In the last few decades, the occurrence of type 2 diabetes has rapidly increased internationally, and it has been estimated that the number of diabetic patients will more than double within 15 years (1). Type 2 diabetes is mainly characterized by the development of increased morbidity and mortality for cardiovascular disease (CVD); thus, it has been suggested that diabetes may be considered a CVD (1). However, diabetes is also characterized by dramatic microangiophatic complications, such as retinopathy, nephropathy, and neuropathy (1).
Recent evidence suggests that glucose overload may damage the cells through oxidative stress (2). This is currently the basis of the “unifying hypothesis” that hyperglycemia-induced oxidative stress may account for the pathogenesis of all diabetic complications (2).
CENTRAL ROLE OF OXIDATIVE STRESS IN THE PATHOGENESIS OF DIABETIC COMPLICATIONS
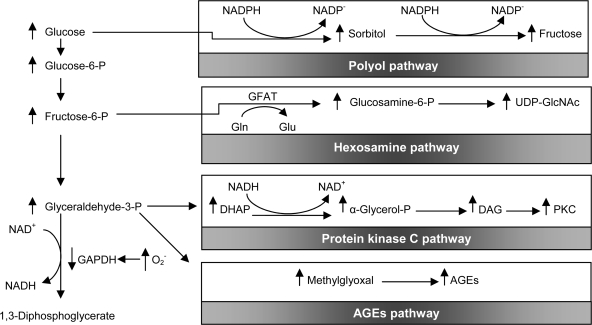
It has been suggested that the following four key biochemical changes induced by hyperglycemia are all activated by a common mechanism—overproduction of superoxide radicals (2): 1) increased flux through the polyol pathway (in which glucose is reduced to sorbitol, reducing levels of both NADPH and reduced glutathione); 2) increased formation of advanced glycation end products (AGEs); 3) activation of protein kinase C (with effects ranging from vascular occlusion to expression of proinflammatory genes), and 4) increased shunting of excess glucose through the hexosamine pathway (mediating increased transcription of genes for inflammatory cytokines). Excess plasma glucose drives excess production of electron donors (mainly -NADH/H+) from the tricarboxylic acid cycle; in turn, this surfeit results in the transfer of single electrons (instead of the usual electron pairs) to oxygen, producing superoxide radicals and other reactive oxygen species (instead of the usual H2O end product). The superoxide anion itself inhibits the key glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GADPH), and consequently, glucose and glycolytic intermediates spill into the polyol and hexosamine pathways, as well as additional pathways that culminate in protein kinase C activation and intracellular AGE formation (Fig. 1).
Figure 1.
Potential mechanism by which hyperglycemia-induced mitochondrial superoxide overproduction activates four pathways of hyperglycemic damage. Excess superoxide partially inhibits the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), thereby diverting upstream metabolites from glycolysis into pathways of glucose overutilization. This results in increased flux of dihydroxyacetone phosphate (DHAP) to diacylglycerol (DAG), an activator of protein kinase C (PKC), and of triose phosphates to methylglyoxal, the main intracellular AGE precursor. Increased flux of fructose-6-phosphate (Fructose-6-P) to UDP-N-acetylglucosamine increases modification of proteins by O-linked N-acetylglucosamine (GIcNAc), and increased glucose flux through the polyol pathway consumes NADPH and depletes glutathione. GFAT, glutamine:fructose-6-phosphate aminotransferase; Gln, glutamine; Glu, glutamate; NAD+, nicotinamide dinucleotide; UDP, uridine diphosphate.
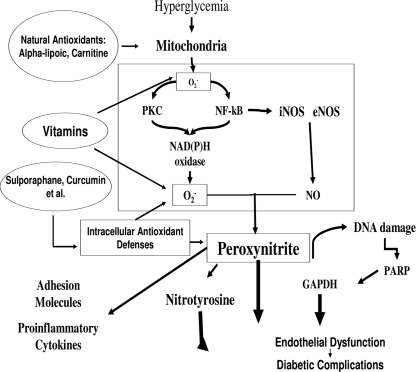
However, superoxide overproduction is also accompanied by increased nitric oxide generation, due to endothelial nitric oxide synthase and inducible nitric oxide synthase uncoupled state (3), a phenomenon favoring the formation of the strong oxidant peroxynitrite, which in turn damages DNA (3). The DNA damage is an obligatory stimulus for the activation of the nuclear enzyme poly(ADP-ribose) polymerase (4). Poly(ADP-ribose) polymerase activation in turn depletes the intracellular concentration of its substrate NAD+, slowing the rate of glycolysis, electron transport, and ATP formation, and produces an ADP ribosylation of the glyceraldehyde-3-phosphate dehydrogenase (4). These processes result in endothelial dysfunction (Fig. 2).
Figure 2.
In the cells, hyperglycemia induces overproduction of superoxide at the mitochondrial level and nitric oxide overproduction through both inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS). Whereas protein kinase C (PKC) and NF-κB are also activated and favor an overexpression of the enzyme NAD(P)H. NAD(P)H generates a great amount of superoxide. Superoxide overproduction, accompanied by increased nitric oxide generation, favors the formation of the strong oxidant peroxynitrite, which in turn damages DNA. DNA damage is an obligatory stimulus for the activation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP). PARP activation in turn reduces the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity. This process results in endothelial dysfunction, which, in turn, contributes to the development of diabetic complications. The new approach to a possible natural antioxidant therapy now takes into account three different possibilities: Vitamins, substances that can balance free radical production at the mitochondrial level, and substances able to induce their own antioxidant defenses in the cells. All these three different substance types are present in vegetables. Adapted from Ceriello (3).
These pathways are confirmed by at least one study on the perfusion for 2 h of isolated rat hearts with solutions of 11.1 mmol/l glucose, 33.3 mmol/l glucose, or 33.1 mmol/l glucose plus glutathione. In the hearts perfused with high glucose concentrations, coronary perfusion pressure was significantly increased; there was a 40% increase in nitric oxide (NO) levels and an upregulation of inducible nitric oxide synthase, but a 300% increase in the production of superoxide species; nitrotyrosine and cardiac cell apoptosis were also significantly increased (5). All these effects were substantially prevented by glutathione, which effectively removes reactive oxygen species including peroxynitrite (5).
However, more recently, evidence from in vitro studies suggests that marked fluctuations in glucose levels, as seen in diabetic patients, have consequences that are even more deleterious than those of continuous high glucose levels and that oxidative stress is convincingly involved. For example, in cultures of human umbilical vein endothelial cells, levels of nitrotyrosine (a marker of oxidative stress), intercellular adhesion molecule-1, vascular cellular adhesion molecule-1, E-selectin, interleukin-6, and 8-hydroxydeoxyguanosine (a marker of oxidative damage of DNA) were all increased after incubation in a medium containing 20 mmol/l glucose, compared with a (interleukin [IL]-6) 5 mmol/l glucose medium, but alternating the two media caused even greater increases (6–8). In addition, intermittent hyperglycemic conditions increased rates of cellular apoptosis and stimulated the expression of caspase-3 (a pro-apoptotic protein), but decreased bcl-2 (an anti-apoptotic protein). These effects were abolished by adding superoxide dismutase (which scavenges free radicals) or inhibitors of the mitochondrial electron transport chain, suggesting that overproduction of free radicals in the mitochondria mediates the apoptotic effects of increased glucose concentrations and fluctuations (9).
OXIDATIVE STRESS IN DIABETES: IN VIVO EVIDENCE
The response-to-injury hypothesis of atherosclerosis states that the initial damage affects the arterial endothelium, in terms of endothelial dysfunction. Notably, today's evidence confirms that endothelial dysfunction, associated with oxidative stress, predicts CVD (10). Indeed, studies show that high glucose concentrations induce endothelial dysfunction in diabetic as well as normal subjects (3). The role of free radical generation in producing the hyperglycemia-dependent endothelial dysfunction is suggested also by studies showing that the acute effects of hyperglycemia are counterbalanced by antioxidants (11,12).
Numerous studies have also noted the effect of hyperglycemia-induced oxidative stress on inflammation. A study in which insulin secretion was blocked, and subjects were maintained at plasma glucose levels of 15 mmol/l for 5 h, found that levels of IL-6, tumor necrosis factor-α, and the proinflammatory cytokine IL-18 rose significantly and returned to baseline within 3 h in the control group (13). However, patients with impaired glucose tolerance had significantly higher tumor necrosis factor-α and IL-6 at baseline levels, and cytokine levels reached substantially higher peaks and stayed elevated for considerably longer than in the control subjects (13). All changes in plasma cytokine levels were abolished by infusion of the antioxidant glutathione, consistent with the hypothesis that hyperglycemia, especially in the form of spikes, is linked to immune activation via an oxidative mechanism (13). Another study matching diabetic patients and healthy control subjects found increases in circulating intercellular adhesion molecule-1 (ICAM-1) in both groups during an oral glucose tolerance test; these increases were also abolished by glutathione (14). Glutathione administered without a glucose load decreased circulating ICAM-1 levels in the diabetic group, but not in the control group, again suggesting that hyperglycemia increases ICAM-1 levels via an oxidative mechanism (14).
More direct evidence for the central role of oxidative stress is derived from clinical studies that measured the markers. For example, among 20 diabetic patients, either a low-carbohydrate or high-carbohydrate meal increased levels of plasma glucose, insulin, triglycerides, and malondialdehyde (a marker for lipid peroxidation) and decreased nonesterified fatty acids and total radical-trapping antioxidant parameter (TRAP) (a global measure of antioxidant capacity in the plasma) (15). However, the high-carbohydrate meal (designed to produce higher postprandial glucose levels) increased glucose and malondialdehyde, decreased TRAP significantly more, and rendered LDL more susceptible to oxidation than did the low-carbohydrate meal (15). The decrease in TRAP highlights the fact that oxidative stress may also ensue from the failure of normal antioxidant defenses: the same group found that during the oral glucose tolerance test, TRAP was reduced from baseline in both well-controlled nonsmoking diabetic and healthy age-matched subjects, as were levels of protein-bound thiol (−sulphur) groups, vitamins C and E, and uric acid (15).
As aforementioned, superoxide anion combines with NO to produce peroxynitrite ion; this species is capable of peroxidating lipoproteins and damaging DNA, which then activates the nuclear enzyme poly (APD-ribose) polymerase, depleting intracellular NAD+ and (among other effects) causing acute endothelial dysfunction (3). In one study involving 12 healthy subjects, infusion of l-arginine (to supply NO) reversed hyperglycemia-induced increases in systolic and diastolic blood pressure, heart rate, plasma catecholamine levels, ADP-induced platelet aggregation, and blood viscosity (16). However, infusing NG-monomethyl-l-arginine, which inhibits the synthesis of endogenous NO, produced effects that were similar to those produced by hyperglycemia. Thus, decreased NO availability may be one mechanism by which hyperglycemia induces hemodynamic and rheological changes in blood (16). It has been shown, however, that unlike normal control subjects, patients with diabetes have significantly elevated fasting nitrotyrosine levels, as well as postprandial increases, after intake of a standard mixed meal; the effect was significantly normalized by insulin aspart (which targets postprandial glucose) but not by regular insulin (17).
Finally, consistent with the recent emerging role of glucose fluctuations, a new study confirms that, in type 2 diabetes, diurnal glucose fluctuations are the most powerful predictors of oxidative stress generation (18).
NEW PERSPECTIVES: OXIDATIVE STRESS AND HYPERGLYCEMIA-INDUCED “METABOLIC MEMORY”
Large randomized studies have established that early intensive glycemic control reduces the risk of diabetic complications, both micro- and macrovascular (19). However, epidemiological and prospective data support a long-term influence of early metabolic control on clinical outcomes (19). This phenomenon has recently been defined as “metabolic memory” (19). Potential mechanisms for propagating this “memory” are the nonenzymatic glycation of cellular proteins and lipids, and an excess of cellular reactive oxygen and nitrogen species, in particular, those that originated at the level of glycated-mitochondrial proteins, perhaps acting in concert with one another to maintain stress signaling (19).
Experimental evidence supporting the concept of “metabolic memory” and its possible link with oxidative stress
Several years ago, there were preliminary reports of the possibility that “hyperglycemic memory” for hyperproduction of fibronectin and collagen in endothelial cells persists after glucose normalization (20). Using the same design, i.e., 14 days in high glucose followed by 7 days of culture in normal glucose, it was shown that, in endothelial cells, an overproduction of free radicals persists after the normalization of glucose and is accompanied by a prolongation of the induction of protein kinase-β, NAD(P)H oxidase, Bax, collagen, and fibronectin, in addition to 3-nitrotyrosine (21). This result suggests that oxidative stress may be involved in the “metabolic memory” effect.
The effect of reinstitution of good glucose control on hyperglycemia-induced increased oxidative stress and nitrative stress was also previously evaluated in the retina of rats maintained in poor glucose control before initiation of good control (22). In diabetic rats, 2 or 6 months of poor control (A1C >11.0%) was followed by 7 months of good control (A1C <5.5%). Reinstitution of good control after 2 months of poor control inhibited elevations in retinal lipid peroxides and NO levels by ∼50%, but failed to have any beneficial effects on nitrotyrosine formation. However, reversal of hyperglycemia after 6 months of poor control had no significant effect on retinal oxidative stress and NO levels. In the same rats, inducible nitric oxide synthase expression and nitrotyrosine levels remained elevated by >80% compared with normal rats, or rats kept in good control for the duration (22). In a similar study, caspase-3 activity in diabetic rats kept in poor control for 13 months was higher than in normal rats (23). Reinstitution of good glycemic control after 2 months of poor control partially normalized the hyperglycemia-induced activation of caspase-3 (to 140% of normal values), whereas reinstitution of good control after 6 months of poor control had no significant effect on the activation of caspase-3. In the same study, nuclear factor (NF)-κB activity was 2.5-fold higher in diabetic rats kept in poor control than in normal rats. Reinstitution of good control after 2 months of poor control partially reversed this increase, but good control after 6 months of poor control had no effect. Initiation of good control soon after induction of diabetes in rats prevented activation of retinal caspase-3 and NF-κB (23). Similar results are available for the kidney. Diabetic rats were maintained in good glycemic control (A1C 5%) soon, or 6 months after, induction of hyperglycemia and were killed 13 months after induction of diabetes (24). For rats in which good control was initiated soon after induction of diabetes, oxidative stress (as measured by the levels of lipid peroxides, 8-hydroxy-2′-deoxyguanosine, and reduced glutathione) and NO in urine and renal cortex were no different from that observed in normal control rats, but when reinstitution of good control was delayed for 6 months after induction of diabetes, oxidative stress and NO remained elevated in both urine and renal cortex (24). These data suggest that hyperglycemia-induced oxidative stress and NO, as well as activation of apoptosis and the NF-κB, can be prevented if good glycemic control is initiated early, but are not easily reversed if poor control is maintained for longer durations. Therefore, these findings suggest a persistence of the hyperglycemia-induced damage in such organs, even after its normalization.
However, if excess reactive species are central in development of hyperglycemia-related diabetic complications, could this excess explain the persistence of the risk for complications, even when the hyperglycemia is reduced or normalized?
The above reported studies suggest that long-lasting effects of hyperglycemia result in increased oxidative stress, while inhibiting oxidative stress has preliminarily been shown to reverse these effects (21). Mitochondrial overproduction of superoxide in hyperglycemia has been suggested as the “unifying hypothesis” for the development of diabetic complications (2). Therefore, it is reasonable that mitochondria are also important players in propagating the “metabolic memory.” Chronic hyperglycemia is thought to alter mitochondrial function through glycation of mitochondrial proteins (25). Levels of methylglyoxal, a highly-reactive α-dicarbonil byproduct of glycolysis, are increased in diabetes (26). Methylglyoxal readily reacts with arginine, lysine, and sulfhydryl groups of proteins (26) in addition to nucleic acids (26), inducing the formation of a variety of structurally identified AGEs, in both target cells and plasma (26). Methylglyoxal has an inhibitory effect on mitochondrial respiration, and methylglyoxal-induced modifications are targeted to specific mitochondrial proteins (26). These premises are important because a recent study has described, for the first time, a direct relationship between the formation of intracellular AGEs on mitochondrial proteins, the decline in mitochondrial function, and the excess formation of reactive species (25). Therefore, mitochondrial respiratory chain proteins that underwent glycation were prone to produce more superoxide, independently from the level of hyperglycemia. The glycation of mitochondrial proteins may be a contributing explanation for the phenomenon of the “metabolic memory.” Glycated mitochondria overproducing free radicals, independently from actual glycemia, can lead to a catastrophic cycle of mitochondrial DNA damage, as well as functional decline, further oxygen radical generation, and cellular injury, maintaining the activation of the pathways involved in the pathogenesis of diabetic complications (27). Furthermore, mitochondrial proteins become damaged or post-translationally modified as a consequence of a major change in a cell's redox status (27). This, on the other hand, may also affect mitochondrially destined proteins that are imported into the mitochondrial outer membrane, inner membrane, or matrix space via specific import machinery transport components (27).
In other words, it may be postulated that in the “metabolic memory,” the cascade of events is the same as that proposed by Brownlee (2). The source of superoxide is still the mitochondria, but that, in addition, the production of reactive species is unrelated to the presence of hyperglycemia, depending on the level of glycation of mitochondrial proteins.
HOW COULD OXIDATIVE STRESS BE REDUCED WITH PHARMACOLOGICAL AND NONPHARMACOLOGICAL INTERVENTIONS?
Antioxidant therapy may be of great interest in diabetic patients. However, the classical antioxidants, such as vitamins E and C, do not appear to be helpful. New insight into the mechanisms leading to the generation of oxidative stress in diabetes are now available. Presumably these findings lead to the discovery and evaluation of new antioxidant molecules, such as super oxide dysmuthase and catalase mimetics, which may, at an early stage, hopefully inhibit the mechanism leading to diabetic complications (3). While waiting for these new and specific compounds, it is reasonable to suggest that substances already available, such as statins, ACE inhibitors, and angiotensin I blockers, should also be used for their effectiveness as “causal and preventive” antioxidants (rev. in 3). However, new intriguing perspectives are arising. Whereas clinical trials with antioxidant vitamins have been unsuccessful in preventing CVD in diabetic and nondiabetic patients, it is well recognized that the consumption of fresh fruit and vegetables is particularly helpful. Foods of plant origin, despite plenty of nutrients, contain many non-nutritional compounds, which may prevent oxidative stress–induced damage. There are at least two hypothetical new beneficial mechanisms that can be related to vegetable consumption: one is the possibility that compounds such as α-lipoic acid and carnitine may regulate free radical over-generation at the mitochondrial level (3), and a second is the possibility of increasing their own intracellular defenses (28). Plants produce thousands of phenolic compounds as secondary metabolites, such as nitrous compounds. Glucosinolates are responsible for the secretion of detoxifying enzymes that remove free radicals from the organism (28). Furthermore, they activate proteins and phase 2 detoxifying enzymes (28). In particular, the consumption of cruciferous vegetables has long been associated with a reduced risk in the occurrence of cancer at various sites, including the prostate, lung, breast, and colon (29). This protective effect is attributed to isothiocyanates present in these vegetables. Sulforaphane, found in broccoli, is by far the most extensively studied to uncover the mechanisms behind this chemoprotection (29). The major mechanism by which sulforaphane protects cells was traditionally thought to be through Nrf2-mediated induction of phase 2 detoxification enzymes that elevate cell defense against oxidative damage and promote the removal of carcinogens (29). However, it is becoming clear that there are multiple mechanisms activated in response to sulforaphane, including suppression of cytochrome P450 enzymes, induction of apoptotic pathways, suppression of cell cycle progression, inhibition of angiogenesis, and anti-inflammatory activity. This new approach appears very promising, and it seems reasonable that it could also be helpful in diabetes (Fig. 2).
CONCLUSIONS
Oxidative stress is convincingly a key pathogenetic factor for diabetic complications. However, despite this strong evidence, the usefulness of antioxidants in preventing such complications is still elusive.
New “antioxidants” are emerging, based on new findings on oxidative stress, in particular on how the oxidative stress is produced by the balance between free radical production and antioxidant defenses. The “new antioxidant” approach includes the possibility of controlling free radical production and of increasing intracellular antioxidant defenses, a concept different from the old one, when antioxidant action meant just scavenging the free radicals already produced. The new view, of course, needs to be proven in clinical trials, but it seems very promising.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The publication of this supplement was made possible in part by unrestricted educational grants from Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, Hoffmann-La Roche, Johnson & Johnson, LifeScan, Medtronic, MSD, Novo Nordisk, Pfizer, sanofi-aventis, and WorldWIDE.
References
- 1. Chaturvedi N: The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract 2007; 76 ( Suppl. 1): S3– S12 [DOI] [PubMed] [Google Scholar]
- 2. Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813– 820 [DOI] [PubMed] [Google Scholar]
- 3. Ceriello A: New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 2003; 26: 1589– 1596 [DOI] [PubMed] [Google Scholar]
- 4. Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C: Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med 2001; 7: 108– 113 [DOI] [PubMed] [Google Scholar]
- 5. Ceriello A, Quagliaro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D: Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 2002; 51: 1076– 1082 [DOI] [PubMed] [Google Scholar]
- 6. Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabó C, Ceriello A: Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost 2004; 2: 1453– 1459 [DOI] [PubMed] [Google Scholar]
- 7. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A: Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of PKC and NAD(P)H-oxidase activation. Diabetes 2003; 52: 2795– 2804 [DOI] [PubMed] [Google Scholar]
- 8. Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A: Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 2005; 183: 259– 267 [DOI] [PubMed] [Google Scholar]
- 9. Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A: Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabete Metab Res Rev 2006; 22: 198– 203 [DOI] [PubMed] [Google Scholar]
- 10. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T: Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673– 2678 [DOI] [PubMed] [Google Scholar]
- 11. Marfella R, Verrazzo G, Acampora R, La Marca C, Giunta R, Lucarelli C, Paolisso G, Ceriello A, Giugliano D: Glutathione reverses systemic hemodynamic changes by acute hyperglycemia in healthy subjects. Am J Physiol 1995; 268: E1167– E1173 [DOI] [PubMed] [Google Scholar]
- 12. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA: Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1996; 97: 22– 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D: Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002; 106: 2067– 2072 [DOI] [PubMed] [Google Scholar]
- 14. Ceriello A, Falleti E, Motz E, Taboga C, Tonutti L, Ezsol Z, Gonano F, Bartoli E: Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res 1998; 30: 146– 149 [DOI] [PubMed] [Google Scholar]
- 15. Ceriello A, Bortolotti N, Motz E, Pieri C, Marra M, Tonutti L, Lizzio S, Feletto F, Catone B, Taboga C: Meal-induced oxidative stress and low-density lipoprotein oxidation in diabetes: the possible role of hyperglycemia. Metabolism 1999; 48: 1503– 1508 [DOI] [PubMed] [Google Scholar]
- 16. Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giunta R, Nappo F, Lucarelli C, D'Onofrio F: Vascular effects of acute hyperglycemia in humans are reversed by L-arginine: evidence for reduced availability of nitric oxide during hyperglycemia. Circulation 1997; 95: 1783– 1790 [DOI] [PubMed] [Google Scholar]
- 17. Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, Marra G, Tonutti L, Taboga C, Motz E: Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care 2002; 25: 1439– 1443 [DOI] [PubMed] [Google Scholar]
- 18. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D: Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349– 1354 [DOI] [PubMed] [Google Scholar]
- 19. Ihnat MA, Thorpe JE, Ceriello A: Hypothesis: the ‘metabolic memory,’ the new challenge of diabetes. Diabet Med 2007; 24: 582– 586 [DOI] [PubMed] [Google Scholar]
- 20. Roy S, Sala R, Cagliero E, Lorenzi M: Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A 1990; 87: 404– 408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ihnat MA, Thorpe JE, Kamat CD, Szabó C, Green DE, Warnke LA, Lacza Z, Cselenyák A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A: Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia 2007; 50: 1523– 1531 [DOI] [PubMed] [Google Scholar]
- 22. Kowluru RA: Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 2003; 52: 818– 823 [DOI] [PubMed] [Google Scholar]
- 23. Kowluru RA, Chakrabarti S, Chen S: Re-institution of good metabolic control in diabetic rats and activation of caspase-3 and nuclear transcriptional factor (NF-kappaB) in the retina. Acta Diabetol 2004; 41: 194– 199 [DOI] [PubMed] [Google Scholar]
- 24. Kowluru RA, Abbas SN, Odenbach S: Reversal of hyperglycemia and diabetic nephropathy: effect of reinstitution of good metabolic control on oxidative stress in the kidney of diabetic rats. J Diabetes Complications 2004; 18: 282– 288 [DOI] [PubMed] [Google Scholar]
- 25. Rosca MG, Mustata TG, Kinter MT, Ozdemir AM, Kern TS, Szweda LI, Brownlee M, Monnier VM, Weiss MF: Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol 2005; 289: F420– F430 [DOI] [PubMed] [Google Scholar]
- 26. Yan SF, Ramasamy R, Schmidt AM: Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab 2008; 4: 285– 293 [DOI] [PubMed] [Google Scholar]
- 27. Zorov DB, Juhaszova M, Sollott SJ: Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 2006; 1757: 509– 517 [DOI] [PubMed] [Google Scholar]
- 28. Jacob C: A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep 2006; 23: 851– 863 [DOI] [PubMed] [Google Scholar]
- 29. Juge N, Mithen RF, Traka M: Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci 2007; 64: 1105– 1127 [DOI] [PMC free article] [PubMed] [Google Scholar]