Abstract
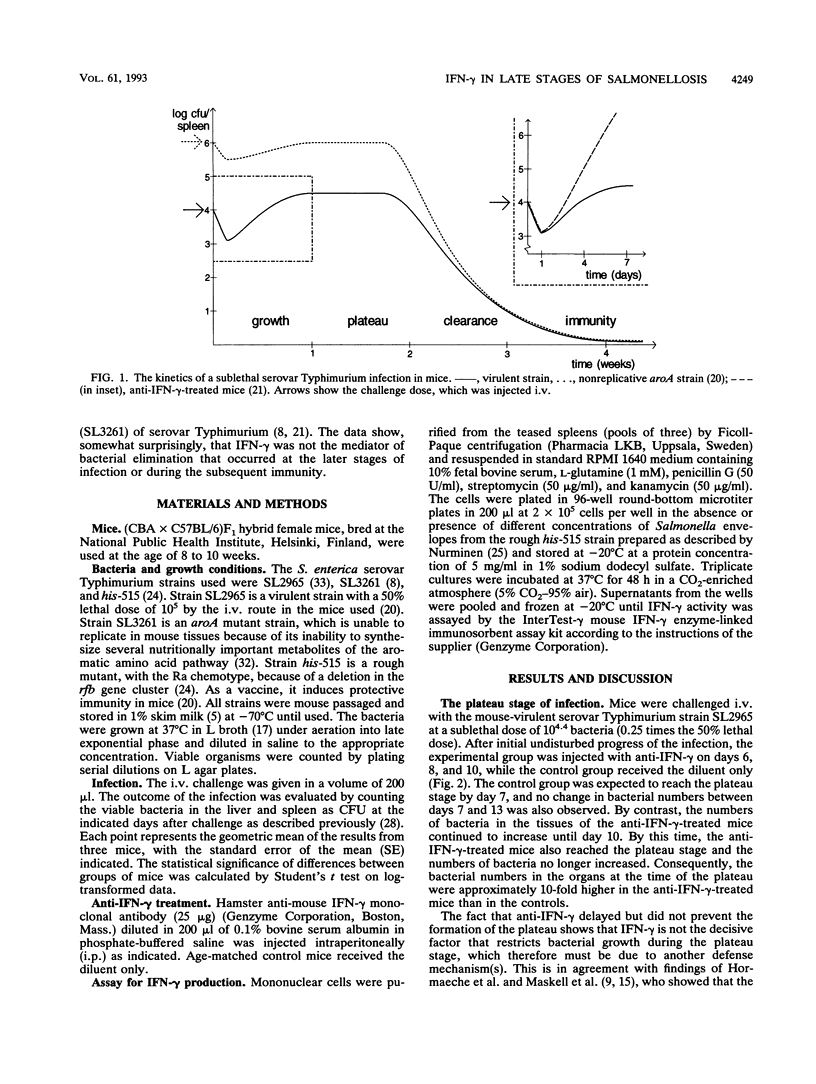
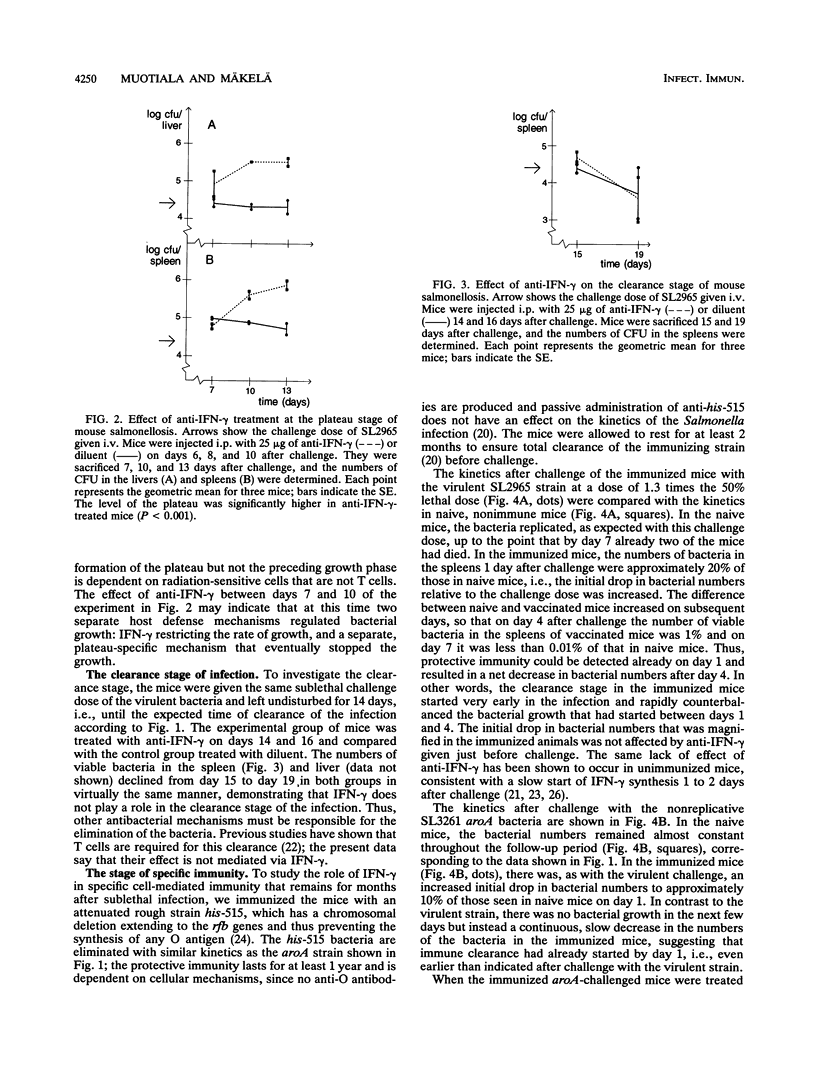
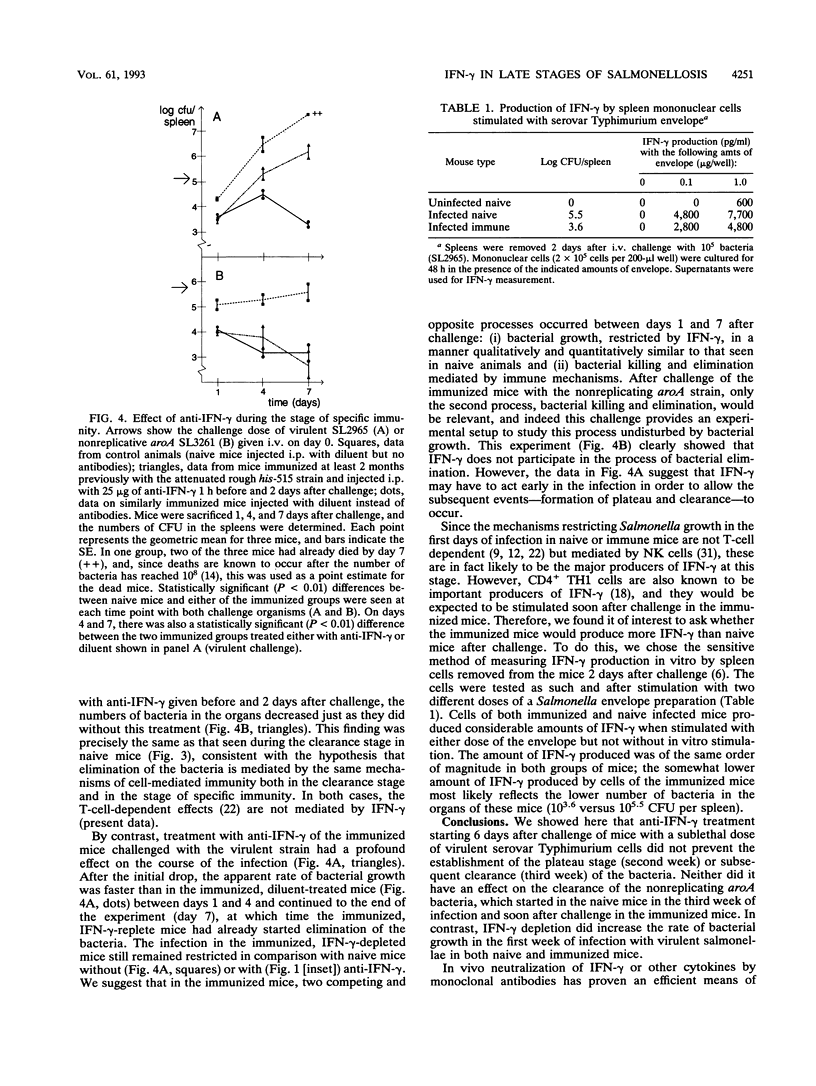
Gamma interferon (IFN-gamma) is an important mediator of natural resistance of mice to Salmonella species during the first week of infection, when it restricts the rate of intracellular growth of the bacteria but does not lead to their killing (A. Muotiala and P. H. Mäkelä, Microb. Pathog. 8:135-141, 1990). We used the experimental mouse salmonellosis model to investigate the role of IFN-gamma in the later stages of a sublethal infection and the ensuing specific immunity. When anti-IFN-gamma was administered starting 6 days after challenge, it did not prevent the cessation of intracellular bacterial growth and the formation of the plateau stage in the second week of infection. In addition, anti-IFN-gamma given 14 and 16 days after challenge did not alter the elimination of the bacteria in the clearance stage in the third week of infection. When mice immunized 2 months previously with live vaccine were infected with virulent salmonellae, depletion of IFN-gamma enhanced the early growth of the bacteria in the same manner as that seen in naive mice. However, when the immunized mice were infected with attenuated aroA bacteria, their clearance started immediately and was unaffected by IFN-gamma depletion, demonstrating that IFN-gamma is not required for the clearance. We conclude that IFN-gamma restricts the rate of intracellular bacterial multiplication in the first week of Salmonella infection in both naive and immune mice but is not a mediator of bacterial clearance in either naive or immunized mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Hall P., Roberts S. J., Briles D. E. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J Immunol. 1990 Apr 15;144(8):3143–3151. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Kumazawa Y., Meding S., Langhorne J., Galanos C. Gamma interferon production in endotoxin-responder and -nonresponder mice during infection. Infect Immun. 1991 Oct;59(10):3484–3491. doi: 10.1128/iai.59.10.3484-3491.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E., Mastroeni P., Arena A., Uddin J., Joysey H. S. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990 Jun;70(2):247–250. [PMC free article] [PubMed] [Google Scholar]
- Hougen H. P., Jensen E. T. Experimental Salmonella typhimurium infections in rats. III. Transfer of immunity with primed lymphocyte subpopulations. APMIS. 1990 Nov;98(11):1015–1021. [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985 Mar;47(3):605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell D. J., Hormaeche C. E., Harrington K. A., Joysey H. S., Liew F. Y. The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microb Pathog. 1987 Apr;2(4):295–305. doi: 10.1016/0882-4010(87)90127-6. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Arena A., Costa G. B., Liberto M. C., Bonina L., Hormaeche C. E. Serum TNF alpha in mouse typhoid and enhancement of a Salmonella infection by anti-TNF alpha antibodies. Microb Pathog. 1991 Jul;11(1):33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Muotiala A. Anti-IFN-gamma-treated mice--a model for testing safety of live Salmonella vaccines. Vaccine. 1992;10(4):243–246. doi: 10.1016/0264-410x(92)90159-h. [DOI] [PubMed] [Google Scholar]
- Muotiala A., Hovi M., Mäkelä P. H. Protective immunity in mouse salmonellosis: comparison of smooth and rough live and killed vaccines. Microb Pathog. 1989 Jan;6(1):51–60. doi: 10.1016/0882-4010(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Muotiala A., Mäkelä P. H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990 Feb;8(2):135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol. 1990 Aug 15;145(4):1265–1269. [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarathinam L., Shaban R. A., Niesel D. W., Klimpel G. R. Interferon gamma (IFN-gamma) production by gut-associated lymphoid tissue and spleen following oral Salmonella typhimurium challenge. Microb Pathog. 1991 Nov;11(5):347–356. doi: 10.1016/0882-4010(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Riikonen P., Mäkelä P. H., Saarilahti H., Sukupolvi S., Taira S., Rhen M. The virulence plasmid does not contribute to growth of Salmonella in cultured murine macrophages. Microb Pathog. 1992 Oct;13(4):281–291. doi: 10.1016/0882-4010(92)90038-p. [DOI] [PubMed] [Google Scholar]
- Saxén H., Nurminen M., Kuusi N., Svenson S. B., Mäkelä P. H. Evidence for the importance of O antigen specific antibodies in mouse-protective Salmonella outer membrane protein (porin) antisera. Microb Pathog. 1986 Oct;1(5):433–441. doi: 10.1016/0882-4010(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Saxén H., Reima I., Mäkelä P. H. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb Pathog. 1987 Jan;2(1):15–28. doi: 10.1016/0882-4010(87)90111-2. [DOI] [PubMed] [Google Scholar]
- Schafer R., Eisenstein T. K. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992 Mar;60(3):791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A. Auxotrophic Salmonella typhi as live vaccine. Vaccine. 1988 Apr;6(2):141–145. doi: 10.1016/s0264-410x(88)80017-3. [DOI] [PubMed] [Google Scholar]
- Sukupolvi S., O'Connor D., Edwards M. F. The traT protein is able to normalize the phenotype of a plasmid-carried permeability mutation of Salmonella typhimurium. J Gen Microbiol. 1986 Aug;132(8):2079–2085. doi: 10.1099/00221287-132-8-2079. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981 May;32(2):490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Dougan G., Chatfield S. N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991 Nov 1;147(9):3161–3164. [PubMed] [Google Scholar]



