It is generally recognized that type 2 diabetes is one of the major challenges of the 21st century. Its prevalence is growing rapidly worldwide, particularly in developing countries (1). It remains the first cause of blindness, end-stage renal disease, and nontraumatic lower-limb amputation and one of the major causes of cardiovascular disease (2,3). Because of its high morbidity and excess mortality, it has become a tremendous burden on health care cost (4).
In the last decade, a number of interventions have been shown to be effective in the prevention of diabetes in high-risk populations with impaired glucose tolerance and/or impaired fasting glucose (5–7). Yet, few countries have adopted policies for the screening and strategies for treating subjects with pre-diabetes. Furthermore, despite the development of newer therapies for treatment of diabetes, the disease is still associated with a high incidence of microangiopathy and macrovascular complications. Any new strategies that could help to curtail the burden of diabetes would be greatly appreciated.
More recently, it was postulated that early insulin replacement in type 2 diabetes, and perhaps even in pre-diabetes, may reduce cardiovascular risk and may even offer protection for the β-cell (8,9). However, a closer look at the available data does not support the hypothesis. The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) study is still ongoing and therefore does not allow any conclusion in pre-diabetes. In type 2 diabetes, however, the published data support neither a beneficial effect of insulin therapy per se on microvascular complications, nor on cardiovascular events and mortality. In addition, the data suggesting that insulin therapy can improve the β-cell response to a glucose challenge could just as well be interpreted as being due to the improvement in glycemic control, rather than to the insulin therapy. And finally, we should not underestimate the adverse events associated with insulin treatment.
INSULIN THERAPY AND MICROVASCULAR COMPLICATIONS
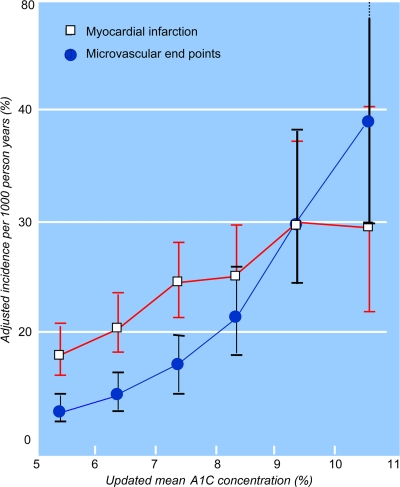
The Kumamoto study was the first prospective randomized controlled trial showing that multiple insulin injection therapy in type 2 diabetes, compared with conventional insulin treatment, was associated with a significant reduction in the onset and progression of retinopathy, nephropathy, and neuropathy in a relatively small number (n = 101) of Japanese patients with type 2 diabetes (10). Nevertheless, the pivotal study on the effect of glycemic control on the progression of microvascular complications in type 2 diabetes remains the U.K. Prospective Diabetes Study (UKPDS). In this landmark study, 4,209 newly diagnosed type 2 diabetic patients were randomized to receive conventional therapy (n = 1,138) or intensive treatment (n = 3,071) (11,12). Those randomized to intensive policy were further randomized to sulfonylurea (n = 1,573) or insulin (n = 1,156), and a small number of overweight patients were randomized to metformin (n = 342). Over a 10-year period, median A1C was 7.0% in the intensive treatment group with sulfonylurea and insulin, compared with 7.9% in the conventional treatment group, an absolute 0.9% reduction. There was no difference in A1C among the various therapies in the intensive group. Compared with the conventional group, the intensive treatment was associated with a relative risk reduction of 12% for any diabetes-related end points (P = 0.029), 25% for microvascular end points (P = 0.0099), 21% for retinopathy at 12 years (P = 0.015), and 33% for albuminuria at 12 years (P = 0.000054) (Table 1) (11). The median A1C for the metformin treatment group was 7.4% compared with 8.0% for the conventional group, a 0.6% difference. This lower A1C was also associated with a significant difference in the risk of any diabetes-related end points (32% risk reduction; P = 0.0023) (12). Furthermore, there was a linear relationship between A1C and the risk of microvascular complications (Fig. 1) (13). For any 1% reduction in A1C, there was an estimated risk reduction of microvascular complications of 37%. Of importance is that the UKPDS authors stated clearly that “no difference in the risk reduction of microvascular clinical end points was seen between the three intensive treatments, and thus, improved glycemic control, rather than any one therapy, is the principal factor” (11). Therefore, we can conclude that, in the UKPDS, early insulin treatment offered any advantage over other therapies in the prevention of microvascular complications.
Table 1.
Effect of intensive glycemic control with sulfonylurea or insulin on diabetes-related complications
| Relative risk reduction (%) | End points | P |
|---|---|---|
| 12 | Any diabetes-related end point | 0.029 |
| 25 | Microvascular end points | 0.0099 |
| 16 | Myocardial infarction | 0.052 |
| 24 | Cataract extraction | 0.046 |
| 21 | Retinopathy at 12 years | 0.015 |
| 33 | Albuminuria at 12 years | 0.000054 |
Figure 1.
The relationship between A1C and the incidence of microvascular end points and myocardial infarction. Reproduced with permission from Stratton et al. (13).
INSULIN THERAPY AND MACROVASCULAR COMPLICATIONS
The data supporting a protective role for exogenous insulin against cardiovascular complications is not more convincing. In fact, a tremendous amount of literature going back 50 years suggests that hyperinsulinemia and/or exogenous insulin injection is associated with increased cardiovascular disease (14). The first study suggesting that intensive insulin treatment could have a beneficial effect on cardiovascular mortality in type 2 diabetes after myocardial infarction was the Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 1 study (15). In this study, 620 subjects admitted for acute myocardial infarction and previously known to have diabetes were allocated to standard treatment plus insulin-glucose infusion for at least 24 h and then randomized to either multiple insulin injections (intensive group) or standard treatment (control group). After a mean follow-up of 3.4 years, there was a relative risk reduction of 28% in the intensive insulin group (P = 0.011). The reduction in mortality was most apparent in those patients who had never been treated with insulin before and who had a lower cardiovascular risk (15). Nonetheless, the DIGAMI 2 study did not confirm the first observation that an early and acutely introduced insulin therapy followed by long-term insulin treatment improves survival rate in type 2 diabetic patients after myocardial infarction, compared with conventional management (16). In this study, 1,253 patients with type 2 diabetes and acute myocardial infarction were randomly assigned to acute insulin-glucose infusion followed by insulin-based long-term glucose control (group 1; n = 474), to insulin-glucose infusion followed by standard glucose control (group 2; n = 473), or to routine metabolic management according to local practice (group 3; n = 306). The primary end point was all-cause mortality. These patients were followed for a median of 2.1 years, and no significant difference was observed in mortality rate among the three groups. In fact, there was no difference in glycemic control between the three groups. However, the epidemiological analysis of the data confirms that the glycemic control was a strong and independent predictor of long-term mortality. Again, this suggests that the blood glucose control is of importance, not the manner in which it is achieved.
A further randomized-controlled trial where insulin therapy in type 2 diabetes was compared with other therapies on cardiovascular disease is the UKPDS (11). Overall, intensive glycemic control was associated with a 16% relative risk reduction in myocardial infarction (hazard ratio [HR] 0.84 [95% CI 0.71–1.0]; P = 0.052). Much has been written about the P value of 0.052; although the purist statisticians interpret this as nonsignificant, the clinicians find it clinically relevant. Even then, insulin treatment did not perform better than the other oral agents in the intensive group. In fact, only metformin as a subgroup showed a significant reduction in myocardial infarction compared with the conventional group (HR 0.61 [0.41–0.89]; P = 0.01), but it was not significantly different from the other intensive treatment, including insulin. Furthermore, the epidemiological analysis of the data showed a linear relationship between the A1C and the risk of myocardial infarction (Fig. 1) (13). For any 1% reduction in A1C, there was a 14% decrease in the risk of myocardial infarction (P < 0.0001). Again, this strongly suggests that glycemic control is essential, independent of how it is achieved. Interestingly, the 10-year follow-up analysis of the UKPDS showed a 9% significant reduction in myocardial infarction in the insulin and/or sulfonylurea group (P = 0.04) and a continued benefit of metformin (33%, P = 0.005) (17). These results emphasized the importance of reducing blood glucose early in the disease, independent of the antidiabetic medications used, and suggest that the benefit is long term.
INSULIN THERAPY AND THE β-CELL
Garvey et al. (18) published an elegant study of 14 poorly controlled type 2 diabetic patients submitted to intensive insulin therapy. All patients were submitted to an intravenous glucose tolerance test before and after 3 weeks of intensive treatment with continuous subcutaneous insulin infusion. After achieving near-normal plasma glucose, continuous subcutaneous insulin infusion resulted in a significant improvement in the first- and second-phase insulin secretion, as well as in C-peptide in response to the intravenous glucose challenge (P < 0.01). This was confirmed by Ryan et al. (19) in 16 newly diagnosed type 2 diabetic patients put on 2–3 weeks of intensive insulin therapy, followed up for 1 year. There was a major improvement in the glucose profile in response to a 75-g oral glucose tolerance test (OGTT) and a small but significant improvement in insulin response immediately after the short-term intensive insulin therapy. At 1 year, good glycemic control was maintained and insulin response to an OGTT was further improved (P < 0.01).
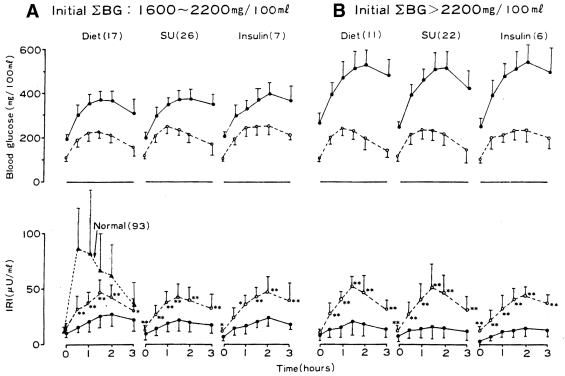
However, Kolterman et al. (20) showed similar observations in type 2 diabetes treated intensively with glyburide. In 17 patients, serum glucose and insulin levels were measured at hourly intervals throughout the day, during which they had a standardized breakfast and lunch. This assessment was carried out before and after 3 months of glyburide therapy. The fasting and the postprandial serum glucose levels were significantly reduced after 3 months of therapy (P < 0.02 and P < 0.001, respectively). Although the fasting serum levels were not significantly different, the postprandial insulin levels were significantly increased in response to the standardized meals after 3 months of glyburide treatment (P < 0.001). Interestingly, Kosaka et al. (21), as early as 1980, had already shown that after intensive treatment in poorly controlled type 2 diabetic patients, the improvement in insulin response to a glucose challenge was independent of the mode of treatment. Eighty-nine poorly controlled patients with type 2 diabetes who attended the outpatient clinic were treated with either diet alone (n = 28), sulfonylurea plus diet (n = 48), or insulin plus diet (n = 13). They were all submitted to a 100-g OGTT before treatment and after 6 months of intensive therapy; for the insulin-treated group, however, the OGTT was done after 2 weeks of treatment to avoid interference with insulin antibodies in the radioimmunoassay. The degree of improvement of insulin response to the OGTT was similar among the three treatment groups, and the glucose tolerance curves were improved to a similar extent (Fig. 2) (21).
Figure 2.
Changes in blood glucose (BG) and plasma insulin (immunoreactive insulin [IRI]) during a 100-g glucose tolerance test before and after treatment with diet only, sulfonylurea (SU), or insulin of overtly diabetic patients (mean ± SD) according to the initial sum of blood glucose. Number in parentheses indicates number of patients before treatment (●) and after treatment (○). Reproduced with permission from Kosaka et al. (21).
It is therefore concluded that intensive glucose treatment in type 2 diabetes will improve the β-cell response to a glucose challenge independently of the antidiabetic medication used. It is believed that this is due to a reduction in glucose toxicity at the β-cell level.
INSULIN THERAPY AND ITS ADVERSE EVENTS
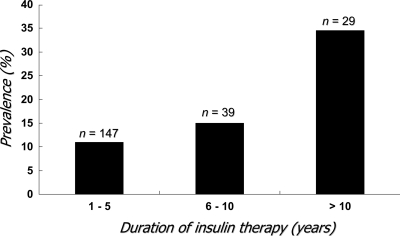
Hypoglycemia is considered to occur less frequently in type 2 diabetes than in type 1 diabetes. However, in recent years, the pursuit of strict glycemic control in type 2 diabetes has encouraged the earlier introduction of insulin and the use of more intensive regimens. This is bound to have a significant impact on the risk of hypoglycemia in these patients. Again, the pivotal study is the UKPDS (11). In this population with newly diagnosed type 2 diabetes, the incidence of any hypoglycemic event in insulin-treated newly diagnosed type 2 diabetic patients was 36.5 per 100 patient-years, at least twice as frequent as in sulfonylurea-treated patients. Although the incidence of severe hypoglycemia was much lower than in type 1 diabetes, it was still 2.3 per 100 patient-years, a four- to sixfold increase compared with the sulfonylurea-treated group. Even more disturbing is that the risk of severe hypoglycemia is far greater with increasing duration of diabetes and insulin therapy. Henderson et al. (22) observed that in those type 2 diabetic patients treated with insulin for over 10 years, the estimated incidence of severe hypoglycemia was 28 episodes per 100 patient-years (Fig. 3). This is nearly half the rate documented during intensive insulin therapy in type 1 diabetes in the Diabetes Control and Complications Trial (23). The risk of severe hypoglycemia should not be underestimated. This does not indicate that we should not start insulin in a timely fashion. But we should try to minimize the occurrence of severe hypoglycemia.
Figure 3.
The prevalence of severe hypoglycemia in relation to the duration of type 2 diabetes. Adapted from Henderson et al. (22).
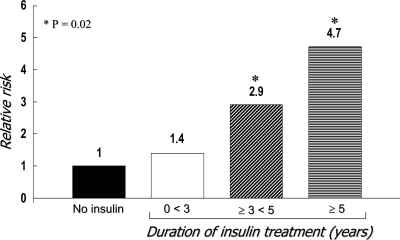
In the UKPDS, intensive insulin therapy was associated with a 5-kg weight gain (11), and this is not just a cosmetic problem. Weight gain is also associated with an increase in cardiovascular risk factors, such as hypertension and dyslipidemia (24). Meigs et al. (24) observed that in 638 obese subjects with the metabolic syndrome, the adjusted risk for cardiovascular disease was 2.13 (1.43–3.18). Furthermore, 6.8% weight loss in type 2 diabetes resulted in a significant improvement of cardiovascular risk factors, such as dyslipidemia and hypertension (25). Finally, in vitro, in vivo, and epidemiological studies suggest that there is a link between insulin concentration and the risk of colorectal cancer. Insulin has been shown to be a growth factor and to stimulate the growth of colorectal cancer precursor cells in animals (26). Clinical studies have shown that high circulating insulin levels are independent predictors of colorectal cancer (27). In addition, type 2 diabetes, a condition associated with hyperinsulinemia, has a 30–40% increased risk of colorectal cancer (28). When endogenous insulin declines, most of these patients will require exogenous insulin injection, thus perpetuating hyperinsulinemia (18). More recently, Yang et al. (29). conducted a retrospective nested case-control study of all patients with a diagnosis of type 2 diabetes (n = 24,918) in the General Practice Research Database from the U.K. between June 1987 and April 2002 and performed follow-up over time for the occurrence of colorectal cancer. The incidence of colorectal cancer in insulin users (n = 3,160) was 197 per 100,000 person-years, compared with 124 per person-years in type 2 diabetic subjects not receiving insulin (n = 21,758). The age- and sex-adjusted HR of colorectal cancer associated with ≥1 year of insulin use was 2.1 (95% CI 1.2–3.4; P = 0.005). The odds ratio (OR) for each incremental year of insulin therapy was an increased risk of 1.21 (95% CI 1.03–1.42; P = 0.02). After 5 years of insulin therapy, the OR was 4.7 (95% CI 1.3–16.7; P = 0.02) (Fig. 4). It was concluded that the chronic use of insulin may increase the risk of colorectal cancer in type 2 diabetic patients (29). Of course, this observation does not confirm a cause-and-effect relationship.
Figure 4.
The relative risk of colorectal cancer in relation to the duration of insulin therapy in type 2 diabetes. Adapted from Yang et al. (29).
CONCLUSIONS
Current evidence clearly indicates that achieving normoglycemia reduces the risk of microvascular complications and strongly suggests that this is probably true also for cardiovascular complications. There is no reliable evidence at the present time to indicate that the earlier use of insulin provides any additional long-term benefit beyond glycemic control. In addition, insulin therapy is associated with adverse effects including hypoglycemia, weight gain, and probably increased risk of colorectal cancer. However, treating to target remains the crucial goal. Insulin must be introduced in a timely fashion, that is, as soon as the oral antidiabetic agents fail to maintain A1C <7.0%.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047– 1053 [DOI] [PubMed] [Google Scholar]
- 2. de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M: Cause-specific mortality in type 2 diabetes: the Verona Diabetes Study. Diabetes Care 1999; 22: 756– 761 [DOI] [PubMed] [Google Scholar]
- 3. Klein R, Klein BEK, Moss SE: Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 1996; 124: 90– 96 [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association: Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care 1998; 21: 296– 309 [DOI] [PubMed] [Google Scholar]
- 5. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343– 1350 [DOI] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M: Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002; 359: 2072– 2077 [DOI] [PubMed] [Google Scholar]
- 8. Gerstein HC, Rosenstock J: Insulin therapy in people who have dysglycemia and type 2 diabetes mellitus: can it offer both cardiovascular protection and beta-cell preservation? Endocrinol Metab Clin North Am 2005; 34: 137– 154 [DOI] [PubMed] [Google Scholar]
- 9. Origin TI, Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J: Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008; 155: 26– 32 [DOI] [PubMed] [Google Scholar]
- 10. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furoyoshi N, Schichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995; 28: 103– 117 [DOI] [PubMed] [Google Scholar]
- 11. U.K. Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837– 853 [PubMed] [Google Scholar]
- 12. U.K. Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854– 865 [PubMed] [Google Scholar]
- 13. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stout RW: Hyperinsulinemia and atherosclerosis. Diabetes 1996; 45 ( Suppl. 3): S45– S46 [DOI] [PubMed] [Google Scholar]
- 15. Malmberg K: Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus: DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 1997; 314: 1512– 1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenstrom A: Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005; 26: 650– 661 [DOI] [PubMed] [Google Scholar]
- 17. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577– 1589 [DOI] [PubMed] [Google Scholar]
- 18. Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG: The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 1985; 34: 222– 234 [DOI] [PubMed] [Google Scholar]
- 19. Ryan EA, Imes S, Wallace C: Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004; 27: 1028– 1032 [DOI] [PubMed] [Google Scholar]
- 20. Kolterman OG, Gray RS, Shapiro G, Scarlett JA, Griffin J, Olefsky JM: The acute and chronic effects of sulfonylurea therapy in type II diabetic subjects. Diabetes 1984; 33: 346– 354 [DOI] [PubMed] [Google Scholar]
- 21. Kosaka K, Kuzuya T, Akanuma Y, Hagura R: Increase in insulin response after treatment of overt maturity onset diabetes mellitus is independent of the mode of treatment. Diabetologia 1980; 18: 23– 28 [DOI] [PubMed] [Google Scholar]
- 22. Henderson JN, Allen KV, Deary IJ, Frier BM: Hypoglycaemia in insulin-treated type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med 2003; 20: 1016– 1021 [DOI] [PubMed] [Google Scholar]
- 23. DCCT Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 [DOI] [PubMed] [Google Scholar]
- 24. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB: Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006; 91: 2906– 2912 [DOI] [PubMed] [Google Scholar]
- 25. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ: Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007; 30: 1374– 1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tran TT, Medline A, Bruce WR: Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev 1996; 5: 1013– 1015 [PubMed] [Google Scholar]
- 27. Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ: Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999; 91: 1147– 1154 [DOI] [PubMed] [Google Scholar]
- 28. Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speizer FE, Giovannucci E: Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst 1999; 91: 542– 547 [DOI] [PubMed] [Google Scholar]
- 29. Yang YX, Hennessy S, Lewis JD: Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 2004; 127: 1044– 1050 [DOI] [PubMed] [Google Scholar]