Abstract
T helper type 17 (Th17) cells are a distinct lineage of T cells that produce the effector molecules IL-17, IL-17F, IL-21 and IL-22. Although the role of Th17 cells in autoimmunity is well documented, there is growing evidence that the Th17 lineage and other IL-17 producing cells are critical for host defense against bacterial, fungal and viral infections at mucosal surfaces. Here we summarize recent progress in our understanding of the function of IL-17 producing cells as a bridge between innate and adaptive immunity against infectious diseases at the mucosa.
Keywords: Th17, Infection, mucosa
Introduction
CD4+ T helper cells are important mediators of adaptive immune responses. Following interaction with antigen presenting cells, T cells receive signals by engagement of the T cell receptor (signal 1), costimulatory molecules (signal 2) and a complex network of cytokine signals (signal 3) and undergo activation and differentiation into effector CD4+ T cells. Their critical role in host defense against infections is clearly manifested in humans with either congenital deficiencies or acquired deficiency (most commonly through HIV infection) of the T-cell lineage. It was recognized shortly after the initial description of AIDS that the primary immunodeficiency was loss of circulating CD4+ T-cells and that the risk of opportunistic infection with many pathogens was directly related to the severity of the CD4+ T-cell deficiency. Four years after the initial clinical description of AIDS, Mossmann and Coffman described the first two CD4+ T-cells subsets based on discrete cytokine profiles1. Th1 effectors produce Interferon-gamma (IFN-γ) and regulate cellular immunity against intracellular infections whereas Th2 cells produce Interleukin (IL)-4, IL-5 and IL-13 and mediate humoral immunity against parasite infections (Figure 1). However this dichotomy of T-cell subsets could not fully explain the infections seen in congenital or acquired absence of CD4+ T-cells such as mucosal Candidiasis, Pneumocystis carinii pneumonia, or some bacterial pneumonias. For example mice deficient in Th1, Th2 responses (or both) are not permissive for P. carinii pneumonia2, a hallmark infection in AIDS patients with low CD4+ T-cell counts. These data suggested other CD4+ T-cell lineages are critical for host defenses against opportunistic infections. Recent compelling evidence has clearly changed this traditional paradigm of Th1/Th2 cell dichotomy to include a third subset of T cells referred to as Th17 cells3-6. Th17 cells produce the cytokines IL-17A (IL-17) 4,5 and IL-17F3, as well as the cytokines IL-217,8 and IL-22 9 10 (Figure 1). This new Th17 cell lineage fills in some of the missing gaps in host immunity not fully explained by the Th1/Th2 paradigm.
Figure 1. Current understanding of T-helper cell differentiation.
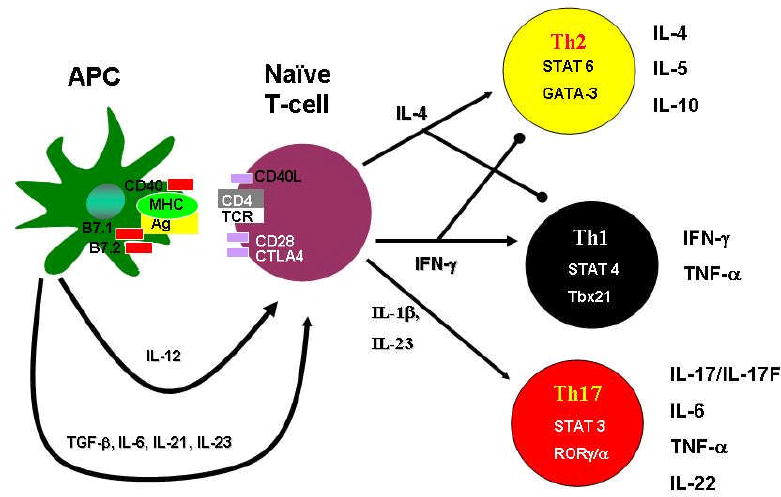
Naïve CD4+ T cells, after activation by signaling via the T-cell Receptor and co-stimulatory molecules such as CD28 and Cytotoxic T lymphocyte-associated protein 4 (CTLA-4), can differentiate into three lineages of effector T helper(Th) cells namely Th1, Th2 or Th17 cells. Th subsets produce different cytokines and have distinct immunoregulatory functions. Th2 cells produce IL-4, IL-5 and IL-10 and promote immunity against parasites such as helminths. Th1 cells produce IFNγ and TNF-α and provide immunity against intracellular pathogens. Th17 cells produce the cytokines IL-17/IL-17F, IL-22, IL-21 and TNF-α and primarily promote immunity against extracellular pathogens.
IL-17 is the prototype of Th17 cytokines and is the best studied of the Th17 cytokines. The receptor for IL-17 (IL-17R) is a Type I transmembrane protein and is ubiquitously expressed on various organs including lung, kidney and spleen11. Cells that express the receptor for IL-17 are leucocytes, epithelial cells, mesothial cells, vascular endothelial cells, keratinocytes and fibroblasts and respond to IL-17R-mediated signaling by production of granulocyte colony-stimulating factor (G-CSF), IL-6, IL-8 and mediate granulopoiesis, neutrophil recruitment and inflammatory responses (reviewed in12). IL-17 was initially identified as a key factor in the induction of inflammation and tissue destruction associated with animal models of autoimmune disease like Multiple Sclerosis, Collagen Induced Arthritis and experimental colitis (reviewed in13,14). Based on the efficacy of targeting IL-17 in pre-clinical models of these chronic inflammatory conditions, IL-17 is currently a promising therapeutic target15. Initial work implicating IL-17 to be protective against extracellular bacterial infections at the lung mucosal surface was through effective recruitment of neutrophils mediated by chemokine induction16-18. More recent advances in our understanding of the complexity of the Th17 cytokines suggests broader effects for these cytokines in protecting against a variety of pathogenic bacteria, fungi and viruses at different mucosal surfaces. In this review we have summarized recent progress in our understanding of the role of Th17 cells and effector cytokines and their function as a bridge between innate and adaptive immunity against infectious diseases at the mucosa.
Innate receptors and signaling pathways involved in induction of Th17 cells during infections
The differentiation of Th17 cells is determined by the exposure to TGF-β, IL-6, IL-21, while IL-23 further stabilizes the commitment of Th17 cells to this lineage (reviewed in19). These polarizing cytokines act on newly primed cells to induce the expression of the transcription factor RORγt and RORα which induces Th17 differentiation20,21. RORγτ also control the expression of IL-23 inducible receptors on newly primed T cells, further expanding their responsiveness to IL-23 to sustain the T lineage specific responses. The gp-130-Stat3 pathway is essential for expression of RORγτ and Th17 development22-24. Recently it has been shown that IL-21 acts downstream of these events to further amplify Th17 cell generation in an autocrine manner7. Additional co factors for the differentiation of Th17 are IL-1β and TNF-α25,26. Although in vitro studies have shown that IL-23 is redundant in the generation of Th17 responses27, in vivo studies in the mouse model 18,28,29 and in humans 30 have shown that IL-23 is critical for maintenance of Th17 responses in vivo. Since over-induction of Th17 cells can impact tissue damage due to induction of inflammatory pathways, the generation of Th17 cells is strictly regulated. For example, cytokines such as IL-2731,32, those belonging to Th1 (IFNγ) and Th2 (IL-4) 4,5 and IL-2 33 tightly regulate the induction of Th17 cells. Endogenous lipid mediators like prostaglandin E2 (PGE2) released under inflammatory conditions promotes Th17 cells differentiation 34 35 36-39 suggesting that external infection-induced mediators can also influence the decision between a Th1/Th2/Th17 and T regulatory cell responses.
Several of these Th17 polarizing cytokines such as IL-23, TGF-β, IL-6 and IL-1β are induced in dendritic cells activated by components of microbes. Several bacteria and its products such as Kleibsiella pneumoniae18,40, Mycobacterium tuberculosis26,41, Helicobacter pylori42, Francisella tularensis43Salmonella enterica44 and Bordetella pertusis45 induce these cytokines through TLR signaling. Further bacterial peptidoglycans can induce the generation of Th17 cells through nucleotide oligomerization domain 2 (NOD2) receptor signaling in dendritic cells25. Viruses such as Herpes simplex virus 46 and fungus and fungal components such as B–glucans26,47, Cryptococcus48, Candida albicans47 and Aspergillus fumigatus49 can all induce some or all of these polarizing cytokines from dendritic cells and play a role in differentiation of Th17 cells. These studies suggest that relative amounts of the polarizing cytokines induced by the pathogen may define the final outcome of differentiation of naïve T cells to Th1, Th2, Th17 or T regulatory cells during infection.
IL-17 as an effector molecule during the innate immune response following infection at mucosal sites
Although most of the recent focus has been on IL-17 produced by CD4αβ T cells, γδ T cells in some cases are more potent producers of IL-17 during the early immune response at mucosal sites following infections. In a pulmonary murine model of Mycobacterium tuberculosis50 and M.bovis BCG51 the major producers of IL-17 in T cells isolated from lungs of infected mice were γδ T cells. This finding was also highlighted in a recent study where γδ T cells were the major source of IL-17 in human tuberculosis patients52. In addition to γδ T cells, subsets of NKT cells 53 and NK cells expressing RORγτ+NKp46+ 54,55 can produce IL-17 and may impact the innate response to infections. Further, the detection of IL-17 mRNA in neutrophils 56 as well as production of IL-17 and IL-22 by Lymphoid-tissue inducer like cells(Lti)57 54,55,58-60 suggests that the role of IL-17 during the innate immune response is largely under-explored. It is likely that the production of IL-17 by innate cells at mucosal sites can serve as a mechanism to provide defense mechanism until the adaptive immune cells are recruited to control the infection.
Th17 cytokines as effector molecules during the immune response to bacterial infections at the mucosa
Early work in establishing a critical role for IL-17 in protective immunity against extracellular bacterial infections was using K.pneumoniae in a respiratory infection model17. IL-17R knockout (KO) mice displayed significant delays in neutrophil recruitment and had greater dissemination of K. pneumoniae. These studies provided early evidence that IL-17R signaling was important for neutrophil-mediated control of pulmonary K. pneumoniae infection by optimal induction of chemokines such as macrophage inflammatory protein-2(MIP-2) and granulocyte colony-stimulating factor (G-CSF) (Figure 2). IL-17 induces granulopoietic factors (G-CSF and stem cell factor) and CXC chemokines CXCL1, CXCL2, and CXCL-5 in fibroblasts and epithelial cells61-63. Furthermore, IL-17 mediated induction of G-CSF also results in differentiation of CD34+ progenitors into neutrophil progentiors61. Therefore, over expression of a recombinant adenovirus encoding IL-17 (AdIL-17) reversed the disease phenotype in mice challenged with K. pneumoniae by induction of chemokines, augmented polymorphonuclear leukocyte recruitment and enhanced bacterial clearance and survival16. Further that IL-17 can induce the expression of human beta defensin-264,65, S100 proteins and chemokines CXCL5, CXCL9, CCL3 65 and CCL-20 66 in lung epithelial cells, suggests that IL-17 can also enhance host defense against pathogens by induction of anti-microbials and chemokines for recruitment of immune cells. The cellular source of IL-17 was soon identified to be CD4 and CD8 T cells40, was dependent on IL-23 and mediated by TLR4 signaling18. More recently IL-22, another effector cytokine produced by Th17 cells has been implicated in induction of host cell antimicrobials and defensins in the respiratory epithelium and is required for early control of K. pneumoniae, in part by regulating the expression of the antimicrobial protein lipocalin-265. Further that IL-17 and IL-22 function synergistically in induction of antimicrobials such as human beta defensin-2 and S100 proteins66, suggests that the Th17 cytokines may have evolved to generate effective host defense mechanisms against extracellular pathogens at mucosal sites. Further studies also show that Th17 cells play protective roles against extracellular bacterial infections in the gut mucosa. Citrobacter rodentum, a naturally occurring mouse pathogen requires the generation of IL-23-dependent protective Th17 cells in the lamina propria for protection67,68. Also, IL-22 contributes to the early host defense against C.rodentium through the direct induction of the Reg family of antimicrobial proteins in colonic epithelial cells29. IL-17 signaling also appears to be host-protective in the oral mucosa, as IL-17R-deficient mice are highly susceptible to infection by the gram-negative anaerobic periodontal pathogen, Porphyromonas gingivalis, due to reduced neutrophil mobilization and recruitment69,70.
Figure 2. Role of Th17 cytokines in protective immunity at the mucosa.

Infection-induced IL-17 and IL-22 can be produced by several immune cells found in mucosal sites. A critical likely target of IL-17 and IL-22 is the mucosal epithelium where IL-17 augments G-CSF and CXC chemokine production resulting in recruitment of neutrophils that contribute to bacterial and fungal clearance at mucosal sites. IL-22 along with IL-17 also augments antimicrobial peptides and epithelial repair function important for control of extracellular fungal pathogens. In the setting of vaccine-induced immunity, Th17 cells can induce the production of ligands for CXCR3 and augment the recruitment of IFNγ producing Th1 cells to control intracellular pathogen growth.
These studies provide clear evidence that Th17 cytokines are critical for protection against a variety of extracellular bacteria and are perfectly poised to control infection either by recruitment of protective cells or induction of anti-microbials during the early immune responses at mucosal sites. However, the Th17 response in some cases can also be a double-edged sword and the balance between protection and pathology may define the outcome of the infection. For example, whooping cough caused by B.pertusis, a gram negative extracellular bacterial infection in the respiratory tract results in persistent cough as one of the hallmarks of the clinical disease. Accumulating evidence suggests that the B.pertusis infection may bias the host response towards the production of Th17 cytokines 35,45 by preferentially inhibiting IL-12 and inducing IL-2345. B.pertusis causes severe respiratory pathology including bronchiectasis while B.parapertusis causes less severe disease pathology71. Interestingly, B.pertusis lipooligosacharide induces potent production of IL-23, IL-1β and IL-6 from DCs and drives a more robust differentiation of naïve CD4 T cells to Th17 cells when compared to DCs activated with LPS from B.parapertusis45. The current emerging hypothesis is that the host bias towards Th17 results following B.pertusis infection results in inflammation and destruction of the airways leading to bronchiectasis and causes persistent cough. This hypothesis is further supported by another cause of bronchiectasis, cystic fibrosis which is highly associated with bronchiectasis due to chronic biofilm infection with P. aeruginosa and elevated IL-17 and IL-22 responses in draining lung lymph node cells65. These disease models may serve as an example for the role of IL-17 in mediating pathology while conferring protection against extracellular bacterial infections in the respiratory mucosa. Another fine example of how a protective Th17 response may culminate in a pathogenic inflammatory response is evident in H.pylori infections in the gut mucosa. H.pyroli is a gram negative bacteria that colonizes the gastric mucosa and induces an inflammatory infiltration of neutrophils associated with robust IL-17 production by CD4 and CD8 T cells72,73. These data suggests that IL-23/IL-17 pathway play a crucial role in defining the ongoing gastric inflammation in H pylori-infected patients.
While a clear protective role for Th17 cytokines in extracellular bacterial infections is emerging, Th17 effector cytokines appear to be dispensable for protection against most studied intracellular bacterial infections at the mucosal sites. The absence of the IL-23/IL-17 axis impacts the formation of granulomas 51 and inflammation following mycobacterial pulmonary infection41. However, IL-23KO mice and IL-17RKO mice are not more susceptible than wild type control mice to M.tuberculosis41,65 and M.bovis BCG pulmonary infections51. Interestingly, pulmonary but not intradermal route of infection with a intracellular bacteria F.tularensis LVS, induces Th17 cells and inhibits Th1 cell generation36. However, whether IL-17 is required for protection against F. tularensis respiratory infection is not known. These studies implicate that the host Th17 effector cytokines have evolved as protective immune mechanisms against extracellular bacteria and are dispensable for primary protection against most intracellular pathogens that require a Th1 pathway for protection.
In contrast, intracellular pathogens that may require both CD4 Th1 cells and neutrophils for protection at mucosal sites may be dependent on IL-23/IL-17 axis for pathogen control (Figure 1). For example, the induction of IL-17 and IL-17F production following acute Mycoplasma pneumonia pulmonary infection is IL-23-dependent and contributes to neutrophil recruitment and mediates protection against the infection74. Also, infection with Salmonella typhimurium, which can exist as an intracellular pathogen, results in induction of IL-17 and IL-22 in the ileal mucosa and the absence of IL-17R signaling results in increased translocation of the bacteria to the mesenteric lymph nodes, reduced induction of chemokines, anti-microbials and reduced neutrophilic recruitment to the ileal mucosa75. These studies show that IL-17 may be critical for the full induction of immune responses that lead to neutrophil influx and contribute to control of bacteria in some intracellular infections.
Using a macaque model of Simian Immunodeficiency Virus (SIV) to study HIV human disease and related complications arising due to bacterial coinfections such as S.typhimurium, it was found that SIV coinfection selectively inhibits Th17 responses elicited by S. typhimurium, probably due to depletion of CD4+ T cells in the ileal mucosa75. This results in an inability of SIV-infected macaques to mount normal mucosal inflammatory response to S. typhimurium infection and allows dissemination of bacteria to the mesenteric lymph node. This data may provides a basis for the observation that people with HIV are at a increased risk of developing bacteremia due to dissemination of bacteria resulting from reduced CD4 Th17 responses.
Th17 cytokines as effector molecules during the immune response to fungal infections at the mucosa
Inflammation is a critical component of the protective host response to fungal infections. However resolution of inflammation is essential for reducing the immunopathology resulting from the infection. Candida albicans infection models appear to reflect this dichotomy. C. albicans is a commensal organism of the oral cavity and gastrointestinal(GI) tract, but can become pathogenic in settings of immunodeficiency. The most commonly used experimental model of Candida infection represents the disseminated form of disease, which in humans occurs as a nosocomial infection with a 40% mortality rate. In mice, IL-17 receptor signaling is highly protective in this setting, acting through recruitment and expansion of neutrophils76. In the oral cavity, Candida causes thrush (oropharyngeal candidiasis, OPC), which occurs in > 90% of HIV+ individuals77. In a mouse model of OPC, Th17 cells and IL-17 receptor signalling but not Th1 cells and IFNγ are necessary for host protection78,79. New data show that Th17-based immunity appears to be mediated primarily through the macrophage mannose receptor80. Patients with Chronic mucocutaneous candidiasis (CMC) produced significantly lower amounts of IL-17 and IL-22 mRNA and protein in vitro following antigen stimulation when compared to healthy individuals, suggesting that Th17 cytokine production correlates with protection81. Even more compelling, patients with autosomal dominant hyper-IgE syndrome (HIES, Job's Syndrome) resulting from a mutation in the STAT-3 gene are extremely susceptible to bacterial infections such as S.aureus and mucocutaneous fungal infections caused by Candida species. In line with a critical role for STAT-3 in driving Th17 cellular responses, these patients do not generate C.albicans- and S.aureus- specific Th17 cellular responses24. Therefore, the mechanism underlying the susceptibility to recurrent fungal infections commonly seen in these patients is likely an inability to mount Th17 responses23,24,82. In contrast, a gastric model of C. albicans infection stimulates severe gut pathology, which is exacerbated by IL-23 and IL-1749. Finally, C. albicans also causes vaginal yeast infections. Interestingly, female HIV+ patients do not experience a high incidence of vaginal Candida infections, despite their high incidence of thrush77. It is believed that the vaginal epithelial cells play a far more critical role in this setting, but the role of IL-17 is not well defined. Therefore, route of entry and site of infection dictate the consequence of Th17-mediated immunity in fungal infections.
In respiratory tract models of fungal infections using Pneumocytis carnii83 and Aspergillus fumigatus84, induction of IL-23 and IL-17 following pathogen challenge is protective, since IL-23KO mice or neutralization of the IL-23/IL-17 axis resulted in impaired clearance of the pathogen. Although these studies suggest that the production of IL-23 and IL-17 is protective during fungal infections, it has been suggested that heightened IL-23 dependent Th17 responses against two major fungal pathogens C. albicans and A.fumigatus mediates neutrophilic inflammation and severe tissue pathology49. Further, phagocytes from patients with Chronic Granulomatous Disease (CGD) lack NADPH oxidase activity and hence the ability to generate reactive oxygen species, resulting in recurrent bacterial and fungal infections. In a mouse model of CGD, pulmonary aspergillosis infection is lethal due to heightened Th17 response and inflammatory lung pathology85. Although lL-17 neutralization was moderately beneficial, complete cure and reversal of the phenotype was only achieved with replacement therapy with a natural kynurenine85. In the context of CGD, the inflammatory pathology mediated by IL-17 is suggested to be pathogenic rather than protective. Therefore it appears that the early induction of IL-23 and IL-17 during fungal infections is protective. However, heightened Th17 responses that follow may result in the generation of pathological rather than protective outcomes during fungal infections.
Th17 cytokines as effector molecules during the immune response to viral infections at the mucosa
CD4 T cells can regulate the adaptive immune responses to viruses by providing help to CD8 and B cells and also by direct anti-viral activity. Documentation of the presence of viral specific CD4 Th17 cells in HIV infection86, cytomegalovirus (CMV) specific IL-17 producing cells in humans86, and in mice 87 suggests that Th17 cells may have a role to play in viral infections. Herpes simplex virus (HSV-1) infection of the cornea results in early induction of IL-2346 and IL-1788 and mice that lack IL-17R showed reduced early infiltration of neutrophils and corneal opacity following HSV infection88. On the contrary, IL-23KO mice showed a more severe disease phenotype to HSV infection, probably as a consequence of increased IL-12 responses and higher numbers of IFNγ producing cells46. Human rhinovirus (HRV) infections are associated with exacerbations of asthma and chronic obstructive pulmonary infiltration and IL-17 was shown to function synergistically with HRV to induce IL-8 from epithelial cells and may contribute to the recruitment of neutrophils, immature DCs and memory T cells to the lung contributing to severe inflammatory profiles seen during viral exacerbations of airway disease89. More recently, neutralization of IL-17 responses during a influenza challenge in mice resulted in increased weight loss and reduced survival of mice90. These studies suggest that the fine balance between protection and pathological manifestations of Th17 responses will define the outcome of viral infections at the mucosa and further research is required to clarify the Th17 cellular balance at the mucosa.
Role of IL-17 in vaccine-induced immunity and immunotherapy
Convincing data show a protective role for IL-17 in immunity to primary infections against extracellular pathogens and fungal infections. However, the role of IL-17 in memory immune responses to infections is less well studied and understood. IL-23 was initially reported to act on memory or activated T cells that express the IL-23 receptor and produce IL-17 91 and therefore it is likely that these cells may have a role to play in vaccine-induced immunity. Although IL-17 responses are induced during primary M.tuberculosis pulmonary infection, it is dispensable for protection against primary challenge with M.tuberculosis41. However, induction of IL-17 during a memory response to M.tuberculosis challenge correlates with protection. Following challenge with M. tuberculosis, protection is associated with the recruitment of protective Th1 cells and production of IFNγ which results in macrophage activation and mycobacterial killing. In this vaccine-induced protection model, Th17 cells upregulate CXCR3 ligating chemokines and accelerate the recruitment of Th1 cells that express the receptor CXCR3 28(Figure 1). Further, peripheral blood of mycobacteria-exposed healthy adults express M.tuberculosis specific memory Th17 cells producing IL-17 or the related cytokine IL-22 92 suggesting that the IL-23/IL-17 axis contribute to protective immunity during tuberculosis. In support for a protective role for IL-23 and IL-17 in immunotherapy against M.tuberculosis, over expression of an recombinant adenovirus expressing IL-23 when delivered prior to M.tuberculosis pulmonary infection results in increased IFN-γ and IL-17 responses in the lung and mediates improved protection compared to control adenovirus treated mice93. In the absence of effective current vaccines against tuberculosis, the protective role for Th17 cells in generation of memory immunity will play a critical role in design of new vaccines against tuberculosis. Further, vaccine-induced protection against another pulmonary pathogen B.pertussis is mediated by IL-17. Th17 cells are induced by vaccination with whole cell pertussis vaccines (Pw) and neutralization of IL-17 reduces protection following a pulmonary challenge with B. pertussis. Contrary to the M.tuberculosis model, in this model IL-17 was suggested to have a protective role via direct activation of macrophages and B. pertussis killing94. In a CD4 T cell dependent, antibody-independent model of vaccine-induced protection following S. pneumoniae challenge, treatment with antiserum to IL-17 resulted in reduced immunity to pneumococcal colonization compared to the control serum treated mice95. In viral vaccine-induced responses, mucosal immunization of mice with rotavirus V6 protein reduces rotavirus fecal shedding and was associated with the presence of memory Th1 and Th17 cells in the intestine96,97. Although it is thought that the Th1 response is the major protective mechanism in this model, it is suggested that the Th17 response may play an indirect protective role. These data suggest a protective role for IL-17 in vaccine-induced immune responses against bacterial, fungal and viral infections. However further studies are required to dissect the direct and indirect pathways and molecular mechanisms involved in protection.
Mucosal immunity versus tissue pathology and inflammation
A protective versus pathogenic role for IL-17 in settings of chronic infections that occur in diseases such as cystic fibrosis (CF), recurrent fungal infections or CGD is currently an area of extensive research. IL-17 induction associated with these diseases may often be pathologic rather than protective, as a result of severe defects in downstream effector functions of responding cells. For example, bacterial infections such as Klebsiella and Pseudomonas that are often associated with CF disease, induce IL-17-dependent neutrophil recruitment and induction of anti-microbial proteins65,98, and under normal conditions this IL-17 dependent response is protective. However in the context of chronic biofilm infection in CF patients, the resulting induction of IL-17 and neutrophil recruitment may not result in bacterial clearance but in inflammation, possibly due to the defective mucociliary clearance mechanisms in CF respiratory epithelial cells98. Similarly, in a mouse model of CGD, A.fumigatus induces IL-17 and recruits high numbers of neutrophils85. However, the lack of NAPDPH oxidase activity and the inability to generate reactive oxygen species by the neutrophils renders this downstream effector IL-17 ineffective, resulting in acute inflammatory responses and inability to clear the pathogen. Likewise in the oral cavity, chronic periodontal infection has been associated with elevated IL-17 levels70. Taken together these data suggest that Th17 mucosal immunity requires functional downstream effector cells, either epithelial cells or neutrophils for effective clearance of the pathogen.
Summary and outlook
Evidence suggests that Th17 cells have evolved to mediate protective immunity against a variety of pathogens at different mucosal sites. Moreover the lack of Th17 responses appears to explain in part some of the immunodeficiency observed in both AIDS and in patients with Job's syndrome, particularly in terms of mucosal infection with C. albicans and some cases of bacterial pneumonia. These data may lead to new avenues of immunotherapy to treat or prevent these infections in these patient groups. Also, the emerging evidence that Th17 cells are crucial players in generation of vaccine-induced protective responses against a variety of pathogens suggests that the incorporation of this knowledge into the design of current and future vaccines against infectious diseases should be an active area of research. It is also becoming apparent that the fine balance between protection and pathological manifestations of Th17 responses will define the outcome of infections and further research is required to define the Th17 cellular balance at mucosal sites.
Table 1. Function of Th17 cells in infectious disease models at the mucosa.
| Organism | Mucosal surface | Role of Th17 cells | Functional relevance |
|---|---|---|---|
| Klebsiella pneumonia | Respiratory | Protective | Induction of chemokines, anti-microbials and neutrophil recruitment 16,17,18,65. |
| Citrobacter rodentum | Gut | Protective | Induction of chemokines and antimicrobials 29,67,68. |
| Porphyromonas gingivalis | Oral cavity | Protective | Neutrophil recruitment 69,70. |
| Bordetella pertusis | Respiratory | Exacerbation | Inflammation and pathology35,45. |
| Helicobacter pylori | Gut | Exacerbation | Inflammatory infiltration of neutrophils 72,73. |
| Mycobacterium tuberculosis Mycobacterium bovis BCG | Respiratory | No effect on protection | Impacts granuloma formation 41,51,65. |
| Mycoplasma pneumonia | Respiratory | Protective | Recruitment of neutrophils 74. |
| Salmonella typhimurium | Gut | No effect on bacterial burden at the site of primary infection | Impacts epithelial barrier function, recruitment of neutrophils and increased bacterial translocation to lymph nodes 75. |
| Candida albicans | Oral cavity | Protective | Induction of chemokines and anti-microbials and neutrophil recruitment 78,79. |
| Candida albicans | Gut | Exacerbation | Increased neutrophilic recruitment 49. |
| Pneumocystis carinii | Respiratory | Protective | Reduced chemokine induction and recruitment of effector CD4 T cells 83. |
| Aspergillus fumigatus | Respiratory | Protective/Exacerbation | Reduced chemokine induction and neutrophil recruitment 84. IL-17 exacerbates disease in NADPH oxidase deficient mice85. |
| Herpes Virus | Respiratory | Exacerbation | Recruitment of neutrophils and pathology 46,88. |
| Human Rhinovirus | Respiratory | Exacerbation | Recruitment of neutrophils and effector T cells and pathology89. |
Acknowledgments
Funding for this project was provided, in part, by the Pennsylvania Department of Health, Tobacco Formula Funding and the National Institutes of Health to JKK, Pennsylvania Department of Health, Tobacco Formula Funding, R00AI075106 from the National Institute Of Allergy And Infectious Diseases and Children's Hospital of Pittsburgh to SAK. SLG was supported by AR054389 and DE018822.
Abbreviations
- IL
Interleukin
- IFN-γ
Interferon gamma
- MIP-2
macrophage inflammatory protein-2
- G-CSF
Granulocyte colony stimulating factor
- CXCR-3
chemokine CXC motif receptor 3
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65:5052–6. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–33. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 7.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung Y, et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–7. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- 10.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–6. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663–8. doi: 10.1016/j.coi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–5. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Ye P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–40. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 17.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel KI, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19:400–8. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008 doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Gerosa F, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–61. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 30.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 31.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 32.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 33.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Khayrullina T, Yen JH, Jing H, Ganea D. In Vitro Differentiation of Dendritic Cells in the Presence of Prostaglandin E2 Alters the IL-12/IL-23 Balance and Promotes Differentiation of Th17 Cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siciliano NA, Skinner JA, Yuk MH. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J Immunol. 2006;177:7131–8. doi: 10.4049/jimmunol.177.10.7131. [DOI] [PubMed] [Google Scholar]
- 36.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76:2651–9. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chizzolini C, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–48. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–12. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 40.Happel KI, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–6. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khader SA, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–95. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell P, et al. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun. 2007;75:810–9. doi: 10.1128/IAI.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butchar JP, et al. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–54. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 44.Siegemund S, et al. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology. 2007;212:739–50. doi: 10.1016/j.imbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Fedele G, et al. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis Differently Modulate Human Dendritic Cell Functions Resulting in Divergent Prevalence of Th17-Polarized Responses. J Immunol. 2008;181:208–16. doi: 10.4049/jimmunol.181.1.208. [DOI] [PubMed] [Google Scholar]
- 46.Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect. 2008;10:302–12. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 48.Siegemund S, Alber G. Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol. 2008;52:417–27. doi: 10.1111/j.1574-695X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 49.Zelante T, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 50.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 51.Umemura M, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 52.Peng MY, et al. Interleukin 17-Producing gammadelta T Cells Increased in Patients with Active Pulmonary Tuberculosis. Cell Mol Immunol. 2008;5:203–8. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–12. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 57.Takatori H, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 59.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 60.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–53. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 63.Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–6. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kao CY, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 65.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang F, et al. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–13. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 67.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 68.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–19. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The IL-17 receptor plays a gender-dependent role in host protection against P. gingivalis-induced periodontal bone loss. Infect Immun. 2008 doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–28. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr Opin Pharmacol. 2007;7:272–8. doi: 10.1016/j.coph.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Luzza F, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–7. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 73.Caruso R, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur J Immunol. 2008;38:470–8. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- 74.Wu Q, et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raffatellu M, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–31. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 77.Dongari-Bagtzoglou A, Fidel PL., Jr The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–77. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- 78.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farah CS, Hu Y, Riminton S, Ashman RB. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol. 2006;21:252–5. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 80.van de Veerdonk FL, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–40. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Eyerich K, et al. Patients with Chronic Mucocutaneous Candidiasis Exhibit Reduced Production of Th17-Associated Cytokines IL-17 and IL-22. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 82.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008 doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–61. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werner JL, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romani L, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 86.Yue FY, et al. Virus-specific interleukin-17-producing CD4+ T cells are detectable in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:6767–71. doi: 10.1128/JVI.02550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arens R, et al. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J Immunol. 2008;180:6472–6. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–8. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 89.Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2007;293:L505–15. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- 90.Hamada H, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–81. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 92.Scriba TJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Happel KI, et al. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–8. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–9. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 95.Malley R, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smiley KL, et al. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) J Virol. 2007;81:3740–8. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McNeal MM, et al. IFN-gamma is the only anti-rotavirus cytokine found after in vitro stimulation of memory CD4+ T cells from mice immunized with a chimeric VP6 protein. Viral Immunol. 2007;20:571–84. doi: 10.1089/vim.2007.0055. [DOI] [PubMed] [Google Scholar]
- 98.Dubin PJ, McAllister F, Kolls JK. Is cystic fibrosis a TH17 disease? Inflamm Res. 2007;56:221–7. doi: 10.1007/s00011-007-6187-2. [DOI] [PubMed] [Google Scholar]


