and compensate for increasing insulin resistance with increasing insulin secretion.
Genetic predisposition to β-cell failure
Data strongly support a genetic predisposition to β-cell failure (15). A genetic subtype of the disease characterized by diagnosis at <25 years of age, β-cell dysfunction, an autosomal dominant mode of inheritance, and heterozygous mutations in β-cell transcription factors has been identified as a common cause of early-onset type 2 diabetes (16). Multiple genetic mutations have been identified, and in some affected individuals, a genetic cause for their disease is recognized (17). However, in most patients in clinical practice, it is impossible to identify a genetic abnormality clinically and environmental factors predominate.
Mechanisms responsible for the decline in insulin secretion
Normal β-cell adaptation to insulin resistance can occur through increased insulin secretion from each β-cell and/or an increase in the β-cell mass. Some individuals have a reduced insulin secretion or reduced β-cell mass but normal glucose levels; they have sufficient insulin sensitivity to ensure adequate insulin secretion. In insulin-resistant subjects or subjects with type 2 diabetes, there is inadequate insulin secretion from each β-cell or an inadequate β-cell mass for the levels of prevailing insulin sensitivity (5).
When blood glucose is elevated, insulin secretion is stimulated and glucagon secretion is suppressed. Conversely, when blood glucose is decreased, insulin secretion should be suppressed and glucagon secretion stimulated. All of these actions are highly glucose dependent and critical to maintain normal glycemia in the face of varying insulin needs. They also provide the classic response to a meal. Although the failing β-cell loses its ability to respond to glucose, not all responses are diminished. Insulin secretion, e.g., in response to amino acid stimulation or through stimulation with other hormones such as glucagon-like peptide 1 (GLP-1), is preserved.
β-Cells maintain their responsiveness in the face of insulin resistance through increased insulin secretion in response to meals as well as through a chronic response by increasing β-cell mass (18). Normal-weight and obese individuals maintain a normal and similar 24-h glucose response to meals. However, the groups differ in their mean insulin secretion, which is significantly higher in obese subjects than in their normal-weight counterparts. In addition, insulin secretion in obese subjects fails to return to baseline between meals (19).
Two acquired defects have been implicated with regard to impaired glucose secretion: glucotoxicity, whereby β-cells become sensitized to the presence of glucose, and lipotoxicity, whereby accumulated fatty acids and their metabolic products deleteriously affect β-cells. In glucotoxicity, chronic hyperglycemia depletes insulin secretory granules from β-cells, lessening the amount of insulin available to be released in response to new glucose stimuli. Lowering glucose levels permits regranulation of β-cells and a better acute insulin response follows. In lipotoxicity, prolonged increases in free fatty acid levels adversely affect the conversion of proinsulin to insulin and eventually affect insulin secretion. Fatty infiltration of pancreatic islets may also contribute to β-cell dysfunction, and pancreatic fat correlates negatively with β-cell function (20). But once diabetes occurs, factors additional to pancreatic fat (perhaps glucose toxicity) account for further β-cell function decline. The concepts of gluco- and lipotoxicity remain hypotheses; the exact mechanisms responsible for impaired β-cell function have yet to be conclusively proved. This concept is being tested further in clinical trials such as ORIGIN (21), where insulin will be used early to eliminate glucotoxicity and determine whether early and maintained normoglycemia will decrease disease progression. The concept of remission in diabetes by elimination of glucotoxicity has actually been tested in a few small studies (22).
In addition to glucose and lipid deposition in the pancreas, another local factor may be the accumulation of amyloid, which has long been associated with the development and progression of type 2 diabetes (23).
Thus, in type 2 adipogenic diabetes, excessive carbohydrate and fat intake causes hyperinsulinemia in association with increased hepatic lipoprotein secretion, adipose tissue growth, and increased free fatty acid levels in genetically susceptible individuals. Together with episodes of postprandial hyperglycemia, elevated free fatty acid levels cause muscle and liver insulin resistance and increase hepatic glucose production. The same stimuli also facilitate β-cell compensation by promoting insulin secretion and biosynthesis as well as β-cell growth. In late stages, however, the progressive rise in insulin resistance, combined with alterations in β-cell gene expression and signaling induced by rising levels of free fatty acids, cause β-cell failure. Overt diabetes occurs as a result of this β-cell decompensation, with altered insulin secretion and apoptosis as possible contributing factors.
β-Cell mass deficits
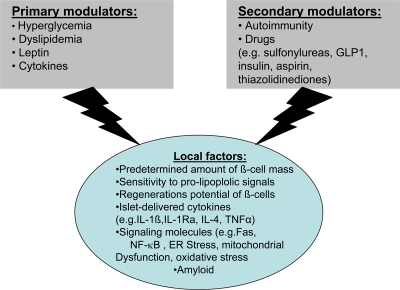
Although β-cell function is paramount, decreasing β-cell mass is an important factor in progression of type 2 diabetes. β-Cell mass is increased by neogenesis, as well as replication and hypertrophy. In individuals who do not have diabetes, these activities are counterbalanced by apoptosis and necrosis, thereby maintaining a balance in β-cell mass. In individuals who are obese or insulin resistant, the number of islets and β-cells, in the presence of increased insulin demand, increase with some degree of hypertrophy (18). Several factors and mechanisms regulate β-cell mass, and only in a minority of diabetic patients does one single etiological factor underlie the failure of the β-cell. The various factors regulating mass are summarized in Fig. 3 (24). In animal models of insulin resistance, there is both replication of existing β-cells and neogenesis from ductal precursor cells. In the Zucker diabetic fatty rat model, Pick et al. (25) determined β-cell mass and replication rates. In nondiabetic but obese rats, the size of the islets increased. In contrast, obese diabetic rats showed slight decreases in islets and in the amount of insulin stained, whereas glucagon was either maintained or increased (25). The β-cell replication rate was significantly greater in Zucker diabetic fatty rats than in either lean control or obese nondiabetic animals. In addition, increased apoptosis, rather than decreased neogenesis, is the major factor responsible for reduction in β-cell mass (25).
Figure 3.
Factors regulating β-cell mass (24).
In human autopsy pancreatic tissue (18), subjects with impaired fasting glucose and type 2 diabetes had a relatively reduced β-cell mass, whether they were lean or obese. Obese subjects without diabetes had an ∼50% increase in relative β-cell volume. Obese subjects with impaired fasting glucose or type 2 diabetes had 40 and 63% deficits, respectively, in relative β-cell volume than obese subjects without diabetes. These in vivo findings suggest that a decreased number of β-cells, rather than a decreased volume of individual cells, causes β-cell volume decrease. Subjects with impaired fasting glucose also had decreased relative β-cell volume, suggesting that this is an early process and mechanistically important in the development of type 2 diabetes (18). In another recent autopsy study, there was a significant curvilinear relation between β-cell volume and fasting blood glucose level, and β-cell deficiency was associated with a steep increase in blood glucose with further decrement in β-cell mass (26).
Abnormalities in the pancreatic islets may also contribute to deficits in β-cell mass with type 2 diabetes. Insulin secretion from islets of organ donors who had diabetes was significantly less than that of control subjects, and islet yield decreased as disease duration lengthened (27).
Imaging studies substantiate that the pancreas declines in size as type 2 diabetes progresses. More than 20 years ago, we used ultrasound to show some decrease in early type 2 disease and a significant decrease in later-stage disease with declining β-cell function and mass (28). Using computerized tomography, Goda et al. demonstrated that pancreatic volume and pancreatic volume index were greatest in the healthy group and lowest in type 1 diabetes, although subjects with type 2 disease did not differ significantly compared with control subjects (29).
Does the amount of pancreatic mass matter or can the residual mass take on the load? An intriguing insight is provided by a study on donors of pancreatic tissue. Hemipancreatectomy for the purpose of organ donation has been associated with a 25% risk of developing abnormal glucose tolerance or diabetes in the year after surgery (30), and 43% of healthy humans who underwent hemipancreatectomy have impaired fasting glucose, impaired glucose tolerance, or diabetes on follow-up. These findings are compatible with the notion that a loss of 50% of pancreatic function is associated with the development of diabetes. Matveyenko et al. (31) studied the effect of an ∼50% deficit in β-cell mass on carbohydrate metabolism in dogs. After partial pancreatectomy, both basal and glucose-stimulated insulin secretion were decreased through the mechanism of a selective ∼50 and ∼80% deficit in insulin pulse mass, respectively. These defects in insulin secretion were partially offset by decreased hepatic insulin clearance (P < 0.05). Partial pancreatectomy also caused an ∼40% decrease in insulin-stimulated glucose disposal. Thus, an ∼50% deficit in β-cell mass can recapitulate the alterations in glucose-mediated insulin secretion and insulin action in humans with IFG and IGT, supporting a mechanistic role of a deficit in β-cell mass in the evolution of IFG/IGT and diabetes.
β-Cell inflammation in type 2 diabetes
Inflammation is not in itself a disease, but a manifestation of disease that may prevent spread of infections or promote organ regeneration. Equally, it may exacerbate disease by tissue destruction due to inflammatory mediators, reactive oxygen species, and complement components (32). Pancreatic islets from type 2 diabetic patients are known to have amyloid deposits, fibrosis, and increased cell death (18,32,33), associated with an inflammatory response. Pancreatic α-cells produce increased IL-1α and other inflammatory factors in response to glucotoxicity and nutrients (32,34,35).
α-Cell pancreatic function
Although the focus herein has been on β-cell function, some attention must be paid to α-cell pancreatic function in the progression of diabetes. Glucose and a variety of hormones and substrates work to regulate glucagon secretion in a coordinated manner, and abnormalities of α-cells may reflect impaired glucose sensing. In type 2 diabetes, relative glucagon hypersecretion occurs at normal and elevated levels of glucose and an impaired response to hypoglycemia. The incretin hormone GLP-1, which promotes assimilation of ingested nutrients via a glucose-dependent stimulation of insulin release, apparently improves α-cell glucose sensing. Thus, GLP-1–based therapies improve α-cell function and may also prove to be useful in improving glycemic control in diabetes (36).
Microvascular and macrovascular complications
Longer survival times and development of type 2 diabetes at younger ages increase the risk of developing duration-dependent complications. In UKPDS 16, 18% of patients, all of whom were presumed to be clinically healthy, had a clinical end point within 6 years of diagnosis.
UKPDS 35 showed highly significant associations between development of diabetes complications, including death, across the broad range of exposure to glycemia, with no evidence of a threshold. Conversely, each 1% reduction in mean A1C was associated with reduction in risk of 21% for any end point related to diabetes (P < 0.0001) (1).
The role of complications on disease progression and failure has not been well studied. A change in insulin sensitivity and clearance is well recognized in renal failure and clearance is well recognized. However, the impact of these changes on the natural history of diabetes itself needs to be studied. Many patients with established complications tend to be poorly controlled, and factors such as glucose toxicity may play a role in disease progression as discussed above. In addition, various cardiovascular drugs such as diuretics and β-blockers may affect β-cell function adversely.
CONCLUSIONS
In type 2 diabetes, β-cells fail to adapt to impaired glucose tolerance. This failure appears to be related to a reduction in insulin secretion per islet as well as a reduction in the total number of islets. Progressive loss of β-cell function and, to a lesser extent, reduced β-cell mass lead to worsening glycemic control and development of complications. Although they lower glucose, current therapies do not completely abolish this progressive loss of β-cell function, and their use is also associated with hypoglycemia and weight gain (Table 2). Thus, the need for additional glucose-lowering therapies that can halt β-cell deterioration without contributing to weight gain continues.
Table 2.
Strategies to decrease/delay disease progression
| Strategy | Possible treatment approach |
|---|---|
| Weight loss | |
| Eliminate glucose toxicity | Early treatment early insulin |
| Eliminate lipotoxicity | Thiazolidinediones, decrease free fatty acids |
| Decrease apoptosis and increase regeneration | Incretin therapies |
Acknowledgments
V.A.F. was supported in part by the American Diabetes Association, National Institutes of Health (ACCORD and TINSAL type 2 diabetes trials), and the Earl Madison Ellis fund and the Tullis-Tulane Alumni Chair in Diabetes supporting diabetes research at Tulane University Health Sciences Center. V.A.F. and Tulane University have Research Support Grants and honoraria for consulting and lectures from GlaxoSmithKline, Novartis, Novo Nordisk, Takeda, Astra-Zeneca, Pfizer, sanofi-aventis, Eli Lilly, Daiichi-Sankyo, and Novartis.
No other potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols GA, Hillier TA, Brown JB: Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care 2007; 30: 228– 233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R: Baltimore Longitudinal Study of Aging The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003; 52: 1475– 1484 [DOI] [PubMed] [Google Scholar]
- 4. Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP: Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes 2004; 53: 160– 165 [DOI] [PubMed] [Google Scholar]
- 5. Weyer C, Tataranni PA, Bogardus C, Pratley RE: Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001; 24: 89– 94 [DOI] [PubMed] [Google Scholar]
- 6. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM: The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study Diabetes 2006; 55: 1114– 1120 [DOI] [PubMed] [Google Scholar]
- 7. Pani LN, Nathan DM, Grant RW: Clinical predictors of disease progression and medication initiation in untreated patients with type 2 diabetes and A1C less than 7%. Diabetes Care 2008; 31: 386– 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33): UK Prospective Diabetes Study (UKPDS) group Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 9. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34): UK Prospective Diabetes Study (UKPDS) group Lancet 1998; 352: 854– 865 [PubMed] [Google Scholar]
- 10. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 [DOI] [PubMed] [Google Scholar]
- 11. Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC: UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years: UK Prospective Diabetes Study (UKPDS) Group Diabet Med 1998; 15: 297– 303 [DOI] [PubMed] [Google Scholar]
- 12. U.K. Prospective Diabetes Study 16: overview of 6 years' therapy of type II diabetes: a progressive disease: U.K. Prospective Diabetes Study Group. Diabetes 1995; 44: 1249– 1258 [PubMed] [Google Scholar]
- 13. Wallace TM, Matthews DR: Coefficient of failure: a methodology for examining longitudinal beta-cell function in type 2 diabetes. Diabet Med 2002; 19: 465– 469 [DOI] [PubMed] [Google Scholar]
- 14. Riddle MC, Rosenstock J, Gerich J: The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26: 3080– 3086 [DOI] [PubMed] [Google Scholar]
- 15. Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE: Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007; 30: 677– 682 [DOI] [PubMed] [Google Scholar]
- 16. Frayling TM, Evans JC, Bulman MP, Pearson E, Allen L, Owen K, Bingham C, Hannemann M, Shepherd M, Ellard S, Hattersley AT: Beta-cell genes and diabetes: molecular and clinical characterization of mutations in transcription factors. Diabetes 50 ( Suppl. 1); 2001: S94– S100 [DOI] [PubMed] [Google Scholar]
- 17. Stride A, Hattersley AT: Different genes, different diabetes: lessons from maturity-onset diabetes of the young. Ann Med 2002; 34: 207– 216 [PubMed] [Google Scholar]
- 18. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 19. Polonsky KS, Given BD, Van Cauter E: Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 1988; 81: 442– 448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M: Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30: 2916– 2921 [DOI] [PubMed] [Google Scholar]
- 21. Origin Trial I, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J: Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008; 155: 26– 32, 32.e1–6 [DOI] [PubMed] [Google Scholar]
- 22. Banerji MA, Chaiken RL, Lebovitz HE: Long-term normoglycemic remission in black newly diagnosed NIDDM subjects. Diabetes 1996; 45: 337– 341 [DOI] [PubMed] [Google Scholar]
- 23. Kahn SE, Andrikopoulos S, Verchere CB: Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes (Review). Diabetes 1999; 48: 241– 253 [DOI] [PubMed] [Google Scholar]
- 24. Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M: Mechanisms of beta-cell death in type 2 diabetes. Diabetes 54 ( Suppl. 2); 2005: S108– S113 [DOI] [PubMed] [Google Scholar]
- 25. Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS: Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998; 47: 358– 364 [DOI] [PubMed] [Google Scholar]
- 26. Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC: Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care 2006; 29: 717– 718 [DOI] [PubMed] [Google Scholar]
- 27. Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF: Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 2004; 53: 624– 632 [DOI] [PubMed] [Google Scholar]
- 28. Fonseca V, Berger LA, Beckett AG, Dandona P: Size of pancreas in diabetes mellitus: a study based on ultrasound. Br Med J Clin Res Ed 1985; 291: 1240– 1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T: Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol 2001; 38: 145– 149 [DOI] [PubMed] [Google Scholar]
- 30. Kumar AF, Gruessner RW, Seaquist ER: Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care 2008; 31: 1639– 1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matveyenko AV, Veldhuis JD, Butler PC: Mechanisms of impaired fasting glucose and glucose intolerance induced by an approximate 50% pancreatectomy. Diabetes 2006; 55: 2347– 2356 [DOI] [PubMed] [Google Scholar]
- 32. Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA: Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31 ( Suppl. 2); 2008: S161– S164 [DOI] [PubMed] [Google Scholar]
- 33. Hull RL, Westermark GT, Westermark P, Kahn SE: Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 3629– 3643 [DOI] [PubMed] [Google Scholar]
- 34. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY: Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002; 110: 851– 860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T: Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev 2008; 29: 334– 350 [DOI] [PubMed] [Google Scholar]
- 36. Dunning BE, Foley JE, Ahren B: Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia 2005; 48: 1700– 1713 [DOI] [PubMed] [Google Scholar]