Summary
Post-menopausal osteoporosis is considered to be an inflammatory process, in which numerous pro-inflammatory and T cell-derived cytokines play a bone-destructive role. IL-17A is the signature cytokine of the pro-inflammatory Th17 population, and plays dichotomous roles in diseases that affect bone turnover. Whereas IL-17A promotes bone loss in rheumatoid arthritis, it is protective against pathogen-induced bone destruction in a periodontal disease model. We used a model of ovariectomy-induced osteoporosis (OVX) in IL-17RAKO mice to evaluate the role of the IL-17A in bone loss caused by estrogen deficiency. Unexpectedly, IL-17RAKO mice were consistently and markedly more susceptible to OVX-induced bone loss than controls. There were no changes in prototypical Th1, Th2 or Th17 cytokines in serum that could account for increased bone loss. However, IL-17RAKO mice exhibited constitutively elevated leptin, which further increased following OVX. Consistently, IL-17A and IL-17F treatment of 3T3-L1 pre-adipocytes inhibited adipogenesis, leading to reduced production of leptin. In addition to its role in regulating metabolism and satiety, leptin can regulate bone turnover. Accordingly, these data show that IL-17A negatively regulates adipogenesis and subsequent leptin expression, which correlates with increased bone destruction during OVX.
Keywords: IL-17, T cells, bone loss, knockout, cytokine
Introduction
Osteoporosis is characterized by low bone mass and loss of structural integrity. In the U.S., 10 million individuals are estimated to have the disease and 34 million have low bone mass, placing them at increased risk. Despite much research effort, the molecular mechanisms involved in bone loss induced by estrogen deficiency remain poorly understood. Many proinflammatory cytokines, including interleukin (IL)-6 and TNF-α, have been implicated in the pathogenesis of osteoporosis. The pro-inflammatory cytokine IL-17A (also known as IL-17) acts upstream of IL-6 and synergizes with TNFα to promote inflammation and bone turnover in rheumatoid arthritis (RA) (reviewed in [1]). However, the role of IL-17A in osteoporosis is unknown.
IL-17A and the related cytokine IL-17F are produced by a newly-discovered subset of T helper lymphocytes, termed Th17 cells [2]. These cells develop in the context of TGF-β, IL-1 and IL-6 and undergo expansion and activation in the response to IL-23. IL-17A and IL-17F can also form heterodimers, and all 3 IL-17 isoforms are produced by γδ T cells, CD8+, NK and LTi (lymphoid tissue inducer) cells. IL-17-producing cells play important roles in host defense, particularly against extracellular pathogens. Conversely, Th17 cells drive immunopathology in autoimmune tissue injury [3]. Of particular relevance to bone diseases, IL-23 and IL-17A both contribute to the bone pathology in various models of inflammatory arthritis. This occurs in part by upregulating the relative ratio of RANKL (receptor activator of NF-κB ligand) to osteoprotogerin (OPG), which promotes osteoclastogenesis [4]. Blocking IL-17A or IL-23 in collagen-induced arthritis (CIA) reduces disease, and IL-17A-deficient mice are resistant to CIA and spontaneous arthritis in IL-1Ra-deficient mice (reviewed in [1]). In addition, Th17 cells express high levels of RANKL, providing a direct mechanism by which they may drive mediate bone destruction [5]. IL-17A induces production of TNF-α and IL-1β in macrophages [6], and synergizes with IL-1β and TNF-α in many cell backgrounds to increase the synthesis of pro-inflammatory effectors such as IL-6, IL-8 and matrix mellaproteinases [7, 8]. IL-1β, IL-6 and TNF-α are all associated with increased osteoclastogenesis following OVX [9-12]. Indeed, IL-6KO and TNF-αKO mice are protected from estrogen deficiency-induced bone loss [10, 13]. Thus, IL-17A and related pro-inflammatory cytokines have potent bone-destructive capacities in RA and other bone-destructive settings.
In contrast, IL-17A and IL-23 are bone-protective in certain situations. For example, bone destruction in periodontal disease (PD) occurs in the alveolar (jaw) bone crest, and is mediated by an exuberant inflammatory reaction following infection with anaerobic bacteria such as Porphyromonas gingivalis. In a mouse model of P. gingivalis-induced periodontal bone loss, the IL-17 receptor (IL-17RA) plays a bone-protective function by enhancing neutrophilic responses, which limit bacterial colonization [14-16]. Interestingly, the effects of IL-17A are more profound in female mice, suggesting a gender-biased activity of this cytokine [14]. There is also evidence that IL-23 inhibits osteoclastogenesis and is required for maintenance of bone mass in mice [17], indicating a bone-protective role for IL-23 that is independent of infection.
Accordingly, depending on context, the IL-23/IL-17A axis plays dichotomous roles in regulating bone. The role of IL-17A in the pathogenesis of postmenopausal osteoporosis is unknown. Osteoporosis is considered to be a pro-inflammatory disease [18] with many parallels to RA [19]. Accordingly, it was compelling to dissect the role of IL-17A and its receptor in the pathogenesis of osteoporosis.
Results
IL-17RA-deficient mice exhibit reduced bone mineral density following ovariectomy
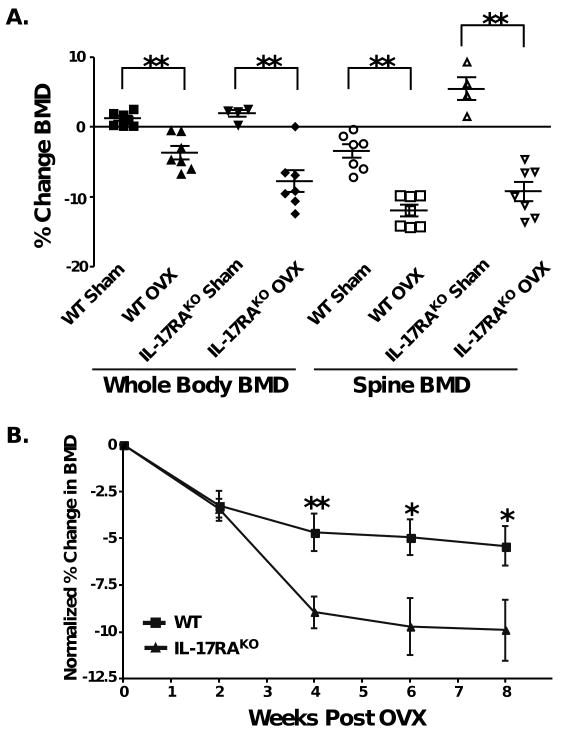
Since IL-17A activity correlates with other cytokines known to promote bone loss in osteoporosis models (e.g., IL-6, TNFα, IL-1β), we postulated that IL-17A would promote systemic bone loss following OVX. To test this hypothesis, OVX-induced bone loss was evaluated in IL-17RA-deficient mice, which cannot respond to IL-17A or IL-17F. First, we measured the baseline bone mineral density (BMD) in wild type (WT) and IL-17RAKO mice using a PIXImusTM DXA (dual X-ray absorptimetry) densitometer at 16 weeks, as mice acquire peak bone density by this age [20, 21]. WT and IL-17RAKO mice showed highly similar baseline BMD (data not shown). Mice were subjected to OVX or sham surgery, and DXA scans were performed 2, 4, 6 and 8 weeks later. Starting at 4 weeks post-OVX and continuing throughout the experiment, WT mice exhibited approximately 5% reduced whole body BMD compared to baseline (Fig 1), comparable to previous studies [22]. In 3 independent experiments, IL-17RAKO mice consistently showed significantly reduced BMD compared to WT, reaching ∼10% bone loss by weeks 6-8 (Fig. 1B and data not shown). DXA measurements of the lumbar spine also showed that IL-17RAKO mice had lower bone mineral density than WT starting at 4 weeks post-OVX and continuing throughout the duration of the experiment (Fig 1A; 6-week data are shown). Although spine BMD measurements were more variable than whole body BMD, the trend to reduced bone loss was statistically significant. Thus, contrary to expectations, IL-17RA signaling protects against estrogen deficiency-induced bone loss.
Figure 1. IL-17RAKO mice exhibit increased bone loss following ovariectomy.
A. Whole body BMD and spine BMD at 6 weeks post-OVX. Data are depicted as percent change from baseline. Solid symbols indicate whole body BMD measurements, open symbols represent spine BMD. This experiment is representative of 3 independent trials. ** p<0.01 by Students t-test (mean ± SD). B. Normalized BMD in WT and IL-17RAKO mice. Data were normalized to sham-surgery at each time point. * p<0.05; ** p<0.01 by students t-Test (mean ± SD). Experiments were performed 3 independent times, and a representative experiments is shown.
Weight gain in IL-17RAKO and WT mice
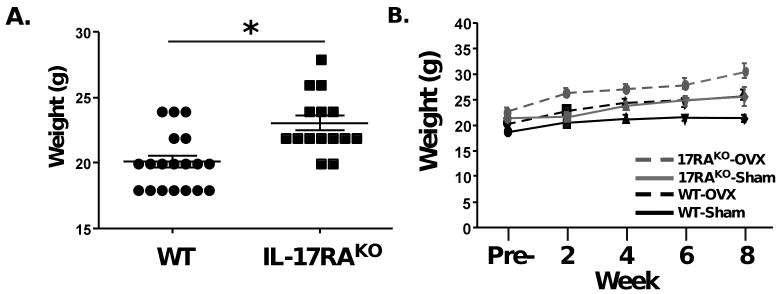
We and others have observed that IL-17RAKO mice weigh more than their WT counterparts. As shown, IL-17RAKO mice at baseline weighed an average of 23.07 g, whereas WT mice weighed an average of 20.1 g (Fig 2A). Following OVX, both WT and IL-17RAKO mice gained weight, as described previously [23]. However, while sham-treated WT mice did not gain weight, IL-17RAKO sham-treated animals did steadily gain weight, similar to WT mice undergoing OVX (Fig 2B). Accordingly, body weight is regulated abnormally in IL-17RAKO mice, which could potentially impact systemic physiological processes.
Figure 2. IL-17RAKO mice show increased weight compared to WT mice.
A. Weight (g) of WT and IL-17RAKO mice taken pre-OVX (includes mice from both Sham and OVX groups). *p<0.001 students t-Test. B. Average weight (g) of each group before OVX (“Pre”) and at 2-week intervals. Data are taken from the mice depicted in Figure 1.
Cytokine profile of WT and IL-17RA knockout mice following OVX
The finding that IL-17RAKO mice show elevated OVX-induced bone loss was quite unexpected, given the pro-inflammatory nature of IL-17A and the bone-destructive capacity of IL-17A and its downstream gene targets in RA. To better understand the mechanistic basis for this difference, we evaluated cytokine levels in sera of IL-17RAKO and WT mice, collected at baseline (1 week before OVX), and at 5 and 8 weeks post-OVX (Table 1). We assessed multiple cytokines based on their known roles in osteoporosis [11, 18, 24] or in Th1, Th2 or Th17 cells [16] (see also Discussion). Surprisingly, no significant differences in most cytokine levels were observed between WT and IL-17RAKO mice (Table 1, data not shown). TGF-β levels increased after OVX in both groups of mice, but no significant differences were observed between WT and IL-17RAKO mice. No significant changes were seen in Th1-associated cytokines such as TNFα and IFNγ or Th2-associated cytokines such as IL-4 (not shown) or IL-13 (Table 1). There were also no significant changes in the levels of IL-17A, and IL-23 was below the limit of detection (data not shown). IL-17F was not examined. This analysis suggests that either IL-17A modulates bone remodeling independently of these cytokines, or else IL-17A may affect cytokines in the local milieu proximal to bone, and such changes are not reflected in the peripheral blood.
Table 1. Serum concentrations of selected cytokines during ovariectomy.
WT or IL-17RAKO mice were subjected to OVX and serum collected at baseline (0 weeks), or 5 and 8 weeks post-OVX. Samples were assayed in by luminex. Data ± SEM are presented. * only 1 sample was available for analysis. N.D., not determinable, because data were below limit of detection.
| Condition | Week | IFNγ | TNFα | IL-13 | TGFβ | IL-17 |
|---|---|---|---|---|---|---|
| WT-Sham | 0 | 240.8±210.7 | 126.9±58.81 | 194.8±74.7 | 331,496±59,399 | 16.2±2.0 |
| 5 | 63.2±24.9 | 20.4±9.5 | 50.6±38.5 | 297,338±80,164 | 21.1±8.2 | |
| 8 | 53.5±27.6 | 17.25±6.3 | 119.6±30.2 | 519,008±50019 | 11.25±4.5 | |
| WT-OVX | 0 | 372.0±206.7 | 25.8±12.8 | 76.47±38.79 | 283,088±68,212 | 18.44±8.5 |
| 5 | 88.6±34.3 | 113.8±75.14 | 40.23±27.0 | 423,442±92,834 | 9.9±2.0 | |
| 8 | 75.1±49.5 | N.D.** | 27.33±14.13 | 609,411±53,835 | 9.3±3.8 | |
| KO-Sham | 0 | 1132±502.8 | 103.6±92.65 | 119.8±114.3 | 118,175±74675 | 32.2±13.4 |
| 5 | 300.2±154.2 | N.D. ** | 106.3±100.8 | 216327±83877 | 7.9±2.5 | |
| 8* | 67.1 | N.D. ** | 5.5 | 248,956±112,655 | 5.5 | |
| KO-OVX | 0 | 412.0±217.8 | 83.79±47.1 | 14.01±8.5 | 359043±105046 | 16.94±6.1 |
| 5 | 310.7±150.6 | N.D. ** | 115±83.3 | 160999±28873 | 12.9±5.1 | |
| 8 | 166.5±139.1 | N.D. ** | 144.7±123.6 | 464,410±13,708 | 10.28±4.3 |
IL-17RKO mice have elevated leptin levels, both at baseline and following OVX
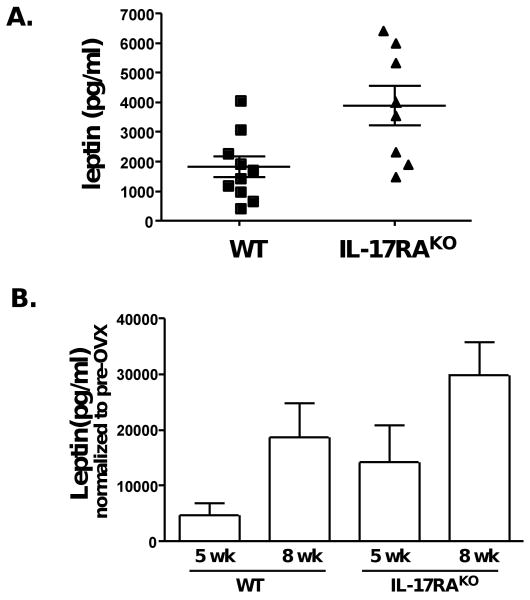
In addition to examining inflammatory and immunoregulatory cytokines, we also evaluated the IL-6-family cytokine leptin. Leptin was included in the study based on the observation that IL-17RAKO mice are consistently overweight (Fig. 2). Leptin was first discovered for its role in controlling satiety and body weight, but has since been implicated in bone metabolism [25, 26]. At baseline, IL-17RAKO mice had elevated leptin in serum compared to WT mice. Specifically, the mean of WT mice (sham and OVX groups combined) was 1820+352 pg/ml, whereas the mean of the IL-17RAKO mice was 3904±669 pg/ml (Table 1, Fig. 3A). At 5 and 8 weeks post-OVX, both WT and IL-17RAKO mice showed higher levels of leptin compared to baseline, but the leptin levels in IL-17RAKO mice were considerably and significantly higher than in the WT mice at both time points (Fig. 3B). The IL-17RAKO mice that underwent sham surgery also showed higher levels of leptin as they aged, suggesting that IL-17RAKO mice have higher leptin levels for reasons in addition to estrogen-deficiency.
Figure 3. Leptin is elevated in IL-17RAKO mice before and after OVX.
A. Baseline levels of leptin are lower in WT mice compared to IL-17RAKO mice. Each data point represents a single animal, and data are from Day 0 of both the Sham and OVX groups. B. Leptin levels normalized to the pre-OVX levels of leptin at 5- and 8-weeks post-OVX, as indicated. Data are taken from the mice depicted in Figure 1.
IL-17 downregulates leptin in 3T3L-1 cells
The role of leptin in bone metabolism is complex and not well understood. Leptin has been shown to cause bone loss through a central pathway in the hypothalamus [27]. Conversely, leptin is bone protective through a peripheral pathway [28, 29]. Since we observed that the accentuated bone loss in the IL-17RAKO mice correlated with elevated leptin, we hypothesized that leptin may act through the central pathway to trigger increased bone loss. According to this model, IL-17 would be expected to inhibit leptin expression. To test this possibility, we assessed the effects of IL-17 on leptin expression in adipocytes. 3T3-L1 pre-adipocyte cells were stimulated to differentiate into adipocytes for 11 days in vitro, and the effects of IL-17RA agonists (IL-17A and IL-17F) on leptin expression was determined. In addition, since IL-17A and IL-17F are known to exhibit potent synergy with TNFα [30], we also evaluated the effects of these cytokines in the presence of low levels (2 ng/ml) of TNFα. Strikingly, IL-17A, IL-17F (to a lesser extent) and TNF-α individually and synergistically suppressed leptin production in differentiated 3T3-L1 cells (Fig 4A). Moreover, this correlated with reduced differentiation, indicated by Oil Red O staining (Fig 4B). This finding indicates that IL-17RA signaling, particularly in concert with TNFα, downregulates adipocyte differentiation and hence inhibits leptin production. Thus, in IL-17RAKO mice, leptin levels are increased, which correlates with higher bone loss post-OVX.
Figure 4. IL-17 signaling negatively regulates adipogenesis and leptin expression.
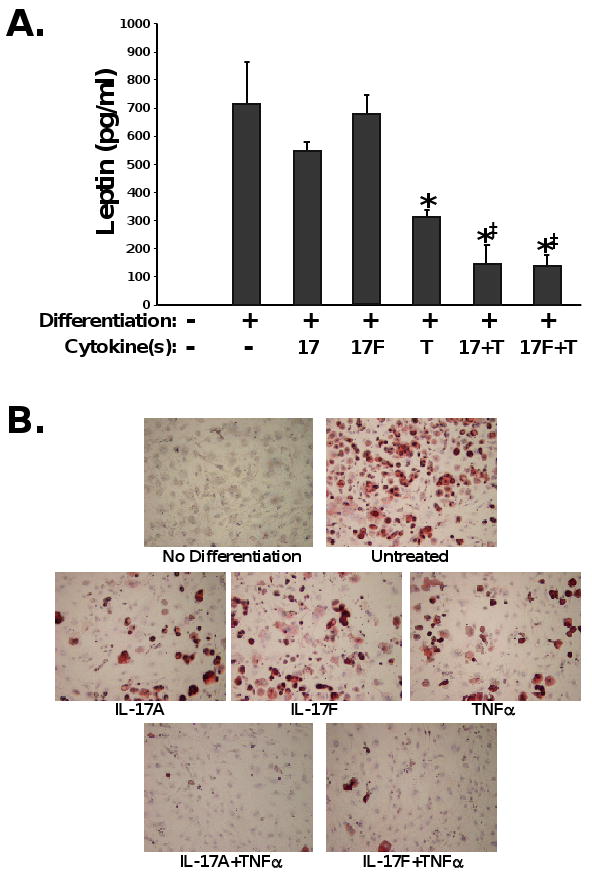
3T3-L1 pre-adipocytes were stimulated for differentiation for 8 days. As indicated, cultures were incubated with TNFα, IL-17A, and/or IL-17F during the adipogenesis culture period. Supernatants were analyzed in triplicate by ELISA. *p<0.05 compared to differentiated sample without cytokines by students t-Test. ‡ p<0.05 compared to TNFα-treated sample by students t-Test. B. Representative images of 3T3-L1 cells differentiated in the presence or absence of the indicated cytokines. Experiments were performed 3 independent times.
Discussion
The mechanisms of estrogen deficiency-induced osteoporosis remain poorly understood. Many cytokines, chemokines and chemokine receptors modulated by IL-17A or acting synergistically with IL-17A have been implicated in the pathogenesis of osteoporosis, which led us initially to predict that IL-17A would be bone-destructive in this context. However, our findings demonstrated that signaling through IL-17RA plays a strongly protective role in OVX-induced bone loss, as IL-17RAKO mice exhibited more bone loss than WT mice (Figure 1). The only cytokine that we found to be implicated in this process is leptin, which is increased in IL-17RAKO mice compared to WT. Leptin plays roles in both bone turnover and weight control [25], and here we show that IL-17 signaling inhibits both adipogenesis and leptin expression. Thus, IL-17RA appears to impact osteoporotic systemic bone loss in a complex and unexpected manner.
Cytokines and bone remodeling
Bone mass is regulated by a coordinated balance between bone-destructive osteoclasts (OCs) and bone-forming osteoblasts (OBs). OC development outpaces OB development, hence favoring bone loss during active bone remodeling. OC differentiation is mediated by the TNFR superfamily cytokine RANKL in combination with M-CSF (Macrophage-Colony Stimulating Factor). RANKL is inhibited by a soluble decoy receptor OPG (osteoprotegerin), which binds to RANK and prevents osteoclastogenesis [31, 32]. Cytokines produced or regulated by T cells can enhance OC formation during inflammation and estrogen deficiency by increasing the RANKL:OPG ratio [24]. Estrogen deficiency also increases the RANKL:OPG ratio through increases in proinflammatory effectors, including IL-1β, IL-6, IL-11, TNF-α, M-CSF and PGE2 [33-35]. IL-17A has been shown to increase RANKL expression and other inflammatory effectors in OBs, further stimulating bone turnover [8, 36]. Conversely, while T cells are a source of pro-osteolytic stimuli, they can also secrete factors that inhibit osteoclast formation, including IFN-γ, GM-CSF, IL-3, OPG, IL-4, IL-10, IL-13, and osteoclast inhibitory lectin (OCIL) [17, 37].
Osteoporosis is a pro-inflammatory state with increased inflammatory cytokines and activated T-cells [19]. A compelling reason to look into the role of IL-17 signaling in osteoporosis was previous evidence regarding the role of IL-6 in this setting. Since IL-17A is a potent inducer of IL-6, especially in combination with TNF-α, we predicted that IL-17A would act upstream of IL-6 to promote osteoporosis. However, our data revealed that IL-17RA signaling is protective in estrogen deficiency-induced bone loss. Although unexpected, this finding is consistent with a recent report that IL-23, a key upstream regulator of IL-17A, is bone protective under physiological conditions. The effects of IL-23 are mediated indirectly through lymphocytes, and GM-CSF is essential for this effect [17]. We did not assess GM-CSF in the present study, but it would be interesting to evaluate this cytokine in the future.
Cytokines and bone loss
To gain insight into the mechanisms underlying IL-17RA-mediated bone loss in OVX, we performed a phenotyping analysis of serum samples at 0, 5 and 8 weeks (Table 1 and data not shown). Surprisingly, there were no major changes in typical Th effector cytokines of the Th1, Th2 and Th17 lineages. With regards to Th1 cytokines, IFNγ was somewhat higher in IL-17RAKO mice, but this was not statistically significant. IFNγ has complex effects on osteoclastogenesis and bone resorption, depending on the system and the model employed. IFNγ has direct anti-osteoclastogenic effects on OC differentiation. However, in vivo the net effect of IFNγ is to stimulate osteoclastogenesis via upregulation of antigen presentation. This process leads to T cell activation and proliferation, with a consequent upregulation of osteoclastogenic cytokines including RANKL and TNFα [38]. In our serum analyses, IFNγ levels were somewhat higher in IL-17RAKO vs. WT at 5 weeks post-OVX (88.6±34.3 pg/ml in WT vs. 310.7±150.6 pg/ml in IL-17RAKO) but this did not reach significance. TNFα is sometimes considered a Th1 cytokine, although it is produced primarily by monocytes. IL-17A and TNFα potently synergize to regulate target gene expression [8]. There are numerous reports evaluating the role of TNFα in OVX-induced bone loss [13, 39-41]. Collectively, these studies suggest that upregulation of TNF-producing cells in the bone marrow is a mechanism by which estrogen deficiency induces bone loss. Indeed, functional blockade of TNFα in mice leads to protection from OVX-induced bone loss [41]. In our samples, levels of TNFα were not significantly different between WT and the IL-17RAKO mice (Table 1). One complication in comparing these prior studies to our findings is the time point at which OVX was performed; whereas we performed surgery at 16 weeks of age, the above-referenced reports performed OVX at 6 weeks, before the onset of peak bone mass in mice. Alternatively, it is possible that TNFα acts locally in the bone marrow while its levels remained unchanged in the serum.
Similarly, Th2 and Th17 cytokines did not vary significantly with OVX in IL-17RAKO mice. IL-4 was undetectable (data not shown), and IL-13 levels were variable. IL-13 is secreted by Th2 cells and regulates inflammatory responses in allergy. IL-13 has been shown to inhibit proliferation and stimulate IL-6 formation in human OBs [42]. However, IL-13 remained unchanged in WT vs. IL-17RAKO (115±83.3 pg/ml in IL-17RAKO vs. 40.23±27.0 pg/ml in WT). TGFβ controls Th17 differentiation, together with IL-6 and IL-1 (reviewed in [3]). Estrogen prevents bone loss through TGFβ signaling in T cells [22]. The authors of that study observed decreased TGFβ following OVX. However in our study, no significant change was observed comparing WT to IL-17RAKO mice (464,410±13,708pg/ml in IL-17RAKO vs. 609,411±53,835pg/ml at 8 weeks post-OVX). As noted, those studies were also performed in much younger mice and hence are not directly comparable to our model. Although IL-17A is often elevated in IL-17RAKO mice [43], we did not find an increase in this cohort, nor did levels change substantially during OVX. IL-23 levels were also undetectable (data not shown). Although IL-23 is implicated together with IL-17 in the pathogenesis of bone destruction in RA, IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for maintenance of bone mass in mice. Thus, despite evidence in the literature that pro-inflammatory Th-derived cytokines are important in OVX-induced bone loss, the prototypical Th effector cytokines did not change in a manner that could explain the increased bone destruction observed in IL-17RAKO mice.
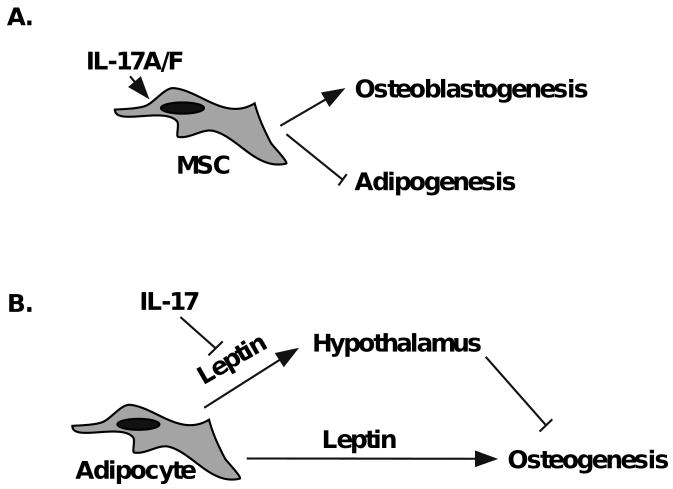
In contrast to the Th effector cytokines, cytokine analysis revealed that IL-17RAKO mice exhibit elevated leptin, which are further enhanced following OVX. This finding suggested that IL-17 may act to suppress leptin expression. Consistently, we found that IL-17A (and the closely related cytokine IL-17F) downregulates adipogenesis and leptin production in differentiated 3T3-L1 adipocytes, an effect that was more pronounced in the presence of TNFα (Fig 4). Consistent with this, a new report shows that IL-17A inhibits adipocyte differentiation and adiponectin expression in a human mesenchymal stem cell (hMSC) system [44]. Moreover, IL-17 accelerates differentiation of hMSCs into osteoblasts [45]. Putting these observations together, it is plausible that the balance of MSC differentiation in IL-17RAKO mice is tipped in favor of adipogenesis rather than osteoblastogenesis during bone remodeling in OVX, thus favoring accelerated bone destruction (Fig 5A).
Figure 5. Models for regulation of bone turnover by leptin and IL-17A.
A. Signaling via IL-17A and IL-17F promote mesenchymal stem cells (MSC) to undergo osteoblastogenesis and simulataneously inhibit adipogenesis Thus, the absence of IL-17RA may limit osteoblast development and drive accelerated bone turnover during OVX. B. Leptin can limit osteogenesis through a central pathway from the hypothalamus. By inhibiting leptin expression, IL-17 may aid in limiting bone turnover during OVX. In IL-17RAKO mice, bone loss is thus enhanced.
This finding was initially surprising, as IL-17RAKO mice are consistently heavier than age- and sex-matched WT mice (Fig 2). However, obesity is often associated with leptin resistance and elevated leptin levels in serum [28], and hence the high basal levels of leptin (Fig 3A) may reflect an altered metabolism that predisposes to obesity in these mice. In humans, osteoporosis negatively correlates with body mass. However, this issue is very different in rodents, where a small body size becomes far less subject to the load-bearing effects of gravity. Although these studies do not directly prove a causative role for leptin in the exacerbated bone loss observed in IL-17RAKO mice, they nonetheless highlight the complexity of the IL-17R signaling pathway in mediating complex physiological events. Counter to this, in a small study of obese humans, IL-17A and IL-23 have been linked to obesity, independent of leptin levels [46]. Thus, further studies are needed to understand the relationship between body mass and bone regulation.
The skeletal effects of leptin are complex and regulated across multiple pathways. Acting centrally through the hypothalamus, leptin exerts opposing effects on cancellous versus cortical bone. Specifically, leptin deficient (ob/ob) mice have higher density of cancellous bone but lower cortical and total bone density [28]. Leptin also exerts effects peripherally through direct action on osteoblasts, resulting in increased bone mass [28]. Based on our data, another plausible explanation is that the predominant effect of leptin in the context of estrogen deficiency-induced osteoporosis occurs through the hypothalamus acting to inhibit osteogenesis. IL-17A normally helps limits production of leptin; thus, its absence in IL-17RAKO mice correlates with reduced bone formation (Fig 5B).
Although this work has focused on the roles of IL-17RA and its ligands IL-17A and IL-17F, there is increasing evidence that IL-17RA acts as a commonly shared subunit within the larger IL-17 receptor family. For example, it was recently shown that IL-17RA is an essential receptor for IL-17E/IL-25 signaling [47]. Moreover, IL-17RA has been reported to pair with IL-17RD in an overexpression system [48], although the cognate ligand for an IL-17RA/IL-17RD complex is as yet unidentified.
Clearly, far more work needs to be done to dissect the molecular role of IL-17RA signaling in estrogen deficiency-induced bone loss. This area is particularly important due to the impending use of anti-cytokine therapies that are designed to block IL-17A or its receptor in autoimmune disease. Since the target populations for RA and osteoporosis have considerable overlap (namely, post-menopausal women), the potential side effects of blocking IL-17A with respect to osteoporosis need to be considered.
Materials and Methods
Mice, ovariectomy and bone densitometry
IL-17RAKO mice on the C57BL/6 background were kindly provided by Amgen (Seattle, WA). C57BL/6 mice were from The Jackson Laboratory (Bar Harbor, ME). All data were generated from female, age-matched littermates (n=7-8). Ovariectomy was performed on 16-week-old mice under general anesthesia. At necropsy (8 post-surgery), uterus atrophy was used to confirm successful ovariectomy retrospectively. Bone densitometry measurements of whole body or spine were taken using PIXImusTM bone densitometer (GE Lunar Medical Systems) under general anesthesia, subtracting contributions from the head. All procedures were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Cytokine profiling
Serum samples were collected by retro-orbital bleeding, or by heart puncture after sacrifice. Samples were analyzed by mutiplex assay using the Thermo Scientific (SearchLightTM) array system.
Adipogenesis studies
3T3-L1 cells were from ATCC (Manassas, VA). Cells were grown to confluence in 6 well plates in maintenance media [High glucose DMEM, Bovine Calf Serum, Pen-Strep, Non-essential amino acids, Pantothenate, Biotin (Invitrogen, Carlsbad CA)]. Two days after reaching confluence, cells were incubated with an adipogenesis cocktail consisting of 0.07mg/ml Insulin (bovine pancreas), 0.0004mg/ml dexamethasone, 0.5mM 3-isobutyl-1-methylxanthine (IBMX), 0.003mg/ml ciglitizone (Sigma, St. Louis MO) with IL-17A (200ng/ml; Peprotech), IL-17F (200ng/ml; Peprotech), TNFα (2ng/ml; Peprotech, Rocky Hill, NJ) for 4 days. Thereafter cells were allowed to load lipid for 4-6 days. Leptin ELISA was performed in triplicate using a kit from R&D Systems(Minneapolis, MN).
Statistical analyses
Data are presented as the means ± SEM. Statistical comparisons were by Student's unpaired two-tailed t test (Graph Pad Prism). P values ≤ 0.05 were considered significant.
Acknowledgments
We thank Dr. Charles O'Brien (University of Arkansas) and Dr. Scott Wersinger (SUNY at Buffalo) for advice regarding OVX methodology. IL-17RAKO mice were generously provided by Amgen. SLG was supported by NIH grants AR054389 and DE019424. LAZ was supported by F31-AI073198-02.
Abbreviations
- OVX
ovariectomy
- RA
rheumatoid arthritis
- RANKL
receptor activator of NF-κB ligand
- OPG
osteoprotogerin
- BMD
bone mineral density
- DXA
dual energy X-ray absorptiometry
- PD
periodontal disease
References
- 1.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 4.Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LA, van den Berg WB. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003;170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jovanovic D, di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1b and TNF-a, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 7.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 9.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas S. Increased osteoclast development after estrogen loss: Mediated by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 10.Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo J. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The Interleukin-17 Receptor Plays a Gender-Dependent Role in Host Protection against Porphyromonas gingivalis-Induced Periodontal Bone Loss. Infect Immun. 2008;76:8. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn JM, Sims NA, Saleh H, Mirosa D, Thompson K, Bouralexis S, Walker EC, Martin TJ, Gillespie MT. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J Immunol. 2008;181:5720–5729. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- 18.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res. 2005;20:1085–1092. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 21.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Qian WP, Dark K, Toraldo G, Lin AS, Guldberg RE, Flavell RA, Weitzmann MN, Pacifici R. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci U S A. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, Cui H, Yamada K, Ishimi Y. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004;53:942–948. doi: 10.1016/j.metabol.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr. 2009;89:991S–997S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karsenty G, Ducy P. The hypothalamic control of bone mass, implication for the treatment of osteoporosis. Ann Endocrinol (Paris) 2006;67:123. doi: 10.1016/s0003-4266(06)72566-5. [DOI] [PubMed] [Google Scholar]
- 27.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 28.Lee NJ, Wong IP, Baldock PA, Herzog H. Leptin as an endocrine signal in bone. Curr Osteoporos Rep. 2008;6:62–66. doi: 10.1007/s11914-008-0011-y. [DOI] [PubMed] [Google Scholar]
- 29.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 30.Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukoc Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 31.Theill L, Boyle W, Penninger J. RANK-L and RANK: T cells, bone loss and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 32.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 33.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 34.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 35.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 36.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 37.Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9:103. doi: 10.1186/ar2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 40.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271:28890–28897. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- 41.Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 42.Frost A, Jonsson KB, Brandstrom H, Ohlsson C, Ljunghall S, Ljunggren O. Interleukin-13 inhibits cell proliferation and stimulates interleukin-6 formation in isolated human osteoblasts. J Clin Endocrinol Metab. 1998;83:3285–3289. doi: 10.1210/jcem.83.9.5127. [DOI] [PubMed] [Google Scholar]
- 43.Nagata T, McKinley L, Peschon JJ, Alcorn JF, Aujla SJ, Kolls JK. Requirement of IL-17RA in Con A induced hepatitis and negative regulation of IL-17 production in mouse T cells. J Immunol. 2008;181:7473–7479. doi: 10.4049/jimmunol.181.11.7473. [DOI] [PubMed] [Google Scholar]
- 44.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, Lee Y, Kim HH. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 46.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 47.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 48.Rong Z, Wang A, Li Z, Ren Y, Cheng L, Li Y, Wang Y, Ren F, Zhang X, Hu J, Chang Z. IL-17RD (Sef or IL-17RLM) interacts with IL-17 receptor and mediates IL-17 signaling. Cell Res. 2008;19:208–215. doi: 10.1038/cr.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]