Abstract
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease which is in part mediated by the migration of monocytes from blood to RA synovial tissue, where they differentiate into macrophages and secrete inflammatory cytokines and chemokines. The T cell cytokine IL-17 is expressed in the RA synovial tissue and synovial fluid. In order to better understand the mechanism by which IL-17 might promote inflammation, its role in monocyte trafficking was examined. In vivo, IL-17 mediates monocyte migration into sponges implanted into severe combined immunodeficient (SCID) mice. In vitro, IL-17 was chemotactic, not chemokinetic, for monocytes at the concentrations detected in the RA synovial fluid. Further, IL-17-induced monocyte migration was mediated by ligation to IL-17 receptor (R) A and C expressed on monocytes and was mediated through p38MAPK signaling. Finally, neutralization of IL-17 in RA synovial fluid or its receptors on monocytes significantly reduced monocyte migration mediated by RA synovial fluid. These observations suggest that IL-17 may be important in recruiting monocytes into the joints of patients with RA, supporting IL-17 as a therapeutic target in RA.
Keywords: IL-17, monocyte, migration, rheumatoid arthritis
INTRODUCTION
IL-17 (also known as IL-17A) is the hallmark cytokine of a novel T helper cell population termed TH-17, which has altered the TH1/TH2 paradigm in immune biology (1, 2). IL-17 is produced by memory CD4+ T lymphocytes. TGF-β, IL-1β, IL-6 and IL-23 are important for the polarization of TH-17 cells from naive human CD4+ T cells, and the absence of TGF-β mediates a shift from a TH-17 profile to a TH1-like profile (3, 4). TH-17 cell polarization is dependent on the transcription factor RORC2, the human homolog of the murine RORγt (3–5). In addition to IL-17, TH-17 cells also produce IL-17F, IL-22, IL-21, IL-26, and the chemokine CCL20 (6–8), all of which may contribute to disease pathogenesis (9).
A number of observations suggest that IL-17 may be important in RA. IL-17 is found in RA synovial fluid and in the T cell rich areas of RA synovial tissue (10, 11). In spite of the observations by others (12), we found that TH-17 cells were significantly increased in RA synovial fluid compared to RA or normal peripheral blood (13). A two-year prospective study analyzing RA synovial tissues demonstrated that IL-17 and TNF-α mRNA levels were synergistic prognostic factors for worse outcomes (14). The effects of IL-17 may be due to its ability to promote inflammation, by inducing cytokines and chemokines (15, 16). Although the direct pro-inflammatory effects of IL-17 are often small when compared to those of IL-1β and TNFα, IL-17 may enhance the effects of other cytokines. Employing RA synovial tissue fibroblasts, IL-17 enhanced IL-1-mediated IL-6 and CCL20/MIP-3α production(17, 18) and the TNFα-induced synthesis of IL-1, IL-6, IL-8 and CCL20 (18, 19). Therefore, a major role of IL-17 may be to amplify the effects of macrophage derived cytokines, and it may therefore be the missing link between T cells in RA joint and the effector phase of RA.
The role of IL-17 in chemotaxis has been examined. The ectopic expression of IL-17 intraarticularly enhanced neutrophil migration into the joints of mice (20). In the rat airway, IL-17 mediates neutrophil recruitment via induction of IL-8 (21). Neutrophil chemotaxis caused by conditioned media from IL-17-stimulated gastric epithelial cells was inhibited by a neutralizing antibody to IL-8 but not to IL-17, suggesting that IL-17 is unable to directly induce neutrophil chemotaxis (22).
In contrast to its effects on neutrophils, little is known about the effect of IL-17 on the recruitment of monocytes. In the current study, therefore, we evaluated the role of IL-17 on monocyte migration. Our results demonstrate that IL-17 recruited monocytes into sponges implanted into SCID mice. In vitro, IL-17 was chemotactic for monocytes at the concentrations detected in the RA synovial fluid. Additionally, neutralizing antibodies to IL-17 or to IL-17 receptor (IL-17R) A or C significantly reduced RA synovial fluid-mediated monocyte migration. Taken together, these observations support the importance of IL-17 in recruiting monocytes to the RA synovial tissue. Hence, therapy directed against IL-17 may reduce inflammation by inhibiting monocyte migration into the joint.
MATERIALS AND METHODS
IL-17 monocyte migration in an in vivo sponge model
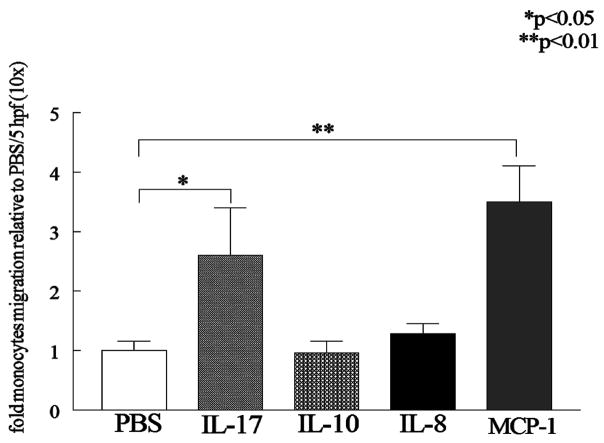
Six week old female SCID mice (NCI, Bethesda, M.D) were anesthetized using an anesthesia machine (IMPACT 6, VetEquip Inc., Pleasanton, CA). Thereafter (8mm ×2mm) sterile sponges soaked (Ivalon, New England, CT) with PBS (50μl) (n=9), human IL-17 (1μg/50μl) (n=5) (R&D Systems), human IL-10 (1μg/50μl) (n=4) (R&D Systems), human IL-8 (1μg/50μl) (n=3) (R&D Systems) or human MCP-1(1μg/50μl) (n=7; positive control) (R&D Systems) were implanted subcutaneously in the back of the mice. Monocytes isolated from the buffy coats of healthy donors (20, 21) were labeled with PKH26 fluorescent dye (Sigma) according to manufacturer’s instruction (22, 23) and successful labeling was determined using a fluorescence microscope. Labeled cells (5 × 106 cells/mouse) were injected intravenously via the tail vein. Three days later, mice were sacrificed and the sponges were retrieved and cells were eluted by forcing 4 ml 0.03% EDTA in PBS through the sponge employing 3ml syringe and then cytospun on a glass slide. Eluted cells from the sponge were stained with Hoechst (nucleus staining). Monocytes were quantified based on the double PKH26 (red) and Hoechst (blue) staining. Migrating labeled monocytes were quantified by counting the number of cells in 5 HPF per slide prepared from cells eluted from each mouse in different treatment groups. The results were analyzed from 45 HPF in the PBS treatment group, 25 HPF in the IL-17 group, 20 HPF in the IL-10 treatment group, 15 HPF in the IL-8 group and 35 HPF in the MCP-1 group. Data is shown as fold increase compared with random migration detected in the PBS group (Fig. 1).
Figure 1. IL-17 recruits monocytes into sponge implants in SCID mice.
Six week old female SCID were anesthetized, and thereafter sterile sponges treated with PBS (50μl) (n=9), human IL-17 (1μg/50μl) (n=5), human IL-10 (1μg/50μl) (n=4), human IL-8 (1μg/50μl) (n=3) or human MCP-1(1μg/50μl) (n=7; positive control) were implanted subcutaneously in the back of the mice. Monocytes were tagged with PKH26 fluorescent dye and were injected intravenously via the tail vein. Three days later, mice were sacrificed and the sponges were retrieved and labeled monocytes eluted quantified by counting number of cells/5 HPFs for each mouse in different treatment groups. Values are the mean ± SE. * represents p <0.05 and ** denotes p <0.01.
Monocyte chemotaxis
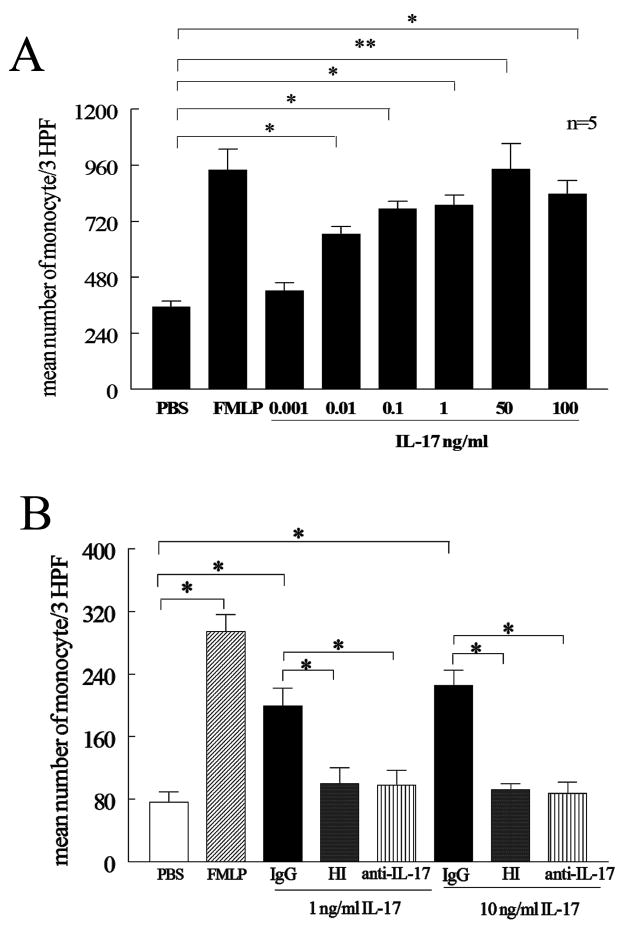
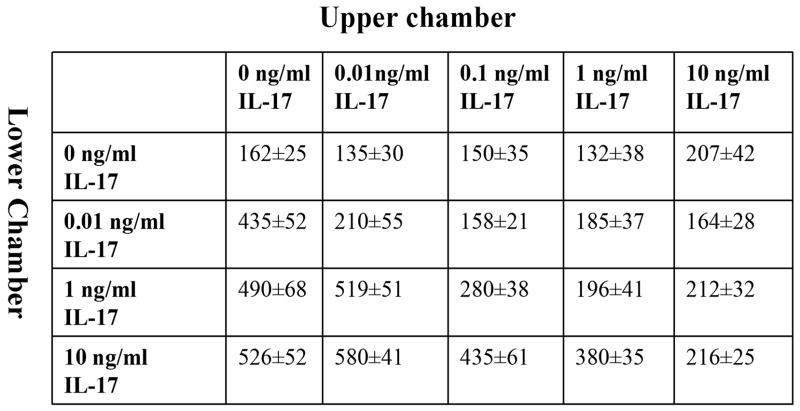
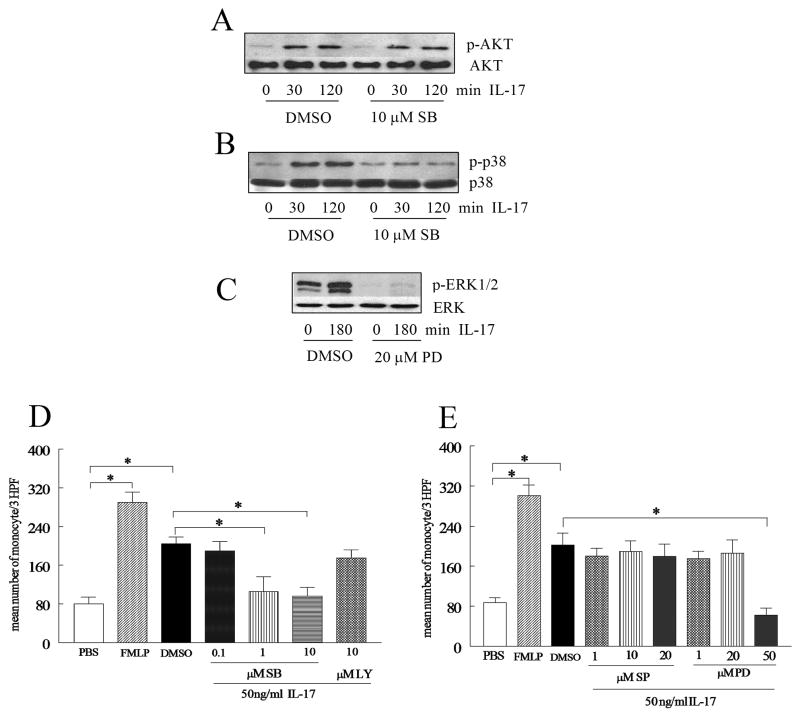
Chemotaxis was performed in triplicate for 2 hours in Boyden chambers (Neuroprobe) with IL-17 concentrations varying from 0.001 to 100 ng/ml (R&D Systems) Formyl-Met-Leu-Phe (FMLP) (100nM) (Sigma) was used as the positive control and PBS as negative control (23). To test specificity of IL-17 induced monocyte migration, monocyte chemotaxis was examined to heat inactivated IL-17 (1 and 10 ng/ml incubated in 100°C for 15min) or neutralization of IL-17 by an anti-IL-17 antibody or IgG control (1 or 10 μg/ml in 37°C) (R&D Systems) for 2–3h. To examine for chemokinesis, a series of checker board experiments were performed by placing increasing concentrations of IL-17 (0, 0.01, 0.1, 1 and 10 ng/ml) together with monocytes in the top chamber, as well as in the bottom chamber. To define which signaling pathways mediated IL-17-induced monocyte chemotaxis, monocytes were pre-incubated with inhibitors to p38 (SB203580; 0.1, 1 and 10 μM), JNK (SP600125; 1, 10 and 20μM), ERK (PD98059; 1, 20 and 50 μM) and PI3K (LY294002; 10 μM) (Calbiochem) for 1 hour. Subsequently, monocyte chemotaxis was performed for 2–3 h.
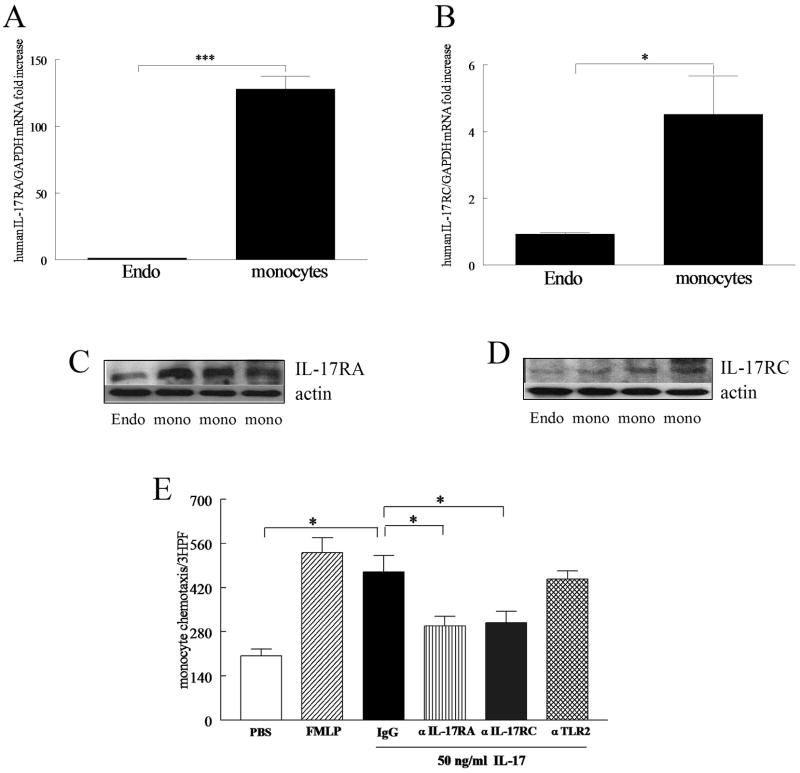
To determine which IL-17 receptors are important for IL-17 monocyte migration in some experiments, antibodies to IL-17 RA (Catalog number: MAB177) and RC (Catalog number: MAB2269; 10 μg/ml) (R&D Systems), Toll like receptor 2 (TLR2, 10 μg/ml) (Imgenex), or isotype control antibody were incubated with the monocytes for 3h prior to adding the cells to the Boyden chambers. Chemotaxis induced by RA synovial fluids was examined following incubation of fluids with control IgG or neutralizing anti-IL-17 antibody (10μg/ml) for 2–3h. The fluids were diluted 1:20 prior to addition to the bottom chambers. To examine whether IL-17 receptors are involved in RA SF induced monocyte migration, monocytes were incubated with antibodies to IL-17 RA and RC (10 μg/ml) as well as isotype control for 2–3 hour prior to adding the RA SF to the bottom chambers. To test the efficacy of IL-17RA and RC,
Cytokine Quantification
Human IL-17 (R&D Systems) ELISA kit was used according to the manufacturers’ instructions.
Characterization of IL-17 signaling pathways in monocytes
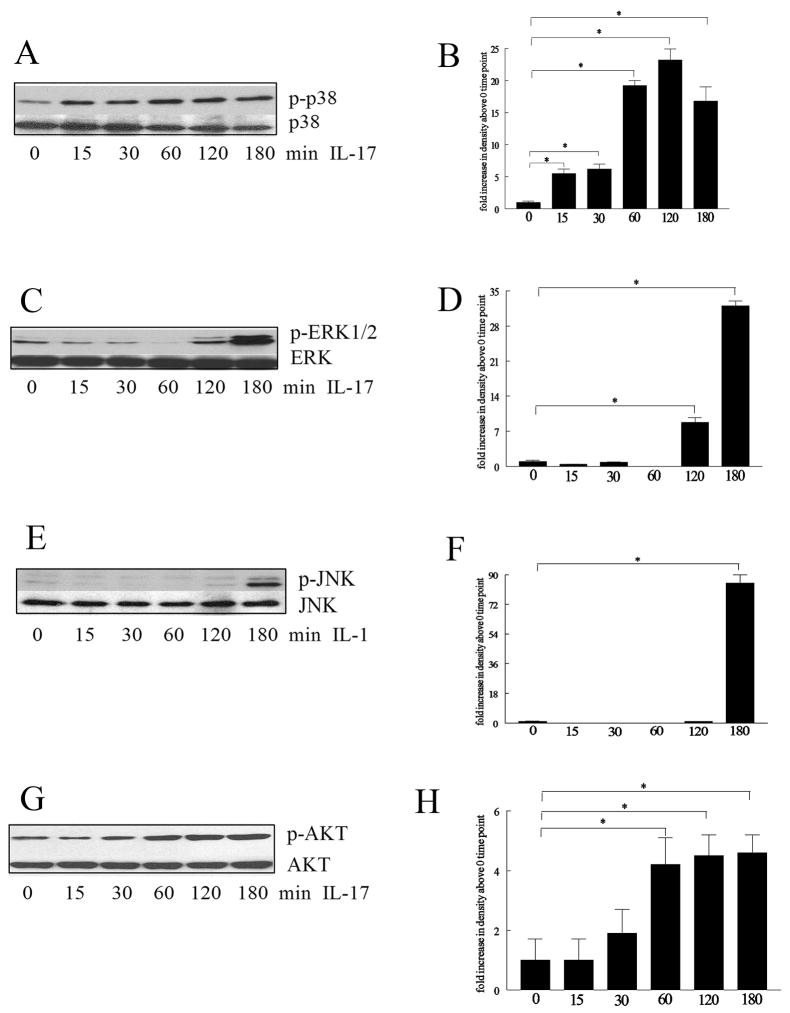
Monocytes (2×106/ml) were untreated or treated with IL-17 (50 ng/ml) for 15 to 180 min. Cell lysates were examined by Western blot analysis as previously described(24). Blots were probed with phospho (p)-ERK, pJNK, p-p38 MAPK, pAKT (Cell Signaling; 1:1000 dilution) overnight and after stripping, were probed with ERK, JNK, p38 or AKT (Cell Signaling; 1:3000 dilution) overnight. Blots were scanned and band intensities determined employing UN-SCAN-IT version 5.1 software (Silk Scientific, Urem, UT). Band intensities correspond to the sum of all pixel values in the segment selected minus the background pixel value in that segment.
To examine whether inhibition of p38 may effect IL-17 activation of PI3K, monocytes were treated with SB203580 10 μM or DMSO an hour prior to IL-17 (50 ng/ml) activation for 0 to 120 min. These cell lysates were then probed for p-p38 MAPK and p-AKT as well as p38 or AKT (Cell Signaling). To verify that 20 μM of PD98059 could effectively inhibit IL-17-induced ERK phosphorylation, monocytes were treated with PD98059 20 μM or DMSO an hour prior to IL-17 (50 ng/ml) activation for 0 or 180 min.
To determine signaling pathways associated with IL-17 monocyte migration, monocytes were pre-incubated with the identified chemical inhibitors for p38 (SB203580; 0.1, 1 and 10 μM) or PI3K (LY294002; 10 μM) as well as JNK (SP600125; 1, 10 and 20 μM) or ERK (PD98059; 1, 20 and 50 μM) and for 1 hour. Subsequently, monocyte chemotaxis was performed for 2–3 h.
Detection of IL-17 receptors
Total cellular RNA for IL-17 RA and RC (Applied Biosystems) were extracted from HMVECs and monocytes using Trizol, and reverse transcription and Real-time RT-PCR were performed as previously described (13, 25). Relative gene expression was determined by the ΔΔCt method.
Employing monoclonal antibodies to human IL-17 RA and RC (R&D Systems, 1μg/ml) the protein expression of these receptors was determined in untreated human microvascular endothelial cells (HMVECs) and monocytes from three donors. Blots were stripped and probed with actin (Sigma, 1:3000 dilution).
Statistical Analysis
The data were analyzed using the 2-tailed Student’s t tests for paired and unpaired samples. P values < 0.05 were considered significant.
RESULTS
IL-17 induces monocyte migration in vivo
In preliminary studies, we observed that IL-17 in vivo resulted in the accumulation of macrophages in the joints of experimental animals (data not shown). Therefore, experiments were performed to determine if IL-17 might promote the recruitment of monocytes in vivo employing sponges placed subcutaneously. After 3 days the number of murine cells that had migrated into the sponges soaked with PBS, MCP-1, IL-8, IL-10 and IL-17 was similar. In contrast, the number of labeled human monocytes that migrated into the sponges was significantly (p < 0.05) increased by IL-17 compared to PBS (Figure 1). The number of monocytes attracted to the positive control, MCP-1, was increased compared to PBS (p < 0.05). In contrast, neither IL-8 nor IL-10 induced monocyte migration into the sponges. Therefore, these observations suggest that IL-17 may be chemotactic for monocytes while IL-8 and IL-10 are not.
IL-17 is chemotactic for monocytes
Next, experiments were performed to determine if IL-17 was directly chemotactic for monocytes. Employing Boyden chambers, IL-17 was chemotactic for monocytes at concentrations ranging from 0.01 ng/ml (p<0.05) to 100 ng/ml (p<0.01) (Figure 2A). Heat inactivation of IL-17, or incubation of IL-17 with neutralizing antibodies to IL-17, suppressed monocyte migration (Figure 2B). Consistent with these data, in Figure 2B, 10 μg/ml of anti-IL-17 neutralized 10 ng/ml of recombinant IL-17, a concentration that was greater than that observed in the synovial fluids. These observations suggest that IL-17 is capable of mediating monocyte migration.
Figure 2. IL-17 induces monocyte migration.
(A) Dose response curve of IL-17-induced monocyte chemotaxis. IL-17 monocyte chemotaxis was performed in a Boyden chemotaxis chamber with varying concentration of IL-17. Values demonstrate mean ± SE from three independent donors performed in triplicate. (B) IL-17-induced monocyte chemotaxis was suppressed by heat inactivating IL-17 (both 1 and 10 ng/ml incubated in 100°C for 15min) or neutralization of IL-17 (1 and 10 ng/ml) by anti-IL-17 antibody or IgG control (10 μg/ml 1h in 37°C) for 2–3h. Values are the mean ± SE from three different experiments. * represents p <0.05 and ** denotes p <0.01.
Next, experiments were performed to determine if the effects of IL-17 were mediated through chemokinesis. The presence of higher concentrations of IL-17 in the upper chamber did not enhance migration of monocytes (Figure 3). Likewise, when the concentrations of IL-17 in the upper and lower chamber were the same, little or no enhancement of migration was observed (Figure 3). Taken together, our results suggest that IL-17 mediates monocyte chemotaxis.
Figure 3. IL-17 does not induce chemokinesis.
A series of checker board experiments were performed by placing increasing doses of IL-17 together with 2×106/ml monocytes in the top chamber, in addition to placing different concentrations of IL-17 in the bottom wells of the chemotaxis chamber. The data presented are representative of 3 independent experiments.
p38 MAPK blockade inhibits IL-17-induced monocyte migration
Experiments were performed to determine the monocyte signaling pathway(s) responsible for monocyte chemotaxis induced by IL-17. Since the monocyte chemotaxis assays were performed for 2 hours, IL-17-activated signaling pathways were analyzed between 0 and 180 minutes. The ability of IL-17 to activate the pathways examined was determined by phosphorylation of MAPK mediators and AKT. The MAPK p38 pathway was activated as early as 15 minutes (Figure 4A and B), followed by AKT at 60 minutes (Figure 4C and D). However ERK and JNK were not activated until 120 and 180 minutes respectively (Figure 4E–F and 4G–H).
Figure 4. IL-17-induced monocyte migration is suppressed by p38 MAPK inhibition.
In order to determine the mechanism of IL-17 in monocytes, cells were stimulated with IL-17 (50 ng/ml) for 0–180 minutes, and the cell lysates were probed for p-p38 (A–B), pERK (C–D), pJNK (E–F), and pAKT (G–H). These results are representative of 3 experiments. Blots were scanned and band intensities determined employing UN-SCAN-IT version 5.1 software (Silk Scientific, Urem, UT). Band intensities correspond to the sum of all pixel values in the segment selected minus the background pixel value in that segment (B, D, F, H). Values demonstrate mean ± SE of three experiments in triplicate. * represents p <0.05.
To demonstrate that inhibition of p38 specifically blocks p38 but not pAKT, monocytes were treated with p38 inhibitor (SB203580 10 μM) or control an hour prior to IL-17 activation. Results from these studies demonstrate that inhibition of p38 MAPK in monocytes had no effect on activation of AKT by IL-17, indicating that p38 MAPK is not upstream PI3K signaling pathway (Figure 5A and B).
Figure 5. IL-17 mediates monocyte migration through p38 MAPK activation.
To examine whether inhibition of p38 may affect IL-17 activation of PI3K, monocytes were also treated with SB203580 10 μM or DMSO an hour prior to IL-17 (50 ng/ml) activation for 0 to 120 min. Thereafter, cells were probed for p-p38 MAPK (A) and pAKT (1:1000 dilution) (B) as well as p38 or AKT (1:3000 dilution). (C) To ensure that 20 μM of PD98059 could effectively inhibit IL-17-induced ERK phosphorylation, monocytes were treated with PD98059 20 μM or DMSO an hour prior to IL-17 (50 ng/ml) activation for 0 or 180 min (experiments were done in duplicates). To determine signaling pathways associated with IL-17 monocyte migration, monocytes were pre-incubated with the identified chemical inhibitors for p38 (SB203580; 0.1, 1 and 10 μM) or PI3K (LY294002; 10 μM) (D) as well as JNK (SP600125; 1, 10 and 20 μM) or ERK (PD98059; 1, 20 and 50 μM) (E) and for 1 hour. Subsequently, monocyte chemotaxis was performed for 2–3h. Values demonstrate mean ± SE of three experiments in triplicate. * represents p <0.05.
In order to determine which of these pathways may contribute to IL-17-mediated chemotaxis, monocytes were then pre-incubated with inhibitors of the ERK, JNK, p38 and PI3K pathways prior to performing the chemotaxis. Only inhibition of the MAPK p38 (1 and 10 μM) pathway significantly reduced IL-17-induced monocyte migration (Figure 5D). Different concentrations of inhibitors of the PI3K (10 μM), ERK(1, 20, 50μM) and JNK (1, 10, 20 μM) pathways were unable to inhibit IL-17-mediated monocyte migration except at the highest concentration of the ERK inhibitor (50 μM) which was toxic for monocytes, as determined by trypan blue staining (Figure 5D and E). None of the other inhibitors were toxic at the concentrations employed. Since 50 μM of ERK inhibitor reduced IL-17 induced monocyte chemotaxis due to its toxic effect on monocytes, and monocyte chemotaxis was not affected by 20 μM of PD98059, experiments were performed to ensure that 20 μM of PD98059 was efficient in blocking IL-17 induced ERK phosphorylation in monocytes (Figure 5C). These results suggest that IL-17 can directly mediate monocyte migration through activating the p38 MAPK pathway.
Monocytes express IL-17 RA and RC which are involved in IL-17 mediated monocyte chemotaxis
Experiments were performed to determine which IL-17R were involved with monocyte migration. Employing real-time RT-PCR we demonstrated that the expression levels of IL-17 RA and RC (Figure 6A and B) on monocytes are significantly higher than that of HMVECs, with IL-17 RA being more the prominent receptor in monocytes compared to IL-17 RC. The Western blot data also confirmed that monocytes express both receptors (Figure 6C and D). We found that both anti-IL-17 RA and RC antibodies are efficient in reducing IL-17-induced IL-6 production in RA synovial tissue fibroblasts (data not shown). Neutralization of monocyte IL-17 receptors by adding anti-IL-17 RA and RC antibodies to monocytes prior to their addition to the Boyden chamber suppressed IL-17-mediated chemotaxis (Figure 6E). Control IgG or antibody to TLR2 did not suppress IL-17-mediated chemotaxis (Figure 6E). These results indicate that both IL-17 receptors contribute to monocyte migration.
Figure 6. Human peripheral blood monocytes express IL-17 RA and RC, which are important for IL-17 mediated monocyte migration.
Real-time RT-PCR (A and B) and Western blot analysis (C and D) were employed to quantify IL-17 RA and RC expression levels in monocytes compared to HMVECs (Endo). mRNA and protein values were normalized to GAPDH or actin content, respectively. IL-17-induced monocyte chemotaxis was suppressed by neutralizing anti-IL-17 receptor antibodies. Monocytes were incubated with mouse anti-human IL-17 RA or RC antibody (10 μg/ml), control IgG (10 μg/ml) and anti-TLR2 antibody (10 μg/ml) for 3h. Thereafter, monocyte chemotaxis was performed in response to IL-17 (50 ng/ml) for 2–3h. Values are the mean ± SE from three different experiments. * represents p <0.05.
IL-17 and its receptors significantly contribute to RA SF-induced monocyte chemotaxis
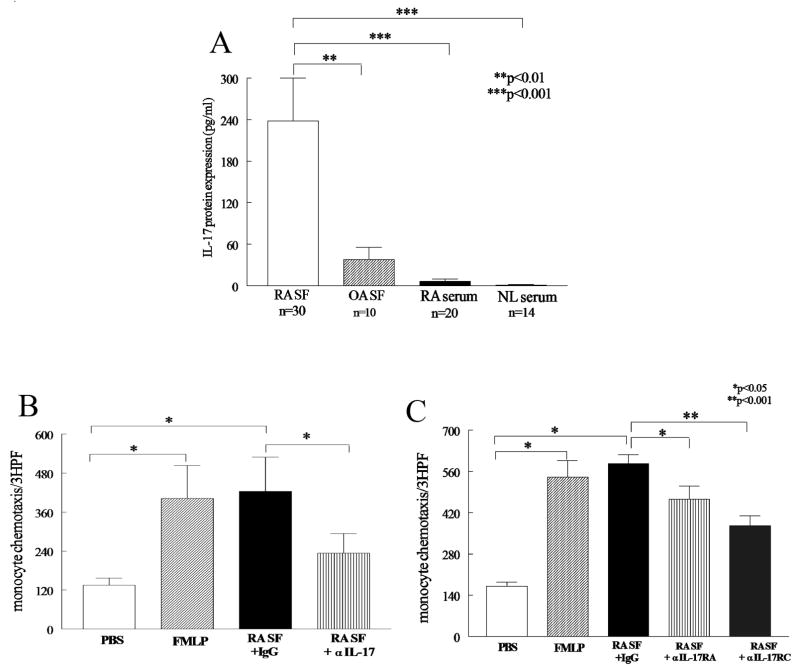
Studies were performed to determine if the IL-17 identified in RA synovial fluid was chemotactic for monocytes. The mean concentration of IL-17 in the 30 RA synovial fluids analyzed was 233 ± 64 pg/ml (Figure 7A), a concentration that was highly chemotactic (Figure 2A). The concentration of IL-17 in the RA synovial fluids was significantly greater than observed in osteoarthritis synovial fluid or RA or normal serum. Confirming our results others have demonstrated that IL-17 levels are markedly increased in RA synovial fluid compared to OA synovial fluid (26, 27). The basal levels of IL-17 were not different in RA pheripheral blood compared to that of OA patients. However peripheral blood cells activated with phytohemaglutinin (PHA) resulted in higher production of IL-17 in RA compared to OA patients(28).
Figure 7. RA synovial fluid-induced monocyte chemotaxis is mediated by IL-17.
(A) The levels of IL-17 were quantified in RA and osteoarthritis (OA) synovial fluid and RA and normal (NL) peripheral blood (PB) by ELISA. To investigate the role of IL-17 in RA SFs (B), anti-IL-17 (10 μg/ml) or control IgG was added to RA synovial fluids from 9 patients (1:20 dilution) (2–3h) prior to performing monocyte chemotaxis in response to the RA SFs. (C) Monocytes were incubated with antibodies to IL-17 RA and RC (10 μg/ml), as well as isotype control for 2–3 h prior to performing monocyte chemotaxis in response to 6 RA SFs. The values presented represent the mean ± SE. * represents p<0.05 and ** denotes p <0.01 and *** denotes p<0.001.
RA synovial fluid was chemotactic for monocytes, similar to the positive control FMLP (Figure 7B). Next, experiments were performed to determine if the chemotaxis mediated by RA synovial fluid was mediated by IL-17. Neutralization of IL-17 employing a monoclonal antibody to IL-17 significantly reduced (40%; p<0.05) monocyte chemotaxis compared to control IgG-treated RA synovial fluids (Figure 7B). Using the current data set there was no significant correlation (r=0.03) between the levels of IL-17 in the RA synovial fluid (up to 500 pg/ml) and the % chemotaxis reduction achieved by employing anti-IL-17 at 10μg/ml. Consistent with these data, in Figure 2B, 10 μg/ml of anti-IL-17 neutralized 10 ng/ml of recombinant IL-17, a concentration that was greater than that observed in the synovial fluids.
Additionally, neutralization of IL-17 RA and RC on monocytes was effective in suppressing RA SF-mediated monocyte migration (Figure 7C). These results suggest that IL-17 and its receptors IL-17 RA and RC may play an important role in migration of monocytes into the joints of patients with RA.
DISCUSSION
In this study we demonstrate that IL-17 promotes monocyte recruitment employing an in vivo sponge model, supporting the novel role of IL-17 in monocyte migration. Therefore, studies were performed to determine if IL-17 might directly mediate monocyte recruitment in vitro and whether a pathogenic role of IL-17 in RA may in part be due to its ability to promote monocyte migration. In the present study in vitro data demonstrates that monocyte migration is mediated by a direct effect of IL-17 on monocytes, since heat inactivation, neutralization of IL-17, and antagonist antibodies to IL-17 RA and RC, abrogate IL-17 mediated monocyte chemotaxis. Further, IL-17 is chemotactic for monocytes at concentrations detected in RA synovial fluid, and neutralization of IL-17 suppressed the monocyte chemotaxis induced with RA synovial fluid.
Previous studies demonstrated that intra-tracheal administration of IL-17 mediated neutrophil migration by inducing the expression of MIP-2 (rat analog of IL-8) (21). Neutralization of MIP-2 abrogated IL-17-induced neutrophil migration into the rat airways, suggesting an indirect effect of IL-17 via chemokine production (21). In contrast to our results with monocytes, IL-17 did not directly mediate neutrophil migration in vitro (22).
Next, experiments were performed to define the pathway(s) mediating IL-17 monocyte chemotaxis. IL-17 activates the p38, ERK, JNK and AKT pathways. The phosphorylation of p38 and AKT occurred within 1 hour, while activation of ERK and JNK was not observed until 2–3 hours. It is possible that IL-17 activation of ERK and JNK is downstream of the PI3K pathway, and therefore phosphorylation of these specific signaling molecules occurs subsequent to activation of PI3K as shown in other cell types (29, 30). Inhibition of each of these signaling pathways demonstrated that only p38 MAPK mediates IL-17-induced monocyte migration. Efforts to employ a more specific means to suppress chemotaxis, employing p38 siRNA, were not successful, since incubation of monocytes with non-specific siRNA interfered with monocyte chemotaxis. Similar to our results with IL-17, CCL2/MCP-1, CCL5/RANTES and CCL3/MIP-1α mediated monocyte chemotaxis was dependent on p38 (31) while the MCP-1-induced endothelial migration was through activation of ERK and PI3K (32). Also similar to the results with IL-17, monocyte migration induced by CCL2/MCP-1, CCL5/RANTES, CCL3/MIP-1α, FMLP was not mediated through PI3K or ERK signaling (33). The effects of p38 activation may be mediated through actin filament reorganization, which is essential for monocyte recruitment (34). HSP27 and lymphocyte specific protein are two substrates activated by p38, and are associated with regulation of actin filament dynamics (35–37). Taken together, p38 MAPK seems to be an important signaling pathway for monocyte migration that is utilized by IL-17, as well as other monocyte chemokines.
Although IL-17 is secreted from TH-17 memory T cells, IL-17 receptor is widely expressed on many cell types including RA synovial tissue fibroblasts and peripheral blood monocytes (10, 11). Employing real-time RT-PCR and Western blot analysis, we demonstrated that both IL-17RA and IL-17RC are expressed by monocytes. Since the monocyte migration induced by the cytokine macrophage migration inhibitory factor (MIF) is mediated through binding to G coupled protein receptor CXCR2 (38), we asked whether monocyte trafficking induced by IL-17 was due to binding to IL-17 RA or RC. IL-17-mediated monocyte chemotaxis was suppressed by neutralization of either IL-17 RA or RC, suggesting that that the effects of IL-17 were not mediated by the promiscuous binding of IL-17 to a classical, the seven trans-membrane domain, chemotactic receptor.
IL-17 contributed to the chemotaxis induced by RA synovial fluid. Neutralizing antibodies to IL-17 or antagonist antibodies to IL-17 RA and RC reduced the RA synovial fluid-induced monocyte migration, suggesting that IL-17 may play an important role in the recruitment of monocytes into RA synovial tissue. Other factors in RA synovial fluid capable of inducing monocyte migration include: CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, CXCL16, CCL20/MIP-3α and CX3CL1/fractalkine (23, 39–42), which are predominately produced by RA synovial tissue fibroblasts and macrophages. In contrast to these chemokines, IL-17 is secreted by RA synovial tissue memory T cells, suggesting a distinct role of T cells in the pathogenesis of RA. In addition to the direct effects on monocyte chemotaxis, IL-17 may also mediate monocyte chemotaxis through induction of monocyte chemokines from RA synovial fibroblasts (CCL7 and CCL20) (43).
In conclusion, we demonstrate that IL-17 induces monocyte migration in vitro and in vivo. IL-17-mediated monocyte migration was dependent on p38 activation and was associated with binding to IL-17 RA and RC on monocytes. Neutralization of IL-17 and its receptors significantly reduced monocyte chemotaxis induced by RA synovial fluid, suggesting that IL-17 may play an important role in monocyte ingress into inflamed RA synovial tissue.
Abbreviations used in this paper
- RA
rheumatoid arthritis
- IFN
interferon
- SCID
severe combined immunodeficient
- CIA
collagen induced arthritis
- FMLP
formyl-Met-Leu-Phe
- MIF
migration inhibitory factor
- TLR
toll like receptor
- HMVEC
human microvascular endothelial cells
Footnotes
This work was supported in part by awards from the National Institutes of Health (AR049353, AR048269 and AR055240) as well as grants from the American College of Rheumatology Within Our Reach.
References
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 3.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008 doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Tato CM, Muul L, Laurence A, O’Shea J. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 8.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamp LK, James MJ, Cleland LG. Interleukin-17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis? Immunology and cell biology. 2004;82:1–9. doi: 10.1111/j.1440-1711.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- 11.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada H, Nakashima Y, Okazaki K, Mawatari T, Fukushi JI, Kaibara N, Hori A, Iwamoto Y, Yoshikai Y. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 13.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 15.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffen SL. Biology of recently discovered cytokines: interleukin-17--a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6:240–247. doi: 10.1186/ar1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–414. [PubMed] [Google Scholar]
- 18.Chabaud M, Page G, Miossec P. Enhancing Effect of IL-1, IL-17, and TNF-alpha on Macrophage Inflammatory Protein-3alpha Production in Rheumatoid Arthritis: Regulation by Soluble Receptors and Th2 Cytokines. J Immunol. 2001;167:6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- 19.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 21.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 22.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 23.Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, Qin S, Koch AE. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 24.Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–8865. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 26.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kageyama Y, Ichikawa T, Nagafusa T, Torikai E, Shimazu M, Nagano A. Etanercept reduces the serum levels of interleukin-23 and macrophage inflammatory protein-3 alpha in patients with rheumatoid arthritis. Rheumatol Int. 2007 doi: 10.1007/s00296-007-0388-4. [DOI] [PubMed] [Google Scholar]
- 28.Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:R139–148. doi: 10.1186/ar1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang LC, Wang JP. Examination of the signal transduction pathways leading to activation of extracellular signal-regulated kinase by formyl-methionyl-leucyl-phenylalanine in rat neutrophils. FEBS Lett. 1999;454:165–168. doi: 10.1016/s0014-5793(99)00717-6. [DOI] [PubMed] [Google Scholar]
- 30.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 31.Ayala JM, Goyal S, Liverton NJ, Claremon DA, O’Keefe SJ, Hanlon WA. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J Leukoc Biol. 2000;67:869–875. [PubMed] [Google Scholar]
- 32.Arefieva TI, Kukhtina NB, Antonova OA, Krasnikova TL. MCP-1-stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–446. doi: 10.1016/j.cyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Fine JS, Byrnes HD, Zavodny PJ, Hipkin RW. Evaluation of signal transduction pathways in chemoattractant-induced human monocyte chemotaxis. Inflammation. 2001;25:61–67. doi: 10.1023/a:1007152903135. [DOI] [PubMed] [Google Scholar]
- 34.Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20:205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- 35.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- 36.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 37.Huang CK, Zhan L, Ai Y, Jongstra J. LSP1 is the major substrate for mitogen-activated protein kinase-activated protein kinase 2 in human neutrophils. J Biol Chem. 1997;272:17–19. doi: 10.1074/jbc.272.1.17. [DOI] [PubMed] [Google Scholar]
- 38.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 39.Tokuhira M, Hosaka S, Volin MV, Haines GK, 3rd, Katschke KJ, Jr, Kim S, Koch AE. Soluble vascular cell adhesion molecule 1 mediation of monocyte chemotaxis in rheumatoid arthritis. Arthritis Rheum. 2000;43:1122–1133. doi: 10.1002/1529-0131(200005)43:5<1122::AID-ANR23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Volin MV, Shah MR, Tokuhira M, Haines GK, Woods JM, Koch AE. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 41.Ruth JH, Haas CS, Park CC, Amin MA, Martinez RJ, Haines GK, 3rd, Shahrara S, Campbell PL, Koch AE. CXCL16-mediated cell recruitment to rheumatoid arthritis synovial tissue and murine lymph nodes is dependent upon the MAPK pathway. Arthritis Rheum. 2006;54:765–778. doi: 10.1002/art.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruth JH, Volin MV, Haines GK, III, Woodruff DC, Katschke KJ, Jr, Woods JM, Park CC, Morel JCM, Koch AE. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, Miossec V, Miossec P. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol. 2008;180:655–663. doi: 10.4049/jimmunol.180.1.655. [DOI] [PubMed] [Google Scholar]