Abstract
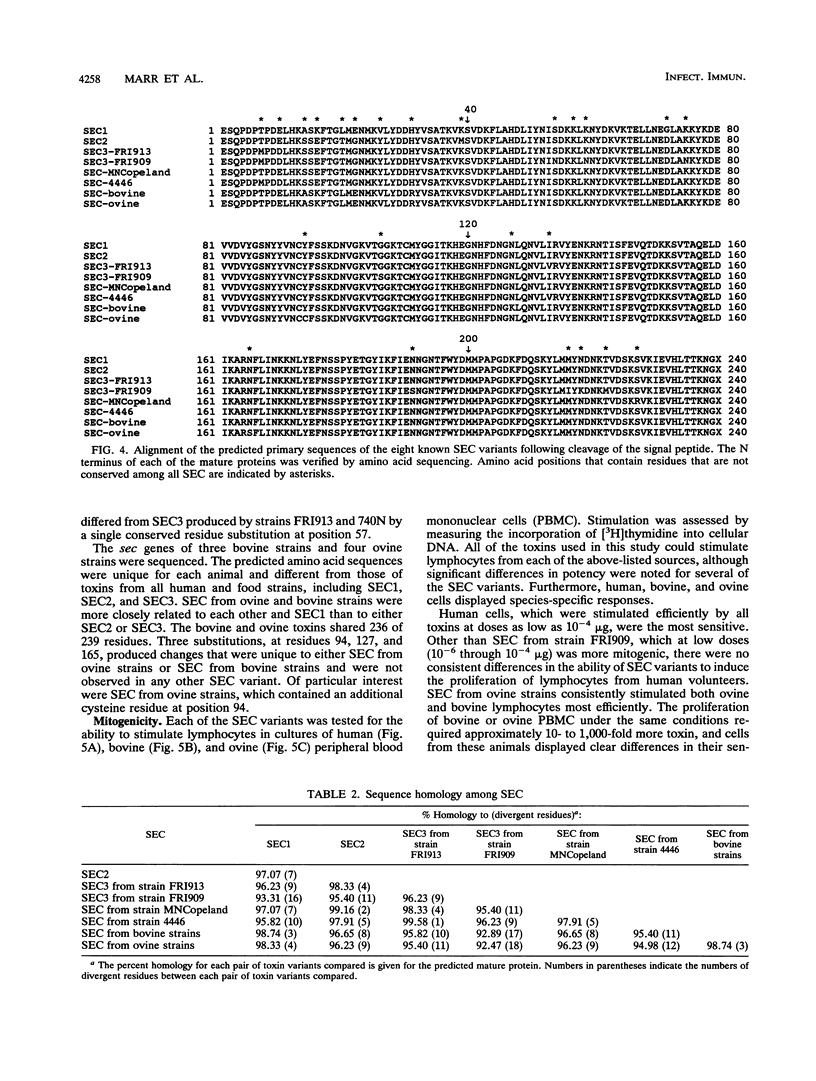
The type C staphylococcal enterotoxins (SEC) are a group of highly conserved proteins with significant immunological cross-reactivity. Although three antigenically distinct SEC subtypes (SEC1, SEC2, and SEC3) have been reported in the literature, we observed that the isoelectric points of SEC from several Staphylococcus aureus isolates are different from those of any of these three subtypes. This observation led us to propose that additional SEC molecular variants exist. For assessment of this possibility, the sec genes from representative human, animal, and food isolates were cloned and sequenced. The toxins encoded by the 18 isolates used in this study included five unique SEC proteins in addition to SEC1, SEC2, and SEC3. Six of the SEC proteins (including SEC1, SEC2, and SEC3) were produced by human and food isolates. Analysis of seven bovine and ovine isolates showed that isolates from each animal species produced a unique host-specific SEC. All of the SEC caused lymphocyte proliferation, although some of the toxins differed in their ability to stimulate cells from several animal species. An explanation for these results, which is supported by our phenotypic analysis of Sec+ staphylococcal isolates, is that toxin heterogeneity is due to selection for modified SEC sequences that facilitate the survival of S. aureus isolates in their respective hosts.
Full text
PDF
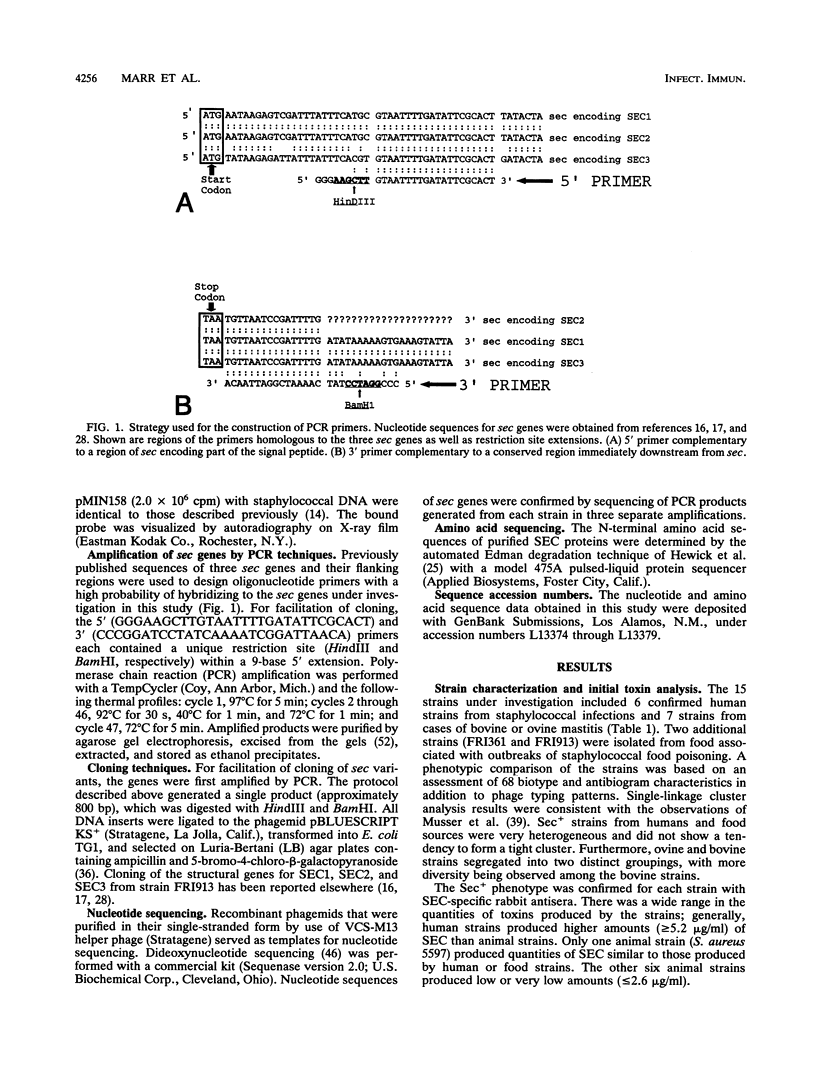




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avena R. M., Bergdoll M. S. Purification and some physicochemical properties of enterotoxin C, Staphylococcus aureus strain 361. Biochemistry. 1967 May;6(5):1474–1480. doi: 10.1021/bi00857a033. [DOI] [PubMed] [Google Scholar]
- Barsumian E. L., Cunningham C. M., Schlievert P. M., Watson D. W. Heterogeneity of group A streptococcal pyrogenic exotoxin type B. Infect Immun. 1978 May;20(2):512–518. doi: 10.1128/iai.20.2.512-518.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll M. S., Borja C. R., Avena R. M. Identification of a new enterotoxin as enterotoxin C. J Bacteriol. 1965 Nov;90(5):1481–1485. doi: 10.1128/jb.90.5.1481-1485.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll M. S., Robbins R. N., Weiss K., Borja C. R., Huang Y., Chu F. S. The staphylococcal enterotoxins: similarities. Contrib Microbiol Immunol. 1973;1:390–396. [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Staphylococcal enterotoxin A is encoded by phage. Science. 1985 Jul 12;229(4709):185–187. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Hovde C. J., Handley J. P., Schlievert P. M. Cross-neutralization of staphylococcal and streptococcal pyrogenic toxins by monoclonal and polyclonal antibodies. Infect Immun. 1988 Feb;56(2):400–404. doi: 10.1128/iai.56.2.400-404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Kreiswirth B. N., Novick R. P., Schlievert P. M. Analysis of toxic shock syndrome isolates producing staphylococcal enterotoxins B and C1 with use of southern hybridization and immunologic assays. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S75–S82. doi: 10.1093/clinids/11.supplement_1.s75. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. doi: 10.1128/iai.57.7.2249-2252.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Expression of staphylococcal enterotoxin C1 in Escherichia coli. Infect Immun. 1987 Feb;55(2):428–432. doi: 10.1128/iai.55.2.428-432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Borja C. R., Bergdoll M. S. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967 May;6(5):1467–1473. doi: 10.1021/bi00857a032. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Ho G., Campbell W. H., Carlson E. Ovine-associated Staphylococcus aureus protein with immunochemical similarity to toxic shock syndrome toxin 1. J Clin Microbiol. 1989 Jan;27(1):210–212. doi: 10.1128/jcm.27.1.210-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hovde C. J., Hackett S. P., Bohach G. A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990 Jan;220(2):329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- Hynes W. L., Weeks C. R., Iandolo J. J., Ferretti J. J. Immunologic cross-reactivity of type A streptococcal exotoxin (erythrogenic toxin) and staphylococcal enterotoxins B and C1. Infect Immun. 1987 Mar;55(3):837–838. doi: 10.1128/iai.55.3.837-838.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. P., Tomai M. A., Schlievert P. M. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986 May;166(2):623–627. doi: 10.1128/jb.166.2.623-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970 Mar 1;131(3):611–622. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Projan S. J., Schlievert P. M., Novick R. P. Toxic shock syndrome toxin 1 is encoded by a variable genetic element. Rev Infect Dis. 1989 Jan-Feb;11 (Suppl 1):S83–S89. doi: 10.1093/clinids/11.supplement_1.s83. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. K., Kreiswirth B. N., Deringer J. R., Projan S. J., Eisner W., Smith B. L., Carlson E., Novick R. P., Schlievert P. M. Nucleotide sequences and biologic properties of toxic shock syndrome toxin 1 from ovine- and bovine-associated Staphylococcus aureus. J Infect Dis. 1992 Jun;165(6):1056–1063. doi: 10.1093/infdis/165.6.1056. [DOI] [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Spero L. Intrinsic and chemically produced microheterogeneity of Staphylococcus aureus enterotoxin type C. Infect Immun. 1975 Jul;12(1):93–97. doi: 10.1128/iai.12.1.93-97.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Schlievert P. M., Chow A. W., Ewan P., Kreiswirth B. N., Rosdahl V. T., Naidu A. S., Witte W., Selander R. K. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci U S A. 1990 Jan;87(1):225–229. doi: 10.1073/pnas.87.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Fox L. K., Hamilton M. J., Davis W. C. Bovine mononuclear leukocyte subpopulations in peripheral blood and mammary gland secretions during lactation. J Dairy Sci. 1992 Apr;75(4):998–1006. doi: 10.3168/jds.S0022-0302(92)77842-4. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Fox L. K., Hamilton M. J., Davis W. C. Suppression of proliferative response of BoCD4+ T lymphocytes by activated BoCD8+ T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis. Vet Immunol Immunopathol. 1993 Mar;36(2):137–151. doi: 10.1016/0165-2427(93)90103-b. [DOI] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Noleto A. L., Khoe G. P., Bergdoll M. S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984 Sep;45(3):625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson J. R., Fox L. K., Hancock D. D., Besser T. E. Evaluation of methods for differentiation of coagulase-positive staphylococci. J Clin Microbiol. 1992 Dec;30(12):3217–3219. doi: 10.1128/jcm.30.12.3217-3219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Blomster D. A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983 Feb;147(2):236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Purification and characterization of staphylococcal pyrogenic exotoxin type B. Biochemistry. 1980 Dec 23;19(26):6204–6208. doi: 10.1021/bi00567a040. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Schoettle D. J., Watson D. W. Purification and physicochemical and biological characterization of a staphylococcal pyrogenic exotoxin. Infect Immun. 1979 Mar;23(3):609–617. doi: 10.1128/iai.23.3.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Cross-reactions between tryptic polypeptides of staphylococcal enterotoxins B and C. J Immunol. 1979 Apr;122(4):1285–1289. [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S. Monoclonal antibodies to staphylococcal enterotoxins B and C: cross-reactivity and localization of epitopes on tryptic fragments. Infect Immun. 1984 Jul;45(1):281–285. doi: 10.1128/iai.45.1.281-285.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. N., Smith C. L., Bohach G. A. Residues 20, 22, and 26 determine the subtype specificities of staphylococcal enterotoxins C1 and C2. Infect Immun. 1992 Feb;60(2):694–697. doi: 10.1128/iai.60.2.694-697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. W. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960 Feb 1;111:255–284. doi: 10.1084/jem.111.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]